Abstract
Objective:
To explore the feasibility and effects of a computer-assisted cognitive rehabilitation intervention, Memory, Attention, and Problem Solving Skills for Persons with MS (MAPSS-MS), for persons with multiple sclerosis (MS) on cognitive performance, memory strategy use, self-efficacy for control of symptoms and neuropsychological competence in activities of daily living (ADL).
Design:
A randomized controlled single-blinded trial with treatment and wait list-control groups.
Setting:
Southwestern United States.
Subjects:
Convenience sample of 61 persons (34 treatment, 27 wait list control) with MS (mean age 47.9 years, SD 8.8).
Intervention:
The eight-week MAPSS-MS intervention program included two components: (a) eight weekly group sessions focused on building efficacy for use of cognitive compensatory strategies and (b) a computer-assisted cognitive rehabilitation program with home-based training.
Outcome Measures:
A neuropsychological battery of performance tests comprising the Minimal Assessment of Cognitive Function in MS (MACFIMS) and self-report instruments (use of memory strategies, self-efficacy for control of MS and neuropsychological competence in ADL) were completed at baseline, 2 months (after classes), and at 5 months.
Results:
Both groups improved significantly (p<.05) over time on most measures in the MACFIMS battery as well as the measures of strategy use and neuropsychological competence in ADL. There was a significant group by time interaction for scores on the measures of verbal memory and use of compensatory memory strategies.
Conclusions:
The MAPSS-MS intervention was feasible and well-accepted by participants. Given the large relative increase in use of compensatory strategies by the intervention group, it holds promise for enhancing cognitive function in persons with MS.
Introduction
According to current estimates, more than 400,000 persons in the United States and 2.5 million worldwide live with multiple sclerosis (MS).1 This unpredictable and generally progressive disease of the central nervous system is characterized by demyelinating lesions in the brain and central nervous system as well as damage and death of axons.2 The effects of MS on cognition, which are thought to occur in 50–75% of persons with MS, have gained increasing recognition as “potentially the most disabling symptom of the disease”. 3 While numerous studies have addressed the emotional and physical impact of MS, little attention has been given to strategies that might help manage the cognitive changes commonly experienced by persons with MS.
Numerous neuropsychological studies with persons with MS have demonstrated deficits in tasks assessing recent memory, attention, information processing (including processing speed), executive functions (including verbal learning), and visuospatial abilities. 3–5 Cognitive impairment seems to have major effects on the lives of persons with MS including influencing role fulfillment in both work and social life.6 Persons with MS with cognitive dysfunction were less likely to be employed, required greater personal assistance and were less likely to engage in social activities7, reported more difficulties parenting, 8 and performed significantly worse on computerized tests of driving skills and had higher rates of motor vehicle crashes than cognitively intact persons with MS and healthy controls.9,10
To date, there have been relatively few studies of cognitive rehabilitation interventions for persons with MS.4 and even fewer that have incorporated computer-training of cognitive skills with a strong randomized clinical trial design. Although not extensive, there is some evidence 11,12,13,14 to suggest that computer-assisted cognitive rehabilitation results in improved neuropsychological performance for persons with MS with subjective concerns or objective evidence of cognitive impairment.
The purpose of this study was to refine and test a novel computer-assisted cognitive rehabilitation intervention, MAPSS-MS (Memory, Attention, & Problem Solving Skills for persons with MS). A group intervention focusing on use of compensatory strategies was paired with an innovative computer-assisted cognitive rehabilitation component that was implemented in the participant’s home.15 We hypothesized that, compared to persons in the wait-list control group, persons with MS who participate in the MAPSS-MS intervention would:
-
➢
Demonstrate significant improvements in cognitive function on the performance measures of memory, language, spatial processing, processing speed, and executive function; and
-
➢
Report significant improvement in self-efficacy for control of MS effects, use of memory strategies, and neuropsychological competence in activities of daily living.
The effects of the intervention on the outcome variables were assessed over a 5-month period, with measurements at baseline, at 2 months (immediately after the MAPSS-MS intervention), and at 5 months (i.e. 3 months after the intervention was complete).
Method
Following approval by our Institutional Review Board, participants were recruited over an 11-month period through physician and health care provider referrals and notices in MS newsletters and publications. A screening interview was conducted with potential participants by phone to ensure that they met the inclusion criteria (being 18 to 60 years of age, able to understand and comply with the study protocol including reading and writing in English, visual acuity with correction sufficient to work on a computer screen, clinically definite MS for at least 6 months that was documented by a physician and stable disease status at the time of study entry). Study staff administered the Perceived Deficits Questionnaires16 by phone and those reporting that they experienced at least 5 problems “sometimes” or more often were eligible to continue in the study. Subjects were excluded if they had other medical causes of dementia, other neurological disorders that might impact cognition, evidence of major psychiatric disorder, or major functional limitations that precluded them from participating in the study.
The persons who were initially screened as “eligible” were told that we would notify them later with exact dates for the baseline neuropsychological testing and the classes. The study was a randomized design with the intervention delivered in small groups over a 15-month period. Twenty to twenty-two persons were available to be scheduled for each of three study cohorts. Prior to the initiation of data collection, the data analyst for the project generated a random number sequence for randomization to intervention and control and placed each assignment in a separate, sealed envelope. Following the completion of informed consent, the baseline neuropsychological tests, and the study questionnaires, the project director opened the next sealed envelope and informed the participants of their random assignments to either the intervention or wait-list control groups.
