Short abstract
Musculoskeletal infection, especially in young children, often presents with non-specific clinical signs and symptoms necessitating early imaging to identify the source of infection. While MRI is the investigation of choice to demonstrate bone infection, it is expensive and often requires a general anaesthetic in the young child. Ultrasound can be a useful tool in the initial assessment due to its easy availability and portable equipment. It does not involve ionising radiation and is used to guide aspiration and drainage procedures. This review explains sonographic features of septic arthritis, osteomyelitis, pyomyositis and soft tissue infection in children and highlights advantages and limitations of sonography when assessing the child with suspected musculoskeletal infection.
Keywords: Musculoskeletal infection, osteomyelitis, septic arthritis, sonography, child
Introduction
The investigation of a child with suspected musculoskeletal infection can present a diagnostic challenge. While older children usually present with systemic symptoms including fever and malaise, young children may show only non-specific signs such as crying, poor feeding or refusal to move a limb. Fever and systemic symptoms can be absent in neonates and inflammatory markers (C-reactive protein, white blood cell count) may be normal due to an immature immune system and with certain infections such as Kingella kingae.1–4 Radiographs are easily available, inexpensive, but insensitive for joint effusion and in the early stages of bone infection.5 Due to its excellent soft tissue contrast, its ability to show bone oedema early and its high sensitivity and specificity, magnetic resonance imaging (MRI) is generally regarded as the imaging modality of choice.6,7 However, it is expensive, not universally available and often requires general anaesthesia or sedation in young children.8 Increasing concerns about high radiation exposure has limited the use of scintigraphy and computed tomography (CT) in recent years.7,9
Sonography offers a cheap, easily available and radiation free alternative. It is however operator dependent, and should be performed and interpreted by a trained professional who is regularly imaging children. Use of a high performance machine is preferable over a handheld or basic scanner. Equipment is portable and images are less susceptible to motion and metal artefact than MRI. Ultrasound is especially useful because the unossified cartilage in a young child helps to visualise the joint and to guide drainage of collections.
Sonography should be performed in a child friendly quiet room with support of the parent or carer. A high frequency linear transducer (at least 10–12 MHz) is ideal for imaging small superficial structures or joints in young children. A lower frequency curvilinear transducer may be required for older children to image a deep intramuscular abscess and extended field of view imaging can be employed to document large lesions.9 Imaging of the opposite joint for comparison can be helpful but detailed anatomical knowledge of each area examined and awareness of physiological changes occurring during growth and development are prerequisites for accurate diagnosis. Sonography should be used as a first line test or in conjunction with radiographs if bone involvement is suspected. It may eliminate the need for further imaging with MRI.
Septic arthritis
Septic arthritis is infection of a joint from haematogenous seeding of organisms into the highly vascular synovium.3 Staphylococcus aureus is most frequently identified, followed by Group A Streptococcus and Streptococcus pneumoniae. Group B Streptococcus and Escherichia coli are recognised in neonates, whereas Neisseria gonorrhoea may occur in adolescents.10 The incidence of septic arthritis in children varies in different regions but ranges between 5 and 12 per 100,000 per year.11 Septic arthritis is usually monoarticular with the hip and knee most commonly affected.11 It may be associated with osteomyelitis due to direct extension of infection from bone into the joint which occurs more frequently in joints where the metaphysis is intra-articular such as the hip or shoulder.6,12 These children usually present with a longer duration of symptoms.12 A quick diagnosis and early treatment is important as joint damage occurs due to bacterial endotoxins, the body’s own inflammatory response and avascular necrosis related to tamponade of nutrient vessels by large joint effusions.3,13,14
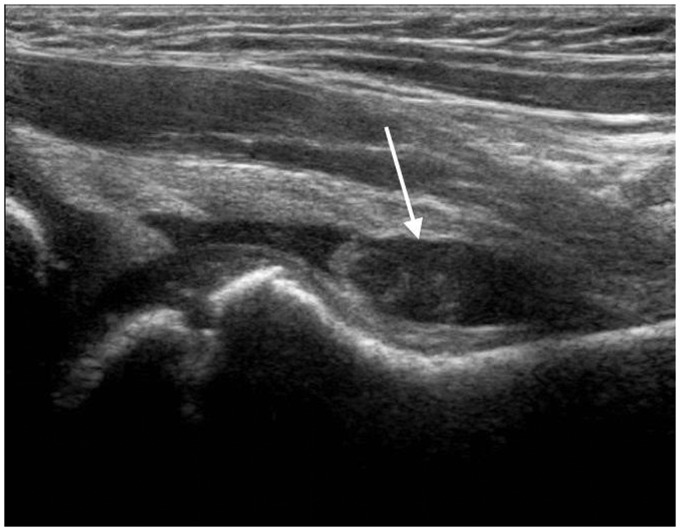
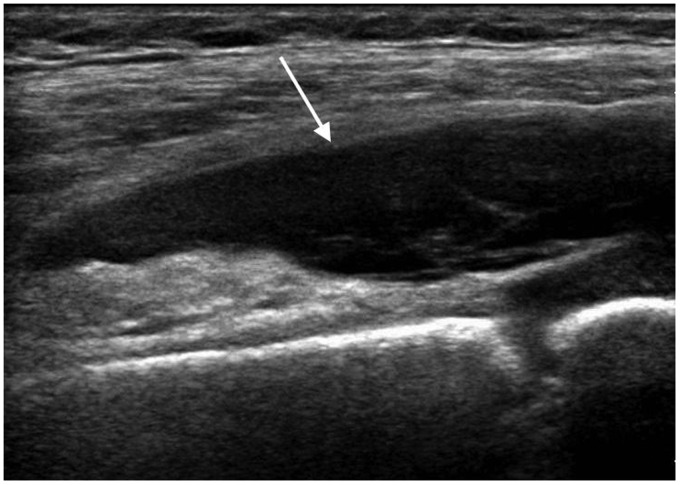
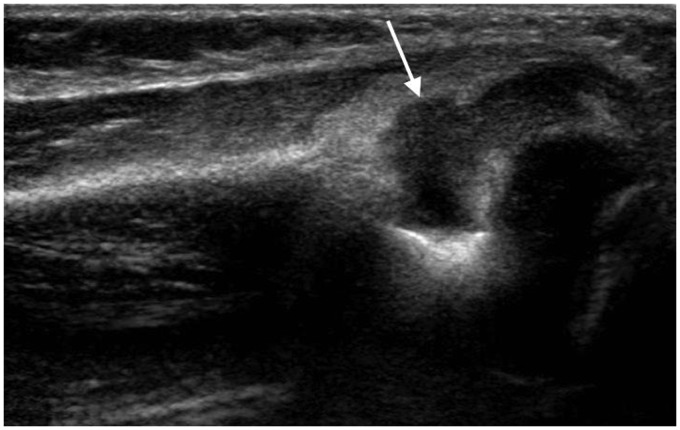
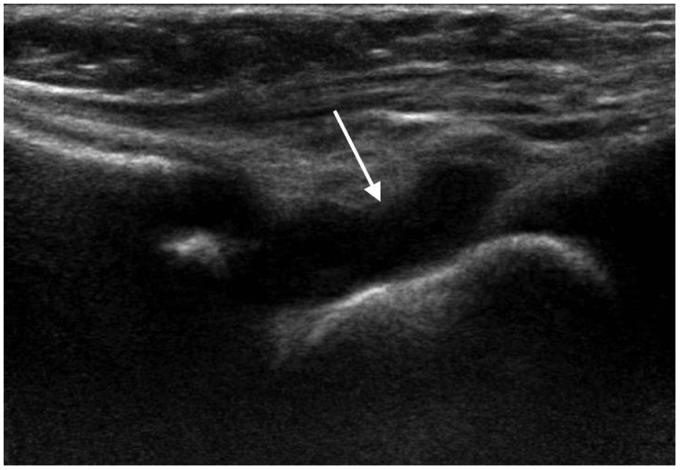
Sonography is usually performed as first line investigation in a young limping child to exclude a hip joint effusion and to investigate any swollen or painful joint.15,16 Ultrasound can detect joint effusions as small as 1–2 ml but cannot distinguish an infected from a non-infected effusion regardless of the size or echogenicity of the fluid.3,12,16 It is useful to compare both sides when deciding if a small amount of fluid may be pathological. While the absence of a joint effusion excludes a diagnosis of septic arthritis, ultrasound may be negative in the first 24 hours after onset of symptoms when a sufficient effusion has not yet accumulated.3,16,17 Fluid preferentially accumulates in different areas of each joint: the suprapatellar recess of the knee, the anterior recess of the hip or the posterior recess of the elbow (Figures 1 to 4). This knowledge should guide the operator to the most appropriate initial scan plane when examining an unsettled child. This includes the suprapatellar region of the knee in a sagittal plane, the anterior aspect of the ankle in a sagittal plane, the posterior aspect of the elbow in a sagittal plane and the anterior aspect of the shoulder including the biceps tendon in a transverse plane. In a cooperative child, a full examination of the joint in multiple planes is suggested. Care should be taken to not mistake the unossified hypoechoic epiphysis in a young child for joint fluid which can be avoided by comparing the affected joint with the opposite site, careful observation of fluid movement and adjusting the gain during scanning.
