Abstract
Alcohol use disorder is a chronic relapsing disease. Maintaining abstinence represents a major challenge for alcohol- dependent patients. Yet the molecular underpinnings of alcohol relapse remain poorly understood. In the present study, we investigated the potential role of the mammalian target of rapamycin complex 1 (mTORC1) in relapse to alcohol-seeking behavior by using the reinstatement of a previously extinguished alcohol conditioned place preference (CPP) response as a surrogate relapse paradigm. We found that mTORC1 is activated in the nucleus accumbens shell following alcohol priming-induced reinstatement of alcohol place preference. We further report that the selective mTORC1 inhibitor, rapamycin, abolishes reinstatement of alcohol place preference. Activation of mTORC1 initiates the translation of synaptic proteins, and we observed that reinstatement of alcohol CPP is associated with increased protein levels of one of mTORC1’s downstream targets, collapsin response mediator protein-2 (CRMP2), in the nucleus accumbens. Importantly, the level of mTORC1 activation and CRMP2 expression positively correlate with the CPP score during reinstatement. Finally, we found that systemic administration of the CRMP2 inhibitor, lacosamide, atten- uates alcohol priming-induced reinstatement of CPP. Together, our results reveal that mTORC1 and its downstream target, CRMP2, contribute to mechanisms underlying reinstatement of alcohol reward seeking. Our results could have important implications for the treatment of relapse to alcohol use and position the Food and Drug Administration approved drugs, rapamycin and lacosamide, for the treatment of alcohol use disorder.
INTRODUCTION
Harmful alcohol use continues to be a major worldwide concern with severe socioeconomic consequences (WHO 2014). The etiology of alcohol dependence remains poorly understood, and only a few treatments are available (for reviews, see Akbar et al. 2018 #133; Heilig & Egli 2006 #73). One of the most troubling aspects of alcohol use disorder (AUD) is relapse to alcohol use that may occur even after several years of abstinence (Moos & Moos 2006). Thus, the high rate of relapse characterizing AUD represents a major challenge, and hence, a better understanding of the mechanisms underlying relapse is of great merit. One strategy to prevent relapse in alcohol addicts could be the attenuation of reward-related memories. Drugs of abuse are thought to usurp normal mechanisms underlying learning and memory processes (Russo et al. 2010; Torregrossa et al. 2011). The mammalian target of rapamycin complex 1 (mTORC1) is required for numerous forms of long-lasting synaptic plasticity, learning and memory (Lipton & Sahin 2014). mTORC1 is a multiprotein complex including the serine and threonine kinase mTOR (Lipton & Sahin 2014). mTORC1 phosphorylates the p70 ribosomal S6 Kinase, which in turn phosphorylates the ribosomal protein S6, as well as the initiation factor 4E binding protein (Lipton & Sahin 2014). These phosphorylation events promote the assembly of the translation initiation complex resulting in the translation of a subset of mRNA to proteins (Saxton & Sabatini 2017). In the central nervous system, activa- tion of mTORC1 triggers the local dendritic translation of synaptic proteins, inducing structural and functional neuroadaptations that in turn participate in synaptic plasticity learning and memory (Buffington et al. 2014; Lipton & Sahin 2014). Accumulating evidence suggest that mTORC1 signaling also plays a central role in the molecular mechanisms underlying addiction (Neasta et al. 2014), and we have generated data implicating mTORC1 in processes that drive alcohol drinking behaviors (Neasta et al. 2010; Barak et al. 2013; Beckley et al. 2016; Liu et al. 2017; Laguesse et al. 2017a). Specifically, we previously reported that excessive alcohol consumption activates mTORC1 in the nucleus accum- bens (NAc) of rodents (Neasta et al. 2010; Beckley et al. 2016; Laguesse et al. 2017a), resulting in the translation of synaptic proteins such as the collapsin response mediator protein-2 (CRMP2) (Liu et al. 2017) and Prosap2-interacting protein 1 (Prosapip1) (Laguesse et al. 2017b). We further have shown that inhibiting the function of these mTORC1 targets attenuates excessive alcohol consumption (Liu et al. 2017; Laguesse et al. 2017b). We also reported that retrieval of memories associated with an alcohol experience increases mTORC1 activity in cortical and amygdalar regions and that systemic or intra-CeA administration of the selective mTORC1 inhibitor, rapamycin, disrupts the reconsolidation of alcohol-related memories (Barak et al. 2013). Finally, in line with the potential important role of mTORC1 in mechanisms underlying AUD, genetic variants in mTOR signaling- related genes predict heavy alcohol consumption in humans (Meyers et al. 2015).
