Short abstract
Introduction
It has long been suggested that ultrasound could be used to measure brain tissue pulsations in humans, but potential clinical applications are relatively unexplored. The aim of this systematic review was to explore and synthesise available literature on ultrasound measurement of brain tissue motion in humans.
Methods
Our systematic review was designed to include predefined study selection criteria, quality evaluation, and a data extraction pro-forma, registered prospectively on PROSPERO (CRD42018114117). The systematic review was conducted by two independent reviewers.
Results
Ten studies were eligible for the evidence synthesis and qualitative evaluation. All eligible studies confirmed that brain tissue motion over the cardiac cycle could be measured using ultrasound; however, data acquisition, analysis, and outcomes varied. The majority of studies used tissue pulsatility imaging, with the right temporal window as the acquisition point. Currently available literature is largely exploratory, with measurements of brain tissue displacement over a narrow range of health conditions and ages. Explored health conditions include orthostatic hypotension and depression.
Conclusion
Further studies are needed to assess variability in brain tissue motion estimates across larger cohorts of healthy subjects and in patients with various medical conditions. This would be important for informing sample size estimates to ensure future studies are appropriately powered. Future research would also benefit from a consistent framework for data analysis and reporting, to facilitate comparative research and meta-analysis. Following standardisation and further healthy participant studies, future work should focus on assessing the clinical utility of brain tissue pulsation measurements in cerebrovascular disease states.
Keywords: Brain tissue displacement, brain pulsatility, transcranial tissue Doppler, ultrasound
Introduction
Following early work in the 1950s using echo-encephalography, it has long been known that the healthy brain pulsates with each cardiac cycle1; however, little is known about how the brain’s pulsations respond to physiological variation and the clinical application of these measurements has yet to be explored.
It is currently believed that brain tissue pulsates due to blood flow variations over the cardiac cycle, following a similar concept to plethysmographic pulsations seen in the limbs. Plethysmography of the limbs has been used for numerous years to monitor digital blood flow and is a measurement of the tissue swelling caused by arterial blood volume in systole exceeding venous drainage.2 The tissue then returns to its original size during diastole and this repeats for each cardiac cycle.
This theory describing the balance between arterial blood volume and venous drainage is supported by numerous magnetic resonance imaging (MRI) findings.3–9 These MRI studies form most of our current knowledge of brain tissue pulsations (BTPs). MRI studies suggest that movement of the brain is largely dominated by a cephalocaudal (top-down) motion4,6,7 and that pulsatile motion originating close to the brain stem propagates outwards to the peripheral brain lobes during ventricular systole.8 Despite the knowledge gained from MRI, ultrasound has become an area of interest due to numerous advantages compared to other imaging modalities, including its non-invasiveness, portability, safety profile, and relatively low cost.
Ultrasound BTP measurements in humans have been investigated by several research groups; however, the nature of these measurements and their usefulness is still unknown. In principle, it could be clinically useful to sensitively measure brain tissue motion, as tissue displacement is likely to be closely linked to tissue stiffness. Neurovascular pathology and acquired or congenital anatomical variants are also likely to affect tissue biomechanics and pulsation characteristics. By improving our understanding of healthy and pathological tissue biomechanics, it may be possible to interpret changes in BTP waveforms in relation to the development of pathology, which in turn could aid medical diagnoses and management.
This systematic review explores and synthesises information from published literature on ultrasound methods for measuring BTPs in humans. To provide an overview of the current status of this technique, the aims of this systematic review will include investigating reported probe positions, extracted motion characteristics, and participant demographics. Also, this review will compare ultrasound measurements to MRI, identify gaps in current knowledge, and propose areas of research interest.
Materials and methods
Search strategy
The protocol for this systematic review was published on the Prospero register on 29 November 2018 (CRD42018114117), prior to data extraction and analysis.
A predesigned systematic database search of MEDLINE OVID (1946–current), SCOPUS (1966–current), Web of Science Core Collection (1970–current), and OpenGrey was conducted using pre-agreed search terms. Searches took place in December 2018.
Search terms included: (Brain OR Cerebral) AND (Tissue) AND (Displace* OR Puls* OR Move* OR Amplitude) AND (Ultrasound) limited to English language articles. No publication type or date limit was applied.
Exclusion criteria
Papers were excluded for the following reasons: studies using phantoms or non-human participants, studies using ultrasound during invasive procedures, and articles without full text access.
