Abstract
Background
Disc degeneration is characterized partly by the degradation in the extracellular matrix (ECM) and excess apoptosis of nucleus pulposus (NP) cells. NLRX1 (nucleotide-binding, leucine-rich repeat containing X1) is different from the other nucleotide-binding-domain and leucine-rich-repeat proteins and mainly located to the mitochondrial. It negatively regulates NF-κB (nuclear factor kappa B) and apoptosis inhibition. However, how NLRX1 is regulated and exerts effects in disc degeneration is unclear. Thus, the study aimed to analyze the effects of NLRX1 on NP cells.
Material/Methods
NLRX1 expression was detected in interleukin (IL)-1β-induced NP cells by western blot and quantitative real-time polymerase chain reaction (qRT-PCR). Then, NLRX1 was overexpressed in IL-1β-induced NP cells to detect apoptosis-related proteins and the extracellular matrix (ECM) by western blot, along with the detection of apoptosis levels using flow cytometry. StarBase predicted miR-423-5p target 3′UTR of NLRX1. Dual luciferase reporter assay showed that miR-423-5p could bind to the 3′UTR of NLRX1. Besides, miR-423-5p significantly affected NLRX1 levels detected by qRT-qPCR.
Results
The miR-423-5p overexpression markedly, and negatively regulated the protective effects of NLRX1 on IL-1β induced NP cells. Thus, our results suggested that miR-423-5p mediated the regulation of NLRX1 to affect apoptosis and ECM levels in IL-1β induced NP cells.
Conclusions
miR-423-5p and NLRX1 could be potential therapeutic targets for patients with disc degeneration.
MeSH Keywords: Apoptosis, Extracellular Matrix, MicroRNAs
Background
Lower back pain is the most common condition seen in orthopedics departments, and it causes mental pain and tremendous economic burden for patients [1]. It has been reported that lower back pain, in about 40% of patients, is due to disc degeneration that is the pathological basis of a series of degenerative spinal diseases [2]. Interleukin (IL)-1β and tumor necrosis factor (TNF)-α are considered important factors leading to disc degeneration [3]. These factors promoted the degradation of extracellular matrix (ECM) and further aggravated disc degeneration [4]. However, IL-1 knockdown was also shown to aggravate disc degeneration instead of delaying ageing [5].
Disc degeneration is characterized partly by the degradation in ECM and excess apoptosis of nucleus pulposus (NP) cells [6]. The main components in ECM are type II collagen and proteoglycan. In healthy discs, the synthesis and decomposition of ECM is kept in balance. Whereas in disc degeneration, cells synthesize increased levels of matrix decomposing enzymes, such as MMPs (matrix metalloproteinases) and ADAMTSs (a disintegrin and metalloproteinase with thrombospondin motifs), while anti-catabolism matrix protease inhibitors presented no balanced increase. Thus, disc degeneration occurs when the decomposition is the priority process. The collagenase MMP-13 mainly acts on fibrous collagen. And matrix lysin protease MMP-3 can hydrolyze a variety of substrates including proteoglycan, gelatin, collagen, and so on. Pro-inflammatory factors can induce the production of MMPs and ADAMTSs to contribute to disc degeneration [7].
NLRX1 (nucleotide-binding, leucine-rich repeat containing X1) is a member of the pattern recognition receptor NLR (nucleotide-binding oligomerization domain [NOD]-like receptor) family, which broadly exists in various animals. NLRX1 plays an important role in inflammatory disease and can trigger related signal pathways to regulate immune inflammatory responses and apoptosis pathways through identifying endogenous and exogenous danger signals [8]. For instance, NLRX1 has been shown to inhibit the activation of NF-κB to reduce apoptosis and inflammation in LPS-induced chondrocytes [9]. In addition, NLRX1 loss was found to increase apoptosis and oxidative stress in metabolic and innate immunity causing tissue impairment and was also found to be a negative regulator of nuclear factor kappa B (NF-κB) signals [10,11]. Thus, NLRX1 may produce effects partly through inflammation and apoptosis pathways. NLRX1 has also been shown to regulate apoptosis via sterile alpha and Toll/interleukin-1 receptor motif containing protein 1 [12]. Moreover, a recent study showed that NLRX1 mediates mitophagy through its LC3-interacting region motif binding to LC3 in cells induced by bacterial pathogens [13].