Study Overview
Self-report and performance data were collected from participants at baseline, 2 months, and 5 months. The staff members conducting neuropsychological assessments were blinded to participants’ group assignment. Given the nature of the design, it was not possible to blind either participants or the group facilitators to treatment allocation once the group sessions began. However, the project director and interventionist who knew individual subjects and their group assignments were not involved in collecting, entering, or analyzing data.
Participants were instructed to complete the questionnaire booklet at home and return it to staff when they came for their neuropsychological tests. Participants received a small incentive ($50) for completing each of the three data collections for a total of $150. Using effect size estimates from prior research with a group wellness intervention for persons with MS,17 a total sample size of 60 was determined to be sufficient to detect statistically significant differences between the two groups (assuming an alpha level of .05) in the performance outcome variables with a moderate effect size .18
Intervention Program
The overall purpose of the MAPSS-MS intervention is to help the individual acquire the highest level of cognitive functioning and functional independence. This was accomplished through teaching the use of compensatory skills, retraining skills (the computer component), and environmental/lifestyle support for cognitive functioning. The MAPSS-MS has two components: (a) eight weekly two-hour group sessions focused on building efficacy for use of cognitive compensatory strategies and (b) a computer-assisted cognitive training program. The home-based computer components19 enabled the participants to engage in practice sessions (minimum of 45 minutes three times per week) without leaving their homes. Translation of skills practiced (e.g. remembering a series of numbers) to everyday issues was a focus of the group sessions.
Details of the intervention components and delivery are provided in Appendix A. The facilitator (interventionist) was a master’s prepared nurse carefully trained prior to the initiation of the study. Each group session was audiotaped and reviewed by the principal or co-investigator for fidelity to both the content and process aspects of the intervention. In addition, electronic logs of participant practice were monitored (which track and which skill) and participants maintained a log of their home practice.
Instruments
The proposed study used both self-report and performance-based measures to provide a more complete understanding of the effects of the proposed intervention. All measures were used at the three data collection points with the exception of demographic information. A Background Information Sheet (BIS) was used to collect information on a variety of demographic (age, gender, education, computer experience, marital status, ethnicity) and disease characteristics. Length of illness was determined by using the subjects’ self-report of the year of diagnosis of MS. Participants completed the Self-Administered Expanded Disability Status Scale (EDSS-S)20 as a measure of functional limitations and impairment due to MS. Scores can range from 0 to 9.5 and the intraclass correlation between patient ratings on the EDSS-S and physician ratings on the EDSS was 0.89.20
Performance Outcomes:
The Minimal Assessment of Cognitive Function in MS (MACFIMS), a recommended battery of neuropsychological tests based on a consensus of the findings of an international conference of experts,21 was used to measure cognitive performance. Benedict and colleagues22 have presented evidence for the construct and concurrent validity of the MACFIMS battery in a sample of 291 persons with MS. The 90-minute battery includes seven well-known and widely used tests covering the five cognitive domains as listed below:
Controlled Oral Word Association Test (COWAT)23 - verbal fluency and word finding;
The Judgment of Line Orientation test (JLO)23 - visuospatial processing;
The California Verbal Learning Test, second edition, (CVLT-II)24 - verbal learning and remembering;
The Brief Visuospatial Memory Test - Revised (BVMT-R)25 - nonverbal learning and memory;
The Paced Auditory Serial Addition Test (PASAT)26 - auditory information processing speed and flexibility as well as calculation abilities;
The Symbol Digit Modalities Test (SDMT)27 - complex scanning and visual tracking; and
The Sorting Test from the Delis-Kaplan Executive Function System (DKEFS)28 - higher executive function skills.
Each measure was administered by a trained rater following standardized testing protocols.
Self-Report Outcomes:
The Control subscale of the MS Self-Efficacy Scale (MSSE-Control)29 was used as a measure of confidence in the ability to manage disease symptoms, reactions to disease-related limitations and the impact of disease on life activities. Respondents were asked to rate each of 9 items from 10 (very uncertain) to 100 (very certain) and scores are summed for a total score ranging from 90 to 900. Sample items include: how certain are you that you can keep your MS symptoms from interfering with your time spent with friends and family; and how certain are you that you can manage your MS symptoms so that you can do the things you enjoy? Internal consistency reliability at the three time points ranged from .93 to .95. The Strategy Subscale of the Multifactorial Memory Questionnaire (MMQ-Strategy)30 was used as a measure of self-assessed use of memory strategies. The 19 items describe various memory aids and strategies such as making to-do lists and writing appointments on a calendar. Respondents indicated the frequency with which each strategy was used during the past two weeks using a 5 point scale (never, rarely, sometimes, often, all the time). Higher scores indicated more frequent use of memory strategies. The internal consistency reliability ranged from .83 to .88. Content validity is supported by expert raters and convergent validity is supported by strong correlations between scores of the MMQ-Strategy and the scores of memory strategies scales on the Metamemory in Adulthood Questionnaire (r=.64) and the Memory Functioning Questionnaire (r=.66).30
The MS Neuropsychological Screening Questionnaire (MSNQ)31 was used as a measure of neuropsychological competence with activities of daily living. Respondents rated how often they experienced each of the 15 items during the last three months. The items include common problems reported by persons with MS (e.g., being easily distracted, difficulty following conversations, forgetting appointments). The Cronbach’s alpha for internal consistency reliability ranged from .94 to .95 and scores were significantly correlated with scores on a battery of neuropsychological tests and measures of whole brain lesion burden and atrophy in prior research.31,32
Analysis of Data
Data analyses were conducted using SPSS, version 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Frequency distributions, means, and standard deviations (SDs) were calculated for the demographic, neurocognitive performance, and other health-related variables. The design of the study was a repeated measures design with three time points (baseline, immediate post-intervention, and 3 months later). Thus we used a 2 × 3 repeated measures analysis of variance (RM ANOVA) utilizing a multivariate approach to avoid the more stringent assumptions of the univariate model. The RM ANOVA procedure partitions variance in outcomes into components related to time, treatment group, and the interaction between time and treatment. The interaction effect is the most useful test of the intervention effects because we can determine if there is significantly greater change over time for the intervention group compared to the control group.33 T-tests were used to compare the change scores (baseline to Time 3) between the two groups. All tests were conducted with an alpha level of p<.05. Separate analyses were conducted for each of the outcome measures.