Figure 1.
Four year old with septic arthritis of the hip. Longitudinal scan of the right anterior hip along the axis of the femoral neck shows a prominent joint effusion (arrow) with associated synovial thickening accumulating in the anterior recess where the capsule is most distensible.
Figure 2.
Five year old with septic arthritis of the knee. Longitudinal scan of the suprapatellar region demonstrates a large joint effusion (arrow).
Figure 3.
Infant of 10 months with septic arthritis of the elbow. Longitudinal scan along the posterior elbow shows a large joint effusion (arrow) in the olecranon fossa.
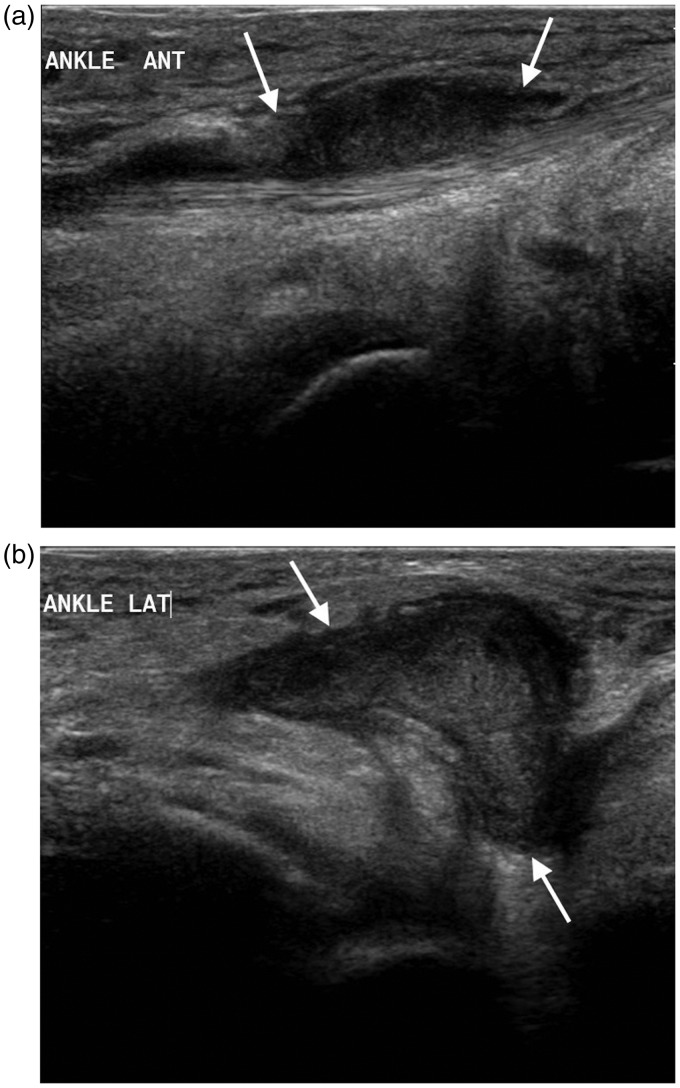
Figure 4.
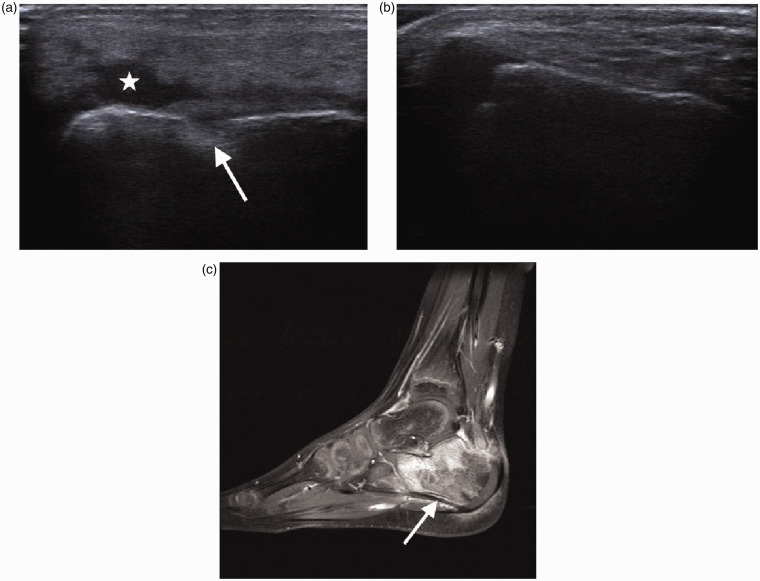
One year old with septic arthritis of the ankle. Longitudinal scan of the anterior ankle demonstrates a large joint effusion (arrow).