MicroRNA-423-5p (miR-423-5p) presents differential expressions in some disease types, such as type 2 diabetes, cancer, and brain metastasis, and regulates cell viability, senescence, and Treg-mediated immune escape [14–19]. In addition, miR-423-5p overexpression promotes inflammation and apoptosis of cardiomyocytes exposed to hypoxia [20]. However, it is still unclear how it may work in disc degeneration diseases. Thus, this study aimed to investigate the roles of miR-423-5p and NLRX1 in human NP cells and to investigate how NLRX1 is regulated.
Material and Methods
Cells
Human NP cells (ScienCell, USA) was cultured at specific medium containing 10% fetal bovine serum (FBS), 1% NP cell growth supplement and 1% penicillin/streptomycin in 5% CO2 at 37°C (ScienCell, USA). When cells adhered to the wall up to 80%, IL-1β (Reprotech, USA) was added into the medium to induce degeneration of NP cells for 24 hours, the concentration of which was 10 ng/mL as described in previous studies [7,21,22].
Western blot
Cell lysate was prepared using radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime Biotechnology, Nanjing, China), and cells were centrifuged at 12 000 rpm for 5 minutes to collect supernatant. Protein concentration was measured with bicinchoninic acid (BCA) assay (Beyotime Biotechnology, Nanjing, China). The proteins (50 μg/each sample) were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membrane. Afterwards, the bands were blocked using 5% skim milk and then washed by TBST (tris-buffered saline and Tween 20) for 3 times. The appropriate primary antibody (Bcl-2, Bax, cleaved caspase-3, cleaved caspase-9, MMPS at 1: 1000 [Abcam, USA]; type II collagen at 1: 1000, aggrecan at 1: 1000, [Herford, Germany], ADAMTS-4 at 1: 500, ADAMTS-5 at 1: 1000 [Sigma-Aldrich, USA], GADPH at 1: 1000 [Santa Cruz, CA, USA]) was added and incubated with the bands at 4°C overnight. The second antibody (1: 10 000, Cell Signaling Technology) was used to bind to the primary antibody after TBST washing the bands for 3 times. Electrochemiluminescence (ECL) was used to develop color (Millipore, USA).
Plasmid transfection
The NLRX1 overexpression plasmids (overexpression-NLRX1) and its negative control (NLRX1-NC) were transfected into cells using Lipofectamine 2000 separately (RiboBio, Guangzhou, China). miR-423-5p mimic was used to mimic its high expression levels in cells. Simultaneously, its negative control (miR-NC), eliminating the influence of the sequence itself on experimental results, was also used. miR-423-5p inhibitor and its negative control (miR-423-5p-inhibitor-NC) were transfected into the cells separately, the final concentration of which was 50 nM. After being transfected by plasmids or miRNAs, the cells were cultured for 24 hours.
Quantitative real-time polymerase chain reaction (qRT-PCR)
After cell was treated by experimental factors, the total RNA was extracted by TRIzol kit (Invitrogen, America). RNA was reversed transcripted into cDNA. miRNA levels were quantified by Hairpin-it™ miRNAs qPCR Quantitation kits (GenePharma, China) and RNU6B was used as a reference. Simultaneously, mRNA levels were detected by the SYBR green PCR Master Mix according to the manufacturer’s instructions (TaKaRa, Japan). GADPH was used as a reference. The relative RNA levels were calculated using 2−ΔΔCt method.
Dual luciferase reporter
The reporter gene plasmid expressing firefly luciferase was used to construct 3′UTR wild-type and luciferase reporter plasmids containing NLRX1 mutant sites (5′ GUAUGAUGGCUUGGUACGGGGAGG 3′) separately (NLRX1-MUT, NLRX1-WT). Luciferase reporter plasmids and miRNA were co-transfected into the cells through different grouping ways, which were cultured for 48 hours at 37°C at 5% CO2. The fluorescence intensity was measured by microplate reader.
