Abstract
There are limited data on the effectiveness of dolutegravir (DTG)-based combination antiretroviral therapy (ART) in real-life settings in southern Africa where HIV-1 subtype C predominates. We report a patient infected with HIV-1 subtype C on DTG-based ART previously exposed to raltegravir who developed multidrug resistance mutations to four antiretroviral classes. There is need for drug resistance monitoring and clinical vigilance to ensure effectiveness of HIV treatment programs even in the era of DTG-based ART.
Maintaining viral load suppression in people living with HIV (PLWHIV) is critical to ensure both the health of PLWHIV and prevent onward transmission of HIV to sexual partners [1–3]. Despite major advances in the development of antiretroviral (ARV) drugs and ARV therapy (ART) treatment guidelines [4–6], low and middle-income countries (LMICs) continue to face challenges such as poor ARTadherence, limited HIV care specialists, drug stock-outs, lack of ancillary healthcare services and so on [7–9]. These barriers to effective ART may lead to development of extensive HIV-1 drug resistance.
Dolutegravir (DTG) has recently been introduced as part of the first-line ART regimen in Botswana and may soon be adopted by other countries in sub-Saharan Africa (SSA) [10,11]. Therefore, there is a need to monitor the development of integrase strand transfer inhibitor (INSTI) resistance mutations. We report a case of a four-class drug-resistant HIV-1 subtype C in a 51-year-old man currently on DTG-based ART with persistent viremia.
Chart reviews of treatment-experienced patients not virologically suppressed while on salvage ART therapy were conducted at a local tertiary hospital as part of the Botswana Epidemiological ART Treatment (BEAT) cohort study. A case-file reported herein was identified and an analysis of the patient’s medical record was conducted with information gathered from September 2003 to November 2017. The patient provided written informed consent to complete the analysis for publication, and the BEAT study is approved by the human research and development council of Botswana.
Genotypic resistance testing (GRT) was performed using Sanger sequencing of the pol and envelope genes. Sequences obtained were assessed for drug resistance mutations using the Stanford University HIV Drug Resistance Database (https://hivdb.stanford.edu), International Antiviral Society USA 2017 mutational list [12] and coreceptor usage was determined using with geno2pheno[coreceptor] [13]. All sequences generated were submitted to GenBank under accession numbers MG989439-MG989443, MH004049 and MH004050.
The patient was initiated on zidovudine (AZT), lamivudine and nevirapine on September 2003 as per the 2002 Botswana National ART guidelines. Apart from persistent viremias, his 14-year follow-up history has been clinically uneventful and devoid of opportunistic infections.
He intermittently achieved virological suppression between September 2003 and April 2014 (six HIV-1 RNA levels <400 copies/ml out of 67 tests) (Fig. 1). His ARTregimens while virologically suppressed were AZT/tenofovir (TDF)/ritonavir-boosted lopinavir from April 2007 and TDF/emtricitabine/ritonavir-boosted darunavir/raltegravir for the remaining aviremic episodes from February 2010. The majority of viral load revealed virological failure with viral load ranging between 2.61 log10 and 5.88 log10 copies/ml (Fig. 1). Nine switches in ARV medications were made from September 2003 to November 2017 (Fig. 1). Adherence to clinic appointments and medications was suboptimal, and he experienced one episode of drug stock-out of ARVs (darunavir) for approximately 2 weeks in March 2012. His social circumstances remained challenging, making it difficult for him to be adherent to his medications despite multiple counseling and adherence support sessions from healthcare providers.
Fig. 1. Plasma viral loads, CD4+ T-cell count and HAART regimens at different time points over a 14-year period for the patient.