Several authors have described clinical decision algorithms evaluating fever, raised CRP >20 mg/l, ESR >40 mm/h, WBC >12 × 109 cells/l and non-weight bearing status to differentiate septic arthritis from transient synovitis, a self-limiting benign process.18–20 However, there is no single investigation or blood test to reliably distinguish the two entities. Many authors regard joint aspiration and culture of the fluid as the definitive investigation if the clinical algorithms point towards septic arthritis or the diagnosis remains unclear.18–21
Osteomyelitis
Osteomyelitis represents infection of the bone and bone marrow with an incidence of approximately 1 in 5000.6,13 Young children are more commonly affected. The most common causative organism is S. aureus, which accounts for 80–90% of infections followed by β-Haemolytic Streptococcus, S. pneumoniae, E. coli and Pseudomonas spp. However, recently other organisms such as K. kingae and aggressive Panton–Valentine leukocidin toxin producing S. aureus strains are more frequently encountered.2,12,22 Infection may occur either from direct or haematogenous spread. The long bones most frequently affected include the femur, tibia and humerus. Other bones affected include pelvis and calcaneus.4
Haematogenous osteomyelitis in children commonly affects the highly vascularised metaphyses of fast-growing long bones allowing easy seeding of bacteria (Figure 5).13 Children less than two years old have more transphyseal vessels entering the epiphyseal circulation from the metaphyses which allows the infection to spread more easily across the physis into the epiphysis and joints.4,6 Epiphyseal osteomyelitis is therefore often observed in neonates and infants but can occur at any age (Figure 6).23 Due to an immature immune system, neonates and young children are prone to multifocal infection (Figure 7).4 In young children, the periosteum is not firmly attached to the cortex leading to prominent subperiosteal collections (Figure 8).6 In the older age group, vascular supply between the epiphysis and metaphysis is separated by the avascular growth plate; it is therefore rare for haematogenous osteomyelitis to spread from the metaphysis to the epiphysis without mechanical disruption of the growth plate.23
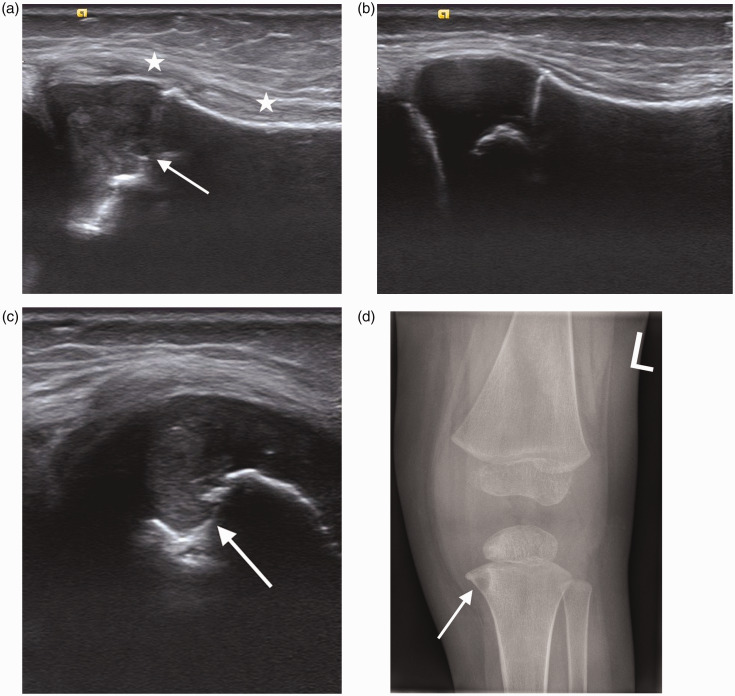
Figure 5.
Two year old with tibial osteomyelitis. (a) Longitudinal scan of the left proximal tibia shows increased echogenicity in the metaphysis and epiphysis (arrow). Marked superficial soft tissue thickening (*) is best appreciated when compared to the normal proximal right tibia in (b). (c) Transverse scan of the proximal tibia demonstrates an echogenic focus and bone destruction in the metaphyseal region (arrow). (d) AP radiograph of the left knee shows the same area of bone destruction (arrow).
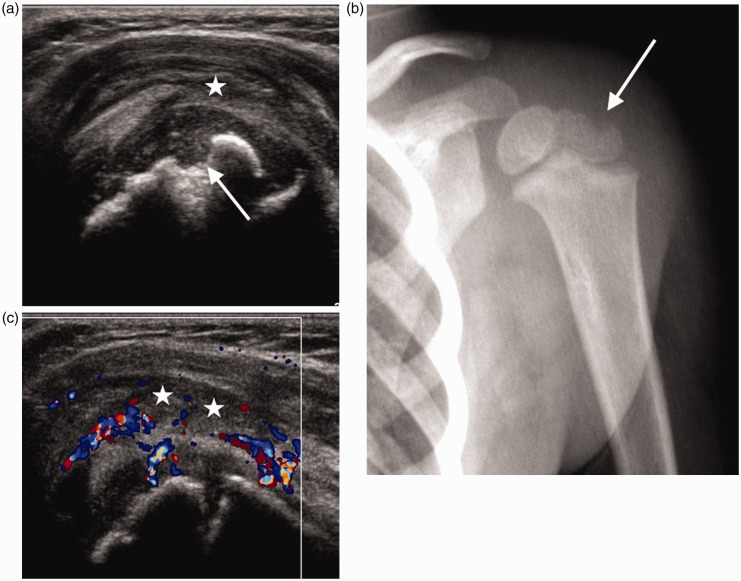
Figure 6.
Infant of 21 months with epiphyseal osteomyelitis of the humeral head. (a) Longitudinal scan of the shoulder demonstrates a defect in the humeral epiphysis (arrow), an echogenic joint effusion (*) and thickening of the joint capsule. (b) AP radiograph of the left shoulder confirms the bone defect seen on sonography (arrow). (c) Longitudinal colour Doppler image shows marked increase in vascularity. Joint effusion (*).
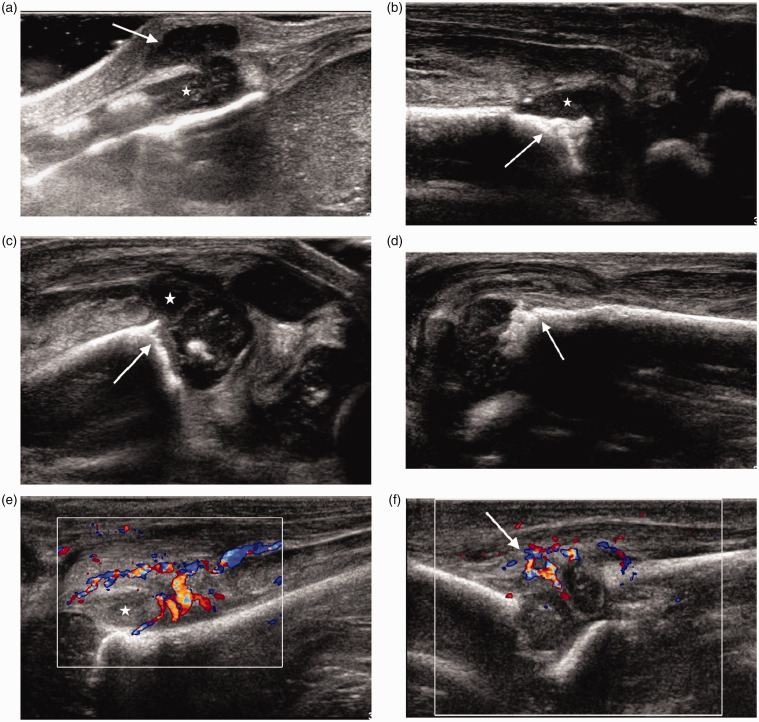
Figure 7.
Neonate of six weeks with multifocal osteomyelitis. (a) Longitudinal scan over the xiphisternum demonstrates a collection (arrow) extending from the cartilage (*) into the superficial soft tissues. (b) Longitudinal scan of the anterior aspect of the left ankle shows bony irregularity (arrow) with a small adjacent collection (*). There was also a joint effusion and synovitis (not shown). (c) Longitudinal scan of the suprapatellar region of the left knee shows an echogenic joint effusion with synovitis and bony irregularity at the femoral metaphysis (arrow). (d) Longitudinal scan along the left proximal humeral metaphysis demonstrates cortical bone irregularity (arrow) in keeping with osteomyelitis. (e) Longitudinal colour Doppler image of the left hip along the femoral neck shows a joint effusion (*) with marked increase in vascularity. (f) Longitudinal colour Doppler image of the left elbow shows increase in vascularity (arrow). There was also a joint effusion better seen in the olecranon fossa (not shown).