Flow cytometry
NP cells were treated with 0.25% trypsin containing no EDTA. Then, the cells were centrifuged at 2000 rpm for 5 minutes and were washed using phosphate-buffered saline (PBS) for 2 times. The cells (1–5×105) were resuspended. The supernatant was discarded by centrifugation for 5 minutes. Annexin V was added to suspended cells. Afterwards, 5 μL Annexin V-FITC was used to bind to phosphatidylserine to detect early apoptotic cells (Beyotime, Shanghai, China). After that, propidium iodide (PI) solution (10 μL) was used to stain dead cells. Flow cytometry (Becton Dickinson, USA) was used to detect cell apoptosis rate.
Statistical analysis
The data were analyzed by Prism software and shown as mean±standard deviation (SD). Statistical method analyzing the effects of treatment factors used one-way ANOVA, accompanied by Dunnett’s t-test. P<0.05 was considered statistically significant.
Results
NLRX1 overexpression decreased cell apoptosis and ECM degradation in IL-1β induced NP cells
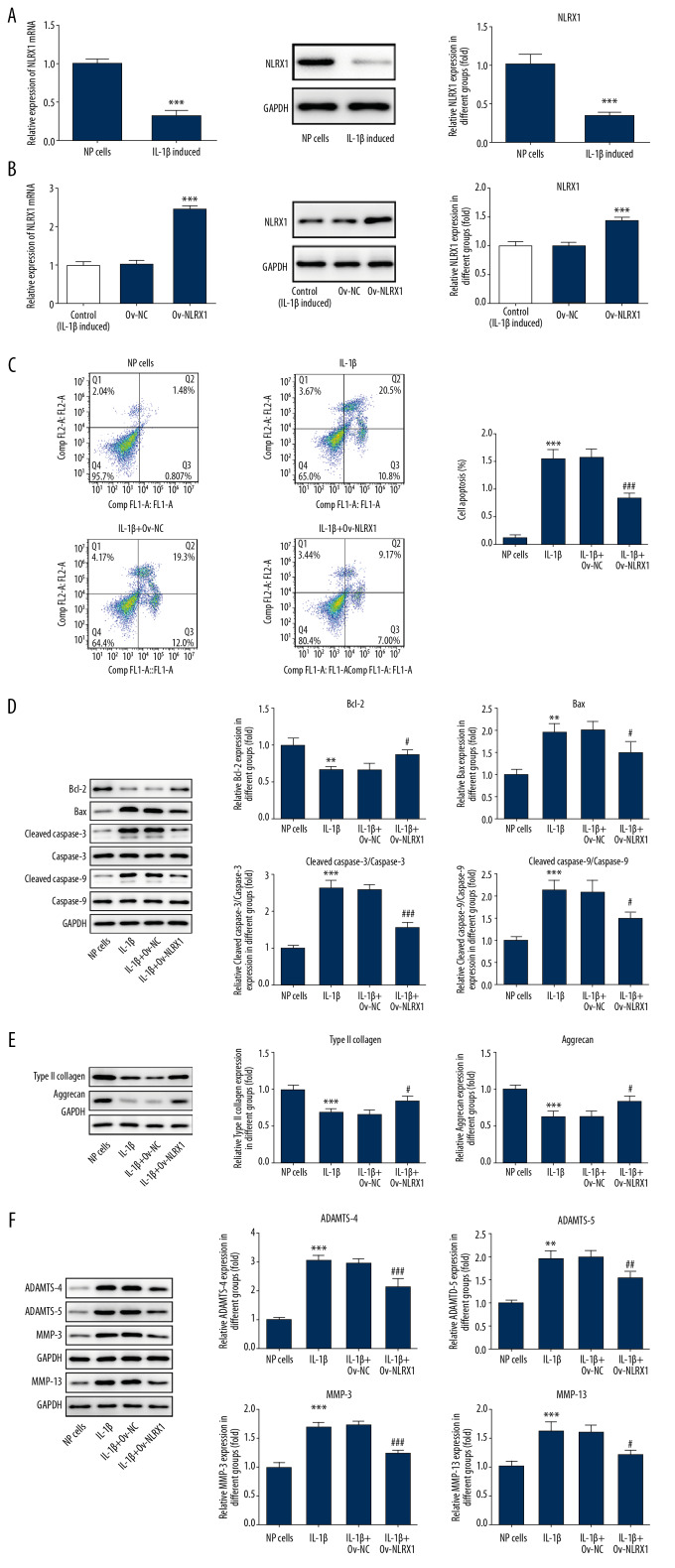
IL-1β was used to establish a disc degeneration cell model. We found that NLRX1 presented decreased levels in IL-1β induced NP cells (Figure 1A). Plasmids overexpressing NLRX1 significantly increased NLRX1 mRNA levels and expression of NLRX1 (Figure 1B). Moreover, apoptosis levels were notably reduced by flow cytometry after transfection (Figure 1C). Furthermore, anti-apoptosis protein Bcl-2 and pro-apoptosis proteins, Bax, cleaved caspase-3, and cleaved caspase-9 levels showed significant changes (Figure 1D). The results suggested that increased NLRX1 inhibited cells apoptosis. Simultaneously, ECM (type II collagen and aggrecan) was increased and matrix decomposing enzymes (MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5) were decreased (Figure 1E, 1F).