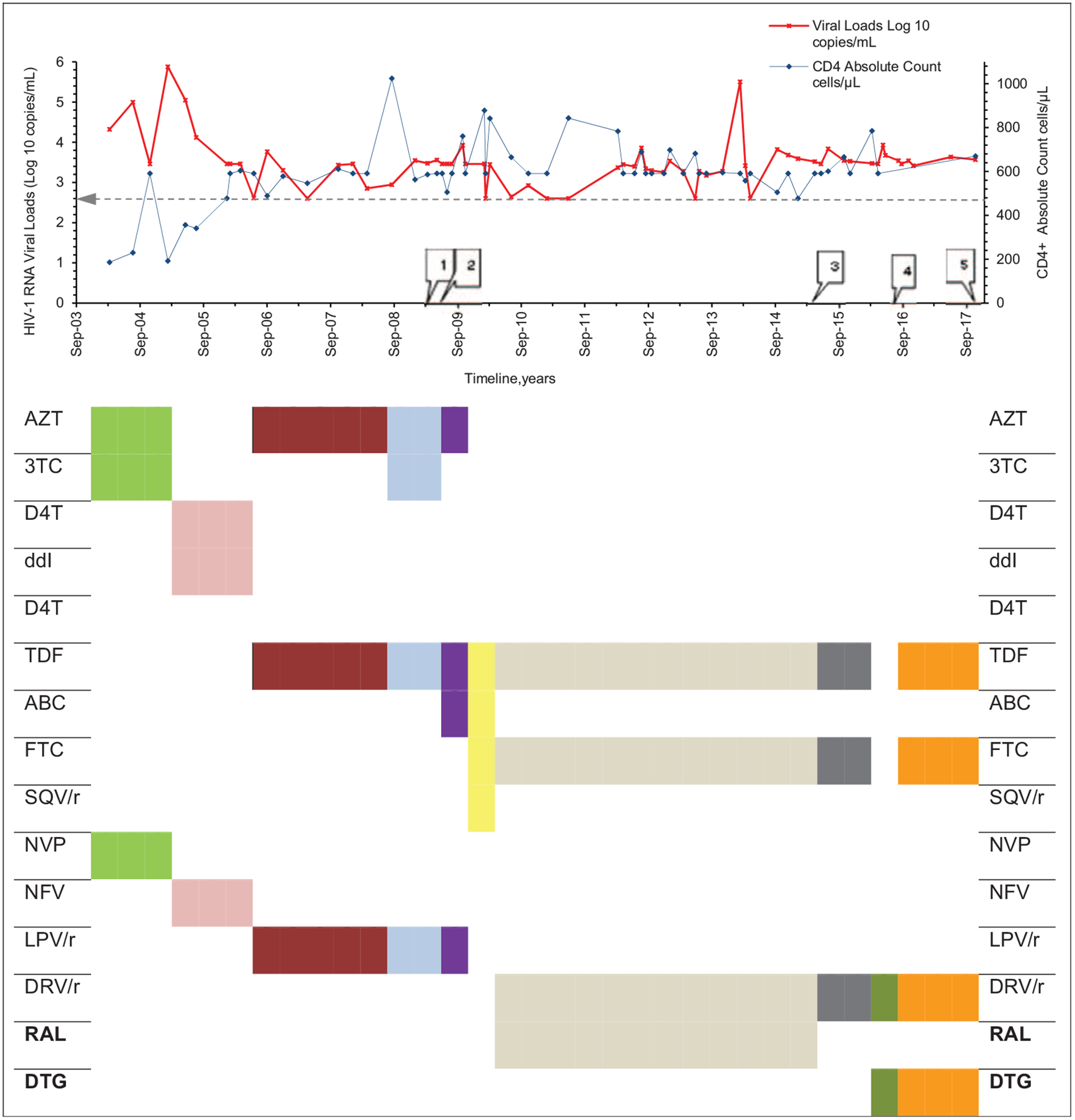
Horizontal line within chart depicts viral load of 400 copies/ml. Numbered rectangular callouts depict when drug resistance testing was done. (1) Mar 2009; †nucleotide reverse-transcriptase inhibitors, D67N, K70R, M184V; nonnucleotide reverse-transcriptase inhibitors, Y181C; protease inhibitors major resistance mutations, V32I, I47V, I54L, I84V, †protease inhibitors accessory resistancemutations, L33F, G73V, L89T; integrase strand transfer inhibitors, not tested. (2) Apr 2009; †nucleotide reverse-transcriptase inhibitors, D67N, K70R, M184V, K219N; †nonnucleotide reverse-transcriptase inhibitors, Y181C; †protease inhibitors major resistance mutations, V32I, I47V, I54L, I84V, protease inhibitors accessory resistance mutations, L33F, G73V, L89T; integrase strand transfer inhibitors, not tested. (3) May 2015; †nucleotide reverse-transcriptase inhibitors, K65KR, D67DN, K70KR, M184MV, K219KHNQ; †nonnucleotide reverse-transcriptase inhibitors, none; †protease inhibitors major resistance mutations, V32VI, I54IL, I84IV, †protease inhibitors accessory resistance mutations, L33LF, G73GV, L89IMT; †integrase strand transfer inhibitors major resistance mutations, E138K, G140A, Q148R, integrase strand transfer inhibitors accessory resistance mutations, none; (4) Aug 2016; †nucleotide reverse-transcriptase inhibitors, D67N, K70R, M184V; †nonnucleotide reverse-transcriptase inhibitors, none; †protease inhibitors major resistance mutations, V32I, I47V, I54L, I84V, †protease inhibitors accessory resistance mutations, L33F, G73V, L89T; integrase strand transfer inhibitors, not tested. (5) Nov 2017; †integrase strand transfer inhibitors major resistance mutations, E138K, G140A, S147G, Q148R, integrase strand transfer inhibitors accessory resistance mutations, T97A; ‡fusion inhibitors, none; entry inhibitors, §R5 tropic; nucleotide reverse-transcriptase inhibitors, not tested; nonnucleotide reverse-transcriptase inhibitors, not tested. 3TC, lamivudine; ABC, abacavir; AZT, zidovudine; d4T, didanosine; ddI, didanosine; DRV/r, ritonivir boosted darunavir; DTG, dolutegravir; EI, entry inhibitor; FI, fusion inhibitor; FTC, emtricitabine; INSTI, integrase strand transfer inhibitor; LPV/r, ritonovir boosted lopinavir; NFV, nelfinavir; NNRTI, nonnucleotide reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; RAL, raltegravir; RM, resistance mutation; SQV/r, ritonivir-boosted saquinavir; TDF, tenofovir; VF, virological failure. Drug resistance mutations interpreted using †Stanford University HIV Drug resistance database; ‡International Antiviral Society USA 2017 mutational list; §coreceptor usage assessed with geno2pheno[coreceptor]. Source: modified version from http://bioafrica.mrc.ac.za/workshops/PDFs/deOliveiraRegaDB.pdf.
