Abstract
Blood vessels are conduits distributed throughout the body, supporting tissue growth and homeostasis by the transport of cells, oxygen and nutrients. Endothelial cells (ECs) form the linings of the blood vessels, and together with pericytes, are essential for organ development and tissue homeostasis through producing paracrine signalling molecules, called angiocrine factors. In the skeletal system, ECs - derived angiocrine factors, combined with bone cells-released angiogenic factors, orchestrate intercellular crosstalk of the bone microenvironment, and the coupling of angiogenesis-to-osteogenesis. Whilst the involvement of angiogenic factors and the blood vessels of the skeleton is relatively well established, the impact of ECs -derived angiocrine factors on bone and cartilage homeostasis is gradually emerging. In this review, we survey ECs - derived angiocrine factors, which are released by endothelial cells of the local microenvironment and by distal organs, and act specifically as regulators of skeletal growth and homeostasis. These may potentially include angiocrine factors with osteogenic property, such as Hedgehog, Notch, WNT, bone morphogenetic protein (BMP), fibroblast growth factor (FGF), insulin-like growth factor (IGF), and platelet-derived growth factor (PDGF). Understanding the versatile mechanisms by which ECs-derived angiocrine factors orchestrate bone and cartilage homeostasis, and pathogenesis, is an important step towards the development of therapeutic potential for skeletal diseases.
Keywords: angiocrine factors, angiogenic factors, bone and cartilage homeostasis, endothelial cells, angiogenesis-osteogenesis coupling
Introduction
Endothelial cells (ECs)-mediated angiogenesis (the sprouting of existing vessels) plays a pivotal role in bone development, growth, and repair 1. Multiple lines of evidence indicate that bone remodelling takes place within vascularized structures, called “bone remodelling compartments” (BRCs) 2, 3. The vascular network is essential for bone formation, metabolism, and repair. Reduced bone vascularity or angiogenesis could lead to impaired bone formation, decreased bone quantity and quality, and reduced healing capacity of bone fracture 1. The reciprocal relationship of the skeleton and vascular network is regulated by complex intercellular crosstalk at the remodelling interface between bone cells (osteoblasts, osteoclasts, and osteocytes) and vascular cells (endothelial cells and pericytes) 2, 4. Bone cell-derived angiogenic factors and ECs -derived angiocrine factors are critical factors, which affect intercellular signalling and maintain homeostatic coupling of angiogenesis-osteogenesis within the bone remodelling microenvironment 5. During embryonic osteogenesis, vascularisation stimulates the replacement of the hypertrophic cartilage core by bone marrow expansion. Endochondral ossification is the process by which bones enlarge and ossify during development, occurring predominantly near the growth plate, whereby cartilage is replaced by vascularized bone tissue 6, and this process is regulated by angiogenic activity 2, 5. ECs are angiogenic progenitors of the subchondral vasculature, which provide the source for vascular expansion and secrete factors to induce late chondrocyte differentiation during endochondral ossification 7. In adult bone, the physiological processes of angiogenesis and osteogenesis are closely coupled, which is essential to maintain bone mass and homeostasis 8, 9. In pathological bone fracture, approximately 10% of human bone fractures fail to heal adequately, which may be caused by the impaired formation of blood vessels and mineralized tissue at the site of injury 10, owing in part to the disrupted intercellular signalling of angiocrine factors 11. However, the expression of angiocrine factors by ECs, and their role in skeletal homeostasis and pathogenesis remain incompletely understood.
Vascular endothelial growth factor (VEGF) signifies a potent angiogenic factor that regulates vascularized skeletal tissue throughout development, and is critical for the coupling of angiogenesis and bone formation 12-14. VEGF derived from osteoblasts appears to stimulate the osteoblastic differentiation of mesenchymal stem cells (MSCs) and bone repair 14, 15. Additional findings indicate that VEGF produced by osteoblasts also affects bone remodelling by stimulating osteoclast differentiation 14. Recently, studies have shown that osteoblasts express numerous angiogenic factors, including chemokine (C-X-C motif) ligand 9 (Cxcl9) 16, Nephronectin (NPNT) 17, EGF-like domain 6 (EGFL6) 18, EGF-like domain 7 (EGFL7) 19 and slit guidance ligand 3 (SLIT3) 20, 21; and osteoclast-like cells too express angiogenic factors, such as platelet- derived growth factor (PDGF)-BB 22 and EGFL7 19, which are involved with the mediation of angiogenesis. Notably, in the skeletal microenvironment, an array of secreted anti-angiogenic factors are also produced including chondromodulin-1 (Chm-1) 23, pigment epithelium-derived factor (PEDF) 24, and connective tissue growth factor (CTGF/CCN2) 25, that co-regulate local vascularization together with angiogenic factors, and play an important balanced role in bone and cartilage homeostasis 26-28.
Reciprocally, blood vessels produce a network of paracrine factors, called angiocrine factors, to regulate skeletal cells, such as osteoclasts, osteoblasts and chondrocytes. Several ECs-derived angiocrine factors involved with the regulation of bone have been identified, either locally within the bone microenvironment, or systemically from distal organs, such as the liver 29-32 (Table 1). Local angiocrine factors, are exemplified by canonical ligands of Notch signalling, Jagged-1 (Jag1) 33 and delta-like-4 (DLL4) 11, 34, which are produced by ECs of the bone marrow vascular niche, and are involved with the regulation of haematopoiesis and the regenerative capacity of bone tissue. Additionally, distal or systemic angiocrine factors have been reported 35, 36. For example, bone morphogenetic protein 2 (BMP2), the well-known regulator of osteoblast differentiation was found to be produced by liver sinusoidal ECs 35, 36.
Table 1.
Examples of angiocrine factors produced locally in the bone microenvironment and by distal organs.