Data extraction
Reviewers (JI and MA) independently removed duplicates and screened the remaining titles and abstracts for relevance to the review’s research question.
Relevant records then had data extracted, including participant and comparison types (healthy or patient), population size, age, sex, measurement outcome (e.g. displacement), ultrasound modality used, ultrasound acquisition point, and main conclusions.
Study quality was assessed using a predefined quality assessment tool (Appendix 1) adapted from an existing tool previously used to review cerebral haemodynamic responses.10 Our adaptations were to ensure relevancy and accuracy in assessing brain tissue displacement (BTD) studies. The quality criteria included 15 domains, each of which were equally weighted and the score was used to determine the risk of bias of the record.
Following data extraction, the two reviewers met and compared their findings. Any discrepancies were discussed, and a consensus arrived at through referring to the full text.
Results
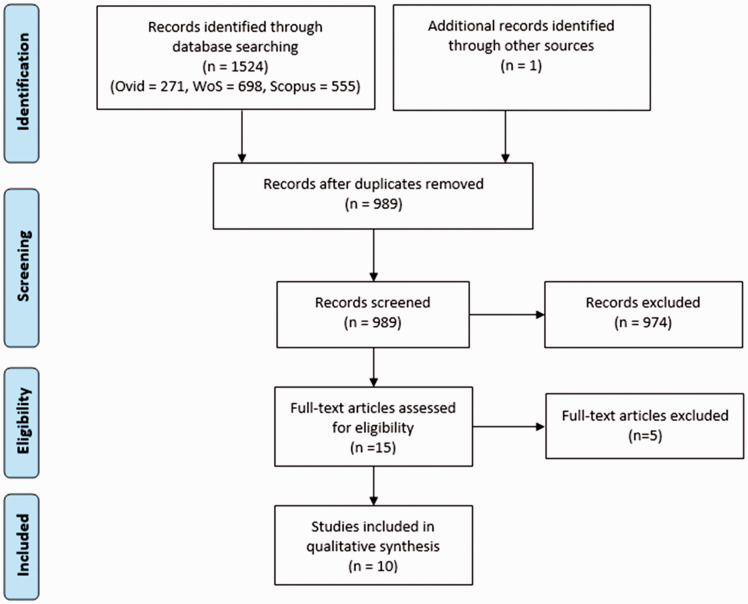
Our database searches identified 1524 records, with one additional record found through cascade referencing. Following removal of duplicates and screening of titles and abstracts for relevance, 15 records remained. These 15 records underwent full text review by both reviewers.
Of the 15 relevant records, three articles were excluded due to being conference proceedings of full papers that were already included. Another record was omitted as it investigated cerebrospinal fluid pulsatility rather than brain tissue pulsatility. Finally, a fifth record was removed as it duplicated another full text article. No records were removed due to being non-full text.
The remaining 10 relevant records comprised nine peer-reviewed original publications11–19 and one conference proceeding.20 The conference proceeding on 2D speckle tracking was included as we found no associated peer-reviewed article. Figure 1 provides a modified version of the PRISMA flowchart, summarising our search process.21 All of the retained records investigated brain tissue pulsatility.
Figure 1.
A modified version of the PRISMA flowchart to summarise the systematic search process.
Study quality
Quality assessment scores for the 10 records ranged from 6/15 for the conference proceeding20 to 14/15.15,16,18 Eight of the records scored over 10 points, which suggests that the majority of studies were of high quality. Appendix 2 summarises the quality assessment for each paper.
As the assessed studies were largely exploratory, it was unclear whether they were adequately powered. Although the results of hypothesis tests were reported by several studies, none reported a sample size estimate prior to the start of data collection. This caused all of the records to lose 1 mark in the quality assessment.
The reviewers noted significant heterogeneity in study design and presentation of basic physiological measurements. Differences existed in ultrasound equipment and methods including probe type (phased-array and capacitive micro-machined ultrasonic transducer (cMUT)), measurement technique (Doppler and speckle tracking), ultrasound frequency (including, 1.82–2.7 MHz), probe position (temporal and occipital windows), and participant position (seated, supine, prone, and standing). Measured structures and reported measures also varied between studies, therefore a quantitative meta-analysis was not possible within this review.