The patient is infected with HIV-1 subtype C clade and, during the period under review, had a median HIV-1 viral load of 3.48 log10 (Q1, Q3: 3.27, 3.68) copies/ml and absolute CD4+ T-cell count of 595 cells/μl (Q1, Q3: 501, 692), respectively. As of November 2017, viral load and CD4+ T-cell count were 3.57 log10 copies/ml and 670 cells/μl, respectively (Fig. 1).
GRT analysis revealed major drug resistance mutations conferring resistance to nucleoside (nucleotide) reverse transcriptase inhibitors (K65R, D67N, K70R, M184V and K219N), nonnucleotide reverse-transcriptase inhibitors (Y181C), protease inhibitors (V32I, I47V, 154L, I84V) and INSTIs (E138K, G140A, S147G, Q148R, T97A). The virus was chemokine receptor 5 tropic and did not have any resistance mutations to enfuvirtide (T-20).
To our knowledge, this is the first report in SSA of a patient with a virus which has developed drug resistance mutations to all the standard ARVs belonging to four classes including INSTIs. Multidrug-resistant mutations likely developed as a result of multiple factors, including suboptimal adherence, inadequate psychosocial support and limited HIV specialist care - all issues encountered frequently in LMICs.
Although in-vitro and in-vivo clinical studies have shown DTG to retain some activity against a virus with INSTI mutations [14,15], this is not always the case in real-life settings as highlighted by this case report. An interesting observation made was the isolated emergence of the INSTI accessory resistance mutation T97A between May 2015 and November 2017 and concurrent rise in viral load from 2888 to 3690 copies/ml between the same time period. Similar observations of viral rebound after sole emergence of T97A mutations have recently been reported [16]. Phenotypic testing, ARV drug tracing levels and directly observed therapy would have been ideal but often these are not available or feasible in LMICs.
The patient may benefit from a regimen that includes two new agents expected to be fully active, such as maraviroc (MVC) or enfuvirtide (T-20) with an optimized background regimen. Currently, MVC and T-20 are not available under the Botswana National HIV Clinical Care Guidelines.
As this patient remains sexually active with significant psychosocial issues, this raises important public health issues including the possible introduction of a multidrug-resistant HIV-1 subtype C strain into the circulating pool.
The case highlights the need for continued monitoring of HIV-1 viral load and the development of robust HIV drug resistance surveillance systems for failing and highly treatment-experienced patients. Managing treatment-experienced patients in LMICs, in which there is often inadequate psychosocial support, lack of clinical HIV care expertise, limited ARV options and drug resistance testing capacity, will remain an ongoing challenge that will need to become a public health priority to ensure the effectiveness of national HIV programs.