| Angiocrine factors | Produced by endothelial cells | Local and/or distal effects | References |
|---|---|---|---|
| In bone microenvironment | Local effect | ||
| Delta-like 4 | Sinusoidal endothelial cells | Hematopoietic cell modulation | 34 |
| Kit-ligand | Sinusoidal endothelial cells | Hematopoietic stem and progenitor cells (HSPCs) | 40 |
| SDF1 | Sinusoidal endothelial cells | Hematopoietic stem and progenitor cells (HSPCs) | 50 |
| Jagged-1 | Sinusoidal endothelial cells | Self-renewal and regenerative capacity of hematopoietic stem cell | 33 |
| Jagged-2 | Sinusoidal endothelial cells | Homeostasis of haematopoietic stem and progenitor cells | 43 |
| Interleukin-33 | Sinusoidal endothelial cells | Expansion of hematopoietic precursor cells, and osteogenic differentiation | 41 |
| Angiocrine factors | Sinusoidal endothelial cells | Long-term hematopoietic stem cells | 29, 30 |
| Angiocrine factors | Sinusoidal endothelial cells | Hematopoietic stem and progenitor cells (HSPCs) | 31 |
| Noggin, BMPs, Jagged-1 | Type-H and L vessels | Osteogenic differentiation | 11 |
| In distal organs | Local and possible distal effect | ||
| BMP2 | Liver sinusoidal endothelial cells | Iron homeostasis of the liver, possible osteogenic differentiation | 35, 36 |
| Wnt2 | Liver sinusoidal endothelial cells | Hepatic regeneration, possible osteogenic differentiation | 57 |
| BMP4 | Thymic endothelial cells | Thymic regeneration, possible osteogenic differentiation | 48 |
Collectively, ECs are postulated to release local or systemic organ-specific angiocrine factors which orchestrate bone and cartilage homeostasis and regeneration. In this review, we will discuss ECs derived angiocrine factors, including their potential roles and mechanisms of action in the regulation of bone and cartilage homeostasis.
Angiocrine factors of the bone microenvironment
ECs are specialized blood vessel cells present in all organ systems and are involved with the regulation of organ development and tissue homeostasis by the production of paracrine factors 37, 38. Bone marrow ECs (BMECs) regulate physiological and regenerative hematopoiesis of the bone marrow niche throughout life by the expression of angiocrine factors, including stem cell factor 1 (SCF1, also called KITL), CXCL12, and Jag1, which signal via pathways, such as NF-κB, Akt and MAPK 33, 39, 40. EC signalling within the bone marrow vascular niche is critically involved in the regulation of hematopoietic stem cell (HSC) function, both at steady state and in disease conditions 39. ECs are thought to coordinate the vital processes of bone marrow, such as osteogenesis, angiogenesis, and hematopoiesis. Recently, a subset of endoglin- expressing ECs was detected in the bone marrow, which were shown to promote the expansion of distinct subsets of hematopoietic precursor cells, ECs, and osteogenic differentiation by the abundant production of interleukin-33 (IL-33) 41.
Angiocrine factor signalling appears to provide an instructive vascular niche which is important for the modulation of the reconstitution of hematopoietic stem and progenitor cells (HSPCs), and the regulation of long-term hematopoietic stem cells (LT-HSCs) of the bone marrow 29, 31, 42. Initial studies found that angiocrine factors expressed by ECs of the vascular niche within the bone marrow microenvironment promoted the self-renewal and proliferation of LT-HSCs 30. Further, the constitutive expression of Notch ligands, such as Jag1 and jagged-2 (Jag2) was found in endothelial cells, suggesting the role of Notch signalling in the regulation of bone marrow HSPCs 30, 43.
Consistently, conditional knockout of Jag1 in ECs in mice impaired the self-renewal and led to the premature exhaustion of an adult HSC population, indicating that Jag1 expressed by the vascular niche in bone marrow regulates hematopoiesis and supports the regenerative capacity of HSCs through a Notch-dependent signalling pathway 33. Further, conditional deletion of the gene encoding Jag2 specific to ECs, indicated that Jag2 might be indispensable for regulating the hematopoietic recovery and reconstitution of HSPCs in response to myelosuppressive conditions, via Notch2-dependent signalling 43. Further, blocking DLL4, a canonical ligand of the Notch pathway family by using anti-DLL4 monoclonal antibodies appears to disturb angiogenic changes and hematopoiesis of the bone marrow 34. These data suggest that activation of Notch signalling by ECs-Jagged family proteins in HSPCs could have therapeutic potential for the enhancement of hematopoietic homeostasis after myelosuppression 43.
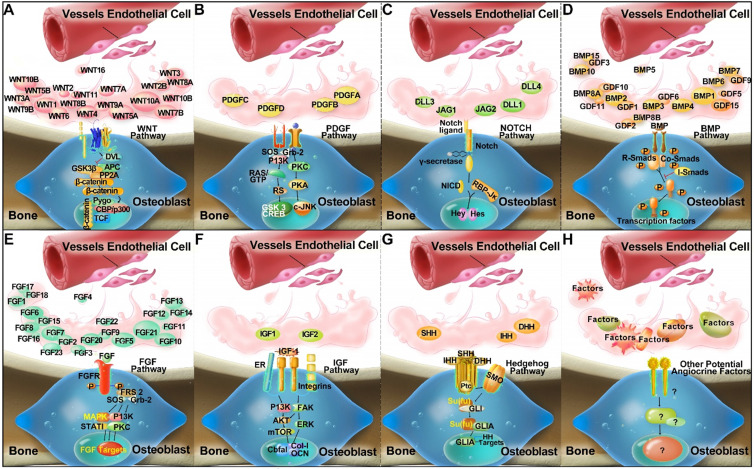
Several established receptor and ligand families appear to be important for the regulation of skeletal homeostasis and tissue regeneration, including Hedgehog, Notch, WNT, BMP, FGF, IGF, and PDGF signalling pathways 44-46. It is postulated that ECs may produce major classes of angiocrine factors at different levels, which act on their respective receptors in the osteogenic lineage cells to modulate skeletal homeostasis (Figure 1). However, it remains to be fully investigated which arrays of ECs derived angiocrine factors from other organs may have regulatory effects on skeletal growth and homeostasis.
Figure 1.
Compendium of molecules (Wnt, PDGF, Notch, BMP, FGF, IGF, and Hedgehog families) which are postulated to mediate crosstalk between ECs and osteoblastic lineage cells, and to activate putative signalling pathways via a paracrine mode of action in the bone microenvironment (A-G) by inference from experimental findings 94-102. In addition, unknown and novel angiocrine factors might be produced by ECs, which are yet to be discovered and require further research (H).
Potential angiocrine factors derived from organs which regulate bone via a systemic route
ECs are defined by organ-specific heterogeneity, which enables them to maintain the vital function of the local vasculature and microenvironment, such as maintenance of the blood-brain barrier, renal filtration, and hepatic clearance 35. Angiocrine factors are specific paracrine growth factors that are secreted by blood vessels, and regulate a wide range of organ development and tissue homeostasis processes, such as the maturation of retinal pigment epithelium for the establishment of the blood-retina barrier 47, thymic regeneration 48, and liver regeneration 49, 50.