Summary of studies
In the literature, tissue pulsatility imaging (TPI) was the most common method of acquiring brain displacement estimates.11–16,18 TPI extends existing Doppler-based echocardiography techniques (used clinically for imaging cardiac motion) to the investigation of brain tissue motion. This uses the same phased-array transducer used in echocardiography to estimate displacement of tissue in the direction of the ultrasound beam within a 2D slice of brain tissue.11
TPI was first applied to measure brain tissue motion by Kucewicz et al.,11 who demonstrated this technique in two healthy subjects by measuring brain displacement during visual stimulation. They found that BTD increased when visual stimuli were applied.11
Kucewicz et al.12 then used TPI to measure the effects of CO2 on BTP in a further four volunteers. They found that pulsations decreased when CO2 levels were lowered by hyperventilation.12
Following this work, other groups started to investigate BTP using TPI in participants with a narrow range of medical conditions.13–15 Desmidt et al.13 found that BTPs were decreased in elderly patients with type 2 diabetes mellitus and depression, compared to non-depressed, elderly patients with diabetes. Later, Desmidt et al.15 studied middle-aged females with depression. They found that BTPs were significantly higher in middle-aged females with depression compared to control subjects or patients in remission.15 Also in 2017, Biogeau et al. found that elderly patients with orthostatic hypotension had lower mean BTPs. These studies suggest that medical conditions may influence BTPs.14
In addition to this work, Ternifi et al.16 compared white matter hyperintensity (WMH) (measured using MRI in nine healthy subjects) with BTD (measured using TPI). Their work suggested that maximum BTP was negatively correlated with WMH volume.16 This is of interest as WMH has also been found to be associated with pathological conditions (such as cognitive impairment and depression).16
Recently, Angel et al.17 studied the relationship between age, cognitive function, and brain tissue pulsatility using TPI in healthy subjects. This study of 39 subjects found that pulsatility decreased with age and was positively correlated with fluid intelligence (such as novel problem solving) measures.17
The latest TPI study was in 2018; Desmidt et al.18 assessed the association between different brain volumes (measured by MRI) and brain pulsatility. TPI was used to estimate tissue pulsatility in 25 healthy, middle-aged females.18 They found a positive correlation between brain pulsatility and the volume of subcortical structures measured using T1 weighted MRI.18 This study also used a conventional transcranial Doppler pulsatility index (TCD-PI). TPI and TCD-PI were both found to be positively correlated with all of the subcortical regions.18
The remaining studies used two alternative ultrasound techniques.19,20 Certon et al.19 designed and tested a cMUT for use in TPI, to measure brain motion through the skull. Performing TPI with this cMUT probe is advantageous as it can use a lower frequency and has been able to overcome bone barriers.19 Amar et al.20 performed 2D speckle tracking to estimate brain parenchyma movement. Measurements were obtained from a medical ultrasound scanner for this study.20 One of the main advantages of the 2D speckle tracking methods used by Amar et al.20 is that it could estimate both mediolateral and anteroposterior brain parenchyma movements. These studies both offer alternatives to TPI, each with their own advantages; however, evaluation of further studies using them is required to determine their practical application to BTP measurements.
Study populations
Populations varied in size (ranging from 1 to 74 participants). Across all of the records, a total of 216 individuals were investigated. Five studies only recruited healthy participants,11,12,16–18 whilst two recruited patients with depression,13,15 and another recruited participants over 80 years of age with orthostatic hypertension.14 Two technical feasibility studies19,20 did not describe the characteristics of their participants.
As a result of the different populations investigated, there are several comparison methods used. Some made comparisons between baseline measurements and an intervention using individuals as their own controls.11,12 Others compared properties between control and non-control populations.13–15,17 Some of the studies compared TPI measurements with results from other modalities such as MRI or TCD blood flow estimates.16,18
Study technical information
Despite most of the studies using TPI, there were wide variations in technical setups, including different ultrasound probes, acquisition points, and participant posture. Despite these, the mechanical probe holders and data analysis methods appeared similar.
Probe variations depended upon the groups performing the research. Kucewicz et al.11,12 used a Terason 2000 4V2 phased array probe during their studies, whilst a majority of the later studies moved to a Siemens PX4-1 phased array probe.13–16,18 Amar et al.20 also used a phased array probe but did not specify the brand. Finally, Angel et al.17 used a linear array ultrasound probe specifically developed by Supersonic Imagine, whilst Certon et al.19 developed their own cMUT ultrasound probe.
The majority of studies accessed the brain through the right temporal bone window,12–16,18–20 or bilaterally.17 Kucewicz et al.11 used an acquisition point in the occipital region. Despite many of the studies using the right temporal window, structures imaged appear to vary. For example, Kucewicz et al.12 recorded motion from structures in both brain hemispheres from a single acquisition point, whilst Angel et al.17 used the temporal windows to visualise six imaging planes.