The analyses of effects of treatment on outcome measures were based on conservative intention to treat methodology. Thus, the final study sample for analysis of the intervention effects included those who had completed the baseline data collection and attended at least one of the classes. Missing values were replaced with the last observation value carried forward if the participant did not complete later measurements. If participants were missing an intermediate value (immediately after the intervention at time 2), we imputed the missing value as the average of times 1 and 3. In addition, baseline scores on the SDMT for 11 participants were invalidated due to testing error. For these 11 individuals, a multiple imputation procedure was used to impute their baseline values using the SPSS add-on module for missing values (SPSS 17.0 version).
Results
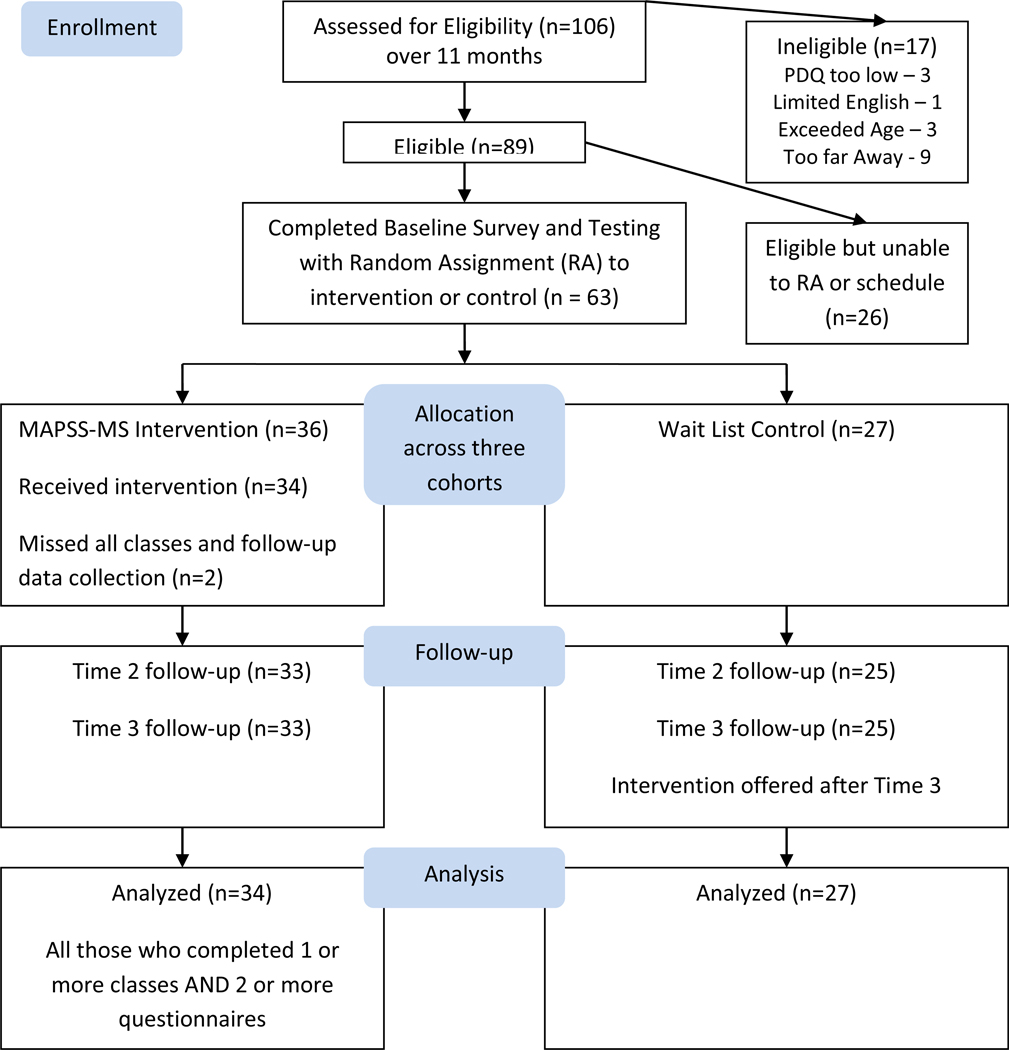
Figure 1 depicts the flow of participants through recruitment and data collection. As seen in Figure 1, 61 persons with MS (34 intervention, 27 control) were included in the analysis. This sample represented 97% of those who completed baseline measures and were randomized to a group. The 61 participants (see Table 1) ranged in age from 24 to 60 years (mean ± SD, 47.95 ± 8.76 years) and the majority were White (n=54, 89%), married (n=40, 66%) and not employed full-time (n=48, 79%). The sample was well-educated, with the majority having completed some post-secondary education.