The reinstatement of drug-seeking behavior in rodents refers to the renewal of a behavior previously reinforced by drugs, by stimuli such as priming, cues, context or by stress (Sanchis-Segura & Spanagel 2006). Wang et al. previously reported that exposure to cocaine-related cues increased mTORC1 activity in the NAc core, but not shell and that specific inhibition of mTORC1 in the NAc core, using rapamycin, reduced reinstatement of cocaine-seeking triggered by cocaine cues (Wang et al. 2010). Furthermore, James et al. reported that intra-NAc shell infusion of rapamycin, during operant self-administration training attenuated reinstatement of cocaine seeking (James et al. 2014).
Here, we sought to determine whether mTORC1 signal- ing also drives the reinstatement of alcohol-seeking be- havior, and if so, we aimed to identify the molecular mechanisms that underlie the behavior.
MATERIALS AND METHODS
Detailed information regarding reagents and preparation of solutions can be found in Data S1
Subjects
Male DBA/2 J mice (Jackson Laboratory, Bar Harbor, Maine, US) were 8 weeks old at the beginning of the experiment and were group-housed (4/cages) in a temperature- and humidity-controlled colony room (22 ± 2°C, relative humidity: 50–60%) under normal 12-hour light/dark cycle (lights on at 07:00 AM) with food and water available ad libitum. Mice were weighed prior to drug administration. All animal procedures were approved by the University of California San Francisco Institutional Animal Care and Use Committee and were conducted in agreement with the Association for Assessment and Accreditation of Laboratory Animal Care.
Conditioned place preference procedures
Acquisition, extinction and reinstatement of alcohol-induced conditioned place preference
The protocol used to acquire alcohol CPP was performed accordingly to (Neasta et al. 2010). Further information can be found in Data S1. The CPP score was calculated as time spent in the drug-paired compartment on the post-acquisition, post-extinction or reinstatement test days minus time spent in the same compartment on the pre-acquisition day.
Rapamycin or lacosamide treatment
Different groups of mice were used to test the effect of treatment with saline and rapamycin or lacosamide on reinstatement of alcohol seeking. Mice conditioned with saline or alcohol were pseudo-randomly divided in two groups with a similar CPP scores on the post-acquisition and post-extinction tests. On day 14, half of each condi- tioned group of the mice was treated (i.p.) with vehicle (sal/veh and alc/veh groups), and the other half with rapamycin (10 mg/kg; sal/rapa and alc/rapa groups) or lacosamide (10 mg/kg; sal/LCM and alc/LCM groups). Three hours (rapamycin experiment) or 90 minutes (lacosamide experiment) later, saline-conditioned and alcohol-conditioned mice received a priming injection of saline or alcohol (0.9 g/kg), and immediately after mice underwent the reinstatement test for 15 minutes. The timing between pre-treatment and reinstatement testing was chosen based on previous studies (Neasta et al. 2010; Liu et al. 2017). Effect of lacosamide on spontaneous locomotor activity
A separate cohort of animals was used to assess the effect of lacosamide on spontaneous locomotor activity. Locomotor activity assessment was conducted in the CPP apparatus and was detected by infrared photobeams. Lacosamide (10 mg/kg, i.p.) or vehicle was administered 90 minutes prior to placing mice in the CPP apparatus. Spontaneous locomotor activity was monitored for 30 minutes.