An interesting question remains as to if the systemic action of angiocrine factors produced by ECs from distal organs might affect bone morphogenesis and remodelling 1, 51. For example, liver sinusoidal ECs (LSEC) are responsible for the hepatic clearance of circulation waste products, and regulate the hepatic vascular niche by producing angiocrine factors, such as BMP2 35. Genetic inactivation of angiocrine BMP2 signalling by LSECs in mice affects iron homeostasis of the liver and whole organism 36. The importance of BMP2 signalling by BMECs in bone and cartilage homeostasis, repair and regeneration is well established 52, 53. Of the BMP family, BMP2 is a prime regulator of postnatal skeletal homeostasis, and BMP2 osteogenic signalling is vital for the reparative and regenerative capacity of bone during fracture healing 52, 54. BMP2-induced osteogenesis is regulated via the activation of receptor-regulated (R-) small mothers against decapentaplegic homologs (Smads) (R-Smads), and transcription factors, such as Runx2, Osx, Dlx5, and Msx2 52, 55. Co-activation of Wnt and BMP2 signalling appears to affect osteogenic activity and the expression of Rux2 and Osx1, indicating the need for investigation of the importance of the possible distal effects of Wnt/BMP2 for skeletal homeostasis and repair 52, 56. LSECs also release inductive angiocrine factors, such as Wnt2 and hepatocyte growth factor (HGF), which stimulate hepatic regeneration 57. Given the key role of BMP2 and Wnt2 in osteogenesis, and the systemic effect of LSEC paracrine signalling of BMP2, it will be interesting to confirm the systemic effect of angiocrine factors on bone. Further investigation of the effect of inactivating BMP2 and Wnt secretion by LSECs on bone health is therefore required. Extracellular vesicles are circulating particles that contain proteins (such as BMPs, Wnts, and VEGF), and nucleic acids (such as microRNAs), and are involved in mediating intercellular communication locally and systemically 58-62. Extracellular vesicles produced by tissue-specific ECs represent a potential means of systemic delivery of angiocrine factors involved with regulating the bone microenvironment requiring further research of their therapeutic effects 63-65. For instance, recent findings indicate that extracellular vesicles from endothelial progenitor cells may have therapeutic potential for the treatment of osteoporosis 63. Further research of tissue-specific EC-extracellular vesicles and their systemic mode of action is required. Thymic endothelial cells appear to critically affect thymus repair and regeneration by BMP4 signalling, indicating the need for further research to investigate the potential for ECs-BMP4 signalling to enhance T cell immunity and skeletal health 48, 66. In responding to metabolic and pathological changes, ECs release angiocrine factors that bind to their respective receptors in a cell type dependent manner to maintain homeostasis and coordinate tissue regeneration. The expression and release of angiocrine factors by ECs is regulated by hypoxia 67-69, and mechanosensing or mechanostretching of ECs in response to blood flow 70. In addition, bone-ECs respond to endocrine signals, including parathyroid hormone (PTH), progesterone (P4), estrogen (E2), IGFs, bFGF, and PDGF 71, 72. Further investigation of the stimuli and signalling pathways affecting the expression of angiocrine factors by tissue- and organ-specific ECs, and their systemic effects is required.
Coupling of angiogenesis and osteogenesis in bone
Although the growth of blood vessels in bone and osteogenesis appear to be coupled, the mechanisms of intercellular crosstalk regulating this relationship are largely undetermined. Specializations of the blood vessel architecture, including EC-tissue specificity, appear to take part in the coupling of angiogenesis and osteogenesis in bone 69, 73, 74.
A specific H-type capillary (CD31hiEmcnhi ECs), was found to couple angiogenesis and osteogenesis, to regulate the metabolic and molecular microenvironment, and to sustain stem cells 69, 73. The H-type capillary (or vessel) is defined by its high expression of CD31 and Endomucin (Emcn), and is located within the metaphysis, near the growth plate, and along the periosteal and endosteal surfaces of the diaphysis, where there is an abundance of surrounding bone marrow osteoprogenitor cells, particularly platelet-derived growth factor receptor β (PDGFR-β) expressing mesenchymal cells 69, 73. H-type vessels appear to coordinate intercellular signalling between osteoblast and osteoclast lineage cells, and to produce factors for the proliferation and differentiation of osteoprogenitor cells, leading to bone formation 73. H-type vessels are an important component of the vascular hematopoietic stem cell niche, and their abundance, together with associated osteoprogenitor cells, appears to decline with age, as indicated by their crucial role in maintaining bone mass in aging mice, and their potential as an effective therapeutic target for osteoporosis and osteoarthritis treatment 69, 73, 75, 76. Further investigation of the molecular signalling by H-type vessels leading to bone formation, and the coupling of angiogenesis and osteogenesis is required 69. For example, stem cell factor (SCF) is a vital modulator of the vascular HSC niche expressed by perivascular cells, and the effect of H-type vessel signalling on SCF expression is unknown 40; and the essential role of Notch signalling by H-type vessels for the maintenance of the vascular stem cell niche remains to be elucidated 30. Factors that appear to regulate the coupling of angiogenesis and osteogenesis, and require further investigation in relation to H-type vessels, include PDGF-BB, SLIT3, hypoxia-inducible factor 1-α (HIF-1α), Notch, and VEGF 73.
Research of the molecular mechanisms mediating endothelial-osteoblast cell crosstalk demonstrates the tissue-specific angiogenesis of bone 11, 73, 75, 77. Inducible genetic disruption of Notch signalling specific to ECs in mice led to an impaired skeletal phenotype characterized by the abnormal development of blood vessels, reduced osteogenesis, shortening of the long bones, chondrocyte defects, and decreased bone mass 11. The impaired skeletal phenotype was found to involve dysregulated Notch signalling from ECs via secreted Noggin, and the delivery of Noggin was able to reverse the skeletal defects of EC-mutant mice 11. Therapeutically, halofuginone was found to attenuate the progression of osteoarthritis (OA) by mitigating articular cartilage and subchondral bone deterioration in a rodent model 74. Halofuginone appears to inhibit aberrant angiogenesis, possibly including H-type capillary formation, and uncoupled bone remodelling in the subchondral bone by reducing osteoclastic bone resorption and Smad2/3-dependent TGF-β signalling 74, 78. Halofuginone treatment is consistently associated with the reduction of expression of OA markers, such as collagen X (ColX), matrix metalloproteinase-13 (MMP13), and A disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS 5) 74, 79. Further, halofuginone appears to increase the expression of OA protective factors, such as lubricin, collagen II and aggrecan 74. TGF-β/Smad pathway signalling is confirmed in the pathogenesis of OA by promoting chondrocyte hypertrophy, cartilage fibrosis, mesenchymal progenitor cell differentiation to osteoblast-lineage cells, and angiogenesis within the subchondral bone by regulating downstream target genes, such as Runx2, MMP13, and ADAMTS 5 80. Therefore, halofuginone appears to disrupt several levels of OA pathogenesis affected by TGF-β signalling, and it is imperative to further investigate the molecular mechanisms of halofuginone treatment for OA in order to develop its therapeutic potential. Together these findings provide a molecular basis for the coupling of angiogenesis and osteogenesis, and highlight the vital role of bone EC-specific angiocrine factors in the bone microenvironment, thus indicating the need for further research of their potential as therapeutic targets.
