Abstract
Rationale: Interleukin 22 (IL-22) is an epithelial survival cytokine that is at present being explored as therapeutic agents for acute and chronic liver injury. However, its molecular basis of protective activities remains poorly understood.
Methods: Here we demonstrate that IL-22 inhibits the deteriorating metabolic states induced by stimuli in hepatocytes. Utilizing cell biological, molecular, and biochemical approaches, we provide evidence that IL-22 promotes oxidative phosphorylation (OXPHOS) and glycolysis and regulates the metabolic reprogramming related transcriptional responses.
Results: IL-22 controls metabolic regulators and enzymes activity through the induction of AMP-activated protein kinase (AMPK), AKT and mammalian target of rapamycin (mTOR), thereby ameliorating mitochondrial dysfunction. The upstream effector lncRNA H19 also participates in the controlling of these metabolic processes in hepatocytes. Importantly, amelioration of liver injury by IL-22 through activation of metabolism relevant signaling and regulation of mitochondrial function are further demonstrated in cisplatin-induced liver injury and steatohepatitis.
Conclusions: Collectively, our results reveal a novel mechanism underscoring the regulation of metabolic profiles of hepatocytes by IL-22 during liver injury, which might provide useful insights from the bench to the clinic in treating and preventing liver diseases.
Keywords: oxidative phosphorylation, glycolysis, mitochondria, reactive oxygen species, lncRNA H19
Introduction
Over the last decades, liver injury has become one of the major causes of illness and mortality with an amazing worldwide prevalence 1. Characterized as hepatocyte damage with severe inflammation, steatosis and fibrosis, liver injury can deteriorate to end-stage cirrhosis and liver failure 2-4. Unfortunately, evidence based-treatment for tackling liver injury is so far unavailable in clinical settings. Moreover, efforts to ameliorate the injury of hepatocytes by regulating their complications, such as intracellular oxidative stress, protein aggregates, and dysfunctional organelles, are far from satisfactory 5-8. Thus, it is of immediate significance to explore effective pharmacological antidotes and strategies for liver injury treatment.
Interleukin-22 (IL-22), which belongs to the interleukin-10 (IL-10) cytokine family, is produced by immune cells, including T cells, NK cells, innate lymphoid cells (ILCs), as well as neutrophils, and directly regulates the function of hepatocytes 9-12. We and others have suggested that IL-22 can protect against liver injury in several mouse models including acetaminophen, T cell or alcohol-induced hepatitis, etc. 13-17. Despite growing evidence suggests that IL-22 is a promising antidote to treat various hepatic disorders, the molecular basis of the liver protective activities of IL-22 needs to be fully characterized. Uncovering the underlying mechanisms of IL-22 is necessary both for understanding how IL-22 acts to prevent liver injury and for identifying critical processes as well as molecular regulators involved in addressing liver injury 18-19.
Altering cellular metabolism by specific proteins or genes can promote mammalian cell and organ repair, suggesting that the metabolic processes during tissue injury are the pivotal component of cellular functions and survive 20-22. Moreover, these works also show that specific inhibition of oxidative phosphorylation (OXPHOS) or glycolysis negates tissue restoring beneficial effects on tissue repair. Our prior researches linking IL-22 to mitochondrial function and activation of STAT3 signaling transduction lead us to inquire whether reprogramming the metabolism of hepatocytes with IL-22 can affect its hepatoprotective capacities. Thus, we hypothesize that IL-22 could beneficially regulate liver homeostasis and hepatocyte function via regulation of metabolic states in stress environmental situations.
In the present work, we first reported that the protective functions of IL-22 were mediated by metabolic reprogramming of hepatocytes. Utilizing cell biological, molecular, and biochemical approaches, we demonstrated that IL-22 opposed the decreased OXPHOS and glycolysis in the damaged hepatocytes induced by injurious stimuli. We further indicated that these metabolic processes were associated with the activation of metabolic signaling pathways, and thereby inhibiting the development of hepatocyte injury possibly via the prevention of mitochondrial dysfunction. Moreover, we investigated the essential roles of STAT3, AMP-activated protein kinase (AMPK), AKT, mammalian target of rapamycin (mTOR) and lncRNA H19 in these metabolic processes in hepatocytes with IL-22. Altogether, these observations highlight the significance of regulating metabolic profiles in hepatocytes for treating and preventing liver injury.
Methods
Reagents and antibodies
Oligomycin, cyanide p-trifluoromethoxyphenyl-hydrazone (FCCP) and rotenone were purchased from Seahorse Biosciences, USA; 2-Deoxy-D-glucose, glucose, palmitic acid (Palm.), rapamycin were purchased from Sigma-Aldrich, USA; Recombinant IL-22 proteins were obtained from Novoprotein, China; dorsomorphin, LY294002, cisplatin (Cisp.), 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1), TRNzol reagent, MitoTracker Green, SYBR Green qPCR mix, MMLV reverse transcriptase were provided by Beyotime Biotechnology, China; ethanol (Eth.), carbon tetrachloride (CCl4) were obtained from Sinopharm, China; MitoTracker Red, Hoechst33342, PKH26, MitoSOX were purchased from Invitrogen, USA. Collagenase IV was obtained from Yeasen, China. Percoll was purchased from GE, USA. Antibodies targeting AKT, p-AKT (S473), mTOR, p-mTOR (S2448), p-STAT3 (Y705), p-PI3K (Tyr199), p-p70S6K, HK-2, c-Myc, Bad, Bcl-2 were obtained from Cell Signaling Technology, USA; Antibodies for Glu1, AMPK, p-AMPKα, IL-22R1, β-Actin and GAPDH were provided by Abcam, USA.
Hepatocyte culture and stimulation
Hepatocytes were isolated from male C57BL/6 mice livers by a nonrecirculating, retrograde perfusion with 0.08% type IV Collagenase and then were purified and collected by the Percoll solution (40%). Human hepatocyte cell line L02 was obtained from Cell Bank of Chinese Academy of Science. Hepatocytes were incubated in standard culture medium containing 10% fetal bovine serum (FBS), Dulbecco's modified Eagle's medium (DMEM) and 1% streptomycin-penicillin, and were grown in a 37oC atmosphere with 5% CO2. We incubated hepatocytes with IL-22 (0.5 μg/mL) for 0.5 h, then 200 mM ethanol or 5 μg/mL cisplatin or 0.25 mM palmitic acid or 10 mM CCl4 for 24 h. In some cases, hepatocytes were cultured in the presence of dorsomorphin (5 μM), LY294002 (20 μM) or rapamycin (50 nM) for indicated times.
Seahorse experiments
We tested hepatocytes using a Seahorse XF96 Extracellular Flux Analyzer, for changes in the oxygen consumption rate (OCR, pmol O2/min) and extracellular acid rate (ECAR, mpH/min) as a measure of OXPHOS and glycolysis respectively. Different compounds were added to the culture media at different time point to target relative metabolic pathways, among which oligomycin functioned as a potent adenosine triphosphate (ATP) synthase inhibitor, the proton gradient uncoupler FCCP utilized to induce maximal OCR, rotenone to block OXPHOS-dependent OCR as the inhibitor of electron-transport chain, glucose to induce glycolysis-dependent lactic acid production whereas 2-deoxy-glucose as a glucose analog to inhibit glycolysis-dependent ECAR. Briefly, hepatocytes were planted overnight on 96-well polystyrene Seahorse plates. Before starting the experiments, we washed and cultured hepatocytes with seahorse assay mediums containing 2 mM glutamine, 1 mM pyruvate and 10 mM glucose (or without glucose for analyzing ECAR) in a 37oC atmosphere without CO2 for 30 min. Experiment results were measured at the indicated time points and after the injection of the under-mentioned inhibitors at optimum concentrations of oligomycin (1.0 µM), FCCP (1.0 µM), rotenone/antimycin A (0.5 μM), glucose (10 mM) and 2-DG (50 mM).
Flow cytometry
Hepatocytes were cultured and stimulated as mentioned above. MitoTracker Red (mitochondrial membrane potential), MitoSOX (mitochondrial ROS) and MitoTracker Green (total mitochondrial mass) staining were performed in accordance with the manufacturer's protocols and previous studies 14-15. Results were obtained by a Beckman Coulter Flow Cytometer (BD Biosciences) and analyzed with CytExpert software.
Gene knockdown
Small interfering RNA (siRNA) was provided by RiboBio (Guangzhou, China). We prepared transfection cocktails by mixing siRNA, (100 pmol) with Lipofectamine RNAiMAX via gentle pipetting and cultured them at room temperature for 30 min. For siRNA gene silencing, we removed the standard culture mediums and then used the transfection cocktails to incubate the hepatocytes (1×106). After 6 hours incubation in an incubator, the transfection cocktails were gently changed by fresh culture mediums. Forty-eight hours after transfection, hepatocytes were treated with IL-22 for the following experiments.