Western blot analysis
Western blot analysis was conducted as described in Laguesse et al. (2017a). Further information can be found in Data S1.
Immunohistochemistry
Immunohistochemistry (IHC) was conducted as de- scribed previously (Ben Hamida et al. 2012). Further in- formation can be found in Data S1.
Data analysis
Biochemical, IHC and CPP results were analyzed using one-way or a two-way analysis of variance (ANOVA). The Newman–Keuls post hoc tests and the method of contrast are used for individual group comparisons. Correlations between CPP scores and either IHC or biochemical data were analyzed using Pearson’s correlation tests. Statistical significance was set at P < 0.05.
RESULTS
Alcohol priming dose induces reinstatement of conditioned place preference to alcohol
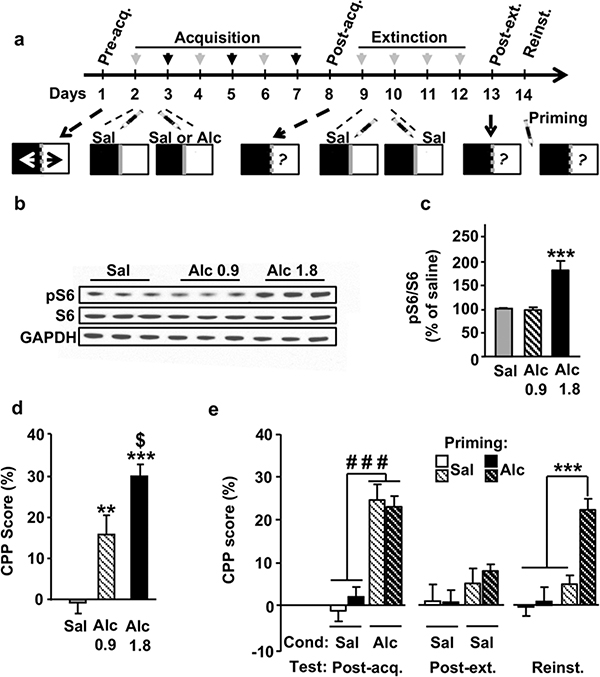
To test whether mTORC1 plays a role in the reinstatement of alcohol reward, we used the Pavlovian-based CPP procedure in which animals develop an association between the rewarding action of a drug and specific environmental cues (Tzschentke 2007). This paradigm is used to study the reinforcing effect of drugs and motivated drug-seeking behaviors (Napier et al. 2013). The experi- mental timeline is illustrated in Figure 1a. For the acquisi- tion phase, a none-hypnotic dose of alcohol (1.8 g/kg), which has previously been shown to induce robust alcohol CPP (Neasta et al. 2010), was used. For the reinstatement test, we used 0.9 g/kg of alcohol as a priming dose. Both doses of alcohol (0.9 and 1.8 g/kg) produced place prefer- ence (Fig. 1d; one-way ANOVA showed a significant effect of the alcohol doses (F(2, 28) = 16.13, P < 0.001), and Newman–Keuls post hoc test showed a significant increase in the CPP score in alcohol treated groups compared with the group conditioned with saline (P < 0.01)); however, the rewarding effects of 0.9 g/kg the dose of alcohol was significantly lower than those induced by the 1.8 g/kg dose (P < 0.05). Acute administration of 1.8 g/kg of alcohol, but not the priming dose of 0.9 g/kg, produced arobust activation of mTORC1 in the NAc, as shown by the increased phosphorylation levels of its downstream target S6 (Fig. 1b–c; One-way ANOVA showed a signifi- cant main effect of alcohol doses (F(2, 14) = 16.23, P = 0.0004), and Newman–Keuls post hoc test showed a significant increase in S6 phosphorylation levels only after injection of alcohol 1.8 g/kg (P < 0.001). Importantly, data shown in Figure 1e demonstrate that priming injection of alcohol reinstated alcoholplace preference in mice conditioned with alcohol. Specifically, on the Post-acquisition test (Fig. 1e left panel), animals spent significantly more time in alcohol-paired compartment versus the saline-paired