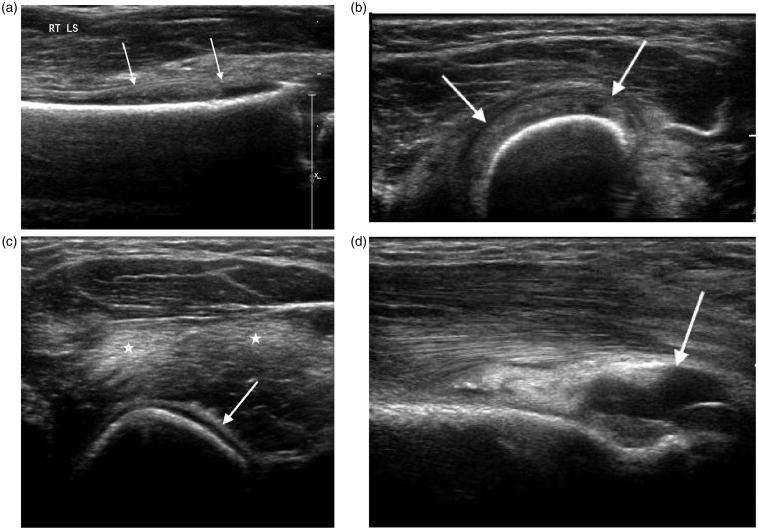
Figure 8.
Two year old with humeral osteomyelitis. (a) Longitudinal and (b) transverse scans of the distal humerus demonstrate an echogenic subperiosteal collection (arrows). (c) Transverse scan of the upper arm demonstrates periosteal reaction (arrow) and associated muscle oedema (*). (d) Longitudinal scan of the posterior elbow shows an associated joint effusion (arrow).
The use of ultrasound in detecting osteomyelitis has been reported in small series.5,24–29 Sonography can demonstrate a range of findings as early as 1–3 days after the onset of symptoms (Figures 5 to 8). Juxtacortical deep soft tissue swelling is the earliest finding visible on ultrasound.24,25,27 This is followed by periosteal thickening or elevation with a thin layer of fluid and subperiosteal abscess formation.24,26,28,30 These findings are usually seen on ultrasound before any change is apparent on radiographs, up to two weeks later.5 Subperiosteal collections are characterised by a hypo- or anechoic lenticular-shaped fluid collection extending along the cortex of the bone. Anechoic collections in continuity with the bone are highly suggestive of osteomyelitis (Figure 9).24,26,27,29 If soft tissue can be identified between the fluid collection and the bone, the collection is likely of non-osseous origin.24 Cortical erosions and cloaca may be demonstrated but are not usually apparent until 7–10 days after onset of symptoms (Figures 7 and 9).24 Reactive or infective joint effusions may also be identified and sonography can be used to guide aspirations (Figures 7 and 8).4,17,24,30
Figure 9.
Six year old with calcaneal osteomyelitis. (a) Longitudinal scan of the lateral aspect of the right calcaneus shows bone destruction (arrow) and a small associated soft tissue collection (*). (b) Normal left calcaneus for comparison. (c) Post-contrast sagittal T1 weighted fat saturated image shows involvement of the whole calcaneus (arrow) which would have been underestimated by sonography.
Colour or power Doppler can demonstrate increased vascularity in the periosteum and surrounding tissues or synovitis in the adjacent joint in keeping with inflammation (Figures 6 and 7).24,27,31 Chao et al.31 report that increased flow on Doppler around the periosteum corresponds to advanced aggressive disease in patients with higher CRP. Ezzat et al.27 have shown that increased flow on Doppler around the periosteum early in the disease process is likely to progress into a subperiosteal collection which can be demonstrated on follow-up sonography. Therefore, Doppler imaging may identify patients that require more aggressive management or surgical intervention.27,31
Ultrasound is most sensitive in suspected acute osteomyelitis of long bones where the tendency to develop periosteal reaction or subperiosteal collections is greatest.30 It cannot assess the bone marrow therefore underestimating the amount of bone involvement, and can also miss collections within the bone (Figure 9).30 Assessment of the pelvis, especially in older children with suspected osteomyelitis, is often difficult.32 Early MRI may be required because presentations can be non-specific, the pelvic bony anatomy is complex and findings of soft tissue oedema and deep pelvic collections can be subtle on ultrasound (Figures 10 and 11).25
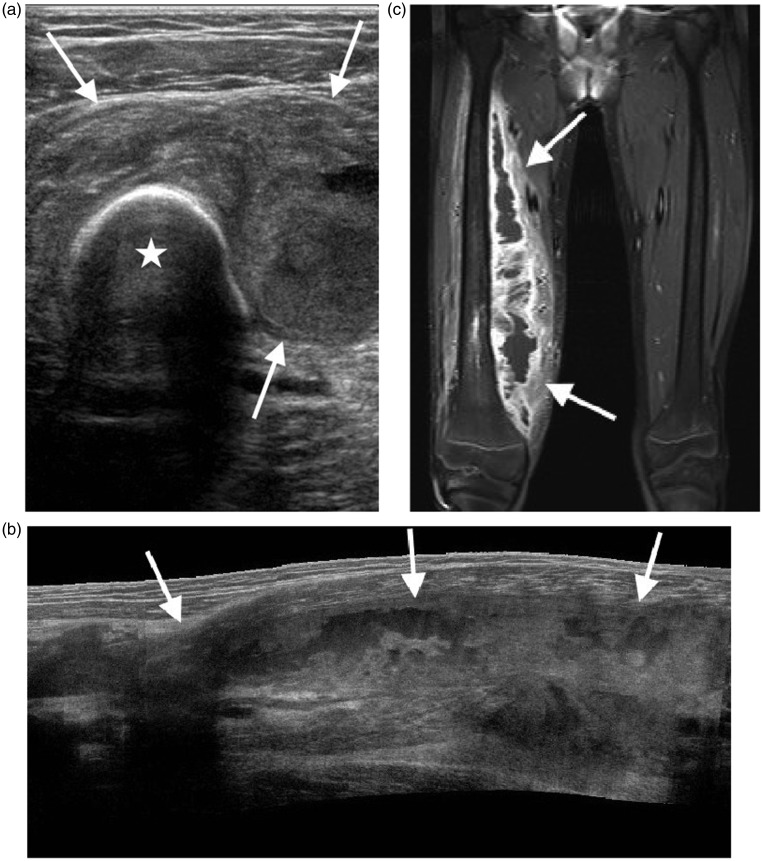
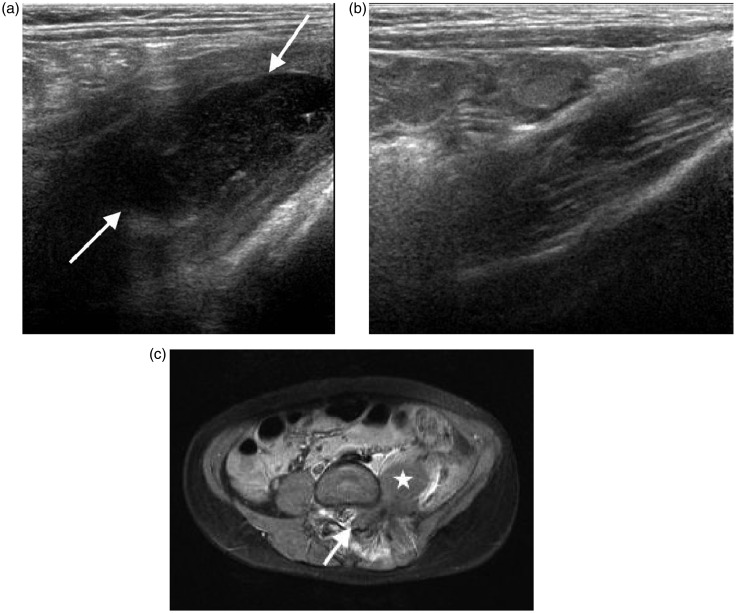
Figure 10.