Figure 1.
(A) NLRX1 expression was detected in IL-β induced NP cells by qRT-PCR and WB. *** P<0.001 versus NP cells. (B) NLRX1 expression was significantly increased by transfecting plasmids overexpressing NLRX1. ** P<0.01; versus Ov-NC. (C) Flow cytometry was used to detect the apoptosis levels of NP cells. (D) Apoptosis-related proteins were measured by WB. (E, F) Related type II EMC (collagen and aggrecan) and matrix decomposing enzymes (MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5) were detected by WB. IL-1β+Ov-NLRX1: the NP cells were treated with IL-1β and plasmids overexpressing NLRX1. IL-1β+Ov-NC: the NP cells were treated with IL-1β and empty plasmids. ** P<0.01 or *** P<0.001; versus NP cells. ### P<0.001, ## P<0.01 or # P<0.05; versus IL-1β+Ov-NC. Data shown as mean±SD. NLRX1 – nucleotide-binding, leucine-rich repeat containing X1; NP – nucleus pulposus; qRT-PCR – quantitative real-time polymerase chain reaction; WB – western blot; ECM – extracellular matrix; MMP – matrix metalloproteinase; ADAMTS – a disintegrin and metalloproteinase with thrombospondin motifs; IL – interleukin; SD – standard deviation.
miR-423-5p binds to NLRX1 3′UTR
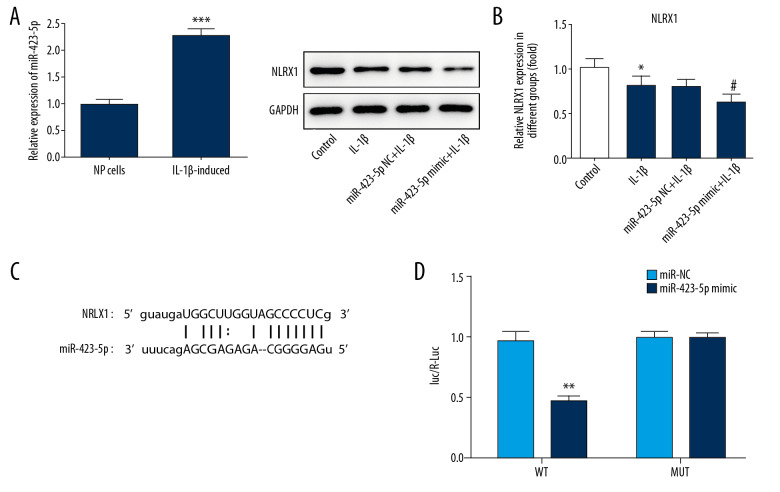
The result showed that IL-1β induced the upregulation of miR-423-5p in NP cells (Figure 2A). In addition, we found that NLRX1 levels are significantly decreased by miR-423-5p mimic treatment (Figure 2B). Then, StarBase database was used to estimate whether there was a targeted connection between miR-423-5p and NLRX1. Bioinformatics predicted the targeted relationship of miR-423-5p and NLRX1 (Figure 2C). Besides, luciferase activities were significantly decreased when plasmids that contains wild type 3′UTR of NLRX1 and miR-423-5p mimic were co-transfected. This suggests that miR-423-5p could bind to NLRX1 mRNA (Figure 2D).
Figure 2.