Immunofluorescence
Hepatocytes grown in microscopy chambers were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 10% bovine serum albumin (BSA) for 60 min. These hepatocytes were then stained with Alexa-488 conjugated anti-GLUT1 antibody at 4°C overnight, washed three times, and stained with PKH26. After washing, hepatocytes were mounted on slides with anti-fade mounting medium containing Hoechst 33342. Pictures were acquired on a confocal microscopy (Zeiss-710, Germany).
RNA-Seq analysis
Total RNA was extracted from cell samples (1×107) using TRNzol reagent (Beyotime Biotechnology, China). Isolated RNA was subjected to library construction utilizing the Illunima mRNA-Seq Prep Kit (Illumina), quantified by Agilent Bioanalyzer 2100 (Agilent Technology, Santa Clara, CA). Sample sequencing was performed on an Illumina HiSeq sequencing system at 50-base read length. The sequencing reads from all samples were trimmed using Cutadapt 1.16 (https://cutadapt.readthedocs.io/en/stable/guide.html) and then aligned to the human (hg19) reference genome by Hisat2 (https://daehwankimlab.github.io/hisat2/). Different gene expression analysis was performed using DESeq2 (http://www.bioconductor.org/packages/release/bioc/html/DESeq2.html) package on Bioconductor using fold change ≥ 2 and P ≤ 0.05 as cut-off. GSEA analyses were performed using GSEA software (https://www.gsea-msigdb.org/gsea/index.jsp).
Immunoblot analysis
The immunoblot analysis was performed as previously stated 13-15. Briefly, we subjected protein samples to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred the separated proteins to polyvinylidene (PVDF) membranes. Subsequently, these membranes were blocked with BSA for 2 hours and then incubated with the primary antibodies at 4°C for 12 hours. After washing four times, these membranes were subjected to horseradish peroxidase (HRP)-conjugated secondary antibody and detected using an enhanced chemiluminescence instrument (Pierce, USA). In situ hybridization was performed using the miRCURY LNA microRNA in situ hybridization kit (Exiqon, Denmark) in accordance with the manufacturer's manual. The images were captured using the confocal microscopy.
Glucose uptake assay
The concentrations of glucose in hepatocyte culture supernatants were detected using the glucose uptake assay kit as the manufacturer's instructions (GAHK20, Sigma). Values were normalized to cell number or tissue mass, as appropriate.
Real-time PCR
Total RNA was obtained from cell samples (1×106) by TRNzol reagent and was transcribed to cDNA by MMLV reverse transcriptase kit. Then the expression levels of mRNA were measured on a BioRad real-time PCR instrument using SYBR green qPCR-mix kit and normalized to GAPDH.
Mice and histological assay
C57BL/6 and BALB/c mice were provided by Slaccas Experimental Animal Co. (Shanghai, China) and were housed in specific pathogen free (SPF) facilities at 22oC with 12 h light/dark cycles. For the drug-induced liver injury model, mice were injected intraperitoneally with cisplatin (20 mg/kg) or saline control. The C57BL/6 mice were fed with normal chow diets or high-fat diets for the indicated times to induce steatohepatitis. For in vivo knockdown of lncRNA H19 test, mice were administrated with adenovirus of H19 shRNA through tail vein (1x1010 virus particles per mouse). The animal experiments were performed following the protocols and procedures approved by the Institutional Animal Care and Use Ethics Committee (IACUE) at Fudan University. ROS, mitochondrial membrane potential, immunohistochemical and histological staining of liver sections were performed as mentioned previously 14.
Statistical Analysis
Results were expressed as means ± standard deviations (SD) unless specified differently. Statistical analyses of experimental results were evaluated using GraphPad Prism 5.0 (LaJolla, USA). Differences were analyzed using one-way of analysis (ANOVA) or Student's t-test. Statistical significance was shown as ***P < 0.001, **P < 0.01 or *P < 0.05.
Results
IL-22 regulates mitochondrial function and glycolysis in hepatocytes on injury factors stimulation
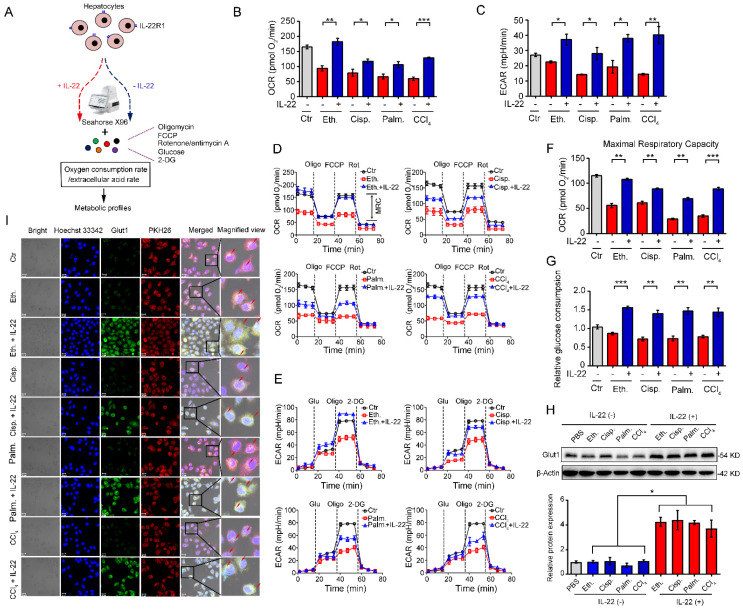
We investigated hepatocytes, for changes in the oxygen consumption rate (OCR), and extracellular acid rate (ECAR), as a measure of OXPHOS and glycolysis, respectively. The damaged hepatocytes, which were stimulated with liver injury factors, became less oxidative and glycolysis, as shown had lower basal OCR and ECAR values (Figure 1A-B). We chose 500 ng/mL of IL-22 for further cell culture experiments, because this concentration led to key signaling transduction activation efficiently and sufficiently without cytotoxic effects (Figure S1). It was noteworthy that IL-22 promoted OXPHOS and glycolysis in these hepatocytes, whereas the metabolic reprogramming effects were completely disarmed by a neutralizing antibody against the IL-22 receptor (IL-22R1) and STAT3-knockdown indicating IL-22 promoted OXPHOS and glycolysis via targeting hepatocytes and activating STAT3 signaling directly. (Figure S1-2). The effects of IL-22 on mitochondrial and glycolytic flux in hepatocytes were further assessed (Figure 1D-E). Just as anticipated, IL-22 reversed the stimuli-induced impairments in maximal respiratory capacity (MRC) and glycolytic flux (Figure 1D-F). These results were also demonstrated by an increase in glucose uptake with the addition of exogenous IL-22 (Figure 1G).
Figure 1.
IL-22 regulates mitochondrial function and glycolysis in hepatocytes. (A) Using Seahorse XF96 Extracellular Flux Analyzer to assess the changes in the oxygen consumption rate and extracellular acid rate of hepatocytes. (B and C) OCR and ECAR in hepatocytes treated with 200 mM ethanol, or 5 μg/mL cisplatin, or 0.25 mM palmitic acid, or 10 mM CCl4 in the absence or presence of IL-22 for 24 h (n = 3). (D and E) Representative curves in the OCR and ECAR of hepatocytes after incubated with oligomycin, glucose, FCCP, rotenone, and 2-DG (n = 3). (F) MRC of hepatocytes evaluated by real time changes in OCR (n = 3). (G) Relative glucose consumption in hepatocytes upon IL-22 treatment (n = 3). (H) Glut1 protein expression upon IL-22 treatment under injury stress. Densitometric values were quantified and normalized to control (PBS) group. (I) Localization and expression of Glut1 (green), nuclear (blue), and plasma membrane (red) in hepatocytes treated as in (B) for 24 h. Scale bars, 20 μm; Student's t test (unpaired); *P < 0.05, **P < 0.01, ***P < 0.001. All data are means ± SD of at least three independent experiments.
Because the kinetics of glucose transporter Glut1 plays a principal role in glucose homeostasis, we therefore sought to test whether IL-22 affects Glut1 expression or translocation from intracellular to the plasma membrane in hepatocytes. The expression and localization of Glut1 were determined by western blot and the presence of co-localization between cell surface (PKH26 for cell membrane labeling, red) and Glut1 (green). These assays indicated that IL-22 promoted the expression of Glut1 (Figure 1H) and translocated it to the plasma membrane (Figure 1I). Collectively, our observations illustrated that IL-22 promoted OXPHOS and glycolysis in the damaged hepatocytes.