Another technical variation included the posture of the participants. Three studies asked participants to adopt a supine position,12,15,18 whilst another three studies had their participants sat upright.13,16,19 Another study used a prone position to facilitate insonation through the occipital window.11 Finally, Biogeau et al.14 altered the posture of the participant, starting in a supine position and then asking the participant to stand. Amar et al.20 and Angel et al.17 did not report the participants’ posture.
Finally, measurements and analysis techniques also varied considerably between studies. Due to multiple measurements being taken and variations occurring within regions, investigators had to either average their data over the region of interest (RoI) or use a principal component analysis (PCA) to manage the data. Currently, it is unclear which method is the most clinically useful.
Most studies focused on measuring mean or maximum brain tissue pulsatility.13–18 Mean BTP (MeanBTP) acquisitions are representative of average tissue displacement across a 2D RoI. Maximum BTP (MaxBTP) describes the largest observed pulsation amplitude within the RoI.14,15,18 However, two articles used a PCA to generate single values relating to the pulsatility of tissue.11,17 Heterogeneity in reported measurements made it difficult to compare findings between studies. This is highlighted in Table 1, which summarises extracted quantities and quantitative estimates from the 10 records.
Table 1.
A summary of the extracted data.
| Publication type | Participant type | Comparison group | Population size | Imaging modality | Acquisition point | Outcome measured | |
|---|---|---|---|---|---|---|---|
| Amar et al.20 | Conferencepaper | Not mentioned | None | Not mentioned | 2D speckle tracking | Right temporal window | BTD (range: 30–50 µm) |
| Angel et al.17 | Journal article | Healthy | Different healthy participants | 39 | TPI | Right and left temporal window | Pulsatility Composite Index |
| Biogeau et al.14 | Journal article | Elderly participants with orthostatic hypotension | No orthostatic hypotension | 39 | TPI | Right temporal window | MaxBTP (range: 100.5–103.2 µm) and MeanBTP (range: 32.8–37.4 µm) |
| Certon et al.19 | Journal article | Healthy | None | 1 | TPI using cMUT | Right temporal window | BTD (80 µm) |
| Desmidt et al.13 | Journal article | Patients with depression and T2DM | Patients without depression with T2DM | 23 | TPI | Right temporal window | MaxBTP (range: 90.1–132.9 µm) and MeanBTP (range: 6.7–10.5 µm) |
| Desmidt et al.15 | Journal article | Patients with midlife depression | Remission group + Control group | 74 | TPI | Right temporal window | MaxBTP (range: 97.5–115.8 µm) and MeanBTP (range: 20.0–24.6 µm), PtoP and RMS |
| Desmidt et al.18 | Journal article | Healthy | Same healthy participants | 25 | TPI | Right temporal window | MaxBTP (34.1 µm) and MeanBTP (7.0 µm) |
| Kucewicz et al.11 | Journal article | Healthy | Baseline | 2 | Functional TPI | Superior to occipital protuberance | BTD |
| Kucewicz et al.12 | Journal article | Healthy | Baseline | 4 | TPI | Right temporal window | BTD (±75 µm) |
| Ternifi et al.16 | Journal article | Healthy | Same healthy participants | 9 | TPI | Right temporal window | Max BTD (80.42 µm) |
| Total population | 216 |
BTD: brain tissue displacement; cMUT: capacitive micro-machined ultrasonic transducer; MaxBTP: maximum brain tissue pulsation; MeanBTP: mean brain tissue pulsation; TPI: tissue pulsatility imaging; T2DM: Type 2 Diabetes mellitus.