(A) miR-423-5p levels were detected by qRT-PCR in IL-β induced NP cells. *** P<0.001; versus NP cells. (B) miR-423-5p mimic significantly affected NLRX1 expression. (C) The binding sites of miR-423-5p and NLRX1 were predicted by StarBase. (D) luciferase reporter assay analyzed whether miR-423-5p bound to the 3′UTR of NLRX1. Data shown as mean±SD. qRT-PCR – quantitative real-time polymerase chain reaction; IL – interleukin; NP – nucleus pulposus; NLRX1 – nucleotide-binding, leucine-rich repeat containing X1; SD – standard deviation.
miR-423-5p overexpression reversed the inhibitory effects of NLRX1 on apoptosis in IL-1β induced NP cells
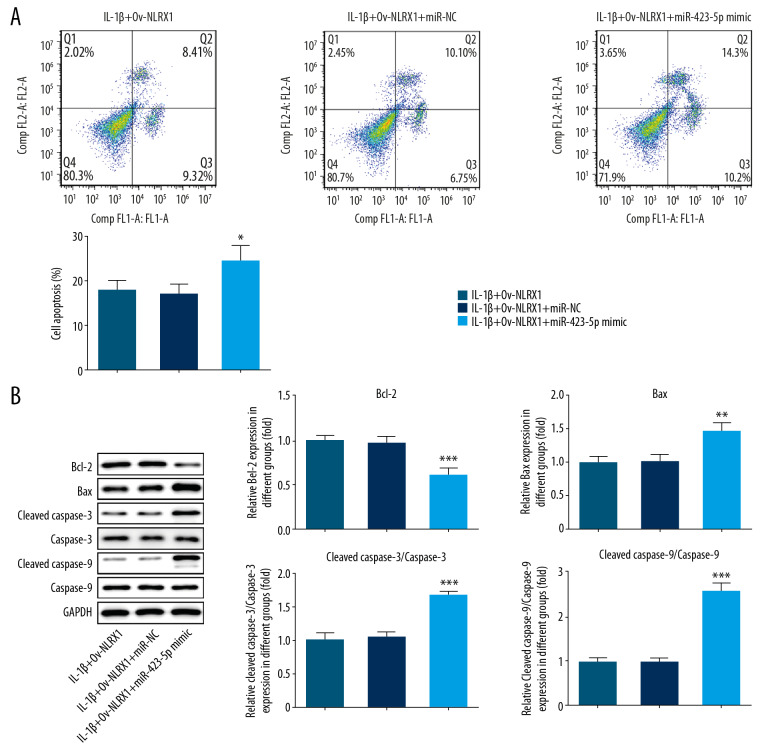
After NLRX1 and miR-423-5p were all overexpressed in IL-1β induced NP cells, the apoptosis levels were significantly reduced compared to overexpressing NLRX1 alone. These results implied that miR-423-5p overexpression decreased the inhibitory effects of NLRX1 on cell apoptosis, which was increased by IL-1β (Figure 3A, 3B).
Figure 3.
(A, B) miR-423-5p regulated the influence NLRX1 on cell apoptosis in IL-1β induced NP cells. * P<0.05, ** P<0.01 or *** P<0.001; versus IL-1β+Ov-NLRX1+miR-NC. Data shown as mean±SD. NLRX1 – nucleotide-binding, leucine-rich repeat containing X1; IL – interleukin; NP – nucleus pulposus; SD – standard deviation.
ECM was markedly reduced by miR-423-5p overexpression
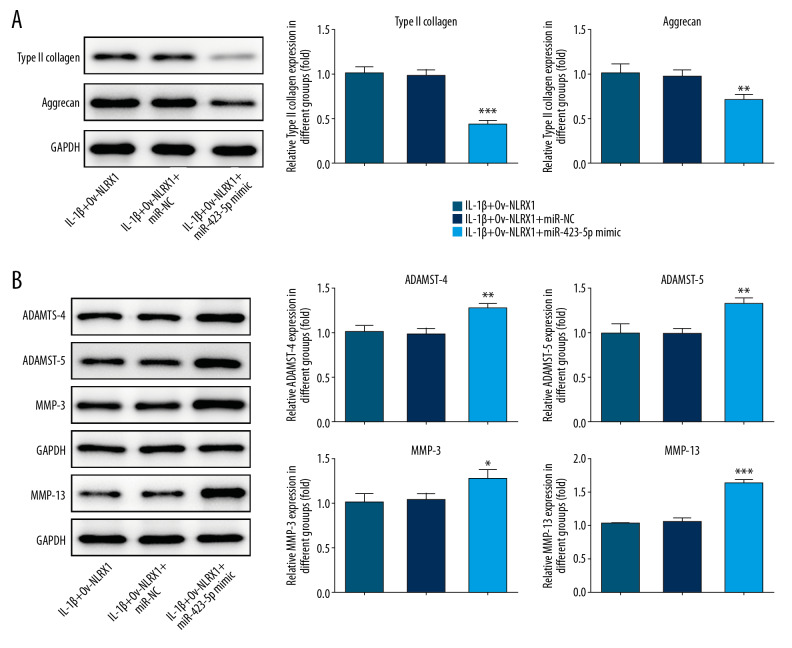
When overexpressing miR-423-5p, we found that ECM was not only reduced, but the matrix degradation enzymes were increased. These results indicated that miR-423-5p decreased the effects of NLRX1 on ECM (Figure 4A, 4B).