Limitations of ultrasound shown in a teenager with chronic osteomyelitis. (a) Transverse image of the right upper thigh shows a large collection (arrows) surrounding the femur (*). Extended field of view imaging (b) and low-frequency images (not shown) failed to fully demonstrate the collection in a way useful for surgical planning. (c) Coronal post-contrast T1 weighted fat saturated MR image better demonstrates the full extent of the collection (arrows).
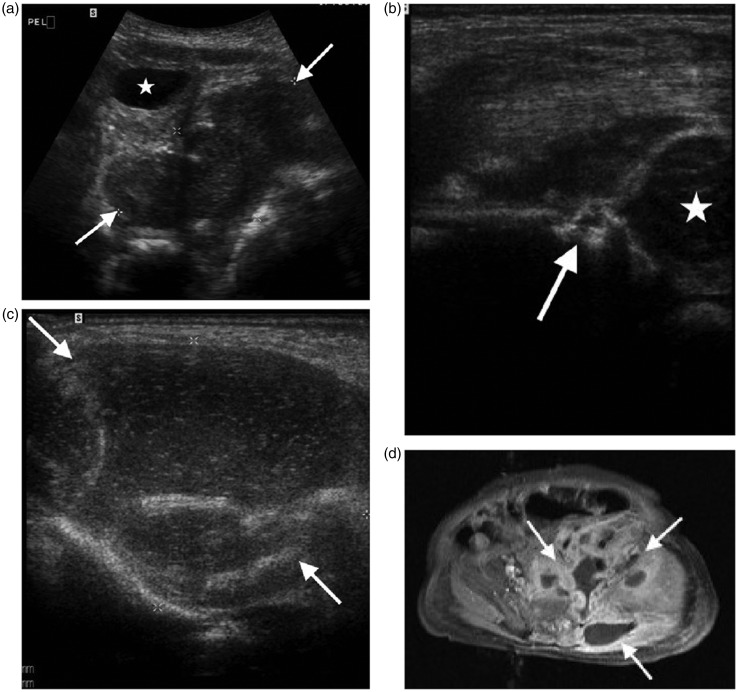
Figure 11.
Neonate of three weeks with pelvic osteomyelitis and soft tissue abscess. (a) Transverse scan through the pelvis shows a large collection (arrows) associated with the left pelvic side wall, bladder (*). (b) Longitudinal scan of the left hip shows bone destruction of the left ilium (arrow), femoral head (*). (c) Transverse scan over the buttock demonstrates a large soft tissue abscess (arrows). (d) Axial post-contrast T1 weighted fat saturated MRI image shows the large collection (arrows) related to the iliac bone extending into the pelvis and buttock, useful for surgical planning.
In children with sickle cell disease, acute pain may be due to vaso-occlusive crisis related bone infarction or osteomyelitis.33 Clinical differentiation can be difficult especially if blood cultures are negative. Ultrasound can help differentiating osteomyelitis from vaso-occlusive crisis if large subperiosteal collections (>4 mm in depth) are detected especially when seen with high CRP and WBC.33–35 William et al.35 suggest that patients with pain and subperiosteal collections of <4 mm require aspiration of the fluid to diagnose osteomyelitis (Figure 12).
Figure 12.
Twelve year old with sickle cell disease and femoral osteomyelitis. (a) Longitudinal extended field of view image of the right femur demonstrates a large subperiosteal collection (arrows). (b) Transverse image shows the eccentric collection (arrow) next to the femur. Wash out of the hip joint effusion (not shown) and drainage of the subperiosteal collections yielded S. aureus.
Deep vein thrombosis (DVT) is a recognised complication of osteomyelitis.36 Localised endothelial damage mediated by surrounding inflammation and release of cytokines that activate the coagulation cascade are thought to play a role, compounded by local oedema compromising venous flow. Clinically, patients complain of rapid onset of intense pain, a greater severity of clinical symptoms, loss of function in the affected limb and extensive soft tissue oedema (Figure 13).36
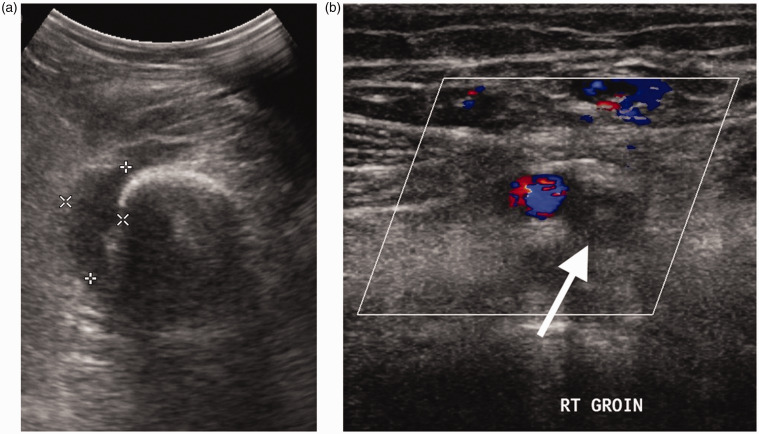
Figure 13.
Teenager with femoral osteomyelitis, septic arthritis and DVT. (a) Transverse scan of the lower thigh demonstrates a collection (callipers) related to the femur. A knee joint effusion (not shown) due to associated septic arthritis was also present. (b) Transverse colour Doppler image of the groin shows a superficial femoral vein (arrow) expanded by thrombus and without colour flow representing DVT.
Pyomyositis
Pyomyositis, an infection of striated muscle, is uncommon as striated muscle is relatively resistant to infection.10 It is caused by S. aureus in over 75% of cases, followed by Mycobacterium tuberculosis, Streptococcus pyogenes and anaerobic bacteria.37–39 Pyomyositis was originally described in tropical climates but penetrating trauma, systemic infections, immunodeficiency syndromes, diabetes, leukaemia and chronic renal failure have accounted for an increase in its incidence worldwide.40,41 Although pyomyositis can affect any muscle, there is a predilection for the large muscles of the pelvis and lower extremity with osteomyelitis and septic arthritis concomitantly present in up to two-thirds of cases.42 Psoas abscesses are a frequent site although multiple areas may be affected.40 Psoas abscesses can be related to bowel disease and appendicitis or spondylodiscitis (Figure 14).
Figure 14.
Four year old with spinal osteomyelitis and psoas abscess presenting with fever and hip pain. (a) Longitudinal scan of the left psoas muscle demonstrates an abscess within the muscle (arrow). (b) Longitudinal scan of the normal right psoas muscle for comparison. (c) Axial post-contrast T1 weighted fat saturated image shows the psoas abscess (*) and an associated extradural collection (arrow) due to osteomyelitis of the posterior spinal elements.
In the early phlegmonous stage of pyomyositis, sonography may identify an increase in muscle volume and oedema with disrupted hyperechoic muscle fibres, hypoechoic septa and generalised hyperaemia.40 In the later suppurative stage, a more discrete, complex fluid collection or abscess can be delineated, surrounded by a thick hyperechoic and hyperaemic wall on colour Doppler imaging (Figure 15).40,43–45 Gas within the abscess is characteristic of infection and manifests as tiny hyperechoic foci with reverberation artefact or ‘dirty shadowing’.46 Dynamic compression aids visualisation of an echogenic liquefied component of the abscess which could be aspirated with ultrasound guidance.47 An enlarged proximal psoas muscle and a focal walled-off collection is best evaluated posterior to the kidneys, from the flank or the lower abdomen (Figure 14).44
Figure 15.