The potential role of pericytes in bone homeostasis
Pericytes, also called Rouget cells or mural cells, are located within the vascular basement membrane and closely encircle ECs in capillaries and micro- vessels 81. Pericytes are distributed throughout the organs of the body, and appear to function principally in maintaining vascular homeostasis and stability, and supporting angiogenesis in a tissue-specific manner 81-83. Studies indicate that sustained communication between pericytes, ECs, and vascular smooth muscle cells (vSMCs) maintains vascular function via Jag1 mediated Notch signalling of the Akt/mTOR pathway 83. The role of pericytes, and angiocrine factors produced by pericytes in regulating bone growth and homeostasis is largely unknown and warrants further investigation.
The role of angiocrine factors in skeletal disease
ECs are fundamentally important in establishing the tissue-specific instructive vascular niche, whose angiocrine factors are critical for the maintenance of regional stem cell populations, and tissue repair and regeneration 30, 40. However, ECs - derived angiocrine factors may be involved in tumour growth and aggressiveness, and might be prime targets for cancer therapy and regenerative medicine 84, 85. Understanding the complex regulation of ECs - derived angiocrine factors in the bone marrow microenvironment will help to pave the way for the discovery of novel approaches to the treatment of diseases, such as leukemia. For instance, the activation of ECs by vascular endothelial growth factor-A (VEGF-A) was found to promote the proliferation of aggressive leukemic cells, and could decrease the efficacy of chemotherapeutic agents targeting leukemic cells 86; whereas inhibiting the activation of ECs by blocking VEGF-receptor 2 (VEGFR 2) signalling might increase the sensitivity of leukemic cells to chemotherapy 86. In acute myeloid leukemia (AML), the leukemia microenvironment, containing BMECs, is postulated to perpetuate refractory disease via a paracrine mechanism, and is a potential therapeutic target 87. Pazopanib, a receptor tyrosine kinase inhibitor (RTKI) of VEGFRs, PDGFRs, and cKit, was shown to be directly cytotoxic to AML cells, and to sensitize AML cells to chemotherapy by eliminating the refractory-disease effect of ECs 87. Combining RTKIs with chemotherapy therefore indicates a potential therapeutic strategy for the prevention of refractory disease and for the treatment of AML, and requires further investigation 87.
Recently, epidermal growth factor-like domain 7 (EGFL7) has been found to be involved in several types of cancers 88, 89. EGFL7 expression is involved with tissue regeneration, and loss of EGFL7 function may result in impaired vessel formation 90. Mechanistically, EGFL7 appears to bind to the extracellular matrix and act in an autocrine manner via its receptor, integrin αVβ3, to increase the motility of ECs during vessel sprouting 91. As an angiocrine factor, which is secreted by bone cells (osteoblasts and osteoclast lineages) and ECs, it may regulate bone homeostasis and cancer development 19, 90, 91, and requires further research to determine its varying roles.
In inflammation, BMECs produce cytokines, such as IL6, tumour-necrosis factor alpha (TNF-α), and interferon-gamma (IFN-γ), which induce NF-κB signalling for the proliferation and differentiation of HSPCs 39. Targeted inhibition of the NF-κB pathway in BMECs in mice following myelosuppressive injury was shown to promote hematopoietic recovery, to protect the BM micro-environment, and to limit damage of the BM vascular niche 39. Further, the transplantation of BMECs with NF-κB inactivation stimulated hematopoietic recovery and protected mice from chemotherapy-induced death by pancytopenia, indicating the potential of BMECs as a cell-based therapeutic approach for the treatment of hematological diseases 39.
In diabetic mice, bone marrow endothelial dysfunction appears to be affected by the disruption of BMEC signalling 92. Altered BMEC signalling, such as the increased activation of RhoA/Rho-associated kinase and Src/vascular endothelial cadherin pathways, and Akt inactivation, appears to dysregulate the expression of angiocrine factors by BMECs and resulted in bone marrow microangiopathy in a rodent model 92.
Additionally, circulating endothelial precursor cells, called endothelial colony-forming cells (ECFCs), represent a potential source of angiocrine factors which may improve the outcome of MSC-based regenerative applications 93. ECFCs paracrine signalling via factor PDGF-BB was found to potentiate regeneration by MSCs, and to improve MSC transplantation 93. MSC engraftment was regulated by signalling from the interaction between PDGF-BB and its receptor, PDGFR-β, and was enhanced by the co-transplantation of ECFCs 93. Further research is needed to investigate the possible application of ECFC-enhanced tissue regeneration in a disease-specific manner.
Future research of angiocrine signalling by ECs, such as BMECs, ECFCs, and the identification of novel factors and their mechanisms of action under pathological conditions, will help us to develop new therapeutic targets and regenerative medical treatments for diseases, including leukemia, osteoporosis, inflammation, and diabetes 32.
Conclusions
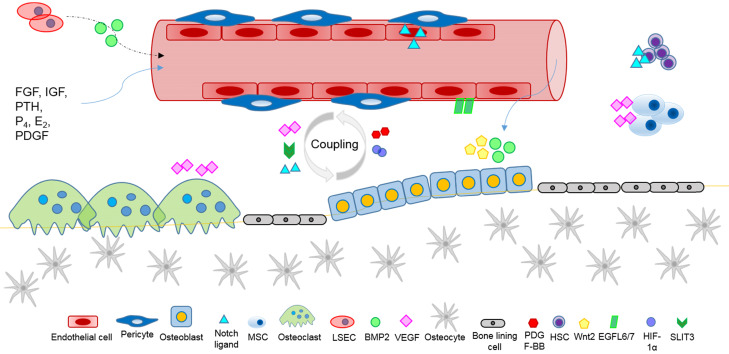
Bone growth, development, homeostasis and repair, require the exchange of oxygen, nutrients, and metabolites with the dense vascular system that surrounds and permeates skeletal tissue, and the anoxic bone microenvironment. Reciprocal crosstalk between skeletal cells (osteoblasts, osteoclasts, osteocytes), blood vessel cells (endothelial cells and pericytes), and bone marrow progenitor cells coordinate the physiological processes of bone growth and remodelling, vascular development, and the vital coupling of angiogenesis and osteogenesis. Angiogenic factors, produced by skeletal cells, affect local vascular ECs and angiogenesis (Figure 2). Conversely, angiocrine factors, secreted by vascular cells locally and systemically, appear to mediate skeletal development, homeostasis, and the recruitment of stem cells during wound healing. Several major classes of ligands, including Hedgehog, Notch, WNT, BMP, FGF, IGF, and PDGF are likely to mediate intercellular crosstalk for the regulation of skeletal homeostasis, and to affect the pathogenesis of skeletal diseases. Angiocrine factor signals of the instructive vascular niche, are involved with the regulation of bone marrow hematopoiesis, enhance the regenerative capacity of HSCs, and help to maintain a healthy bone marrow microenvironment. Future research elucidating the complex crosstalk of angiogenic and angiocrine signals, will help to shed new light on ECs metabolism, organ-specific vascular networks and tissue homeostasis, and advance our knowledge and capability to target vasculature disorders in skeleton, such as impaired fracture healing, osteonecrosis and cancer.