IL-22 upregulates the metabolic reprogramming related transcriptional responses
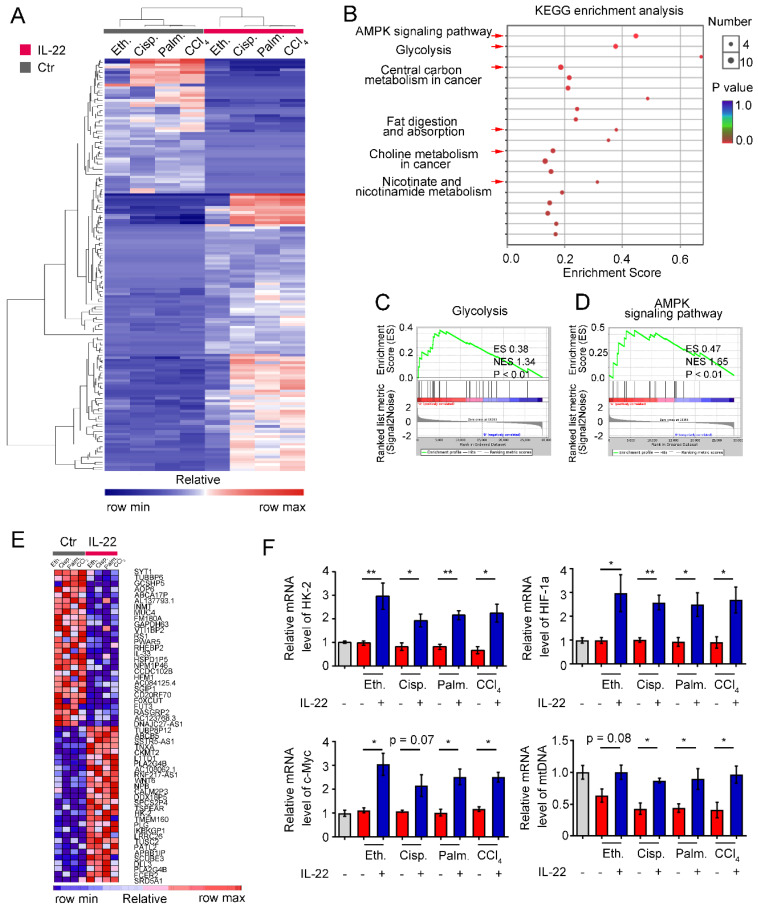
To identify the transcriptional processes elicited by IL-22, we performed RNA sequencing analysis (RNA-seq) on hepatocytes by comparing injury factors-challenged groups to IL-22 plus injury factors-challenged groups at 6 h. Gene expression profiles identified 162 genes whose transcriptional levels were uniquely changed by IL-22 treatment (P < 0.01), indicating that IL-22 plays a vital role in gene expression (Figure 2A). We next carried out Kyoto Encyclopedia of Genes and Genomes analysis (KEGG) and Gene Set Enrichment Analysis (GSEA) to recognize specific processes connected with IL-22 treatment and genotype. In the significantly upregulated transcripts, our data showed that only in IL-22-treated groups, but not control groups, an obvious enrichment of upregulated pathways associated with glycolysis, AMPK signaling pathway, nicotinate and nicotinamide metabolism, and choline metabolism in cancer was present in addition to expected processes (e.g., fat digestion and absorption, and central carbon metabolism in cancer; Figure 2B-D and Figure S3). To further explore our hypothesis, we evaluated the specifically down- and up-regulated genes and suggested a tremendous number of upregulated transcripts well-known to play an important part in metabolic pathways (Figure 2E). Consistently, real-time PCR assay also showed that metabolic enzymes (c-Myc, HK-2), α subunits of the hypoxia-inducible factors (HIF-1α) and mitochondrial DNA involved in cellular metabolism, cell survival and mitochondrial homeostasis (Figure 2F) were obviously upregulated by IL-22 treatment 20-22. Therefore, we indicated that the upregulation of genes correlated to cellular metabolism in hepatocytes was associated with IL-22 regulated metabolic reprogramming.
Figure 2.
IL-22 regulates metabolic reprogramming-related transcriptional responses. (A) Heat map of the remarkably altered genes in hepatocytes with injury factors-challenged groups versus IL-22 plus injury factors-challenged groups. (B) Kyoto Encyclopedia of Genes and Genomes analysis (KEGG) of IL-22 targets in hepatocytes with IL-22-protected and -nonprotected groups. (C and D) KEGG of glycolysis and AMPK signaling pathway in IL-22-protected and -nonprotected hepatocytes. (E) Heat map of top altered genes from hepatocytes with IL-22 treatment. (F) Relative mRNA expression level of HK-2, HIF-1α, c-Myc and mtDNA in the absence or presence of IL-22 treatment (n = 3); Student's t test (unpaired); *P < 0.05, **P < 0.01. All data are means ± SD of at least three independent experiments.
IL-22 prevents the generation of dysfunctional mitochondria and mitochondrial ROS via AMPK-associated signaling mechanism
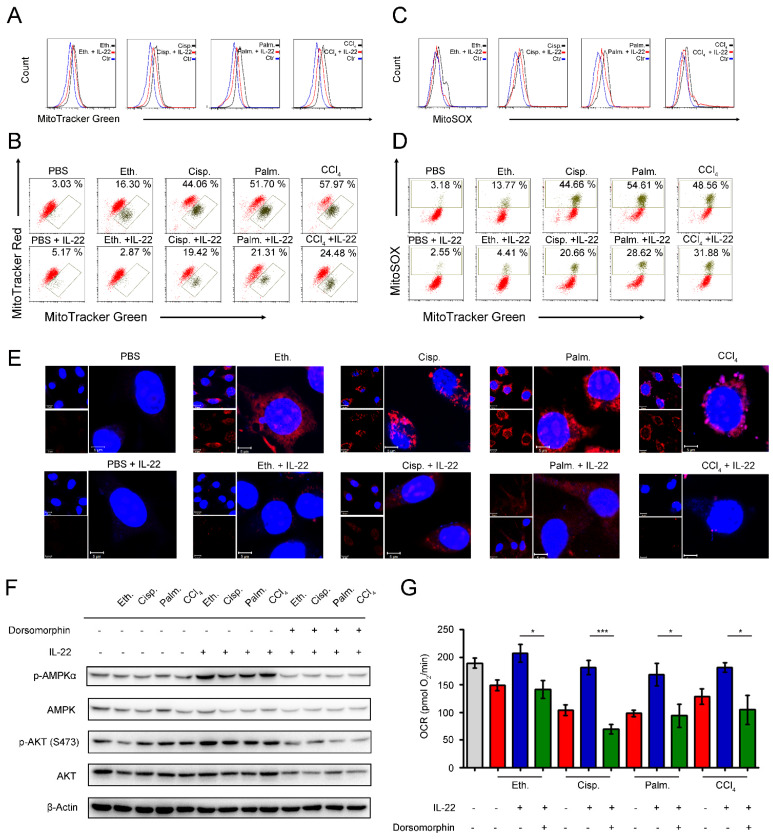
We next inquired whether the metabolic reprogramming effects of IL-22 in the damaged hepatocytes are due to the altered mitochondrial function. The hepatocytes were stained with MitoTracker Green to track the mitochondrial content. Our data suggested that the damaged hepatocytes had increased mitochondrial mass after exposure to injurious stimuli by comparison with normal hepatocytes (Figure 3A). The phenomenon was not associated with greater hepatocytes size, because the damaged hepatocytes had similar sizes with normal cells, but rather was attributed to increased intracellular complexity indicated by the side scatter signal (SSC) (Figure S4). To differentiate between dysfunctional mitochondria and respiring mitochondria, we then stained hepatocytes with MitoTracker Red (mitochondrial membrane potential-dependent stain), and found an increase in dysfunctional mitochondria (with lower MitoTracker Red and higher MitoTracker Green) in hepatocytes after exposure to injurious stimuli (Figure 3B). Of particular note, exogenous IL-22 was able to maintain mitochondrial fitness in damaged hepatocytes observed by decreased abnormal mitochondrial mass and dysfunctional mitochondria (Figure 3A-B).
Figure 3.
IL-22 prevents generation of dysfunctional mitochondria and mitochondrial ROS via AMPK-associated signal mechanism. Hepatocytes were treated with PBS, 200 mM ethanol, or 5 μg/mL cisplatin, or 0.25 mM palmitic acid, or 10 mM CCl4 in the absence or presence of IL-22 for 24 h (n = 3) (A) Mitochondrial mass was stained with MitoTracker Green and assessed by flow cytometry. Mitochondrial ROS and membrane potential were assessed in hepatocytes stained with MitoSOX (C), MitoTracker Green and MitoTracker Red (B), or MitoTracker Green and MitoSOX (D), respectively. (E) Confocal images indicated mitochondrial ROS production in hepatocytes stained with MitoSOX. (F) Western blot analysis suggested that IL-22 induced AMPK/AKT activation in hepatocytes, which could be inhibited by Dorsomorphin. (G) OCR in hepatocytes was measured in the absence or presence of indicated inhibitors and IL-22 for 24 h. Student's t test (unpaired); *P < 0.05, **P < 0.01, ***P < 0.001. All data are means ± SD of at least three independent experiments.