compartment. Two-way ANOVA showed a significant main effect of Conditioning (F(1, 30) = 39.69, P < 0.0001), no effect of Groups (F(1, 30) = 0.21,P = 0.6) and no interaction between the two factors (F(1, 30) = 0.25, P = 0.62). Following analysis using method of contrast indicated that alcohol-conditioned animals exhibited a significantly higher CPP compared with saline-treated animals (Ps < 0.0001). During the Extinction phase, the acquired alcohol CPP was extinguished throughout 4 days with saline injection prior to counterbalanced confinement to the unpaired and alcohol-paired compartment. As shown in Figure 1e (middle panel), on the Post-extinction test, no significant difference was observed in the CPP scores between mice conditioned with saline and those conditioned with alcohol. Two-way ANOVA showed no significant effects of Conditioning (F(1, 30) = 1.313, P = 0.26), Groups (F(1, 30) = 0.001, P = 0.96) and interaction between the two factors (F(1, 30) = 0.09, P = 0.76). Finally, as shown in Figure 1e (right panel), on the reinstatement test day, all mice received a priming injection of 0.9 g/kg of alcohol or saline, and CPP scores were determined after 15 minutes of free ambulation in the CPP apparatus. Two-way ANOVA showed a significant main effect of Conditioning (F(1, 30) = 15.11, P < 0.001), a significant main effect of Priming (F(1, 30) = 16.77, P < 0.001) and a significant interaction between the two factors (F(1, 30) = 4.507, P = 0.04). Newman–Keuls post hoc tests detected a significant difference between the alcohol-conditioned and alcohol-primed mice (Alc/Alc group) and all the other groups (P < 0.001).
Figure 1. Alcohol priming induces reinstatement of alcohol place preference. (a) Experimental timeline depicting the acquisition, extinction and reinstatement of alcohol-induced CPP.
(b–c) Mice were systemically administered with saline or alcohol (0.9 or 1.8 g/kg). One hour after the i.p. injection, the NAc were dissected and phosphorylation levels of S6 were determined by western blot analysis. ImageJ was used for optical density quantification. Data are expressed as the mean ratio ± SEM of phospho-S6 to S6 and are expressed as percentage of the saline control. (d) CPP score on the post-acquisition test. During the conditioning phase (6 d), Mice were administered (i.p.) by alcohol (0.9 or 1.8 g/kg) or saline solution and were then confined in the drug-paired or non-drug-paired compartment. One day after the sixth session, the post-acquisition test was con- ducted for 15 minutes. (e) CPP score of the post-acquisition (Post-acq.), post-extinction (Post-ext.) and reinstatement (Reinst.) tests. In each test day, mice were placed in the central neutral area and allowed to explore