Figure 1.
Flow Chart of Intervention
Table 1.
Sample Demographics (N=61)
| Characteristic | Categories | Intervention Group (n=34) |
Wait list Control Group (n=27) |
Total (n=61 |
|---|---|---|---|---|
| n(%)* | n(%)* | n(%)* | ||
| Gender | Male | 5 (15%) | 2 (7%) | 7 (11%) |
| Female | 29 (85%) | 25(93%) | 54(89%) | |
| Age | 20–35 years | 3 (9%) | 3 (11%) | 6 (10%) |
| 36–50 years | 14 (41%) | 13 (48%) | 27 (44%) | |
| 51–60 years | 17(50%) | 11 (41%) | 28 (46%) | |
| Education | High School Grad | 13 (38%) | 7 (26%) | 20 (33%) |
| Associate Degree | 3 (9%) | 2 (7%) | 5 (8%) | |
| Bachelors Degree | 8 (24%) | 11 (41%) | 19 (31%) | |
| Graduate Degree | 10 (29%) | 7 (26%) | 17 (28%) | |
| Race/Ethnicity | White | 29 (85%) | 25 (93%) | 54 (89%) |
| African American | 2 (6%) | - | 2 (3%) | |
| Multiple categories | - | 2 (7%) | 2 (3%) | |
| Other | 3 (9%) | - | 3 (5%) | |
| Marital Status | Married | 21 (62%) | 19 (70%) | 40 (66%) |
| Un-married | 13 (38%) | 8 (30%) | 21(34%) | |
| Employment Status | Full-time | 7 (21%) | 6 (22%) | 13 (21%) |
| Part-time | 5 (15%) | 8 (30%) | 13 (21%) | |
| Unemployed | 22 (65%) | 13 (48%) | 35 (58%) |
Percentage totals may not add to 100% due to rounding.
On average, this sample had mild to moderate impairment from MS as EDSS scores ranged from 2.5 to 8.5 with a mean of 5.2 ± 1.23. The length of time since diagnosis with MS ranged from 1 to 29 years (mean ± SD, 12.2 ± 7.4 years). To ensure that the demographic and illness characteristics associated with the intervention and control groups were comparable, we used independent sample t-tests for continuous variables to test for differences between the two groups at baseline. The only significant baseline difference on the neuropsychological battery was significantly higher control group scores on the DKEFS (p<.05). There were no statistically significant differences on the self-report measures, or in age, years of education, or time since diagnosis.
Overall, participation in the intervention was high. The mean number of classes attended was 6.1 (SD=1.4) for intervention participants. The majority of the participants (79 – 82% each week) met or exceeded the minimum times of practice sessions each week of the prescribed tracks, as well as met or exceeded the minimum number of minutes of required practice (67–82% each week).
Table 2 provides the means and standard deviations for the intervention and control groups on the major study variables. An examination of the pattern of scores across time periods and between groups revealed that the intervention group typically had greater gains over time than the comparison group. As seen in Table 3, the change scores from baseline to Time 3 (5 months) were significantly different between the two groups for two of the measures - the CVLT-Total (a performance measure of learning and memory) and the Strategy Subscale of the Multifactorial Memory Questionnaire (the self-report of the frequency of use of compensatory memory strategies).
Table 2.
Mean scores by time for wait list control and intervention groups with time and group by time effects
| Scale | Group | Time 1 Baseline |
Time 2 | Time 3 | F (df) Time Effect |
F (df) Group by Time Effect |
|---|---|---|---|---|---|---|
| CVLT Total | Control | 50.3±12.2 | 50.2±12.1 | 53.8±14.3 | 26.07*** | 4.25* |
| Intervention | 49.9±11.3 | 52.2±12.3 | 58.4±13.6 | (2,118) | (2,118) | |
| CVLT Delay | Control | 10.9±4.0 | 10.7±4.1 | 11.4±4.1 | 5.02** | 1.90 |
| Intervention | 11.3±3.8 | 12.3±3.6 | 12.5±4.1 | (2,118) | (2,118) | |
| BVMT Total | Control | 24.3±8.0 | 24.1±7.8 | 24.6±6.9 | 1.32 | 0.54 |
| Intervention | 23.5±6.5 | 23.8±7.6 | 24.9±6.0 | (2,118) | (2,118) | |
| BVMT Delay | Control | 8.8±3.2 | 9.1±3.1 | 8.8±2.8 | 0.22 | 0.42 |
| Intervention | 9.5±2.4 | 9.33.0 | 9.3±2.1 | (2,118) | (2,118) | |
| JLO | Control | 26.1±4.6 | 26.2±5.2 | 27.4±4.2 | 6.72** | 1.24 |
| Intervention | 26.6±3.9 | 27.5±3.6 | 27.8±3.9 | (2,118) | (2,118) | |
| SDMT | Control | 46.3±13.7 | 48.1±14.0 | 50.6±13.1 | 11.86*** | 1.86 |
| Intervention | 44.6±10.4 | 49.6±11.1 | 49.7±12.7 | (2,118) | (2,118) | |
| PASAT - 3 second | Control | 41.9±12.4 | 46.7±11.2 | 47.2±10.7 | 13.47*** | 0.98 |