Loss of mitochondrial membrane potential is well known to be connected with the mitochondrial ROS production 23. We therefore asked whether the IL-22 mediated mitochondrial protective effects in damaged hepatocytes were associated with inhibition of mitochondrial ROS. As evaluated by mitochondria-specific ROS dye MitoSOX, we found that exogenous IL-22 potently inhibited the increased MitoSOX signal, which was correlated with mitochondrial content, indicating IL-22 repressed the ROS generation from dysfunctional mitochondria (Figure 3C-D). These results corresponded to the live-hepatocytes fluorescence imaging, wherein the ROS accumulation could be blocked by IL-22 (Figure 3E). Our observations indicated that IL-22 improves hepatocellular mitochondrial function by reducing mitochondrial ROS, indicating ROS accumulation and the inability to generate and transfer energy are the primary mechanisms linking mitochondrial dysfunction and liver injury 6, 14-15.
AMPK plays a key role in the cellular metabolic process via promoting catabolism to restore metabolic homeostasis, for instance, OXPHOS, glycolysis, fatty acid uptake and glucose uptake 24-25. We observed that exogenous IL-22 induced AMPK and AKT activation in hepatocytes, suggesting that the effect of IL-22 on mitochondrial functions might AMPK/AKT associated signaling mechanism (Figure 3F). In support of this idea, we pretreated hepatocytes with dorsomorphin (an AMPK inhibitor). As expected, the addition of IL-22 failed to maintain mitochondrial fitness and the activation of AKT, which indicated the critical role of AMPK and AKT was one of the target molecule of IL-22-AMPK pathway (Figure 3G). Altogether, these findings indicated that the cytokine IL-22 prevented dysfunctional mitochondria in hepatocytes via AMPK-associated signaling mechanism.
IL-22 maintains mitochondrial function and integrity through activation of mTOR signaling transduction
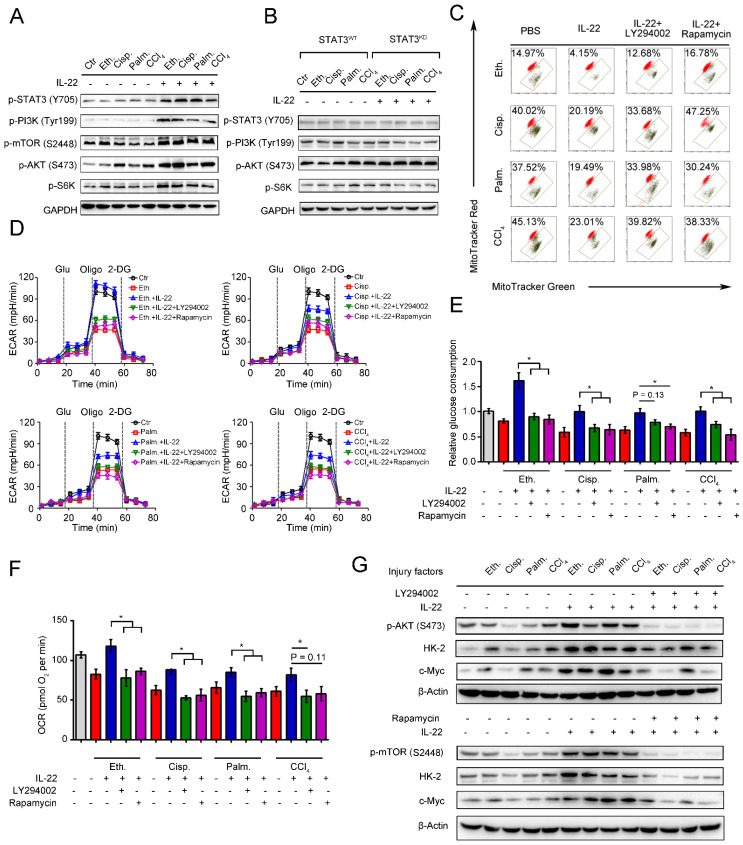
mTOR is the crucial signaling hub connecting cell growth and survival, and the activation of mTOR regulates lipid synthesis, mitochondrial function and glucose metabolism 26-27. According to the observed effects of IL-22 on mitochondrial function and cell metabolism, we tested whether IL-22 controls the activity of mTOR signaling transduction. In support of a view that mTOR might regulate metabolic homeostasis during IL-22 treatment, the addition of exogenous IL-22 to the damaged hepatocytes led to mTOR signaling transduction activation, as evidenced by increased phosphorylation of the upstream and downstream proteins such as STAT3, PI3K, S6K, S6 (Figure 4A). Moreover, the activation of mTOR signaling transduction was inhibited in hepatocytes lacking STAT3, which suggested that IL-22 activated mTOR signaling pathway via STAT3 (Figure 4B and S5).
Figure 4.
IL-22 maintains mitochondrial fitness through activation of mTOR signaling. Hepatocytes were stimulated with or without (control) 200 mM ethanol, or 5 μg/mL cisplatin, or 0.25 mM palmitic acid, or 10 mM CCl4 in the absence or presence of IL-22, rapamycin, or LY294002 for indicated times (n = 3). (A and B) Comparison of mTOR signaling activation in hepatocytes or STAT3-WT and STAT3-KD hepatocytes was assessed by western blot analysis. (C) Mitochondrial membrane potentials were assessed in hepatocytes stained with MitoTracker Red and MitoTracker Green. (D) Real time changes in the ECAR of hepatocytes after incubating with glucose, oligomycin, and 2-DG (n = 3) were analyzed. (E) Relative glucose consumption was measured for LY294002 or rapamycin treated hepatocytes at 24 hours. (F) Basal respiration capacity (OXPHOS) of hepatocytes was measured. (G) Comparison of glycolytic enzymes expression in hepatocytes versus LY294002 and rapamycin treated hepatocytes. Student's t test (unpaired); *P < 0.05, **P < 0.01, ***P < 0.001. All data are means ± SD of at least three independent experiments.
We next asked whether the activation of mTOR signaling transduction by IL-22 was attributed to maintaining mitochondrial function and integrity during injury factors stimulation, which could cause mitochondrial dysfunction. We incubated hepatocytes with LY294002 and rapamycin to directly blocked mTOR signaling pathways during IL-22 treatment and investigated their oxygen consumption and mitochondrial function. Surprisingly, LY294002 and rapamycin inhibited the improved mitochondrial fitness induced by IL-22 treatment (Figure 4C). Furthermore, co-treatment with LY294002 or rapamycin also block enhanced glycolytic flux, glucose consumption, OXPHOS and glycolytic enzymes expression, which indicated that IL-22 promoted hepatocellular metabolism through activating mTOR signaling transduction (Figure 4D-4G). Taken together, these results suggested that activation of mTOR signaling transduction by IL-22 treatment could result in maintained mitochondrial function and integrity.