both compartments of the apparatus for 15 minutes. In the reinstate- ment test day, mice previously conditioned with saline- (Sal) or alcohol- (Alc, 1.8 g/kg) received a priming injection of alcohol (0.9 g/kg, i.p.) or saline immediately prior to the beginning of the test session. Data are represented as mean percentage ± SEM of time spent in the drug-paired compartment during the post-acquisition, post-extinction and reinstatement tests minus time spent in the same compartment during the pre- acquisition session. (b–c) n = 5, (d) n = 10–11, (e) n = 13–18. (c) ***P < 0.001 versus all other groups. (d) **P < 0.01 and ***P < 0.001 versus saline-conditioned mice; $P < 0.05 Alc 0.9 versus Alc 1.8. (e) ###P < 0.001 versus saline-conditioned mice; ***P < 0.001 versus all other groups. CPP, conditioned place preference
Alcohol priming-induced reinstatement of alcohol place preference activates mammalian target of rapamycin complex 1 in the nucleus accumbens shell
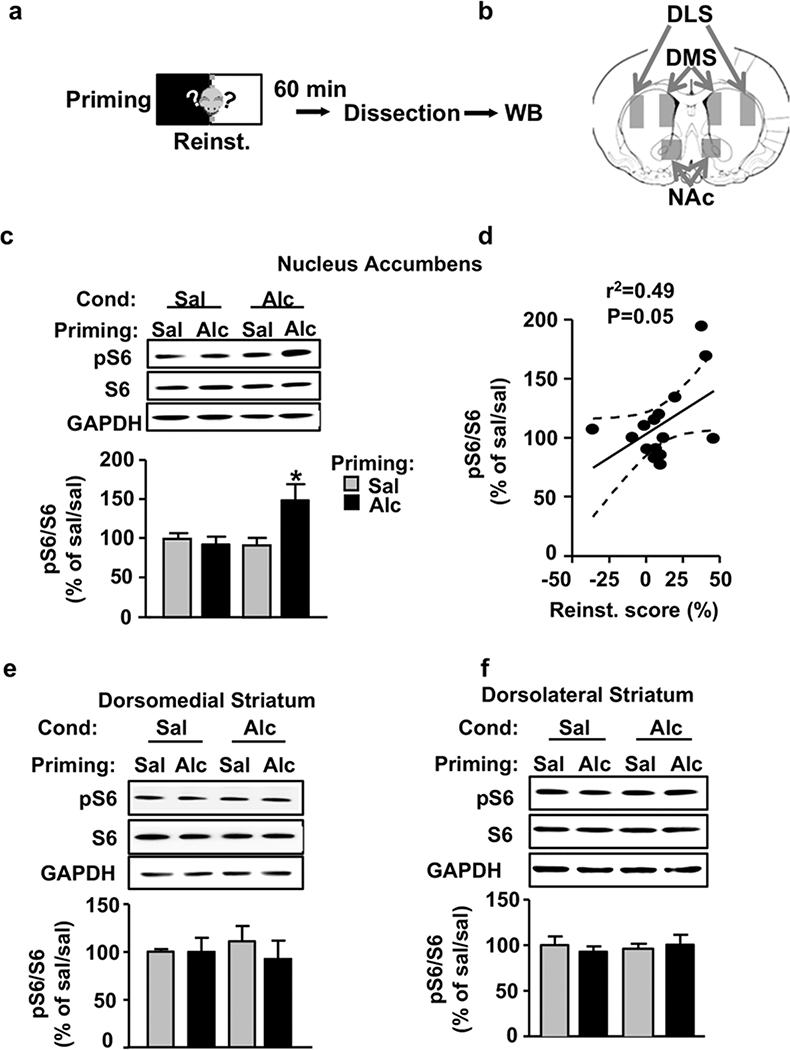
The NAc plays a major role in context–reward associa- tions and guiding motivated behaviors (Everitt 2014), and we previously reported that excessive alcohol drink- ing activates mTORC1 in the NAc (Neasta et al. 2010; Beckley et al. 2016; Laguesse et al. 2017a). In addition, reinstatement of cocaine seeking increases mTORC1 activity in the NAc (Wang et al. 2010). Therefore, we hy- pothesized that reactivation of previously acquired mem- ories by alcohol priming activates mTORC1 signaling in the NAc. To this end, an independent cohort of mice underwent the same paradigm as described in Figure 1 a. One hour following the end of the reinstatement test (Fig. 2a), mTORC1 activity in the NAc, dorsolateral striatum (DLS) and dorsomedial striatum (DMS) (Fig. 2b) were determined by analyzing the phosphorylation levels of S6 (Buffington et al. 2014). As shown in Figure 2c, 1 hour after reinstatement, mTORC1 activity was signifi- cantly increased in the NAc of Alc/Alc mice compared with all other groups. Two-way ANOVA showed no significant effect of Conditioning (F(1, 12) = 3.56, P = 0.083), no significant effect of Priming (F(1, 30) = 3.73, P = 0.077) and a significant interaction between the two factors (F(1, 12) = 6.1542, P = 0.029). Newman–Keuls post hoc tests detected a significant difference between Alc/Alc group and all the other groups (P < 0.05). Interestingly, the reinstatement scores were positively correlated with the observed increased phosphorylation of S6 (Fig. 2d; Pearson correlation, r2 = 0.249, P = 0.05). In contrast, no change in mTORC1 activity following alcohol conditioning or priming was observed in the dorsal striatum (Fig. 2e–f). Specifically, S6 phosphorylation was unaltered by priming