Figure 2.
Angiocrine factors mediate intercellular communication within the bone microenvironment affecting the regulation of skeletal homeostasis and repair, involving the coupling of angiogenesis and osteogenesis, and systemic paracrine signals.
Acknowledgments
This work was partly supported by a research grant from the National Natural Science Funding of China (81802235), Zhejiang Experimental Animal Science and Technology Project of China (2018C37112), Wenzhou basic science research plan project (Y20180033), and Key Laboratory of Orthopaedics of Zhejiang Province, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University Supported by Key Laboratory of Orthopaedics of Zhejiang Province (Grant No: ZJGK1801Z), and Shanxi Key Research and Development Program (International Science and Technology Cooperation) 201803D421050. Drs. Sipin Zhu and Chuan Xiang were visiting scholars to UWA. This study was supported in part by grants from the Australian Health and Medical Research Council (NHMRC, No. 1107828, 1127156, 1163933). Professor Vicki Rosen was an Australia-Harvard Fellow to the School of Biomedical Sciences, the University of Western Australia.
Author contribution
Sipin Zhu and Jiake Xu conducted literature search and drafted the manuscript. Samuel Bennett, Vincent Kuek, Chuan Xiang, Huazi Xu provided evaluation and assistance in the process of drafting and revision of the manuscript. Vicki Rosen and Jiake Xu conceptualized and supervised the study and revised the manuscript.
Abbreviations
- BMEC
bone marrow endothelial cell
- BMP
bone morphogenetic protein
- BRC
bone remodelling compartment
- CXCL
C-X-C motif ligand
- EC
endothelial cell
- EGF
epidermal growth factor
- E2
estrogen
- FGF
fibroblast growth factor
- HSC
hematopoietic stem cell
- HSPC
hematopoietic stem and progenitor cell
- IGF
insulin-like growth factor
- LSEC
liver sinusoidal endothelial cell
- MAPK
mitogen-activated protein kinase
- MSC
mesenchymal stem cell
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PDGF
platelet-derived growth factor
- P4
progesterone
- TGF
transforming growth factor
- VEGF
Vascular endothelial growth factor
References
- 1.Brandi ML, Collin-Osdoby P. Vascular biology and the skeleton. J Bone Miner Res. 2006;21:183–92. doi: 10.1359/JBMR.050917. [DOI] [PubMed] [Google Scholar]
- 2.Chim SM, Tickner J, Chow ST, Kuek V, Guo B, Zhang G. et al. Angiogenic factors in bone local environment. Cytokine Growth Factor Rev. 2013;24:297–310. doi: 10.1016/j.cytogfr.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Kular J, Tickner J, Chim SM, Xu J. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem. 2012;45:863–73. doi: 10.1016/j.clinbiochem.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Pastrama MI, Scheiner S, Pivonka P, Hellmich C. A mathematical multiscale model of bone remodeling, accounting for pore space-specific mechanosensation. Bone. 2018;107:208–221. doi: 10.1016/j.bone.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Zhu S, Yao F, Qiu H, Zhang G, Xu H, Xu J. Coupling factors and exosomal packaging microRNAs involved in the regulation of bone remodelling. Biol Rev Camb Philos Soc. 2018;93:469–80. doi: 10.1111/brv.12353. [DOI] [PubMed] [Google Scholar]
- 6.Knuth C, Kiernan C, Wolvius E, Narcisi R, Farrell E. Understanding tissue-engineered endochondral ossification; towards improved bone formation. Eur Cell Mater. 2019;37:277–91. doi: 10.22203/eCM.v037a17. [DOI] [PubMed] [Google Scholar]
- 7.Babarina AV, Mollers U, Bittner K, Vischer P, Bruckner P. Role of the subchondral vascular system in endochondral ossification: endothelial cell-derived proteinases derepress late cartilage differentiation in vitro. Matrix Biol. 2001;20:205–13. doi: 10.1016/s0945-053x(01)00132-9. [DOI] [PubMed] [Google Scholar]
- 8.Gerber HP, Ferrara N. Angiogenesis and bone growth. Trends Cardiovasc Med. 2000;10:223–8. doi: 10.1016/s1050-1738(00)00074-8. [DOI] [PubMed] [Google Scholar]
- 9.Carlevaro MF, Cermelli S, Cancedda R, Descalzi Cancedda F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci. 2000;113:59–69. doi: 10.1242/jcs.113.1.59. [DOI] [PubMed] [Google Scholar]
- 10.Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11:45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–80. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu K, Olsen BR. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30–8. doi: 10.1016/j.bone.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–8. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 14.Hu K, Olsen BR. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest. 2016;126:509–26. doi: 10.1172/JCI82585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berendsen AD, Olsen BR. How vascular endothelial growth factor-A (VEGF) regulates differentiation of mesenchymal stem cells. J Histochem Cytochem. 2014;62:103–8. doi: 10.1369/0022155413516347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang B, Wang W, Li Q, Wang Z, Yan B, Zhang Z. et al. Osteoblasts secrete Cxcl9 to regulate angiogenesis in bone. Nat Commun. 2016;7:13885. doi: 10.1038/ncomms13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuek V, Yang Z, Chim SM, Zhu S, Xu H, Chow ST. et al. NPNT is expressed by osteoblasts and mediates angiogenesis via the activation of extracellular signal-regulated kinase. Sci Rep. 2016;6:36210. doi: 10.1038/srep36210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chim SM, Qin A, Tickner J, Pavlos N, Davey T, Wang H. et al. EGFL6 promotes endothelial cell migration and angiogenesis through the activation of extracellular signal-regulated kinase. J Biol Chem. 2011;286:22035–46. doi: 10.1074/jbc.M110.187633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chim SM, Kuek V, Chow ST, Lim BS, Tickner J, Zhao J. et al. EGFL7 is expressed in bone microenvironment and promotes angiogenesis via ERK, STAT3, and integrin signaling cascades. J Cell Physiol. 2015;230:82–94. doi: 10.1002/jcp.24684. [DOI] [PubMed] [Google Scholar]