| Intervention | 42.6±10.7 | 45.2±11.2 | 47.4±9.6 | (2,118) | (2,118) | |
| PASAT - 2 second | Control | 31.5±9.8 | 34.9±11.5 | 38.1±9.8 | 24.11*** | 1.60 |
| Intervention | 28.4±8.7 | 34.0±9.3 | 34.2±9.8 | (2,118) | (2,118) | |
| COWAT | Control | 33.4±12.7 | 35.3±11.7 | 36.4±12.0 | 3.77* | 0.25 |
| Intervention | 34.3±11.3 | 35.8±10.6 | 36.1±10.7 | (2,118) | (2,118) | |
| DKEFS-Descriptive | Control | 38.9.1±11.6 | 38.8±12.3 | 41.7±10.5 | 13.03*** | 0.92 |
| Intervention | 34.6±8.6 | 34.9±11.1 | 39.6±8.7 | (2,118) | (2,118) | |
| DKEFS - Sort | Control | 10.21±3.0 | 10.1±3.2 | 10.9±2.7 | 12.00*** | 0.49 |
| Intervention | 9.2±2.3 | 9.2±2.7 | 10.2±2.1 | (2,118) | (2,118) | |
| Self-Efficacy | Control | 527.45± 186.04 | 540.19± 203.25 | 534.26± 201.06 | 1.48 | 0.66 |
| Intervention | 517.13 ±168.44 | 553.24± 167.58 | 557.72± 157.84 | (2,118) | (2,118) | |
| Memory Strategy | Control | 41.3± 10.46 | 40.43± 9.34 | 41.15± 10.65 | 4.72* | 7.23** |
| Intervention | 37.68 ±11.06 | 43.63± 11.32 | 43.12± 11.93 | (2,118) | (2,118) | |
| MSNQ | Control | 28.62 ±12.54 | 27.92± 11.11 | 26.15± 11.56 | 5.50** | 0.20 |
| Intervention | 31.18± 11.12 | 29.68± 10.74 | 28.41± 11.13 | (2,118) | (2,118) |
Note. All F tests use the Wilks Lambda statistic.
p < .05;
p < .01;
p < .001
CVLT - California Verbal Learning Test ; BVMT - Brief Visuospatial Memory Test - Revised; JLO - Judgment of Line Orientation Test; SDMT - Symbol Digit Modalities Test; PASAT - Paced Auditory Serial Addition Test; COWAT - Controlled Oral Word Association Test; DKEFS - Delis-Kaplan Executive Function System; MSNQ - MS Neuropsychological Screening Questionnaire
Table 3.
Change score (Baseline to Time 3) means, standard deviation, and statistical significance
| Measure | Intervention Group (n=34) Mean and S.D. |
Wait List Control Group (n=27) Mean and SD |
P value |
|---|---|---|---|
| CVLT Total | 8.41 ± 6.52 | 3.48 ± 6.50 | 0.005** |
| CVLT Delay | 1.24 ± 2.28 | 0.52 ± 1.95 | 0.20 |
| BVMT Total | 1.41 ± 4.02 | 0.19 ± 5.25 | 0.31 |
| BVMT Delay | −0.15 ± 1.58 | 0.00 ± 1.90 | 0.74 |
| JLO | 1.21 ± 2.68 | 1.33 ± 2.83 | 0.86 |
| SDMT | 5.03 ± 7.50 | 4.25 ± 7.66 | 0.69 |
| PASAT - 3 second | 4.85 ± 5.97 | 5.33 ± 9.81 | 0.81 |
| PASAT - 2 second | 5.88 ± 6.48 | 6.59 ± 7.47 | 0.69 |
| COWAT | 1.76 ± 7.18 | 3.04 ± 6.66 | 0.48 |
| DKEFS-Descriptive | 5.03 ± 7.99 | 2.81 ± 6.29 | 0.24 |
| DKEFS - Sort | 1.18 ± 2.12 | 0.70 ± 1.54 | 0.34 |
| MS Self Efficacy | 40.59 ± 114.70 | 6.81 ± 111.09 | 0.25 |
| MSNQ Score | −2.77 ± 8.02 | −2.47 ± 4.91 | 0.86 |
| Memory Strategy | 5.44 ± 7.16 | −0.15 ± 7.67 | 0.005** |
p < .05,
p < .01
CVLT - California Verbal Learning Test ; BVMT - Brief Visuospatial Memory Test - Revised; JLO - Judgment of Line Orientation Test; SDMT - Symbol Digit Modalities Test; PASAT - Paced Auditory Serial Addition Test; COWAT - Controlled Oral Word Association Test; DKEFS - Delis-Kaplan Executive Function System; MSNQ - MS Neuropsychological Screening Questionnaire
The RM ANOVA analysis revealed that there were significant time effects observed for six of the seven neuropsychological performance tests, as well as the two self-report measures of use of compensatory strategies, and for the MSNQ (the measure of neuropsychological competence with activities of daily living). These significant time effects indicate that scores changed significantly over the five months for participants in both the MAPSS-MS and the wait-list control groups. Two significant group x time interactions were observed. The interaction effect was significant for scores on the MMQ-Strategy, the self-report measure of the frequency of using compensatory memory strategies (F=7.23, p<.01) and the CVLT-Total, the measure of verbal memory (F=4.25, p<0.05). The effect size in the ANOVAs (partial eta squared) was generally small (.044 or less) for five of the seven neuropsychological tests, as well as the MSSE-Control and the MSNQ. However, a medium effect size (partial eta squared 0.06 or greater) was observed for the CVLT and the SDMT - the measures of verbal memory and complex scanning and visual tracking. There was a large interaction effect (partial eta square of .20) for the measure of compensatory memory strategies.