Risk of bias
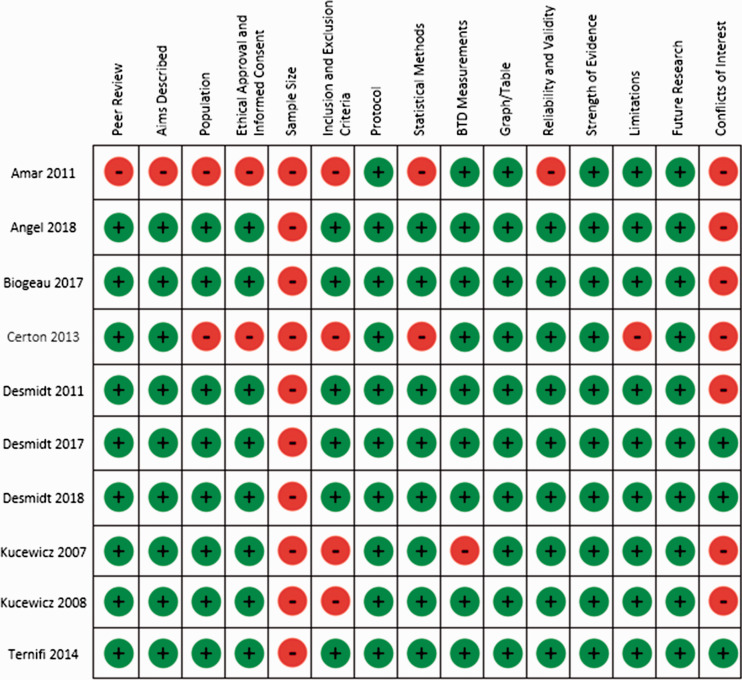
Risk of bias was assessed in the records using the quality assessment tool. Most of the studies11–18 were of high quality (with a quality assessment score >10) and disclosed no conflicts of interest so were deemed to have a low risk of bias. Two studies19,20 had low quality assessment scores of 8 and 6, respectively. Reasons for these low scores include a lack of reporting of ethical considerations for participant recruitment and absence of participant information for technical and feasibility studies (Figure 2).
Figure 2.
Risk of bias summary for the 10 records.
Another source of bias to consider is publication bias. Particularly in emerging research areas, such as this, there is a risk of publication bias when searching the literature. This is due to limited understanding and likely biased publication of the existing data. This can arise from authors not publishing their findings (particularly if they are non-significant), but also from journals being reluctant to publish emerging techniques and technologies. Whilst this systematic review did attempt to avoid this bias by searching grey literature (using OpenGrey), publication bias is still likely to be present.
Discussion
This systematic review identified 10 studies relevant to the measurement of brain tissue motion using ultrasound. Despite using varying techniques and measuring different outcomes, the main finding of this review is that BTD can be successfully measured in humans using ultrasound. Measured values varied greatly between studies, with MaxBTP ranging from 34.1 to 132.9 µm, whilst MeanBTP ranges include 6.7–37.4 µm. These values appear similar to pulsatile motion measurements obtained in MRI studies.22
Three main types of techniques were utilised; however, the most well developed appeared to be TPI, which comprises the majority of current literature.
Cerebral vasoreactivity
This review identified several records suggesting that BTPs are affected by cerebral vasoreactivity.11,12,14 Kucewicz et al.11 identified that BTPs increase with vasodilation. Kucewicz et al.12 also found that BTPs decrease with vasoconstriction. This correlation between blood vessel diameter and BTPs supports the theory that BTPs are linked to vascular haemodynamics, hinting that medical conditions affecting cerebral blood flow might result in changes in the pulsation amplitude. This notion is supported by the findings of Biogeau et al.14 who reported decreased pulsatility in patients with orthostatic hypotension. The findings of these three studies suggest that BTP measurements could be of clinical use; however, a wider range of medical conditions (including stroke and carotid stenosis) need to be investigated with larger, appropriately powered studies to draw any conclusions regarding clinical utility.
Brain structure and cerebral pathophysiology
This review also found evidence to suggest that BTPs are affected by brain structure and cerebral pathophysiology. Ternifi et al.16 identified a negative correlation between BTPs (measured using ultrasound) and WMH volume, measured using MRI. The findings from this study are some of the first to establish a link between a structural pathology and BTPs. They also explain the incongruent findings of the effect of depression on BTPs.13,15 Desmidt et al.13 found that later-life depression was associated with decreased BTPs in patients with diabetes, but midlife depression was associated with increased BTPs.15 These results can be rationalised by WMHs being more likely to have been present in the older patient cohort in the 2011 study. Potentially, WMHs were responsible for the change seen in elderly participants rather than the depression pathophysiology.15 The combination of these findings is interesting as they complement each other and introduce a new dimension to this field of research. Moving forward from this, it would be advantageous to investigate whether there is a defined level of WMHs associated with significant BTP changes for use in WMH screening.