Figure 4.
(A, B) NLRX1 significantly increased ECM and reduced the matrix degradation enzyme levels. * P<0.05, ** P<0.01 or *** P<0.001; versus IL-1β+Ov-NLRX1+miR-NC. Data shown as mean±SD. NLRX1 – nucleotide-binding, leucine-rich repeat containing X1; ECM – extracellular matrix; IL – interleukin; NP – nucleus pulposus; SD – standard deviation.
Conclusions
Apoptosis of NP cells and degradation of ECM could be significantly inhibited by NLRX1 overexpression. However, miR-423-5p binding to 3′UTR of NLRX1 mRNA reduced its translation. Thus, in our study, miR-423-5p significantly decreased apoptosis and degradation of ECM in IL-1β induced NP cells via NLRX1. IL-1β significantly increased the activation of mitochondria-mediated caspase-9 and apoptotic executor caspase-3, anti-apoptosis Bcl-2 levels and reduced pro-apoptotic Bax levels. This study showed that miR-423-5p was involved in regulating mitochondria-mediated apoptosis pathways [23]. Furthermore, disc degeneration could be alleviated by suppressing the apoptosis of intervertebral disc cells. However, the abnormal apoptosis of NP cells is the main cause of disc degeneration [24,25]. Thus, NLRX1 and miR-423-5p could be potential targets for treating intervertebral disc degeneration.
IL-1β and its receptor antagonist have been implicated in accelerating disc degeneration and increasing apoptosis [6]. NLRX1 has been shown to be involved in regulating innate immune response and apoptosis, which interact with NF-κB, TBK1, and TRAF6 in mitochondria [26]. In our study, NLRX1 exerted significantly protective effects in IL-1β induced NP cells through decreasing apoptosis and MMPs (MMP-3 and MMP-9) and ADAMTSs (ADAMTS-4 and ADAMTS-5). Another study indicated that NLRX1 reduces apoptosis through suppressing the mitochondrial antiviral signaling molecule/he RIG-I-like helicase [27]. In addition, the cause of NLRX1 depletion leading to apoptosis have been shown to be mitochondrial impairment and excess oxidative stress [10]. However, how NLRX1 affects apoptosis in IL-1β induced NP cells needs further study.
In cells exposed to chronic inflammation, pro-inflammatory factors have been shown to markedly increase, along with decreased NLRX1 [28]. This suggests that there might be certain connections between IL-1β and NLRX1. In our study, IL-1β decreased NLRX1 expression in NP cells. Simultaneously, miR-423-5p was increased. Thus, IL-1β increases miR-423-5p levels, decreases NLRX1 expression, and then contributes to disc degeneration. Studies have shown that miRNA plays a vital role in regulating related signal pathways in disc degeneration [29,30]. Moreover, nanoparticle delivery of related miRNA inhibitors in intervertebral disc degeneration mice was found to notably increase ECM, reduce NP cell apoptosis, and improve disc degeneration [31]. Thus, miR-423-5p could be a potent therapy target in improving disc degeneration of patients. In our study, ADAMTS4 and ADAMTS5 were significantly increased in NP cells. A previous study showed that the level of ADAMTS4 positive cells were not consistent with level of its inhibitor. ADAMTS4 inhibition might be effective in ameliorating disc degeneration [32].