- 20.Koh JM. Osteoclast-derived SLIT3 is a coupling factor linking bone resorption to bone formation. BMB Rep. 2018;51:263–4. doi: 10.5483/BMBRep.2018.51.6.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu R, Yallowitz A, Qin A, Wu Z, Shin DY, Kim JM. et al. Targeting skeletal endothelium to ameliorate bone loss. Nat Med. 2018;24:823–33. doi: 10.1038/s41591-018-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L. et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20:1270–8. doi: 10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klinger P, Surmann-Schmitt C, Brem M, Swoboda B, Distler JH, Carl HD. et al. Chondromodulin 1 stabilizes the chondrocyte phenotype and inhibits endochondral ossification of porcine cartilage repair tissue. Arthritis Rheum. 2011;63:2721–31. doi: 10.1002/art.30335. [DOI] [PubMed] [Google Scholar]
- 24.Song N, Zhong J, Zhang J, Yu J, Li J, Qi J. et al. Pigment epithelium derived factor play a positive role in bone mineralization of osteoblasts derived from diabetic patients. Gene. 2017;627:563–8. doi: 10.1016/j.gene.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Nishida T, Emura K, Kubota S, Lyons KM, Takigawa M. CCN family 2/connective tissue growth factor (CCN2/CTGF) promotes osteoclastogenesis via induction of and interaction with dendritic cell-specific transmembrane protein (DC-STAMP) J Bone Miner Res. 2011;26:351–63. doi: 10.1002/jbmr.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broadhead ML, Akiyama T, Choong PF, Dass CR. The pathophysiological role of PEDF in bone diseases. Curr Mol Med. 2010;10:296–301. doi: 10.2174/156652410791065345. [DOI] [PubMed] [Google Scholar]
- 27.Zhu S, Qiu H, Bennett S, Kuek V, Rosen V, Xu H, Chondromodulin-1 in health, osteoarthritis, cancer, and heart disease. Cell Mol Life Sci. 2019. pp. 1–10. [DOI] [PMC free article] [PubMed]
- 28.Shimo T, Kubota S, Yoshioka N, Ibaragi S, Isowa S, Eguchi T. et al. Pathogenic role of connective tissue growth factor (CTGF/CCN2) in osteolytic metastasis of breast cancer. J Bone Miner Res. 2006;21:1045–59. doi: 10.1359/jbmr.060416. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi H, Butler JM, O'Donnell R, Kobayashi M, Ding BS, Bonner B. et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046–56. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT. et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–64. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gori JL, Butler JM, Kunar B, Poulos MG, Ginsberg M, Nolan DJ. et al. Endothelial cells promote expansion of long-term engrafting marrow hematopoietic stem and progenitor cells in primates. Stem Cells Transl Med. 2017;6:864–76. doi: 10.5966/sctm.2016-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramasamy SK, Kusumbe AP, Itkin T, Gur-Cohen S, Lapidot T, Adams RH. Regulation of hematopoiesis and osteogenesis by blood vessel-derived signals. Annu Rev Cell Dev Biol. 2016;32:649–75. doi: 10.1146/annurev-cellbio-111315-124936. [DOI] [PubMed] [Google Scholar]
- 33.Poulos MG, Guo P, Kofler NM, Pinho S, Gutkin MC, Tikhonova A. et al. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 2013;4:1022–34. doi: 10.1016/j.celrep.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remedio L, Carvalho T, Caiado F, Bastos-Carvalho A, Martins D, Duarte A. et al. Context- and cell-dependent effects of Delta-like 4 targeting in the bone marrow microenvironment. PLoS One. 2012;7:e52450. doi: 10.1371/journal.pone.0052450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsavszky V, Ulbrich F, Singh S, Diett M, Sticht C, Schmid CD. et al. GATA4 and LMO3 balance angiocrine signaling and autocrine inflammatory activation by BMP2 in liver sinusoidal endothelial cells. Gene. 2017;627:491–9. doi: 10.1016/j.gene.2017.06.051. [DOI] [PubMed] [Google Scholar]
- 36.Koch PS, Olsavszky V, Ulbrich F, Sticht C, Demory A, Leibing T. et al. Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis. Blood. 2017;129:415–9. doi: 10.1182/blood-2016-07-729822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316–25. doi: 10.1038/nature17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witjas FMR, van den Berg BM, van den Berg CW, Engelse MA, Rabelink TJ. Concise review: The endothelial cell extracellular matrix regulates tissue homeostasis and repair. Stem Cells Transl Med. 2019;8:375–82. doi: 10.1002/sctm.18-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poulos MG, Ramalingam P, Gutkin MC, Kleppe M, Ginsberg M, Crowley MJ. et al. Endothelial-specific inhibition of NF-kappaB enhances functional haematopoiesis. Nat Commun. 2016;7:13829. doi: 10.1038/ncomms13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–62. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenswil KJG, Jaramillo AC, Ping Z, Chen S, Hoogenboezem RM, Mylona MA. et al. Characterization of endothelial cells associated with hematopoietic niche formation in humans identifies IL-33 as an anabolic factor. Cell Rep. 2018;22:666–78. doi: 10.1016/j.celrep.2017.12.070. [DOI] [PubMed] [Google Scholar]
- 42.Butler JM, Rafii S. Generation of a vascular niche for studying stem cell homeostasis. Methods Mol Biol. 2012;904:221–33. doi: 10.1007/978-1-61779-943-3_18. [DOI] [PubMed] [Google Scholar]
- 43.Guo P, Poulos MG, Palikuqi B, Badwe CR, Lis R, Kunar B. et al. Endothelial jagged-2 sustains hematopoietic stem and progenitor reconstitution after myelosuppression. J Clin Invest. 2017;127:4242–56. doi: 10.1172/JCI92309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wehner D, Weidinger G. Signaling networks organizing regenerative growth of the zebrafish fin. Trends Genet. 2015;31:336–43. doi: 10.1016/j.tig.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Fujiwara M, Ozono K. [Cytokines and osteogenesis] Clin Calcium. 2014;24:845–51. [PubMed] [Google Scholar]
- 46.Midha S, Murab S, Ghosh S. Osteogenic signaling on silk-based matrices. Biomaterials. 2016;97:133–53. doi: 10.1016/j.biomaterials.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 47.Benedicto I, Lehmann GL, Ginsberg M, Nolan DJ, Bareja R, Elemento O. et al. Concerted regulation of retinal pigment epithelium basement membrane and barrier function by angiocrine factors. Nat Commun. 2017;8:15374. doi: 10.1038/ncomms15374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wertheimer T, Velardi E, Tsai J, Cooper K, Xiao S, Kloss CC, Production of BMP4 by endothelial cells is crucial for endogenous thymic regeneration. Sci Immunol. 2018. 3. [DOI] [PMC free article] [PubMed]