Acknowledgements
We thank our patients and staff of Princess Marina Hospital Infectious Disease Care Clinic (PMH IDCC). We thank the Botswana Harvard HIV Reference Laboratory staff for their hard work and dedication, Botswana Harvard Partnership and Ministry of Health and Wellness for their collaboration. We thank Thongbotho Mphoyakgosi, Tshenolo Ntsipe, Segomotso Maphorisa, Mompati Mogwele and Wonderful T. Chonga for their outstanding laboratory assistance.
The current work was supported through the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative (grant no. DEL-15-006). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (grant no. 107752/Z/15/Z) and the UK government. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government.
SM was supported by the Fogarty International Center and National Institute of Mental Health, of the National Institutes of Health under Award Number D43 TW010543. SM and SG were partially funded by Wellcome Trust DELTAS Initiatives/Sub-Saharan Africa Network for TB/HIV Research Excellence (SANTHE) (107752/Z/15/Z). The funders had no role in the study design, data collection and decision to publish, or in the preparation of the manuscript.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: UNAIDS; 2014. http://www.unaid-s.org/sites/default/files/media_asset/90-90-90_en.pdf. [Accessed 3 February 2018] [Google Scholar]
- 2.Cohen M, Chen Y, McCauley M, Gamble T, Hosseinipour M, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365 (Suppl 3): 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, Van Lunzen J, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016; 316:171–181. [DOI] [PubMed] [Google Scholar]
- 4.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services; 2006. http://www.aidsinfo.nih.gov/ContentFiles/Adult. [Accessed 18 January 2018] [Google Scholar]
- 5.EACS guidelines version 9.0; October 2017. http://www.eacsociety.org/files/guidelines_9.0-english.pdf. [Accessed 3 February 2018].
- 6.Waters L, Churchill D, Ahmed N, Angus B, Boffito M, Bower M, et al. BHIVA guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy; 2015. (2016 interim update). http://www.bhiva.org/documents/Guidelines/Treatment/2016/treatment-guidelines-2016-interim-update.pdf. [Accessed 3 February 2018]. [DOI] [PubMed]
- 7.Wallis CL, Godfrey C, Fitzgibbon JE, Mellors JW. Key factors influencing the emergence of human immunodeficiency virus drug resistance in low- and middle-income countries. J Infect Dis 2017; 216:851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croome N, Ahluwalia M, Hughes LD, Abas M. Patient-reported barriers and facilitators to antiretroviral adherence in sub-Saharan Africa. AIDS 2017; 31:995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bijker R, Jiamsakul A, Kityo C, Kiertiburanakul S, Siwale M, Phanuphak P, et al. Adherence to antiretroviral therapy for HIV in sub-Saharan Africa and Asia: a comparative analysis of two regional cohorts. J Int AIDS Soc 2017; 20:21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Transition to New Antiretrivirals in HIV Programmes, HIV Treatment Policy Brief. Geneva: World Health Organization: July 2017. http://www.who.int/hiv/pub/toolkits/transition-to-new-arv/en. [Accessed 3 February 2018] [Google Scholar]
- 11.Unitaid. New high-quality antiretroviral therapy to be launched in South Africa, Kenya and over 90 low- and middle-income countries at reduced price; 21 September 2017. http://www.who.int/hiv/media-centre/news/high-quality-arv-reduced-price/en/. [Accessed 14 October 2017]
- 12.Wensing A, Calvez V, Gunthard H, Johnson VA, Paredes R, Pillay D, et al. 2017 Update of the drug resistance mutations in HIV-1. Top Antivir Med 2017; 24:6–14. [PMC free article] [PubMed] [Google Scholar]
- 13.Lengauer T, Sander O, Sierra S, Thielen A, Kaiser R. Bioinformatics prediction of HIV coreceptor usage. Nat Biotechnol 2007; 25:1407–1410. [DOI] [PubMed] [Google Scholar]
- 14.Castagna A, Maggiolo F, Penco G, Wright D, Mills A, Grossberg R, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis 2014; 210:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi M, Yoshinaga T, Seki T, Wakasa-Morimoto C, Brown KW, Ferris R, et al. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother 2011; 55:813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuriakose S, George J, Dee N, Stoll P, Agan B, Dewar R, et al. High level resistance to dolutegravir (DTG) after emergence of T97A mutation [abstract 543] In: 25th Conference on Retroviruses and Opportunistic Infections (CROI). Boston; 4–7 March 2018. p. 199, abstract#543. [Google Scholar]