Thus, this study showed that NLRX1 could be considered the therapy target for suppressing ADAMTS.
Footnotes
Source of support: Departmental sources
References
- 1.Joud A, Petersson IF, Englund M. Low back pain: Epidemiology of consultations. Arthritis Care Res (Hoboken) 2012;64(7):1084–88. doi: 10.1002/acr.21642. [DOI] [PubMed] [Google Scholar]
- 2.Cimmino MA, Ferrone C, Cutolo M. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25(2):173–83. doi: 10.1016/j.berh.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Markova D, Anderson DG, et al. TNF-alpha and IL-1beta promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286(46):39738–49. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponnappan RK, Markova DZ, Antonio PJ, et al. An organ culture system to model early degenerative changes of the intervertebral disc. Arthritis Res Ther. 2011;13(5):R171. doi: 10.1186/ar3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorth DJ, Shapiro IM, Risbud MV. A new understanding of the role of IL-1 in Age-related intervertebral disc degeneration in a murine model. J Bone Miner Res. 2019;34(8):1531–42. doi: 10.1002/jbmr.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, Liao Z, Wang K, et al. Targeting the IL-1beta/IL-1Ra pathways for the aggregation of human islet amyloid polypeptide in an ex vivo organ culture system of the intervertebral disc. Exp Mol Med. 2019;51(9):110. doi: 10.1038/s12276-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S, Li L, Zhu L, et al. Aucubin inhibits IL-1beta- or TNF-alpha-induced extracellular matrix degradation in nucleus pulposus cell through blocking the miR-140-5p/CREB1 axis. J Cell Physiol. 2019;234(8):13639–48. doi: 10.1002/jcp.28044. [DOI] [PubMed] [Google Scholar]
- 8.Nagai-Singer MA, Morrison HA, Allen IC. NLRX1 Is a multifaceted and enigmatic regulator of immune system function. Front Immunol. 2019;10:2419. doi: 10.3389/fimmu.2019.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma D, Zhao Y, She J, et al. NLRX1 alleviates lipopolysaccharide-induced apoptosis and inflammation in chondrocytes by suppressing the activation of NF-kappaB signaling. Int Immunopharmacol. 2019;71:7–13. doi: 10.1016/j.intimp.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Stokman G, Kors L, Bakker PJ, et al. NLRX1 dampens oxidative stress and apoptosis in tissue injury via control of mitochondrial activity. J Exp Med. 2017;214(8):2405–20. doi: 10.1084/jem.20161031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coutermarsh-Ott S, Simmons A, Capria V, et al. NLRX1 suppresses tumorigenesis and attenuates histiocytic sarcoma through the negative regulation of NF-kappaB signaling. Oncotarget. 2016;7(22):33096–110. doi: 10.18632/oncotarget.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Killackey SA, Rahman MA, Soares F, et al. The mitochondrial Nod-like receptor NLRX1 modifies apoptosis through SARM1. Mol Cell Biochem. 2019;453(1–2):187–96. doi: 10.1007/s11010-018-3444-3. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Yao Y, Qiu X, et al. Listeria hijacks host mitophagy through a novel mitophagy receptor to evade killing. Nat Immunol. 2019;20(4):433–46. doi: 10.1038/s41590-019-0324-2. [DOI] [PubMed] [Google Scholar]
- 14.Gottmann P, Ouni M, Saussenthaler S, et al. A computational biology approach of a genome-wide screen connected miRNAs to obesity and type 2 diabetes. Mol Metab. 2018;11:145–59. doi: 10.1016/j.molmet.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Z, Zhao H, Feng X, et al. Long Non-coding RNA FENDRR Acts as a miR-423-5p sponge to suppress the Treg-mediated immune escape of hepatocellular carcinoma cells. Mol Ther Nucleic Acids. 2019;17:516–29. doi: 10.1016/j.omtn.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du L, Jiang X, Duan W, et al. Cell-free microRNA expression signatures in urine serve as novel noninvasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Oncotarget. 2017;8(25):40832–42. doi: 10.18632/oncotarget.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang C, Liu Z, Chang X, et al. NR2F1-AS1 regulated miR-423-5p/SOX12 to promote proliferation and invasion of papillary thyroid carcinoma. J Cell Biochem. 2020;121(2):2009–18. doi: 10.1002/jcb.29435. [DOI] [PubMed] [Google Scholar]