- 49.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M. et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang XJ, Olsavszky V, Yin Y, Wang B, Engleitner T, Ollinger R, Schledzewski K, Koch PS, Rad R, Schmid RM, Friess H, Goerdt S, Huser N, Geraud C, von Figura G, Hartmann D. Angiocrine Hepatocyte Growth Factor Signaling Controls Physiological Organ and Body Size and Dynamic Hepatocyte Proliferation to Prevent Liver Damage during Regeneration. Am J Pathol. 2020;190:358–371. doi: 10.1016/j.ajpath.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Streeten EA, Brandi ML. Biology of bone endothelial cells. Bone Miner. 1990;10:85–94. doi: 10.1016/0169-6009(90)90084-s. [DOI] [PubMed] [Google Scholar]
- 52.Rosen V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009;20:475–80. doi: 10.1016/j.cytogfr.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 53.Li WM, Huang WQ, Huang YH, Jiang DZ, Wang QR. Positive and negative hematopoietic cytokines produced by bone marrow endothelial cells. Cytokine. 2000;12:1017–23. doi: 10.1006/cyto.1999.0678. [DOI] [PubMed] [Google Scholar]
- 54.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L. et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 55.Ryoo HM, Lee MH, Kim YJ. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene. 2006;366:51–7. doi: 10.1016/j.gene.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Salazar VS, Ohte S, Capelo LP, Gamer L, Rosen V. Specification of osteoblast cell fate by canonical Wnt signaling requires Bmp2. Development. 2016;143:4352–67. doi: 10.1242/dev.136879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z. et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–5. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang-Doran I, Zhang CY, Vidal-Puig A. Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends Endocrinol Metab. 2017;28:3–18. doi: 10.1016/j.tem.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nahar NN, Missana LR, Garimella R, Tague SE, Anderson HC. Matrix vesicles are carriers of bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF), and noncollagenous matrix proteins. J Bone Miner Metab. 2008;26:514–9. doi: 10.1007/s00774-008-0859-z. [DOI] [PubMed] [Google Scholar]
- 61.Njock MS, Cheng HS, Dang LT, Nazari-Jahantigh M, Lau AC, Boudreau E. et al. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood. 2015;125:3202–12. doi: 10.1182/blood-2014-11-611046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jansen F, Li Q, Pfeifer A, Werner N. Endothelial- and immune cell-derived extracellular vesicles in the regulation of cardiovascular health and disease. JACC Basic Transl Sci. 2017;2:790–807. doi: 10.1016/j.jacbts.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu J, Yang J, Zheng Y, Chen X, Fang S. Extracellular vesicles from endothelial progenitor cells prevent steroid-induced osteoporosis by suppressing the ferroptotic pathway in mouse osteoblasts based on bioinformatics evidence. Sci Rep. 2019;9:16130. doi: 10.1038/s41598-019-52513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vonk LA, van Dooremalen SFJ, Liv N, Klumperman J, Coffer PJ, Saris DBF. et al. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics. 2018;8:906–20. doi: 10.7150/thno.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin H, Chen CY, Liu YW, Tan YJ, Deng ZL, Yang F. et al. Synechococcus elongatus PCC7942 secretes extracellular vesicles to accelerate cutaneous wound healing by promoting angiogenesis. Theranostics. 2019;9:2678–93. doi: 10.7150/thno.31884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ciofani M, Zúñiga-Pflücker JC. The thymus as an inductive site for T lymphopoiesis. Annu Rev Cell Dev Biol. 2007;23:463–93. doi: 10.1146/annurev.cellbio.23.090506.123547. [DOI] [PubMed] [Google Scholar]
- 67.Stegen S, Carmeliet G. The skeletal vascular system - Breathing life into bone tissue. Bone. 2018;115:50–8. doi: 10.1016/j.bone.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 68.Alvarez-Martins I, Remedio L, Matias I, Diogo LN, Monteiro EC, Dias S. The impact of chronic intermittent hypoxia on hematopoiesis and the bone marrow microenvironment. Pflugers Arch. 2016. [DOI] [PMC free article] [PubMed]
- 69.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–8. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lorenz L, Axnick J, Buschmann T, Henning C, Urner S, Fang S. et al. Mechanosensing by beta1 integrin induces angiocrine signals for liver growth and survival. Nature. 2018;562:128–32. doi: 10.1038/s41586-018-0522-3. [DOI] [PubMed] [Google Scholar]
- 71.Collin-Osdoby P. Role of vascular endothelial cells in bone biology. J Cell Biochem. 1994;55:304–9. doi: 10.1002/jcb.240550306. [DOI] [PubMed] [Google Scholar]
- 72.Streeten EA, Ornberg R, Curcio F, Sakaguchi K, Marx S, Aurbach GD. et al. Cloned endothelial cells from fetal bovine bone. Proc Natl Acad Sci U S A. 1989;86:916–20. doi: 10.1073/pnas.86.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peng Y, Wu S, Li Y, Crane JL. Type H blood vessels in bone modeling and remodeling. Theranostics. 2020;10:426–36. doi: 10.7150/thno.34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cui Z, Crane J, Xie H, Jin X, Zhen G, Li C. et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-beta activity and H-type vessel formation in subchondral bone. Ann Rheum Dis. 2016;75:1714–21. doi: 10.1136/annrheumdis-2015-207923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kusumbe AP, Ramasamy SK, Itkin T, Mae MA, Langen UH, Betsholtz C. et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature. 2016;532:380–4. doi: 10.1038/nature17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang J, Yin H, Rao SS, Xie PL, Cao X, Rao T. et al. Harmine enhances type H vessel formation and prevents bone loss in ovariectomized mice. Theranostics. 2018;8:2435–46. doi: 10.7150/thno.22144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singh A, Veeriah V, Xi P, Labella R, Chen J, Romeo SG, Angiocrine signals regulate quiescence and therapy resistance in bone metastasis. JCI Insight. 2019. 4. [DOI] [PMC free article] [PubMed]
- 78.Mu W, Xu B, Ma H, Li J, Ji B, Zhang Z. et al. Halofuginone attenuates osteoarthritis by rescuing bone remodeling in subchondral bone through oral gavage. Front Pharmacol. 2018;9:269. doi: 10.3389/fphar.2018.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mu W, Xu B, Ma H, Ji B, Zhang Z, Li J. et al. Halofuginone attenuates articular cartilage degeneration by inhibition of elevated TGFbeta1 signaling in articular cartilage in a rodent osteoarthritis model. Mol Med Rep. 2017;16:7679–84. doi: 10.3892/mmr.2017.7549. [DOI] [PubMed] [Google Scholar]