injections of alcohol in the DMS [Fig. 2e; two- way ANOVA showed no significant effects of Conditioning (F(1, 12) = 0.015, P = 0.904), Priming (F(1, 12) = 0.4221, P = 0.528) and interaction between the two factors (F(1, 12) = 0.3792, P = 0.549)], or in the DLS (Fig. 2f; two-way ANOVA showed no significant effects of Conditioning (F(1, 12) = 0.0541, P = 0.82), Priming (F(1, 12) = 0.03, P = 0.864) and interaction between the two factors (F(1, 12) = 0.488, P = 0.498). Together, these data suggest that mTORC1 is specifically activated in the NAc following priming-induced reinstatement of alcohol seeking.
Figure 2. mTORC1 is activated in the nucleus accumbens during reinstatement of alcohol place preference.
(a) Mice underwent acquisition, extinction and reinstatement of alcohol place preference as depicted in Figure 1. DMS, DLS and NAc of mice were dissected 60 minutes after the end of the reinstatement test. (b) Schematic drawing of a coronal section of the mouse brain showing the sectioned DMS, DLS and NAc at bregma DV = +1.10/+0.70. (c–f) Phosphorylation level of S6 by saline- (Sal) or alcohol- (Alc) in conditioned and primed mice was determined by western blot analysis in the NAc (c), DMS (e) and DLS (f). ImageJ was used for optical density quantification. Data are expressed as the mean ratio ± SEM of phospho-S6 to S6 and are expressed as percentage of the control sal/sal group. (d) Scatter plot showing the relationship between CPP score on the reinstatement test and phospho-S6 in the NAc. Centerline is the linear regression and dashed lines are the 95% confidence interval. n = 4, *P < 0.05 versus all other groups. CPP, conditioned place preference; DLS, dorsolateral striatum; DMS, dorsomedial striatum; mTORC1, mammalian target of rapamycin complex 1; NAc, nucleus accumbens
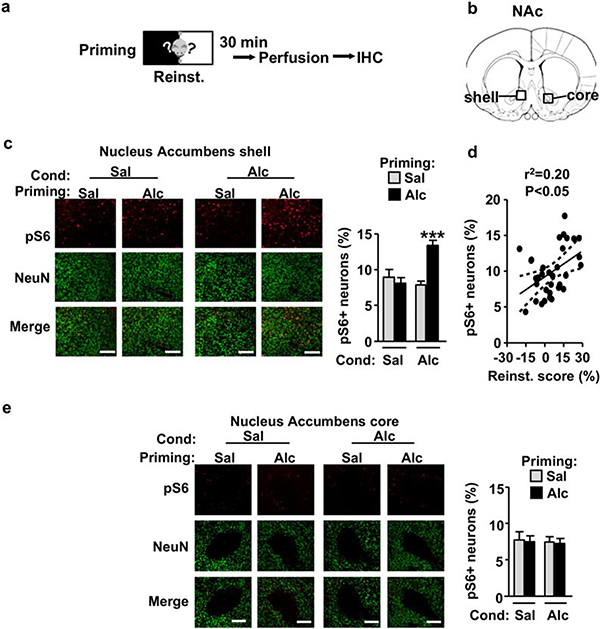
The NAc is divided into the core and shell subregions, which are known to play distinct roles in motivation pro- cesses and modulation of alcohol seeking by context (Janak & Chaudhri 2010; Corbit et al. 2016). We previ- ously showed that intake of large quantities of alcohol activates mTORC1 specifically in the NAc shell but not the core of rodents (Beckley et al. 2016; Laguesse et al. 2017a). In order to determine whether mTORC1 is differentially regulated in the NAc core and shell by reinstatement of alcohol seeking, we analyzed the immunoreactivity of phospho-S6 in the two NAc regions 30 minutes after the end of reinstatement test (Fig. 3a–b). As shown in Figure 3c–d, reinstatement of the alcohol- induced CPP promoted the activation of mTORC1 specifically in the NAc shell. Two-way ANOVA showed a significant main effect of Conditioning (F(1, 30) = 6.68, P = 0.014), a significant main