The induction of lncRNA H19 by IL-22 activates mTOR signaling transduction
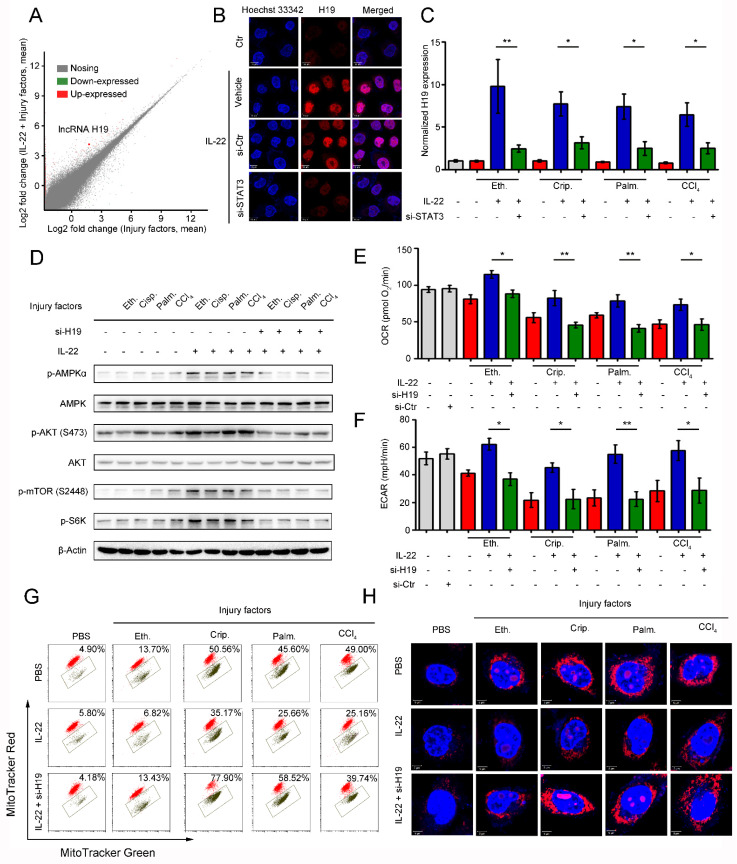
We next sought to decipher how IL-22 activates mTOR signaling transduction. Because the activation is STAT3 dependent, the underlying mechanisms should demand transcription. To test our hypothesis, we performed gene expression analysis in hepatocytes using RNA-seq and assessed if IL-22 transcriptionally controls metabolic reprogramming, which might attribute to the activation of mTOR signaling transduction. We observed that IL-22 regulated a large number of genes, which participated in varying biological processes. Of note, from among the lncRNAs, lncRNA H19 was remarkably induced by IL-22 during injury factors stimulation (Figure 5A). This result was also demonstrated by in situ hybridization assays and quantitative real-time PCR (Figure 5B-C and S6), and it required STAT3. Previous study demonstrated IL-22 activates expression of lncRNA H19 in intestinal epithelial cells (IECs), which is required for IECs healing and regeneration 28. In the current study, we hypothesize that IL-22 drives metabolic reprogramming to maintain mitochondrial fitness and treat liver injury through lncRNA H19. To further explore the key roles of lncRNA H19 in hepatocyte metabolic states, we carried out the following series of experiments. Firstly, we found that IL-22 failed to activate AMPK/AKT/mTOR signaling transduction in hepatocytes lacking lncRNA H19 (Figure S7), indicating that the activation of mTOR signaling by IL-22 was lncRNA H19-dependent (Figure 5D and S7). Secondly, we observed that genetic silencing of lncRNA H19 attenuated the protective effects of IL-22 on restoring the changes in the profiles for OCR and ECAR (Figure 5E-F) and the accumulation of dysfunctional mitochondria with loss of mitochondrial membrane potential and increased ROS generation after exposure to injurious stimuli (Figure 5G-H), suggesting lncRNA H19 is a key target of IL-22. These data collectively suggested that the activation of mTOR signaling transduction via lncRNA H19 plays a critical role in dysfunctional mitochondria elimination in hepatocytes after exposure to injurious stimuli.
Figure 5.
LncRNA H19 mediates the links between IL-22/IL-22R1 and the AMPK/AKT/mTOR signaling pathways. (A) Bland-Altman plot illustrating relative gene expression in hepatocytes stimulated with or without (control) ethanol, or cisplatin, or palmitic acid, or CCl4 in the absence or presence of IL-22 for the indicated times. (B) Confocal images suggested lncRNA H19 overexpression in hepatocytes after IL-22 treatment, which required STAT3. Control-siRNA (si-Ctr) treated mice as a vehicle control group (n = 5). (C) Real time PCR analysis suggesting that IL-22 induced lncRNA H19 overexpression in hepatocytes, which could be prevented by STAT3 knockdown (n = 3). (D) Comparison of AMPK/AKT/mTOR signaling activation in hepatocytes versus si-H19 treated hepatocytes (lncRNA H19 knockdown). (E and F) Basal OCR and ECAR in hepatocytes at the absence or presence of IL-22, or si-H19 for 24 h (n = 3). (G) Dysfunctional mitochondria were assessed in hepatocytes stained with MitoTracker Green and MitoTracker Red. (H) Confocal images suggested mitochondrial ROS production in hepatocytes stained with MitoSOX. Student's t test (unpaired); *P < 0.05, **P < 0.01, ***P < 0.001. All data are means ± SD of at least three independent experiments.
IL-22 attenuates hepatic oxidative stress, mitochondrial dysfunction and damage in vivo
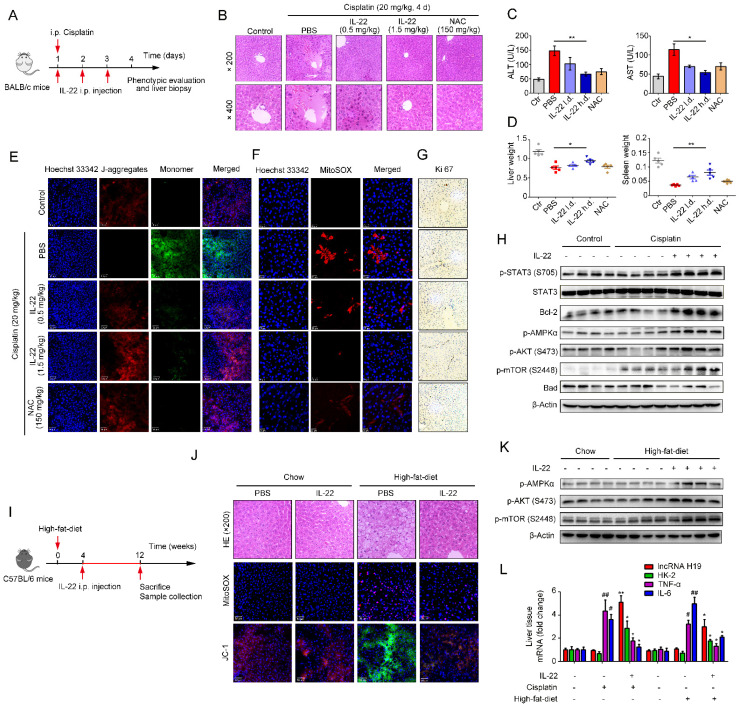
Previously, we have suggested that IL-22 exerts protective potency by inhibiting ROS accumulation and preventing mitochondrial dysfunction 14-15. To study the present model in vivo, we further explored mitochondrial fitness and mTOR signaling transduction in cisplatin-induced liver injury. Similar to injury factors-stimulated hepatocytes in vitro, animals challenged by cisplatin experienced various symptoms of hepatic injury, including hepatocyte slice degeneration and necrosis (Figure 6B and S8A), elevation of alanine aminotransferase (ALT) and aspartate amino-transferase (AST) levels (Figure 6C), weight reduction of livers and spleens (Figure 6D), accumulated mitochondria with loss of mitochondrial membrane potential (MMP) (JC-1 green monomer, Figure 6E), and increased ROS levels (Figure 6F). However, these effects were ameliorated in IL-22-treated mice. Mechanistically, we found that IL-22 upregulated Bcl-2 and the phosphorylation of AMPK/AKT/mTOR signaling pathways (Figure 6H and S9A). Meanwhile, Ki67, a marker of cellular proliferation, was downregulated by cisplatin stimulation, whereas the percentage of Ki67-positive cells was increased with IL-22 treatment (Figure 6G and S10).
Figure 6.
IL-22 attenuates hepatic oxidative stress, mitochondrial dysfunction and damage in vivo. (A) Schematic diagram of the animal experimental protocols to assess the effects of IL-22 (0.5 mg/kg and 1.5 mg/kg) in cisplatin (20 mg/kg) induced liver injury. N-acetyl-L-cysteine (NAC, 150 mg/kg) as a positive control group and PBS as a vehicle control group (n = 5). Representative HE (B), MitoSOX (E), JC-1 (F), and Ki-67 staining (G) images of the liver sections were presented. (C and D) Serum AST levels, serum ALT levels, liver weights and spleen weights were measured (H) Comparison of AMPK/AKT/mTOR activation in liver extracts from IL-22-treated mice versus control subjects was assessed by western blot analysis. (I) Schematic diagram of the animal experimental protocols to assess the effects of IL-22 (2.5 mg/kg) in high-fat-diet fed mice (n = 8). PBS treated mice as a vehicle control group (n = 5). (J) Representative HE, MitoSOX, and JC-1 staining images of the liver sections were presented. (K) Liver extracts were subjected to western blot analysis with various antibodies as indicated. Western blotting suggested that IL-22 induced AMPK/AKT/mTOR activation in liver extracts from IL-22-treated mice. (L) The mRNA expression levels of the indicated genes in the liver (n = 3). Student's t test (unpaired); *P ≤ 0.05 and **P ≤ 0.01 compared with cisplatin-treated and HFD-fed mice. #P ≤ 0.05 and ##P ≤ 0.01 compared with PBS-treated and chow diet fed mice. All data are means ± SD of at least three independent experiments.