The work by Desmidt et al.18 supports the hypothesis that brain structure affects BTPs. Their research found that brain pulsatility was significantly associated with subcortical brain volumes (e.g. the caudate nucleus).18 This work helps to explain factors that may affect BTPs (something to be considered in future studies). For future work, it would be important to systematically quantify expected variances in BTP measurements between individuals, and with potential confounders, such as age, to allow a detailed comparison of BTP variability associated with subcortical volume changes. Identification of factors affecting BTPs will allow adjustment for confounders in future research.
Angel et al.17 found that BTPs decrease with increasing age. This is a major finding as it assists our understanding of how BTPs vary between healthy participants; however, further work is needed to establish the origins of BTPs at physiological and biomechanical level. It may be that changes are due to subcortical volume changes associated with age (as proposed by Desmidt et al.18), or there could be other changes with age due to cerebrovascular disease. Once the cause is identified, applications may emerge targeted at detecting these changes. Angel et al.17 also reported a positive correlation between BTPs and neurocognitive measures, suggesting a potential place for BTP measurement in monitoring neurocognitive health, such as in the care of patients with cognitive impairment.
Limitations
All of the current literature included in this review demonstrate that brain tissue movement can be measured using ultrasound; however, there is still little known about brain motion in the general population or at a fundamental biomechanical level. As existing studies are small and use different measurement outcomes, it is difficult to make direct comparisons or to form a general consensus regarding clinical applicability. Studies are likely to be underpowered, despite showing significant p-values in some group comparisons. Whilst some study designs avoid this by using individuals as their own controls, the results are difficult to generalise.
The current literature is also limited by the absence of any studies investigating intra- or inter-observer variability. Properly conducted methodological trials and technical validation studies are fundamental in assessing and validating new techniques.
Upon review of the current literature, it has also become aware that some of the reporting is limited. Due to a lack of detail in equipment specifications, setup, and analysis methods in some records, it would be hard for a reader to replicate the studies. Ideally, all records should thoroughly detail their methods so that others can repeat the study to verify findings and to demonstrate reproducibility.
Limitations at review level include the limit of only including English language articles, caused by resource availability. This means that some records may not have been retrieved and reviewed. A further limitation of this review is the inability to perform a meta-analysis. This is due to the heterogeneity of the records and the non-standardised reporting measures.
Future work and clinical relevance
By acknowledging the plethysmographic theory of BTPs, there is great potential for BTP measurements to have clinical relevance. As BTPs rely on cerebral blood flow, it can be hypothesised that interruption of this (such as in stroke or traumatic brain injury) could result in altered brain tissue pulsatility.
Future work identifying differences in brain tissue pulsatility between healthy participants and patients could inform the use of BTP measurement devices as diagnostic tools. It could be expected that brain tissue would behave differently in the presence of physiological and biomechanical changes associated with pathology. If identified, differences in conditions (such as stroke or traumatic brain injury) could then be applied as a clinical diagnostic tool.
To ensure that these clinical studies are generalisable and reliable, the studies should use sufficient sample sizes of patients with demographic-matched healthy comparators. Where possible, established clinical parameters (such as radiological imaging reports or clinical severity scores) should also be reported and correlated to BTP measurements. As a minimum standard, all studies should clearly state their recruitment and measurement methodologies to allow others to reproduce their work.
As ultrasound-based devices are often cheaper, more portable, and less resource intensive than current imaging techniques, these could improve patient care by reducing the onset-to-treatment time. Furthermore, these devices could be used in remote areas where resources and infrastructure for other imaging modalities (such as CT or MRI) are not available.
However, prior to investigation of clinical utility, standardisation across the research field is required to unite the work being undertaken. Firstly, a standardised outcome measure is required. Whilst it may be argued that selecting a standardised outcome measure is difficult in this novel research field, unifying reported measures could assist considerably in advancing the field and preventing duplication of work. When selecting this standardised measure, technical variations between acquisition devices should be considered. Based on the current literature, BTD, MaxBTP, and MeanBTP are the most frequently reported quantitative measures of BTP, but their definitions in terms of spatial and temporal averaging are not clearly defined. Additional standardising of reporting could also include specifying the RoI, the size and depths investigated, and measurements for individuals. This reporting better illustrates the methods to the reader and could be used to inform power calculations as the variances between individuals would be known.
Following this standardisation, future work investigating both healthy participants and patients with medical conditions can take place. Research is required into healthy participants as a paucity of data exists on typical, healthy participant values. Whilst many existing ultrasound studies were based on healthy subjects, their sample sizes were often small (1–74 subjects). Future work studying a larger cohort of healthy participants would be beneficial in establishing variability of BTPs for sample size estimation in the design of future research and to provide reference data for clinical comparison. In addition, future studies should ensure that the healthy participant population is representative of the general population, including a wide variety of ages and ethnicities.