effect of Priming (F(1, 30) = 8.229, P = 0.007) and a significant interaction between the two factors (F(1, 30) = 15.28, P < 0.001). Newman– Keuls post hoc test detected a significant increase of phospho-S6 in Alc/Alc group compared with all other groups (P < 0.001). In addition, a positive correlation was observed between phospho-S6 labeling and the reinstatement scores (Fig. 3d, Pearson regression, r2 = 0.2, P = 0.0089). However, no change in phospho- S6 immunoreactivity was observed in the NAc core (Fig. 3e, two-way ANOVA showed no effects of Conditioning (F(1, 30) = 0.098, P = 0.755), Priming (F(1, 30) = 0.068, P = 0.796) and interaction between the two factors (F(1, 30) = 0.0005, P = 0.982)). These results suggest that priming-induced reinstatement of alcohol seeking activates mTORC1 specifically in the NAc shell.
Figure 3. mTORC1 activation following priming-induced reinstatement of alcohol-CPP is restricted to the NAc shell.
(a) Mice underwent ac- quisition, extinction and reinstatement of alcohol place preference as depicted in Figure 1. Mice were euthanized 30 minutes after the end of the reinstatement test. (b) Schematic drawing of a coronal section of the mouse brain showing the shell and core portions of the NAc. (c–e) Phospho-S6 levels in the NAc shell (c) and core (e) (bregma DV = +1.10/+0.70) by saline- (Sal) or alcohol- (Alc) conditioned and primed mice were determined by immunohistochemistry. Left panels, Representative images of mouse NAc shell (c) and core (e) labeled with phospho-S6 in red and NeuN in green. Scale bar 100 μm. Right panels, Phospho-S6 labeled neurons are expressed as percentage of NeuN positive cells. (d) Scatter plot showing the relationship between CPP score on the reinstatement test and phospho-S6+ neurons in the NAc shell. Centerline is the linear regression and dashed lines are the 95% confidence interval. Sal/Sal n = 7, Sal/Alc n = 9, Alc/Sal n = 7, Alc/Alc n = 10. ***P < 0.001 versus all other groups. CPP, conditioned place preference; mTORC1, mammalian target of rapamycin complex 1; NAc, nucleus accumbens
Inhibition of mammalian target of rapamycin complex 1 by systemic administration of rapamycin prevents priming-induced reinstatement of alcohol seeking
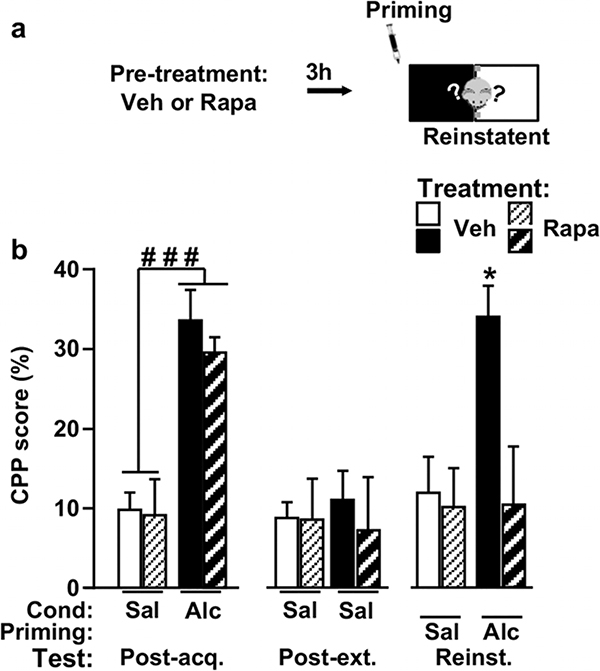
Next, we tested whether inhibition of mTORC1 is suffi- cient to suppress alcohol priming induced-reinstatement of alcohol place preference by using the selective mTORC1 inhibitor, rapamycin (Dowling et al. 2010). To do so, mice underwent the acquisition and extinction of alcohol CPP according to the schedule shown in Figure 1a. Next, on the reinstatement test day, animals received an i.p. administration of rapamycin 3 hours be- fore the alcohol priming