Eight year old with multifocal pyomyositis due to neutropenia while receiving treatment for leukaemia. (a) Longitudinal extended field of view image of the upper lumbar region demonstrates an abscess in the right paraspinal muscles (arrows). (b) Transverse colour Doppler image of the abscess demonstrates a thick echogenic rind with increased vascularity surrounding a hypoechoic centre (*). Several further abscesses were identified in the gluteus and quadriceps muscles (not shown).
Sonography can underestimate the extent of muscle inflammation in the early stages where subtle disruption of muscle fibres and change in echogenicity are the only signs.40 Concerns for pyomyositis in deep pelvic muscles with possible coexistent osteomyelitis and spondylodiscitis are better evaluated with MRI.32
Soft tissue infection and post-operative complications
As soft tissue infection commonly occurs by direct extension through broken skin, trauma and surgery are common predisposing factors. Immunocompromised patients are at increased risk.
Cellulitis and soft tissue abscess
Cellulitis is an inflammatory process of the skin and superficial soft tissues which preferentially affects the extremities in children. S. aureus and S. pyogenes are the commonest causative organisms.10,30
Cellulitis remains a clinical diagnosis but ultrasound findings progress over time and correlate well with clinical findings and serum CRP.48 In the early stages, thickening and increased echogenicity of the skin and subcutaneous tissues are seen (Figure 16). As early as 1–2 days after the onset of symptoms, an advanced stage with distortion of the tissues and layering of fluid between hyperechoic fatty lobules can be observed, giving a ‘cobblestone’ appearance.48 These findings are non-specific and similar appearances may be seen in non-infective subcutaneous oedema.30 Localised abscess formation can be identified with sonography and indicates the late stage.10,48 Abscesses can demonstrate a hypo- or hyperechoic centre. An abscess with a hyperechoic centre may be mistaken for a solid lesion but dynamic assessment to show movement of material within it aids diagnosis and helps assess the need for aspiration.44 Increased flow on power Doppler within the wall of an abscess but not the centre supports the diagnosis.43,45
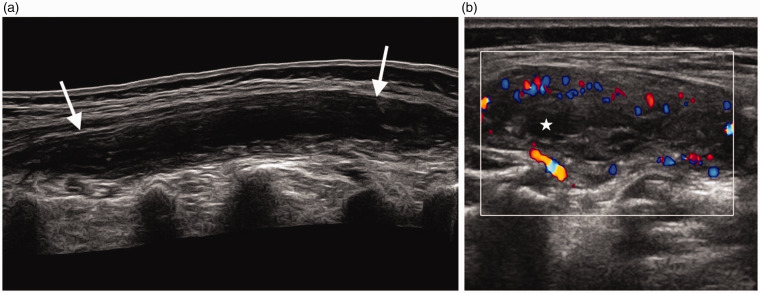
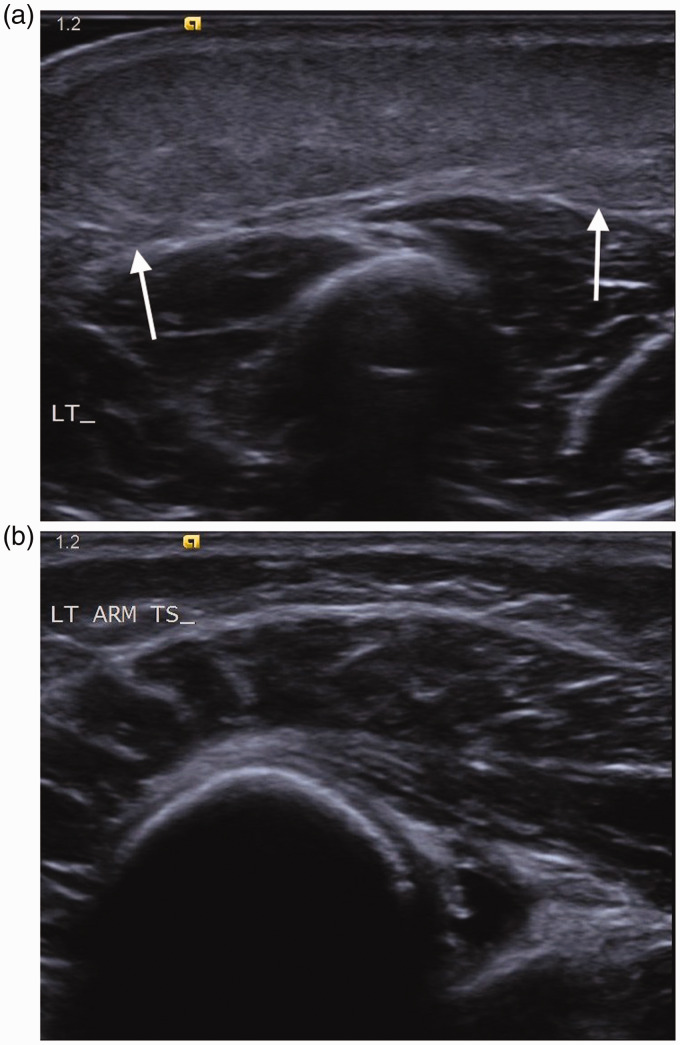
Figure 16.
Infant of 13 months with cellulitis. (a) Transverse scan of the upper arm demonstrates soft tissue oedema and thickening (arrows) excluding an abscess. (b) Transverse scan of the arm distal to the visible area of redness demonstrates normal superficial soft tissues.
Necrotising fasciitis, severe soft tissue infection, can be difficult to diagnose in its early stages when similar findings to cellulitis are seen.10,37 In advanced stages, ultrasound will demonstrate thickening of the fascia with surrounding layered fluid collections.37 If necrotising fasciitis is suspected clinically, cross-sectional imaging may be needed to guide acute intervention.
Infective tenosynovitis
Tenosynovitis represents infection and/or inflammation of the tendon sheath with S. aureus and S. pyogenes being the most common causative organisms.30 Infective tenosynovitis is usually suspected clinically and patients present with raised inflammatory markers.
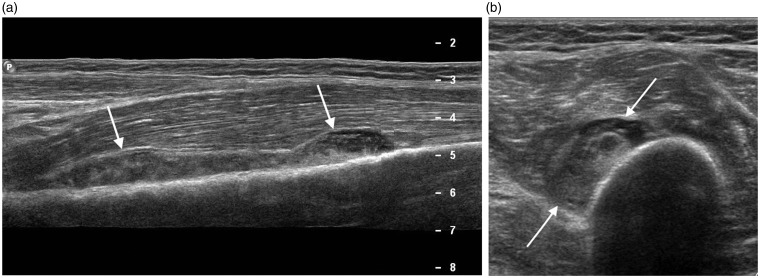
Ultrasound findings of infective tenosynovitis include thickening of the tendon and tendon sheath. Colour Doppler can demonstrate increased vascularity of the tendon sheath and less commonly within the tendon itself. Care should be taken as applying excessive transducer pressure during colour Doppler interrogation may eliminate neovascularity, a supportive feature of infective tenosynovitis.38 The use of colour Doppler can therefore help to distinguish tendon sheath thickening from an effusion which may require drainage under ultrasound guidance. More pronounced tendon sheath fluid collections are a feature of severe suppurative tenosynovitis (Figure 17).30 Thickening, oedema and hyperaemia of the adjacent subcutaneous tissues can indicate peritendinous spread of infection.