Conclusion
In conclusion, this systematic review has identified 10 papers investigating BTD measurements using ultrasound. All of the papers successfully measured brain pulsations.
Whilst measurements have been successfully obtained, typical values and the role of confounding factors have yet to be identified and quantified for a general healthy population. Limited research has been conducted into the effects of medical conditions (including orthostatic hypotension and depression) on BTD; however, clinical applicability of these measurements has yet to be explored.
Following our review, we recommend that a large healthy population is explored to collect reference data for the general population. Furthermore, a study should be conducted to identify and define the ideal standardised measurement for all future studies. This would involve presenting the available outcome measures and justifying which is most useful and representative of the data. We also highlight that future areas of interest could include the investigation of BTPs in major structural damage of the brain and the effects of blood pressure and cerebral haemodynamics on BTPs.
Supplemental Material
Supplemental material, ULT894601 Supplemental Material2 for Ultrasound measurement of brain tissue movement in humans: A systematic review by Jonathan Ince, Meshal Alharbi, Jatinder S Minhas and Emma ML Chung in Ultrasound
Supplemental material, ULT894601 Supplemental Material1 for Ultrasound measurement of brain tissue movement in humans: A systematic review by Jonathan Ince, Meshal Alharbi, Jatinder S Minhas and Emma ML Chung in Ultrasound
Acknowledgements
N/A
Declaration of Conflicting Interests
The author(s) declared following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Whilst the investigators are researching the area of brain pulsatility, they have no conflicts of interest to declare. EC has recently entered a collaboration agreement with Nihon Kohden, Japan.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This systematic review was internally funded. JI is funded by the Wolfson Foundation intercalated awards programme. MA is funded by the King Saud bin Abdulaziz, University for Health Sciences, Riyadh, Saudi Arabia. JSM is funded by a Dunhill Medical Trust Research Training Fellowship (RTF97/0117). EMLC has previously received funding for investigation of tissue displacement using Doppler ultrasound techniques from the Science and technologies Facilities Council (STFC, UK), Engineering and Physical Sciences Research Council (EPSRC, UK), University of Leicester, UK, Nihon Kohden (Japan), and the Institute for Physics and Engineering in Medicine (IPEM, UK).
Ethics Approval
Ethical approval was not required for this review of the literature.
Guarantor
EMLC.
Contributors
JI and MA conducted the literature search, qualitative analysis and prepared the manuscript. JI, MA, JSM and EMLC wrote the manuscript. All authors approved the final version of the manuscript.
ORCID iDs
Jonathan Ince https://orcid.org/0000-0003-2008-8372
Jatinder S Minhas https://orcid.org/0000-0002-0576-9105
Appendix 1. The pre-agreed quality assessment tool
Appendix 2. Summary table of the quality assessment scores
| Amar et al.20 | Angel et al.17 | Biogeau et al.17 | Certon et al.19 | Desmidt et al.13 | Desmidt et al.15 | Desmidt et al.18 | Kucewicz et al.11 | Kucewicz et al.12 | Ternifi et al.16 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Peer review | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Aims Described | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Population | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ethical approvaland informed consent | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Sample size | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Inclusion and exclusion criteria | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ |
| Protocol | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Statistical methods | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| BTD measurements | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Graph/Table | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Reliability and validity | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Strength of evidence | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Limitations | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Future research | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Conflicts of interest | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ |
| Totals | 6 | 13 | 13 | 8 | 13 | 14 | 14 | 11 | 12 | 14 |
References
- 1.Leksell L. Echo-encephalography. I. Detection of intracranial complications following head injury. Acta Chir Scand 1956; 110: 301–315. [PubMed] [Google Scholar]
- 2.Strandness DE, Sumner DS. Hemodynamics for surgeons, New York: Grune & Stratton, 1975.