Hence, IL-22 inhibited the hepatocyte apoptosis and increased hepatocyte regeneration. To further evaluate the molecular basis of the liver protective activities of IL-22 on steatohepatitis, mice were fed with high-fat-diet and then treated with IL-22 (2.5 mg/kg) or PBS during high-fat-diet feeding for indicated times (Figure 6I). We also suggested that IL-22 treatment significantly ameliorated high-fat-diet-induced hepatocellular necrosis, injury, steatosis, ROS accumulation, and mitochondrial dysfunction via activation of AMPK-AKT-mTOR signaling pathways (Figure 6J-K, S8B and S9B). Consistent with our above observations, real-time PCR assays also revealed that IL-22 distinctly induced lncRNA H19 and glycolytic enzyme (HK-2) expression. Furthermore, we showed that IL-6 and TNF-α in the liver were significantly alleviated by IL-22 treatment (Figure 6L).
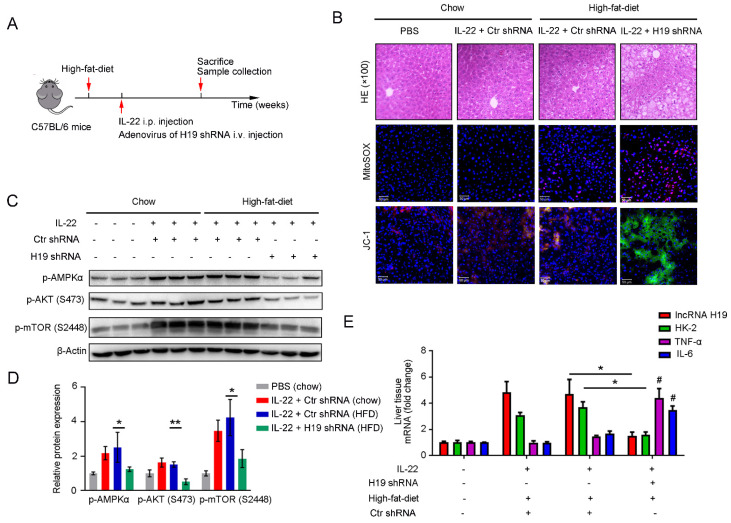
To further investigate whether the beneficial effects of IL-22 in liver are mediated via lncRNA H19, we treated mice with H19 shRNA adenovirus to knockdown lncRNA H19 during high-fat-diet feeding as indicated time (Figure 7A). We suggested that the protective activities of IL-22 on high-fat-diet-induced hepatocellular necrosis, injury, steatosis, ROS accumulation and mitochondrial dysfunction and the activation of AMPK-AKT-mTOR signaling were significantly inhibited by lncRNA H19 knockdown (Figure 7B-D and S8C). Moreover, real-time PCR demonstrated that lncRNA H19 knockdown reversed the induction of glycolytic enzyme (HK-2) and the inhibition of IL-6 and TNF-α by IL-22 in the liver (Figure 7E). Collectively, these findings demonstrated that IL-22 prevented liver injury through activation of mTOR signaling transduction, inhibition of ROS accumulation and mitochondrial function regulation via lncRNA H19.
Figure 7.
IL-22 attenuates hepatic oxidative stress, mitochondrial dysfunction and damage through the induction of lncRNA H19. (A) Schematic diagram of the animal experimental protocols to assess the effects of IL-22 (2.5 mg/kg) and H19 shRNA (1x1010 virus particles per mouse) in high-fat-diet fed mice (n = 8). PBS and Control shRNA (Ctr shRNA) treated mice as a vehicle control group (n = 5). (B) Representative HE, MitoSOX, and JC-1 staining images of the liver sections were presented. (C) Western blotting suggested that IL-22 induced AMPK/AKT/mTOR activation in liver via the induction of lncRNA H19. (D) Densitometric values were quantified and normalized to control group (n = 3; mean ± SD; *P < 0.05, **P < 0.01). The values of control group were set to 1. (E) The mRNA expression levels of the indicated genes in the liver (n = 3). *P ≤ 0.05 compared with H19 shRNA-treated mice. #P ≤ 0.05 compared with IL-22-treated and high-fat-diet fed mice. All data are means ± SD of at least three independent experiments.
Discussion
Multiple lines of in vitro and in vivo studies have suggested that IL-22 has significant protective effects against liver injury 13-17. However, the roles of IL-22 in cell metabolism processes, particularly in hepatocytes and liver, have not been extensively investigated. Previously studies showed that IL-22 ameliorated endoplasmic reticulum (ER) stress and ROS accumulation induced by various stimuli in beta cells via inhibition of oxidative stress associated genes and upregulation of antioxidant gene transcription 13-15, 29. A recent study suggested that IL-22 ameliorated high fat diet-induced nonalcoholic steatohepatitis via upregulating the expression of the antioxidant gene metallothionein 30. These findings provided context to our investigation that IL-22 was capable of protecting liver injury by regulating essential metabolic processes. In the current study, we first demonstrated that IL-22 drives a metabolic adaptive reprogramming to maintain mitochondrial fitness, which in turn regulated hepatocellular metabolic processes. Further data indicated that IL-22 was capable of upregulating lncRNA H19, AMPK, mTOR and AKT in hepatocytes, key regulators of cell proliferation and epithelial wound healing, to eliminate dysfunctional mitochondria and opening up a new arena for IL-22 mediated mechanisms of action 28, 31. Moreover, we have suggested the activation of related signaling transduction could induce Bcl-2 expression and inhibit Bad, TNF-α and IL-1β expression, which could ameliorate the apoptosis and necrosis of hepatocytes to prevent the liver failure (Figure 6H, 6L and 7E). Together, these data illustrated that the induction of metabolic reprogramming in hepatocytes could attribute to IL-22-mediated liver protective activities. Future studies will concentrate on using more animal models to confirmed the novel mechanism of IL-22 in vivo.
Cell metabolism is a promising targetable signaling transduction for treating tissue injury diseases. For instance, increased flux via glycolysis has been suggested as a protection mechanism of cell damage, which is associated with enhancing mitochondrial function through compensating impaired energy generation 22, 32-33. Moreover, the upregulation of OXPHOS in renal tubular endotheliocyte prevents ischemic reperfusion injury, which may thus heighten the emerging therapeutic approach 34. To our best knowledge, we first suggested that rather than switching from mitochondrial OXPHOS to glycolysis, IL-22 drove a metabolic reprogramming of hepatocytes via promoting the activities of both. The activation of glycolysis by IL-22 via the facilitation of glycolytic protein expression and glucose uptake indicated that IL-22 could alter the metabolic profiles associated with the hepatocyte injury. These results are consistent with the tissue repair function of Lin28 and also accord with the observed data from the damaged pinnal tissues, where cellular metabolism is needed for tissue function, but if these processes are inhibited, then the tissue protection efficacy is negated 21.
Emerging evidence suggests that mitochondria are the heart of organelles that integrate cell metabolism and cell fate 24-25, 35. In the present investigation, our findings indicate that IL-22-pretreated hepatocytes maintained mitochondrial fitness with the increased glycolysis and OXPHOS in hepatocytes on injury factors stimulation. Of note, AMPK, a vital energy sensor, regulates cell metabolism to sustain organism homeostasis 36. Particularly, AMPK also prevents cell or tissue damage by alleviating mitochondrial dysfunction and inhibiting cell apoptosis 37-38. Consistent with these studies, our results suggesting that IL-22 induces the activation of AMPK signaling demonstrate that IL-22 has more straight-forward actions in sustaining mitochondrial function, which are significant to maintaining hepatocyte respiratory capacity. Additionally, IL-22 signaling pathways through STAT3 may play direct roles in preserving mitochondrial integrity, as these have been demonstrated formerly that the activation of STAT3 signaling exists in mitochondria and has essential roles in the electron transport chains 39. Moreover, STAT3 has been revealed to potentiate glucose metabolism and accelerate glycolysis. Thus, we can conclude with complete confidence that IL-22 administration alters the metabolic reprogram of hepatocyte in vitro and in vivo. Future therapeutic and mechanistic researches will concentrate on exploring additional targets of IL-22 that may take part in liver protection.
There is growing recognition that mTOR plays a central role in cell metabolism 40. In this study, our results illustrate that IL-22 activates mTOR signaling through STAT3, which demonstrate that IL-22 regulates hepatocyte metabolic profiles by means of engaging the controlling of mTOR associated signaling transductions. These data support our aforementioned investigations in multiple ways. Firstly, mTOR signaling transduction is vital to regulate the promotion of OXPHOS and glycolysis in numerous tissues 41-43. This accords with our observations indicating an exaggerated enhancing glycolysis and OXPHOS in IL-22 pretreated hepatocytes after exposure to injurious stimuli, where the mTOR signaling is activated by IL-22. Secondly, the activation of AMPK-AKT signaling is universally known to induce mTOR signaling, demonstrating a possible mechanism for metabolic reprogramming of hepatocytes by IL-22. Thirdly, it has also been suggested that mTOR signaling activation potentiates glycolytic enzymes expression, likely HK-2, c-Myc 44. Our findings therefore indicate a stream-lined mechanism for the molecular basis of the liver protective potentials of IL-22, where IL-22 leads to the induction of mTOR activation, causing the upregulation of glycolytic enzymes, as suggested in Figure 4E-G we observed. There are some issues yet to be addressed, such as if IL-22 also regulates other metabolic processes linked with its hepatoprotective activities via promoting the mTOR associated signaling transductions, and need further research.