Figure 17.
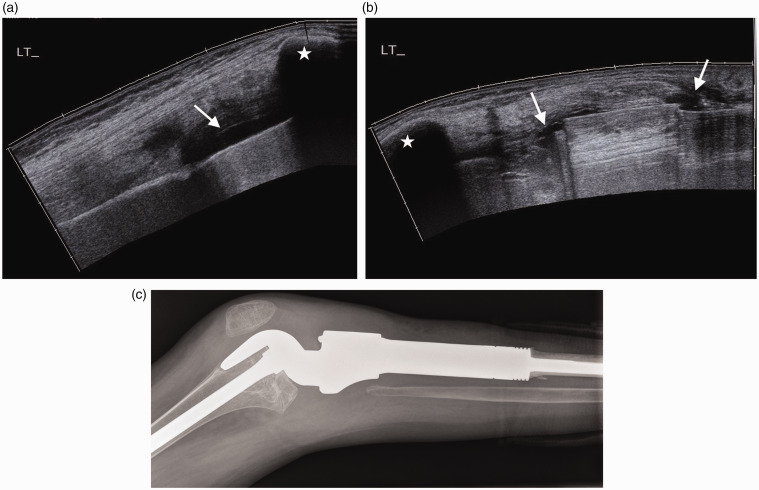
Eleven year old with soft tissue and tendon infection after surgery for tarsal coalition. (a) Longitudinal image of the anterior ankle demonstrates echogenic fluid around the tibialis anterior tendon (arrows) in keeping with tendonitis. (b) Longitudinal image of the lateral ankle shows a separate abscess containing echogenic fluid (arrows).
Infected prostheses and post-operative soft tissue infection
The ferromagnetic material of metal prostheses creates beam-hardening artefact on CT and blooming susceptibility artefact on MRI, thus limiting assessment of patients with suspected post-operative complications involving metal fixation. Artefact from metalwork on ultrasound is less noticeable, but a tightly curved metal prosthetic surface results in scattering and refraction of the ultrasound beam creating posterior acoustic shadowing.39 Hypoechoic fluid collections, subcutaneous oedema and increased vascularity of the adjacent soft tissues on colour Doppler may indicate soft tissue infection (Figure 18). In the early post-operative stages, haematoma appears as ovoid or spherical hypoechoic fluid collections with internal echoes and septations, and can mimic an abscess. Over time, it becomes anechoic and reduces in size. Similarly, periprosthetic soft tissue oedema, evident immediately following surgery, should reduce with time. A follow-up ultrasound scan can therefore help distinguish infection from reactive oedema.49 Juxtacortical non-encapsulated fluid collections in direct contact with the bone have been described as an indirect sign of active bone infection. Cortical discontinuity may be related to post-operative change but cortical breach with increase in vascularity on Doppler in this area strongly suggests active bone infection.50 In patients with an arthroplasty, distension of the pseudocapsule is more frequent with infection. Periosteal hyperaemia is a sign of post-operative reactivated osteomyelitis whereas periosteal thickening alone is a non-specific finding (Figure 18).50
Figure 18.
Teenager with joint infection post joint reconstruction for osteosarcoma. (a) Longitudinal extended field of view scan of the suprapatellar and (b) infrapatellar regions demonstrate fluid (arrows) which is also tracking along the prosthesis. Patella (*) (c) Lateral radiograph of the knee for comparison demonstrates the contour of the prosthesis.
Conclusion
Ultrasound can be a very valuable imaging modality in the diagnosis and follow-up of musculoskeletal infection in children if the operator is aware of its limitations and can interpret findings in the clinical context to direct interventions or further investigation as necessary.
Acknowledgements
None.
Contributors
MS and JKK wrote the draft of the manuscript and selected the images. CH and SOR contributed to the clinical sections of the manuscript. All authors reviewed and approved the final version of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval
As a review article, no approval is required. No specific approval from an authorising body was obtained. Our hospital has a policy that patients can opt out of having their imaging used for teaching purposes and no imaging of patients having used this right has been included.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor
JKK.
ORCID iD
Jeannette K Kraft https://orcid.org/0000-0003-2705-1302
References
- 1.Basmaci R, Ilharreborde B, Bonacorsi S, et al. Septic arthritis in children with normal initial C-reactive protein: clinical and biological features. Arch Pediatr 2014; 21: 1195–1199. [DOI] [PubMed] [Google Scholar]
- 2.Williams N, Cooper C, Cundy P. Kingella kingae septic arthritis in children: recognising an elusive pathogen. J Child Orthop 2014; 8: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal A, Aggarwal AN. Bone and joint infections in children: septic arthritis. Indian J Pediatr 2016; 83: 825–833. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A, Aggarwal AN. Bone and joint infections in children: acute hematogenous osteomyelitis. Indian J Pediatr 2016; 83: 817–824. [DOI] [PubMed] [Google Scholar]
- 5.Riebel TW, Nasir R, Nazarenko O. The value of sonography in the detection of osteomyelitis. Pediatr Radiol 1996; 26: 291–297. [DOI] [PubMed] [Google Scholar]
- 6.Karmazyn B. Imaging approach to acute osteomyelitis in children: an update. Semin Ultrasound CT MRI 2010; 31: 100–106. [DOI] [PubMed] [Google Scholar]
- 7.Pineda C, Espinosa R, Pena A. Radiographic imaging in osteomyelitis: the role of plain radiography, computed tomography, ultrasonography, magnetic resonance imaging and scintigraphy. Semin Plast Surg 2009; 23: 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barberie JE, Wong AD, Cooperberg PL, et al. Extended field-of-view sonography in musculoskeletal disorders. AJR 1998; 171: 751–757. [DOI] [PubMed] [Google Scholar]
- 9.Shah NB, Platt SL. ALARA: is there a cause for alarm? Reducing radiation risk from computed tomography scanning in children. Curr Opinion Pediatr 2008; 20: 243–247. [DOI] [PubMed] [Google Scholar]
- 10.Robben SGF. Ultrasonography of musculoskeletal infections in children. Eur Radiol 2004; 14: 65–77. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Arias M, Balsa A, Mola EM. Septic arthritis. Best Pract Res Clin Rheumatol 2011; 25: 407–421. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery NI, Rosenfeld S. Pediatric osteoarticular infection update. J Pediatr Orthop 2015; 35: 74–81. [DOI] [PubMed] [Google Scholar]
- 13.Offiah A. Acute osteomyelitis, septic arthritis and discitis: differences between neonates and older children. Eur J Radiol 2006; 60: 221–232. [DOI] [PubMed] [Google Scholar]
- 14.Krieg AM. A possible cause of joint destruction in septic arthritis. Arthritis Res 1999; 1: 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarwar ZU, DeFlorio R, Catanzano TM. Imaging of nontraumatic acute hip pain in children: multimodality approach with attention to the reduction of medical radiation exposure. Semin Ultrasound CT MRI 2014; 35: 394–408. [DOI] [PubMed] [Google Scholar]
- 16.Plumb J, Mallin M, Bolte RC. The role of ultrasound in the emergency department evaluation of the acutely painful pediatric hip. Pediatr Emerg Care 2015; 31: 54–58. [DOI] [PubMed] [Google Scholar]