DISCUSSION
This exploratory study of the MAPSS-MS intervention provided initial evidence that this intervention can assist persons with MS to develop compensatory strategies to manage cognitive symptoms. Although there were only two significant interaction effects (use of compensatory strategies and verbal memory), the observed effects were medium-large. In addition, almost all changes in scores over time were statistically significant and in the desired direction - including greater self-efficacy, more frequent use of compensatory strategies, and improved performance on neuropsychological tests. Overall, the MAPSS-MS group had greater improvements than the control group on six of the seven neuropsychological tests and on all three of the self-report measures. However, the observed differences between groups were small and most of the differences in change scores between groups were not statistically significant.
The large effect size observed for the significant time x group interaction (indicating a significant effect of the intervention over time) observed in the scale measuring use of compensatory memory strategies is not surprising. Building efficacy and skills for these strategies was the key focus of the group component of the intervention. Anecdotally, participants reported multiple examples of how use of new strategies allowed them to function more easily in their daily lives. For example, they learned that they were able to learn and retain information more easily when there were fewer distractions present. It is notable that the mean use of strategies actually increased from the end of the actual group sessions (Time 2) when strategy use was being constantly reinforced to Time 3 when individuals were “on their own.” This finding suggests that performance accomplishment (experiencing success in use of one behavior) may have transferred to use of other strategies. In fact, learning and using these strategies may have contributed to the medium effects seen in improvement in the CVLT scores, since many of the strategies recommended and practiced (e.g. clustering of words) could generalize to the follow-up testing sessions.
While there was not a significant impact on scores of the measure of self-efficacy, this may be because the instrument used in this study includes many other MS symptoms that were not addressed in this intervention. Data provided in the homework logs (comments, minutes and times practiced) supported the feasibility of the home computer training and are reported in more depth elsewhere.15
Findings from this exploratory randomized controlled trial of the MAPSS-MS intervention must be interpreted with caution because of the convenience sample and the potential for selection bias. Although participants were easily recruited for the study, in fact “perceived cognitive deficits” were required in order to be eligible for the study. Thus, this sample may have been highly motivated to learn and use compensatory strategies. Treatment contamination is very difficult if not impossible to control in community-based studies. Given that participants could not be blinded to their assignment once they were assigned to a group, it is possible that persons assigned to the wait-list control group may have independently sought out information on compensatory cognitive strategies through the Internet or text information. There are also numerous online cognitive training programs available to the public. Such proactive efforts may explain, in part, why persons in both groups made significant gains over time. In addition, the design of the study with a multi-component intervention limits the ability to determine if one or both components were responsible for the change in outcomes and the relatively small sample limited the power to find statistically significant effects in many of the analyses.
It is also important to note that the inclusion criteria for this study was a perceived moderate level of cognitive difficulty rather than an objective performance measure of cognitive impairment on a neuropsychological test. We would argue that perception of cognitive deficits is, in fact, the most appropriate inclusion criteria as it considers the individuals’ experience of their level of functioning as it interacts with the demands in their environments. Persons in demanding vocational or other life circumstances may be in fact ‘impaired’ in their roles if they lose even small amounts of their cognitive functional abilities, and compensatory strategies are essential to support their functioning. In addition, many persons with MS - including those in this study - are well-educated, and thus it is likely that these individuals would have had preexisting high performance on various tests. It is likely that such individuals might be facing substantial limitations and disability in their everyday lives and still not screen as cognitively impaired based on failure on tests.
To our knowledge, this is the first study to integrate the powerful effects of group interventions to build self-efficacy for new compensatory behaviors with individual home-based computer training. With this innovative approach to improve cognitive functioning, participants had the opportunity in the group setting to develop the knowledge and skills necessary to enact strategies that could support their cognitive functioning. Participants then engaged in structured practice exercises on the computer that allowed them to build cognitive skills, practicing at home at times they have the most energy and that are more convenient. While most tests of cognitive interventions with persons with MS have limited outcomes with respect to performance on structured neuropsychological tests,4 we added additional outcome measures to assess self-efficacy for management of MS, use of compensatory strategies, and neuropsychological competence in activities of daily living to determine whether the intervention effects on neuropsychological tests translate into the everyday lives of persons with MS.
Although performance-based measures (e.g. neuropsychological tests) are often assumed to be superior to self-reported ones, no extensive empirical evidence exists to support this assumption. Sullivan et al.16 argue that while self-reports of cognitive functioning often correlate poorly with neuropsychological tests, the ultimate utility of self-report measures of cognitive function and other constructs lies in the information they add to performance outcomes, rather than the degree to which they are similar. In a direct comparison of self-report and performance measures of basic and instrumental ADLs, Meyers and colleagues34 found that relative to self-report questionnaires, performance measures were not psychometrically superior or more acceptable to participants. It seems likely that neuropsychological performance tests and self-reports of cognitive functioning (or problems with such) may in fact be measuring somewhat different constructs - how one operates in a complex demanding world versus how one performs on a structured exercise/test in a controlled environment. Unfortunately, this study is limited by the lack of a strong outcome reflecting cognitive functioning in everyday life.