Among several regulators of mTOR signaling that have been exported, we first suggest that the expression of lncRNA H19 is remarkably upregulated by IL-22 in hepatocytes and that the IL-22-STAT3-lncRNA H19 signaling pathway is significant for the activation of mTOR and the preservation of mitochondrial fitness during hepatocyte damage. In agreement with previous studies, we show that lncRNA H19 affects the upstream regulators of mTOR signaling, such as AMPK, AKT proteins, thereby activates mTOR and induces the expression of a subset of metabolic reprogramming regulators in hepatocytes 30, 45-47. Accordingly, we find that lncRNA H19 plays a central role in the inhibition of mitochondrial ROS accumulation and dysfunctional mitochondria elimination in hepatocytes. Further investigations are necessary to explore the underlying mechanism of STAT3-induced lncRNA-H19 expression and fully characterize the in vivo functions of lncRNA H19 during liver injury and other liver diseases.
Conclusions
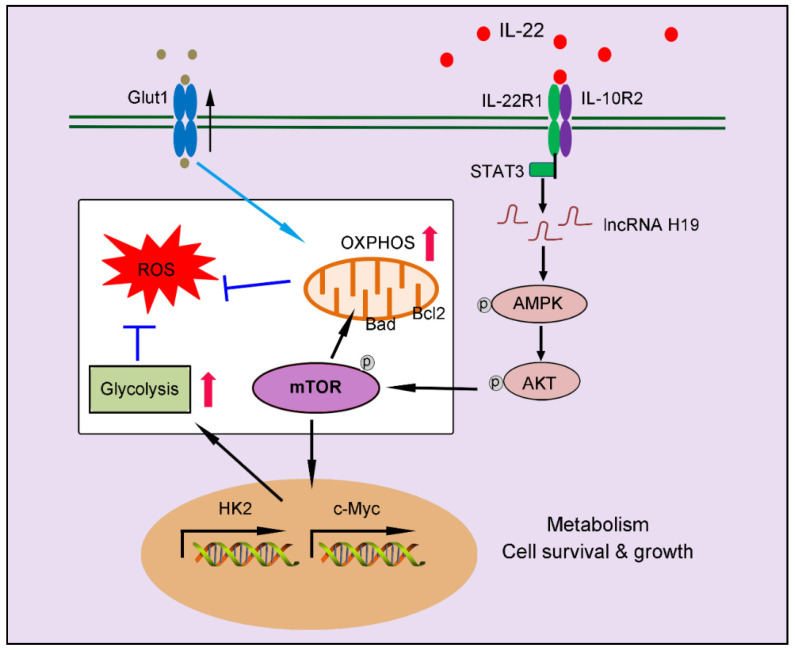
In summary, our findings indicate a crucial role of IL-22 in regulating hepatocellular metabolism through a metabolic reprogramming to maintain mitochondrial fitness and treat liver injury. We put forward that these metabolic regulations by IL-22 are vital to control of hepatocellular function and survive. Lacking their mediators (such as, inhibited lncRNA H19, AMPK, AKT or mTOR activation) can lead to loss of metabolic reprogramming capacities of IL-22 and abnormal mitochondrial fitness, as shown in damaged hepatocytes from IL-22-pretreated cells, which have restrained related signaling transductions (Figure 8). Notably, the liver injury by ROS accumulation owing to dysfunctional mitochondria in animal models was significantly ameliorated by IL-22 via activation of mTOR signaling. Our data are particularly implication to the area for several reasons. Firstly, the controlling of mTOR, a conservative metabolic regulator, by the IL-22-STAT3-lncRNA H19-AMPK-AKT-mTOR axis has not been previously demonstrated. Secondly, therapeutic targeting of these metabolic processes in hepatocytes therefore can be directly favorable to the prevention or treatment of liver injury diseases. Thirdly, with recombinant IL-22 proteins at present being explored as potential therapeutic strategies in human diseases as severe alcoholic hepatitis, graft-versus-host disease and inflammatory bowel disease, our research may have momentous clinical significances 18-19, 37. Additionally, a phase Ⅱ-b trial of IL-22 reveals that IL-22 is free from important side effects and associated with a high rate of improvement in liver disease, highlighting its promising therapeutic opportunities 48-49. However, constitutive or chronic overexpression of IL-22 can have pathogenic properties, for instance in some liver disorders, psoriasis, rheumatoid arthritis and cancers, which require special attention 50-51.
Figure 8.
Our works demonstrate a critical role of IL-22 in regulating hepatocellular metabolism to treat liver injury via activating STAT3-lncRNA H19-AMPK-AKT-mTOR axis. These findings describe a novel mechanism underscoring the regulation of metabolic states of hepatocytes by IL-22 during liver injury with potentially broad therapeutic insights.
Supplementary Material
Supplementary figures and table.
Acknowledgments
Our researches were supported by National Key Basic Research Program of China (No. 2015CB931800) and National Natural Science Foundation of China (No. 81773620, 81573332) and China Postdoctoral International Exchange Program.
Abbreviations
- IL-22
interleukin 22
- OXPHOS
oxidative phosphorylation
- mTOR
mammalian target of rapamycin
- ROS
reactive oxygen species
- IL-10
interleukin-10
- ILCs
innate lymphoid cells
- OCR
oxygen consumption rate
- ECAR
extracellular acid rate
- FCCP
cyanide p-trifluoromethoxyphenyl-hydrazone
- CCl4
carbon tetrachloride
- FBS
fetal bovine serum
- BSA
bovine serum albumin
- IL-22R1
IL-22 receptor
- MRC
maximal respiratory capacity
- KEGG
Kyoto Encyclopedia of Genes and Genomes analysis
- SSC
side scatter signal
- AMPK
AMP-activated protein kinase
- ALT
alanine aminotransferase
- AST
aspartate amino-transferase
- ER
endoplasmic reticulum
References
- 1.Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J. et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. doi: 10.1186/s12916-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villanueva MT. Liver disease: conscious uncoupling in NASH. Nat Rev Drug Discov. 2017;16:238–9. doi: 10.1038/nrd.2017.60. [DOI] [PubMed] [Google Scholar]
- 3.Kupferschmidt K. Europe's new hepatitis problem. Science. 2016;353:862–3. doi: 10.1126/science.353.6302.862. [DOI] [PubMed] [Google Scholar]
- 4.Cubero FJ, Zoubek ME, Hu W, Peng J, Zhao G, Nevzorova YA. et al. Combined activities of JNK1 and JNK2 in hepatocytes protect against toxic liver injury. Gastroenterology. 2016;150:968–81. doi: 10.1053/j.gastro.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czech MP. Obesity notches up fatty liver. Nat Med. 2013;19:969–71. doi: 10.1038/nm.3293. [DOI] [PubMed] [Google Scholar]
- 6.Zhou B, Kreuzer J, Kumsta C, Wu L, Kamer KJ, Cedillo L. et al. Mitochondrial permeability uncouples elevated autophagy and lifespan extension. Cell. 2019;177:299–314. doi: 10.1016/j.cell.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cubero FJ, Peng J, Liao L, Su H, Zhao G, Zoubek ME. et al. Inactivation of caspase 8 in liver parenchymal cells confers protection against murine obstructive cholestasis. J Hepatol. 2018;69:1326–34. doi: 10.1016/j.jhep.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Wang W, Wang X, Zhao J, Xiao L, Gui W. et al. Creg in Hepatocytes ameliorates liver ischemia/reperfusion injury in a TAK1-dependent manner in mice. Hepatology. 2019;69:294–313. doi: 10.1002/hep.30203. [DOI] [PubMed] [Google Scholar]
- 9.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–90. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 10.Mielke LA, Jones SA, Raverdeau M, Higgs R, Stefanska A, Groom JR. et al. Retinoic acid expression associates with enhanced IL-22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation. J Exp Med. 2013;210:1117–24. doi: 10.1084/jem.20121588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aden K, Rehman A, Falk-Paulsen M, Secher T, Kuiper J, Tran F. et al. Epithelial IL-23R signaling licenses protective IL-22 responses in intestinal inflammation. Cell Rep. 2016;16:2208–18. doi: 10.1016/j.celrep.2016.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–59. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zai W, Chen W, Wu Z, Jin X, Fan J, Zhang X. et al. Targeted interleukin-22 gene delivery in the liver by polymetformin and penetratin-based hybrid nanoparticles to treat nonalcoholic fatty liver disease. ACS Appl Mater Interfaces. 2019;11:4842–57. doi: 10.1021/acsami.8b19717. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Luan J, Wei G, Zhang X, Fan J, Zai W. et al. In vivo hepatocellular expression of interleukin-22 using penetratin-based hybrid nanoparticles as potential anti-hepatitis therapeutics. Biomaterials. 2018;187:66–80. doi: 10.1016/j.biomaterials.2018.09.046. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Zhang X, Fan J, Zai W, Luan J, Li Y. et al. Tethering Interleukin-22 to apolipoprotein A-I ameliorates mice from acetaminophen-induced liver Injury. Theranostics. 2017;7:4135–48. doi: 10.7150/thno.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–42. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 17.