- 17.Gordon JE, Huang M, Dobbs M, et al. Causes of false-negative ultrasound scans in the diagnosis of septic arthritis of the hip in children. J Pediatr Orthop 2002; 22: 312–316. [PubMed] [Google Scholar]
- 18.Kocher MS, Mandiga R, Zurakowski D, et al. Validation of a clinical prediction rule for differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg 2004; 86-A: 1629–1635. [DOI] [PubMed] [Google Scholar]
- 19.Caird MS, Flynn JM, Leung YL, et al. Factors distinguishing septic arthritis from transient synovitis of the hip in children J Bone Joint Surg 2006; 88-A: 1251–1257. [DOI] [PubMed] [Google Scholar]
- 20.Sultan J, Hughes PJ. Septic arthritis and transient synovitis of the hip in children: the value of clinical prediction algorithm. J Bone Joint Surg 2010; 92-B: 1289–1293. [DOI] [PubMed] [Google Scholar]
- 21.Taekema HC, Landham PR, Maconochie I. Towards evidence based medicine for paediatricians. Distinguishing between transient synovitis and septic arthritis in the limping child: how useful are clinical prediction tool? Arch Dis Child 2009; 94: 167–168. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan SL. Recent lessons for the management of bone and joint infections. J Infect 2014; 68: 551–556. [DOI] [PubMed] [Google Scholar]
- 23.Gilbertson-Dahdal DG, Wright JE, Kruoinski E, et al. Transphyseal involvement of pyogenic osteomyelitis is considerably more common than classically taught. AJR 2013; 203: 190–195. [DOI] [PubMed] [Google Scholar]
- 24.Azam Q, Ahmad I, Abbas M, et al. Ultrasound and colour Doppler sonography in acute osteomyelitis in children. Acta Orthop Belg 2005; 71: 590–596. [PubMed] [Google Scholar]
- 25.Mah ET, LeQuesne GW, Gent RJ, et al. Ultrasonic signs of pelvic osteomyelitis in children. Pediatr Radiol 1994; 24: 484–487. [DOI] [PubMed] [Google Scholar]
- 26.Abiri MM, Kirpekar M, Ablow RC. Osteomyelitis: detection with US. Radiology 1998; 172: 509–511. [DOI] [PubMed] [Google Scholar]
- 27.Ezzat T, El-Hamid AA, Mostafa M, et al. Early diagnosis of acute osteomyelitis in children by high-resolution and power Doppler sonography. Egypt J Radiol Nucl Med 2011; 42: 233–242. [Google Scholar]
- 28.Howard CB, Einhorn M, Dagan R, et al. Ultrasound in diagnosis and management of acute haematogenous osteomyelitis in children. J Bone Joint Surg 1993; 75: 79–82. [DOI] [PubMed] [Google Scholar]
- 29.Nath AK, Sethu AU. Use of ultrasound in osteomyelitis. Br J Radiol 1992; 65: 649–652. [DOI] [PubMed] [Google Scholar]
- 30.Chau CLF, Griffith JF. Musculoskeletal infections: ultrasound appearances. Clin Radiol 2005; 60: 149–159. [DOI] [PubMed] [Google Scholar]
- 31.Chao HC, Lin SJ, Huang YC, et al. Colour Doppler ultrasonographic evaluation of osteomyelitis in children. J Ultrasound Med 1999; 18: 729–734. [DOI] [PubMed] [Google Scholar]
- 32.Weber-Chrysochoou C, Corti N, Goetschel P, et al. Pelvic osteomyelitis: a diagnostic challenge in children. J Pediatr Surg 2007; 42: 553–557. [DOI] [PubMed] [Google Scholar]
- 33.Inusa BPD, Oyewo A, Brokke F, et al. Dilemma in differentiating between acute osteomyelitis and bone infarction in children with sickle cell disease: role of ultrasound. PLoS One 2013; 8: e65001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Booz MM, Hariharan V, Aradi AJ, et al. The value of ultrasound and aspiration in differentiating vaso-occlusive crisis and osteomyelitis in sickle cell disease patients. Clin Radiol 1999; 54: 636–639. [DOI] [PubMed] [Google Scholar]
- 35.William RR, Hussein SS, Jeans WD, et al. A prospective study of soft-tissue ultrasonography in sickle cell disease patients with suspected osteomyelitis. Clin Radiol 2000; 55: 307–310. [DOI] [PubMed] [Google Scholar]
- 36.Bouchoucha S, Benghachame F, Trifa M, et al. Deep vein thrombosis associated with acute haematogenous osteomyelitis in children. Orthop Traumatol Surg Res 2010; 96: 890–893. [DOI] [PubMed] [Google Scholar]
- 37.Chao HS, Kong MA, Lin TY. Diagnosis of necrotising fasciitis in children. J Ultrasound Med 1999; 18: 277–281. [DOI] [PubMed] [Google Scholar]
- 38.Hodgson RJ, O’Connor PJ, Grainger AJ. Tendon and ligament imaging. Br J Radiol 2012; 85: 1157–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibbon WW, Long G, Barron DA, et al. Complications of orthopedic implants: sonographic evaluation. J Clin Ultrasound 2001; 30: 288–299. [DOI] [PubMed] [Google Scholar]
- 40.Trusen A, Beissert M, Schultz G, et al. Ultrasound and MRI features of pyomyositis in children. Eur Radiol 2002; 13: 1050–1055. [DOI] [PubMed] [Google Scholar]
- 41.Belli LC, Reggiori A, Cocozza E, et al. Ultrasound in tropical pyomyositis. Skelet Radiol 1992; 21: 107–109. [DOI] [PubMed] [Google Scholar]
- 42.Guillerman RP. Osteomyelitis and beyond. Paediatr Radiol 2013; 43: 193–203. [DOI] [PubMed] [Google Scholar]
- 43.Arslan H, Sakarya ME, Bozkurt M, et al. The role of power Doppler sonography in the evaluation of superficial soft tissue abscesses. Eur J Ultrasound 1998; 8: 101–106. [DOI] [PubMed] [Google Scholar]
- 44.Royston DD, Cremin BJ. The ultrasonic evaluation of psoas abscess (tropical pyomyositis) in children. Pediatr Radiol 1994; 24: 481–483. [DOI] [PubMed] [Google Scholar]
- 45.Breidahl WH, Newman JS, Taljanov MS, et al. Power Doppler sonography in the assessment of musculoskeletal fluid collections. AJR 1995; 166: 1443–1446. [DOI] [PubMed] [Google Scholar]
- 46.Loyer EM, DuBrow RA, David CL, et al. Imaging of superficial soft-tissue infections: sonographic findings in cases of cellulitis and abscess. AJR 1996; 166: 149–152. [DOI] [PubMed] [Google Scholar]
- 47.Loyer EM, Kaur H, David CL, et al. Importance of dynamic assessment of the soft tissues in the sonographic diagnosis of echogenic superficial abscesses. J Ultrasound Med 1995; 14: 669–671. [DOI] [PubMed] [Google Scholar]
- 48.Chao HC, Lin SJ, Huang YC, et al. Sonographic evaluation of cellulitis in children. J Ultrasound Med 2000; 19: 743–749. [DOI] [PubMed] [Google Scholar]
- 49.Chun KA, Cho KH. Postoperative ultrasonography of the musculoskeletal system. Ultrasonography 2015; 34: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balankia AP, Papakonstantinou O, Kontopoulou CJ, et al. Gray-scale and color Doppler ultrasonographic evaluation of reactivated post-traumatic/postoperative chronic osteomyelitis. Skeletal Radiol 2009; 38: 363–369. [DOI] [PubMed] [Google Scholar]