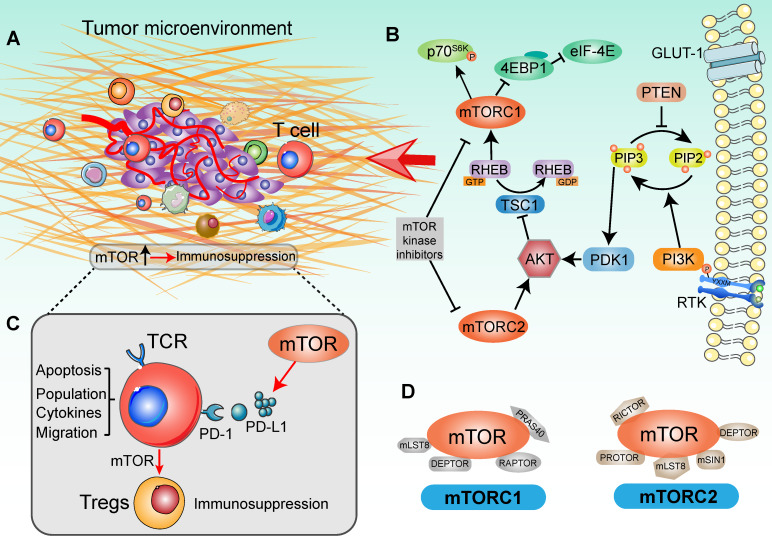
Figure 1.
Signaling cascade with mTOR activation in a tumor immune setting. (A) Tumor immune microenvironment. Tumor anatomy presents tumor foci environmental characteristics, including tumor core and immune environment. (Adapted with permission from 35, copyright 2012 Fridman). (B) mTOR-based immune cells strategy was presented in the context of the PI3K/AKT/mTOR signaling network. For the upstream, extracellular cytokines or chemokines stimulation lead to the recruitment of PI3 kinase (PI3K), thereby phosphorylating phosphatidylinositol 4,5-bisphosphate (PIP2) at the 3' position to generate phosphatidylinositol 3,4,5-trisphosphate (PIP3). In this catalytic node, PTEN plays an opposite role to PI3K. Activated PIP3 leads to the recruitment of AKT in signaling cascade by PDK1. Alternatively, mTORC2 is involved in regulating lipid biosynthesis downstream of AKT. AKT activation negatively modulates TSC1, which shapes the GAP activity for RHEB-GTP and increases RHEB-GTP accumulation. This promoted the function of mTORC1. In tumor immune environment, mTORC1 phosphorylates 4EBP1 and S6K to activate critical drivers of global protein translation, inflammation response, cell proliferation and infiltration. Additionally, many immunologic inputs also play a critical role in regulating mTOR signaling activity. Rapamycin and other mTOR inhibitors potently affect both mTOR complexes in an inhibitory manner. (C) The function of mTOR in T cell-based immunosuppression. mTOR promotes the PD-L1 expression to recognize the T-cell immune checkpoint, subsequently decreasing the tumor-infiltrating T cells activity. Accompany with the mTOR function, naïve T cells differentiate into Tregs and contribute to immunosuppression. (D) Two mTOR signaling complexes with specific substrates. mTOR is associated with two distinct sets of adapter proteins. mTORC1 complex is composed of the mLST8, DEPTOR, RAPTOR and PRAS40; and mTORC2 complex is composed of PROTOR, DEPTOR, RICTOR, mLST8, mSIN1.