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R. et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao B, Xiang X. Interleukin-22 from bench to bedside: a promising drug for epithelial repair. Cell Mol Immunol. 2019;16:666–7. doi: 10.1038/s41423-018-0055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aden K, Tran F, Ito G, Sheibani-Tezerji R, Lipinski S, Kuiper JW. et al. ATG16L1 orchestrates interleukin-22 signaling in the intestinal epithelium via cGAS-STING. J Exp Med. 2018;215:2868–86. doi: 10.1084/jem.20171029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN. et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shyh-Chang N, Zhu H, Yvanka de Soysa T, Shinoda G, Seligson MT, Tsanov KM. et al. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell. 2013;155:778–92. doi: 10.1016/j.cell.2013.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He L, Gomes AP, Wang X, Yoon SO, Lee G, Nagiec MJ. et al. mTORC1 promotes metabolic reprogramming by the suppression of GSK3-dependent Foxk1 phosphorylation. Mol Cell. 2018;70:949–60. doi: 10.1016/j.molcel.2018.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406–17. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito Y, Chapple RH, Lin A, Kitano A, Nakada D. AMPK protects leukemia-initiating cells in myeloid leukemias from metabolic stress in the bone marrow. Cell Stem Cell. 2015;17:585–96. doi: 10.1016/j.stem.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Guan KL. mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol. 2019;21:63–71. doi: 10.1038/s41556-018-0205-1. [DOI] [PubMed] [Google Scholar]
- 27.Sciarretta S, Forte M, Frati G, Sadoshima J. New insights into the role of mTOR signaling in the cardiovascular system. Circ Res. 2018;122:489–505. doi: 10.1161/CIRCRESAHA.117.311147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geng H, Bu HF, Liu F, Wu L, Pfeifer K, Chou PM. et al. In inflamed intestinal tissues and epithelial cells, interleukin 22 signaling increases expression of H19 long noncoding RNA, which promotes mucosal regeneration. Gastroenterology. 2018;155:144–55. doi: 10.1053/j.gastro.2018.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasnain SZ, Borg DJ, Harcourt BE, Tong H, Sheng YH, Ng CP. et al. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med. 2014;20:1417–26. doi: 10.1038/nm.3705. [DOI] [PubMed] [Google Scholar]
- 30.Hwang S, He Y, Xiang X, Seo W, Kim SJ, Ma J. et al. Interleukin-22 ameliorates neutrophil-driven nonalcoholic steatohepatitis through multiple targets. Hepatology. 2019 doi: 10.1002/hep.31031. doi: 10.1002/hep.31031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Y, Wu F, Zhou J, Yan L, Jurczak MJ, Lee HY. et al. The H19/let-7 double-negative feedback loop contributes to glucose metabolism in muscle cells. Nucleic Acids Res. 2014;42:13799–811. doi: 10.1093/nar/gku1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li FL, Liu JP, Bao RX, Yan G, Feng X, Xu YP. et al. Acetylation accumulates PFKFB3 in cytoplasm to promote glycolysis and protects cells from cisplatin-induced apoptosis. Nat Commun. 2018;9:508. doi: 10.1038/s41467-018-02950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi W, Keenan HA, Li Q, Ishikado A, Kannt A, Sadowski T. et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med. 2017;23:753–62. doi: 10.1038/nm.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potente M, Carmeliet P. The link between angiogenesis and endothelial metabolism. Annu Rev Physiol. 2017;10:43–66. doi: 10.1146/annurev-physiol-021115-105134. [DOI] [PubMed] [Google Scholar]
- 35.Khacho M, Harris R, Slack RS. Mitochondria as central regulators of neural stem cell fate and cognitive function. Nat Rev Neurosci. 2019;20:34–48. doi: 10.1038/s41583-018-0091-3. [DOI] [PubMed] [Google Scholar]
- 36.Yang YM, Han CY, Kim YJ, Kim SG. AMPK-associated signaling to bridge the gap between fuel metabolism and hepatocyte viability. World J Gastroenterol. 2010;16:3731–42. doi: 10.3748/wjg.v16.i30.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saberi B, Ybanez MD, Johnson HS, Gaarde WA, Han D, Kaplowitz N. Protein kinase c (pkc) participates in acetaminophen hepatotoxicity through c-jun-n-terminal kinase (jnk)-dependent and -independent signaling pathways. Hepatology. 2014;59:1543–54. doi: 10.1002/hep.26625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mo R, Lai R, Lu J, Zhuang Y, Zhou T, Jiang S. et al. Enhanced autophagy contributes to protective effects of IL-22 against acetaminophen-induced liver injury. Theranostics. 2018;8:4170–80. doi: 10.7150/thno.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T. et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–7. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–22. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das S, Morvan F, Jourde B, Meier V, Kahle P, Brebbia P. et al. ATP citrate lyase improves mitochondrial function in skeletal muscle. Cell Metab. 2015;21:868–76. doi: 10.1016/j.cmet.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Morita M, Gravel SP, Hulea L, Larsson O, Pollak M, St-Pierre J. et al. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle. 2015;14:473–80. doi: 10.4161/15384101.2014.991572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai J, Zhou Q, Chen J, Rexius-Hall ML, Rehman J, Zhou G. Alpha-enolase regulates the malignant phenotype of pulmonary artery smooth muscle cells via the AMPK-Akt pathway. Nat Commun. 2018;9:3850. doi: 10.1038/s41467-018-06376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang SH, Li J, Dong FY, Yang JY, Liu DJ, Yang XM. et al. Increased serotonin signaling contributes to the warburg effect in pancreatic tumor cells under metabolic stress and promotes growth of pancreatic tumors in mice. Gastroenterology. 2017;153:277–91. doi: 10.1053/j.gastro.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Park KS, Mitra A, Rahat B, Kim K, Pfeifer K. Loss of imprinting mutations define both distinct and overlapping roles for misexpression of IGF2 and of H19 lncRNA. Nucleic Acids Res. 2017;45:12766–79. doi: 10.1093/nar/gkx896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geng T, Liu Y, Xu Y, Jiang Y, Zhang N, Wang Z. et al. H19 lncRNA promotes skeletal muscle insulin sensitivity in part by targeting AMPK. Diabetes. 2018;67:2183–98. doi: 10.2337/db18-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zai W, Chen W, Han Y, Wu Z, Fan J, Zhang X. et al. Targeting PARP and autophagy evoked synergistic lethality in hepatocellular carcinoma. Carcinogenesis. 2019 doi: 10.1093/carcin/bgz104. doi: 10.1093/carcin/bgz104. [DOI] [PubMed] [Google Scholar]
- 48.Arab JP, Sehrawat TS, Simonetto DA, Verma VK, Feng D, Tang T. et al. An open label, dose escalation study to assess the safety and efficacy of IL-22 agonist F-652 in patients with alcoholic hepatitis. Hepatology. 2019 doi: 10.1002/hep.31046. doi: 10.1002/hep.31046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang TT, Li YY, Li JJ, Wang K, Han Y, Dong WY. et al. Liver-heart crosstalk controls IL-22 activity in cardiac protection after myocardial infarction. Theranostics. 2018;8:4552–62. doi: 10.7150/thno.24723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng D, Wang Y, Wang H, Weng H, Kong X, Martin-Murphy BV. et al. Acute and chronic effects of IL-22 on acetaminophen-induced liver injury. J Immunol. 2014;193:2512–8. doi: 10.4049/jimmunol.1400588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kleinschmidt D, Giannou AD, McGee HM, Kempski J, Steglich B, Huber FJ. et al. A protective function of IL-22BP in ischemia reperfusion and acetaminophen- induced liver injury. J Immunol. 2017;199:4078–90. doi: 10.4049/jimmunol.1700587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and table.