While statistically significant effects are of primary interest to researchers, meaningful clinical effects are most important for practice. The effects of the interventions on the outcome variables, measured at 5 months post-baseline (3 months after completion of the intervention), were small-medium for all outcomes except use of memory strategies and CVLT scores which had a medium-large effect. While these small and limited effects on neuropsychological tests are somewhat disappointing, they are consistent with the reports in the literature.4,13,14 The small effects may due be to insufficient strength of the intervention - however, one might also question whether an intervention that promoted compensation and adaptation would be expected to induce changes in performance on neuropsychological tests. While there is some initial evidence to support the possibility of neuroplasiticity in persons with MS,11,12 it is unlikely that a modest intervention would change the underlying impairment reflected in neuropsychological tests.
Since this intervention focused on changes in behavior in the context of cognitive impairment, its impact on the underlying impairment as measured by the neuropsychological battery of tests may be of limited clinical significance. However, the 14% improvement in scores on the use of memory strategies for the MAPSS-MS group (compared to 0% change in the wait-list control group) reflects the focus and impact of the intervention on compensation and adaptation and is likely to have clinically significant beneficial effects on the functioning of persons with MS. This effect is particularly notable in that it persisted three months after the intervention ended.
Given the frequency of difficulties with cognitive functioning among persons with MS and the lack of well-established cognitive rehabilitation interventions, future studies with larger samples are needed to further test and refine the MAPSS-MS intervention. The addition of a strong functional outcome measure is essential to explore whether the intervention generates improvement of cognitive functioning in ‘real world’ activities.
Supplementary Material
Clinical Messages.
The 2-month MAPSS-MS intervention produced statistically and clinically significant improvements in use of compensatory strategies and verbal memory among persons with MS experiencing moderate cognitive deficits.
The MAPSS-MS intervention had small-medium effects on neuropsychological tests of cognitive domains most often affected by MS.
Acknowledgement
We gratefully acknowledge the assistance of Hsiu-Rong Cheng and Lynn Chen with data entry and analysis, and Krista Schafer-Merchant, Sherry Morgan, Brittany Thorne, Vanessa Avina, Stephanie Chapman, Dawn Karbowski, and Vonnette Austin-Wells with data collection.
Funding Acknowledgement
The work was supported by the National Institutes of Health, National Institute of Nursing Research 1R21NR011076. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
Footnotes
Competing Interests - none declared
Contributor Information
Alexa K. Stuifbergen, School of Nursing, The University of Texas at Austin, Austin, TX.
Heather Becker, School of Nursing, The University of Texas at Austin, Austin, TX.
Francisco I. Perez, Baylor College of Medicine, Houston, TX.
Janet Morison, School of Nursing, The University of Texas at Austin, Austin, TX.
Vicki Kullberg, School of Nursing, The University of Texas at Austin, Austin, TX.
Ana Todd, School of Nursing, The University of Texas at Austin, Austin, TX.
REFERENCES
- 1.World Health Organization & Multiple Sclerosis International Foundation. [cited 2008] Available from http://www.msif.org/en/about_msif/what_we_do/atlas_of_ms/.html. [Google Scholar]
- 2.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S and Bö L. Axonal transection in the lesions of multiple sclerosis. N Eng J Med 1998; 338(5): 278–285. [DOI] [PubMed] [Google Scholar]
- 3.Pierson SH and Griffith N. Treatment of cognitive impairment in multiple sclerosis. Behav Neurol 2006; 17(1): 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien AR, Chiaravalloti N, Goverover Y and DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: a review of the literature. Arch Phys Med Rehabil 2008; 89(4): 761–769. [DOI] [PubMed] [Google Scholar]
- 5.Goretti B, Portaccio E, Zipoli V, et al. Impact of cognitive impairment on coping strategies in multiple sclerosis. Clin Neurol Neurosurg 2010; 112(2): 127–130. [DOI] [PubMed] [Google Scholar]
- 6.Amato MP, Zipoli V, Portaccio E. Cognitive changes in multiple sclerosis. Expert Rev Neurother 2008; 8(10): 1585–1596. [DOI] [PubMed] [Google Scholar]
- 7.Rao SM, Leo GJ, Ellington L, Nauertz T, Bernardin L and Unverzagt F. Cognitive dysfunction in multiple sclerosis II. Impact on employment and social functioning. Neurology 1991; 41: 692–696. [DOI] [PubMed] [Google Scholar]
- 8.Shevil E and Finlayson M. Perceptions of persons with multiple sclerosis on cognitive changes and their impact on daily life. Disabil Rehabil 2006; 28: 779–788. [DOI] [PubMed] [Google Scholar]