- 80.Shen J, Li S, Chen D. TGF-β signaling and the development of osteoarthritis. Bone Res. 2014;2:14002. doi: 10.1038/boneres.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferland-McCollough D, Slater S, Richard J, Reni C, Mangialardi G. Pericytes, an overlooked player in vascular pathobiology. Pharmacol Ther. 2017;171:30–42. doi: 10.1016/j.pharmthera.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H. et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–53. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kerr BA, West XZ, Kim Y-W, Zhao Y, Tischenko M, Cull RM. et al. Stability and function of adult vasculature is sustained by Akt/Jagged1 signalling axis in endothelium. Nat Commun. 2016;7:10960. doi: 10.1038/ncomms10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cao Z, Ding B-S, Guo P, Lee Sharrell B, Butler Jason M, Casey Stephanie C. et al. Angiocrine factors deployed by tumor vascular niche induce B cell lymphoma invasiveness and chemoresistance. Cancer Cell. 2014;25:350–65. doi: 10.1016/j.ccr.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pedrosa AR, Trindade A, Carvalho C, Graca J, Carvalho S, Peleteiro MC. et al. Endothelial Jagged1 promotes solid tumor growth through both pro-angiogenic and angiocrine functions. Oncotarget. 2015;6:24404–23. doi: 10.18632/oncotarget.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poulos MG, Gars EJ, Gutkin MC, Kloss CC, Ginsberg M, Scandura JM. et al. Activation of the vascular niche supports leukemic progression and resistance to chemotherapy. Exp Hematol. 2014;42:976–86. doi: 10.1016/j.exphem.2014.08.003. e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drusbosky L, Gars E, Trujillo A, McGee C, Meacham A, Wise E. et al. Endothelial cell derived angiocrine support of acute myeloid leukemia targeted by receptor tyrosine kinase inhibition. Leuk Res. 2015;39:984–9. doi: 10.1016/j.leukres.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dudvarski Stankovic N, Bicker F, Keller S, Jones DT, Harter PN, Kienzle A, EGFL7 enhances surface expression of integrin alpha5beta1 to promote angiogenesis in malignant brain tumors. EMBO Mol Med. 2018. 10. [DOI] [PMC free article] [PubMed]
- 89.Hong G, Kuek V, Shi J, Zhou L, Han X, He W. et al. EGFL7: Master regulator of cancer pathogenesis, angiogenesis and an emerging mediator of bone homeostasis. J Cell Physiol. 2018;233:8526–37. doi: 10.1002/jcp.26792. [DOI] [PubMed] [Google Scholar]
- 90.Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T. et al. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–8. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- 91.Nikolic I, Stankovic ND, Bicker F, Meister J, Braun H, Awwad K. et al. EGFL7 ligates alphavbeta3 integrin to enhance vessel formation. Blood. 2013;121:3041–50. doi: 10.1182/blood-2011-11-394882. [DOI] [PubMed] [Google Scholar]
- 92.Mangialardi G, Katare R, Oikawa A, Meloni M, Reni C, Emanueli C. et al. Diabetes causes bone marrow endothelial barrier dysfunction by activation of the RhoA-Rho-associated kinase signaling pathway. Arterioscler Thromb Vasc Biol. 2013;33:555–64. doi: 10.1161/ATVBAHA.112.300424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin RZ, Moreno-Luna R, Li D, Jaminet SC, Greene AK, Melero-Martin JM. Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc Natl Acad Sci U S A. 2014;111:10137–42. doi: 10.1073/pnas.1405388111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herath TDK, Larbi A, Teoh SH, Kirkpatrick CJ, Goh BT. Neutrophil-mediated enhancement of angiogenesis and osteogenesis in a novel triple cell co-culture model with endothelial cells and osteoblasts. J Tissue Eng Regen Med. 2018;12:e1221–e36. doi: 10.1002/term.2521. [DOI] [PubMed] [Google Scholar]
- 95.Ma B, Dohle E, Li M, Kirkpatrick CJ. TLR4 stimulation by LPS enhances angiogenesis in a co-culture system consisting of primary human osteoblasts and outgrowth endothelial cells. J Tissue Eng Regen Med. 2017;11:1779–91. doi: 10.1002/term.2075. [DOI] [PubMed] [Google Scholar]
- 96.Villars F, Bordenave L, Bareille R, Amédée J. Effect of human endothelial cells on human bone marrow stromal cell phenotype: role of VEGF? J Cell Biochem. 2000;79:672–85. doi: 10.1002/1097-4644(20001215)79:4<672::aid-jcb150>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 97.Dohle E, Fuchs S, Kolbe M, Hofmann A, Schmidt H, Kirkpatrick CJ. Sonic hedgehog promotes angiogenesis and osteogenesis in a coculture system consisting of primary osteoblasts and outgrowth endothelial cells. Tissue Eng Part A. 2010;16:1235–7. doi: 10.1089/ten.tea.2009.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Akeno N, Robins J, Zhang M, Czyzyk-Krzeska MF, Clemens TL. Induction of vascular endothelial growth factor by IGF-I in osteoblast-like cells is mediated by the PI3K signaling pathway through the hypoxia-inducible factor-2alpha. Endocrinology. 2002;143:420–5. doi: 10.1210/endo.143.2.8639. [DOI] [PubMed] [Google Scholar]
- 99.Kim J-M, Shin H-I, Cha S-S, Lee CS, Hong BS, Lim S. et al. DJ-1 promotes angiogenesis and osteogenesis by activating FGF receptor-1 signaling. Nat Commun. 2012;3:1296. doi: 10.1038/ncomms2313. [DOI] [PubMed] [Google Scholar]
- 100.Saleh FA, Whyte M, Genever PG. Effects of endothelial cells on human mesenchymal stem cell activity in a three-dimensional in vitro model. Eur Cell Mater. 2011;22:242–57. doi: 10.22203/ecm.v022a19. discussion 57. [DOI] [PubMed] [Google Scholar]
- 101.Michalicka M, Boisjoli G, Jahan S, Hovey O, Doxtator E, Abu-Khader A. et al. Human bone marrow mesenchymal stromal cell-derived osteoblasts promote the expansion of hematopoietic progenitors through beta-catenin and Notch signaling pathways. Stem Cells Dev. 2017;26:1735–48. doi: 10.1089/scd.2017.0133. [DOI] [PubMed] [Google Scholar]
- 102.Deng C, Zhu H, Li J, Feng C, Yao Q, Wang L. et al. Bioactive scaffolds for regeneration of cartilage and subchondral bone interface. Theranostics. 2018;8:1940–55. doi: 10.7150/thno.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]