- 18.Sun G, Ding X, Bi N, et al. MiR-423-5p in brain metastasis: Potential role in diagnostics and molecular biology. Cell Death Dis. 2018;9(10):936. doi: 10.1038/s41419-018-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu B, Ding GY, Fu PY, et al. NOD-like receptor X1 functions as a tumor suppressor by inhibiting epithelial-mesenchymal transition and inducing aging in hepatocellular carcinoma cells. J Hematol Oncol. 2018;11(1):28. doi: 10.1186/s13045-018-0573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu X, Lu X. MiR-423-5p inhibition alleviates cardiomyocyte apoptosis and mitochondrial dysfunction caused by hypoxia/reoxygenation through activation of the wnt/beta-catenin signaling pathway via targeting MYBL2. J Cell Physiol. 2019;234(12):22034–43. doi: 10.1002/jcp.28766. [DOI] [PubMed] [Google Scholar]
- 21.Ge J, Yan Q, Wang Y, et al. IL-10 delays the degeneration of intervertebral discs by suppressing the p38 MAPK signaling pathway. Free Radic Biol Med. 2020;147:262–70. doi: 10.1016/j.freeradbiomed.2019.12.040. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Wang H, Yang H, et al. Tumor necrosis factor-alpha- and interleukin-1beta-dependent matrix metalloproteinase-3 expression in nucleus pulposus cells requires cooperative signaling via syndecan 4 and mitogen-activated protein kinase-NF-kappaB axis: Implications in inflammatory disc disease. Am J Pathol. 2014;184(9):2560–72. doi: 10.1016/j.ajpath.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He H, Feng M, Xu H, et al. Total triterpenoids from the fruits of Chaenomeles speciosa exerted gastroprotective activities on indomethacin-induced gastric damage via modulating microRNA-423-5p-mediated TFF/NAG-1 and apoptotic pathways. Food Funct. 2020;11(1):662–79. doi: 10.1039/c9fo02322d. [DOI] [PubMed] [Google Scholar]
- 24.Zhu C, Jiang W, Cheng Q, et al. Hemeoxygenase-1 suppresses IL-1beta-induced apoptosis through the NF-kappaB pathway in human degenerative nucleus pulposus cells. Cell Physiol Biochem. 2018;46(2):644–53. doi: 10.1159/000488632. [DOI] [PubMed] [Google Scholar]
- 25.Yang S, Zhang F, Ma J, Ding W. Intervertebral disc ageing and degeneration: The antiapoptotic effect of oestrogen. Ageing Res Rev. 2020;57:100978. doi: 10.1016/j.arr.2019.100978. [DOI] [PubMed] [Google Scholar]
- 26.Chu X, Wu S, Raju R. NLRX1 Regulation following acute mitochondrial injury. Front Immunol. 2019;10:2431. doi: 10.3389/fimmu.2019.02431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang MJ, Yoon CM, Kim BH, et al. Suppression of NLRX1 in chronic obstructive pulmonary disease. J Clin Invest. 2015;125(6):2458–62. doi: 10.1172/JCI71747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castano-Rodriguez N, Kaakoush NO, Goh KL, et al. The NOD-like receptor signalling pathway in Helicobacter pylori infection and related gastric cancer: A case-control study and gene expression analyses. PLoS One. 2014;9(6):e98899. doi: 10.1371/journal.pone.0098899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan Y, Zhao L, Xie W, et al. Serum miRNAs are potential biomarkers for the detection of disc degeneration, among which miR-26a-5p suppresses Smad1 to regulate disc homeostasis. J Cell Mol Med. 2019;23(10):6679–89. doi: 10.1111/jcmm.14544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng X, Zhang L, Zhang K, et al. Circular RNA VMA21 protects against intervertebral disc degeneration through targeting miR-200c and X linked inhibitor-of-apoptosis protein. Ann Rheum Dis. 2018;77(5):770–79. doi: 10.1136/annrheumdis-2017-212056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji ML, Jiang H, Zhang XJ, et al. Preclinical development of a microRNA-based therapy for intervertebral disc degeneration. Nat Commun. 2018;9(1):5051. doi: 10.1038/s41467-018-07360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204(1):47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]