- 9.Schultheis MT, Garay E and DeLuca J. The influence of cognitive impairment on driving performance in multiple sclerosis. Neurology 2001; 56: 1089–1094. [DOI] [PubMed] [Google Scholar]
- 10.Schultheis MT, Garay E, Millis SR and Deluca J. Motor vehicle crashes and violations among drivers with multiple sclerosis. Arch Phys Med Rehabil 2002; 83(8): 1175–1178. [DOI] [PubMed] [Google Scholar]
- 11.Sastre-Garriga J, Alonso J, Renom M, et al. A functional magnetic resonance proof of concept pilot trail of cognitive rehabilitation in multiple sclerosis. Mult Scler 2011; 17: 457–467. [DOI] [PubMed] [Google Scholar]
- 12.Hildebrandt H, Lanz M, Hahn HK, et al. Cognitive training in MS: effects and relation to brain atrophy. Restor Neurol Neurosci 2007; 25: 33–43. [PubMed] [Google Scholar]
- 13.Solari A, Motta A, Mendozzi L, et al. Computer-aided retraining of memory and attention in people with multiple sclerosis: A randomized, double-blind controlled trial. J Neurol Sci. 2004; 222(1–2): 99–104. [DOI] [PubMed] [Google Scholar]
- 14.Tesar N, Bandion K and Baumhackl U. Efficacy of a neuropsychological training programme for patients with multiple sclerosis - a randomized controlled trial. Wien Klin Wochenschr 2005; 117(21–22): 747–754. [DOI] [PubMed] [Google Scholar]
- 15.Stuifbergen AK, Becker H, Morgan S, Morrison JD and Perez F. Home-based computer-assisted cognitive training: feasibility and perceptions of persons with MS. Int J MS Care (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan JIL, Edgley K and DeHoux E. A survey of multiple sclerosis, part 1: perceived cognitive problems and compensatory strategy use. Can J Rehabil 1990; 4(2): 99–105. [Google Scholar]
- 17.Stuifbergen AK, Becker H, Blozis S, Timmerman G and Kullberg V. A randomized clinical trial of a wellness intervention for women with multiple sclerosis. Arch Phys Med Rehabil 2003; 84(4): 467–476. [DOI] [PubMed] [Google Scholar]
- 18.Friedman H. Simplified determinations of statistical power, magnitude of effect and research sample sizes. Educ Psych Meas 1982; 42(2): 521–526. [Google Scholar]
- 19.Bracy OL, Oakes AL, Cooper RS, Watkins D, Watkins M, Brown DE, et al. The effects of cognitive rehabilitation therapy techniques for enhancing the cognitive/intellectual functioning of seventh and eighth grade children. Int J Cognit Tech 1999; 4(1): 19–27. [Google Scholar]
- 20.Bowen J, Gibbons L, Gianas A and Kraft GH. Self-administered Expanded Disability Status Scale with functional system scores correlates well with a physician-administered test. Mult Scler 2001; 7: 201–206. [DOI] [PubMed] [Google Scholar]
- 21.Benedict RH, Fischer JS, Archibald CJ, et al. Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol 2002; 16(3): 381–397. [DOI] [PubMed] [Google Scholar]
- 22.Benedict RH, Cookfair D, Gavett R, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 2006; 12(4): 549–558. [DOI] [PubMed] [Google Scholar]
- 23.Benton AL, Sivan AB, Hamsher K, Varnery NR and Spreen O. Contributions to neuropsychological assessment. 2nd ed New York: Oxford University Press; 1994. [Google Scholar]
- 24.Delis DC, Kaplan E, Kramer JH. California verbal learning test manual. 2nd ed, adult version. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 25.Benedict RH. Brief visuospatial memory test - revised: professional manual. Odessa, FL: Psychosocial Assessment Resources, Inc; 1997. [Google Scholar]
- 26.Gronwall DM. Paced auditory serial addition task: a measure of recovery from concussion. Percept Mot Skills 1977; 44: 367–73. [DOI] [PubMed] [Google Scholar]
- 27.Smith A. Symbol digit modalities test manual. Los Angeles, CA: Western Psychological Services; 1982. [Google Scholar]
- 28.Delis DC, Kramer JH, Kaplan E and Ober BA. Delis-Kaplan executive function system. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- 29.Schwartz CE, Coulthard-Morris L, Zeng L and Retzlaff R. Measuring self-efficacy in people with multiple sclerosis: a validation study. Arch Phys Med Rehabil 1996; 77(4): 394–398. [DOI] [PubMed] [Google Scholar]
- 30.Troyer AK and Rich JB. Psychometric properties of a new metamemory questionnaire for older adults. J Gerontol B Psychol Sci Soc Sci 2002; 57: 19–27. [DOI] [PubMed] [Google Scholar]
- 31.Benedict R, Cox D, Thompson LL, Foley F. Weinstock-Guttman B and Munschauer F. Reliable screening for neuropsychological impairment in multiple sclerosis. Mult Scler 2004; 10: 675–678. [DOI] [PubMed] [Google Scholar]
- 32.Benedict RH and Zivadinov R. Predicting neuropsychological abnormalities in multiple sclerosis. J Neur Sci 2006; 245(1–2): 67–72. [DOI] [PubMed] [Google Scholar]
- 33.Stuifbergen AK, Blozis SA, Becker HA, et al. A randomized controlled trial of a wellness intervention for women with fibromyalgia syndrome. Clin Rehabil 2010; 24: 305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyers AM, Holliday PJ, Harvey KA and Hutchinson KS. Functional performance measures: Are they superior to self-assessments? J Gerontol 1993; 48(5): M196–206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.