- 3.Greitz D, Wirestam R, Franck A, et al. Pulsatile brain movement and associated hydrodynamics studied by magnetic resonance phase imaging. Neuroradiology 1992; 34: 370–380. [DOI] [PubMed] [Google Scholar]
- 4.Enzmann DR, Pelc NJ. Brain motion: measurement with phase-contrast MR imaging. Radiology 1992; 185: 653–660. [DOI] [PubMed] [Google Scholar]
- 5.Feinberg DA, Mark AS. Human brain motion and cerebrospinal fluid circulation demonstrated with MR velocity imaging. Radiology 1987; 163: 793–799. [DOI] [PubMed] [Google Scholar]
- 6.Poncelet BP, Wedeen VJ, Weisskoff RM, et al. Brain parenchyma motion: measurement with cine echo-planar MR imaging. Radiology 1992; 185: 645–651. [DOI] [PubMed] [Google Scholar]
- 7.Soellinger M, Ryf S, Boesiger P, et al. Assessment of human brain motion using CSPAMM. J Magn Reson Imaging 2007; 25: 709–714. [DOI] [PubMed] [Google Scholar]
- 8.Zhong X, Meyer CH, Schlesinger DJ, et al. Tracking brain motion during the cardiac cycle using spiral cine-DENSE MRI. Med Phys 2009; 36: 3413–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soellinger M, Rutz AK, Kozerke S, et al. 3D cine displacement-encoded MRI of pulsatile brain motion. Magn Reson Med 2009; 61: 153–162. [DOI] [PubMed] [Google Scholar]
- 10.Salinet ASM, Haunton VJ, Panerai RB, et al. A systematic review of cerebral hemodynamic responses to neural activation following stroke. J Neurol 2013; 260: 2715–2721. [DOI] [PubMed] [Google Scholar]
- 11.Kucewicz JC, Dunmire B, Leotta DF, et al. Functional tissue pulsatility imaging of the brain during visual stimulation. Ultrasound Med Biol 2007; 33: 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kucewicz JC, Dunmire B, Giardino ND, et al. Tissue pulsatility imaging of cerebral vasoreactivity during hyperventilation. Ultrasound Med Biol 2008; 34: 1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desmidt T, Hachemi ME, Remenieras J, et al. Ultrasound brain tissue pulsatility is decreased in middle aged and elderly type 2 diabetic patients with depression. Psychiatry Res Neuroimaging 2011; 193: 63–64. [DOI] [PubMed] [Google Scholar]
- 14.Biogeau J, Desmidt T, Dujardin P, et al. Ultrasound tissue pulsatility imaging suggests impairment in global brain pulsatility and small vessels in elderly patients with orthostatic hypotension. J Stroke Cerebrovasc Dis 2017; 26: 246–251. [DOI] [PubMed] [Google Scholar]
- 15.Desmidt T, Brizard B, Dujardin P, et al. Brain tissue pulsatility is increased in midlife depression: a comparative study using ultrasound tissue pulsatility imaging. Neuropsychopharmacology 2017; 42: 2575–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ternifi R, Cazals X, Desmidt T, et al. Ultrasound measurements of brain tissue pulsatility correlate with the volume of MRI white-matter hyperintensity. J Cereb Blood Flow Metab 2014; 34: 942–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angel L, Bouazzaoui B, Isingrini M, et al. Brain tissue pulsatility mediates cognitive and electrophysiological changes in normal aging: evidence from ultrasound tissue pulsatility imaging (TPI). Brain Cogn 2018; 123: 74–80. [DOI] [PubMed] [Google Scholar]
- 18.Desmidt T, Andersson F, Brizard B, et al. Ultrasound measures of brain pulsatility correlate with subcortical brain volumes in healthy young adults. Ultrasound Med Biol 2018; 44: 2307–2313. [DOI] [PubMed] [Google Scholar]
- 19.Certon D, Ternifi R, Boulme A, et al. Low frequency cMUT technology: application to measurement of brain movement and assessment of bone quality. IRBM 2013; 34: 159–166. [Google Scholar]
- 20.Amar MEH, Ternifi R, Remenieras JP. Brain tissue motion estimation: 2D speckle tracking using synthetic lateral phase technique. In: The 7th international Workshop on Systems, Signal Processing and their Applications (WoSSPA), 2011.
- 21.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weaver JB, Pattison AJ, McGarry MD, et al. Brain mechanical property measurement using MRE with intrinsic activation. Phys Med Biol 2012; 57: 7275–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, ULT894601 Supplemental Material2 for Ultrasound measurement of brain tissue movement in humans: A systematic review by Jonathan Ince, Meshal Alharbi, Jatinder S Minhas and Emma ML Chung in Ultrasound
Supplemental material, ULT894601 Supplemental Material1 for Ultrasound measurement of brain tissue movement in humans: A systematic review by Jonathan Ince, Meshal Alharbi, Jatinder S Minhas and Emma ML Chung in Ultrasound