treatment (saline or alcohol) (Fig. 4a). Immediately after the alcohol prime administration, mice were placed in the CPP apparatus and were given free access to the whole apparatus for 15 minutes (Fig. 4a). As expected, a robust CPP was ob- served in animals conditioned with alcohol compared with those treated with saline. Specifically, as shown in Figure 4b (left panel), in the Post-acquisition test, two- way ANOVA showed a significant main effect of alcohol Conditioning (F(1, 48) = 43.57, P < 0.0001), no effect of Group (F(1, 48) = 0.47, P = 0.49) and no interaction between the two factors (F(1, 48) = 0.23, P = 0.63). Following analysis using method of contrast indicated that alcohol-conditioned animals exhibited a significantly higher CPP compared with saline-treated animals (P’s < 0.001). The acquired alcohol CPP was then extinguished during the extinction phase. As shown in Figure 4b (middle panel), in the Post-extinction test, all groups showed similar levels of place preference to the target compartment. Two-way ANOVA showed no signif- icant effects of Conditioning (F(1, 48) = 0.01, P = 0.9), Group (F(1, 48) = 0.18, P = 0.66) and interaction between the two factors (F(1, 48) = 0.13, P = 0.71). Finally, as shown in Figure 4b (right panel), in the reinstatement test day, treatment with rapamycin abolished the alcohol priming-induced reinstatement of alcohol place prefer- ence. Two-way ANOVA showed a significant main effect of Treatment (F(1, 48) = 5.5, P = 0.023), a significant main effect of Priming (F(1, 48) = 4.25, P = 0.045) and significant interaction between the two factors (F(1, 48) = 4.1, P = 0.049). Newman–Keuls post hoc test showed a significant difference between vehicle and rapamycin treatment in alcohol-primed mice (P < 0.05). Together, our results reveal that systemic ad- ministration of rapamycin efficiently prevents the reinstatement of alcohol seeking induced by a priming injection of alcohol.
Figure 4. Systemic administration with the mTORC1 inhibitor, rapamycin, blocks priming-induced reinstatement of alcohol place preference.
(a) Mice underwent acquisition and extinction of alcohol place preference as depicted in Figure 1. On day 14, mice were pre-treated with vehicle (Veh) or rapamycin (Rapa, 10 mg/kg, i.p.). Three hours later, mice received a priming injection of saline or alcohol (0.9 g/kg, i.p.) and underwent the reinstatement test. (b) CPP scores on the post-acquisition (Post-acq.), post-extinction (Post-ext.) and reinstatement (Reinst.) tests. Data are rep- resented as mean percentage ± SEM of time spent in the drug-paired compartment during the tests minus time spent in the same compartment on the pre-acquisition session. Veh/Sal n = 13, Rapa/Sal n = 14, Veh/Alc n = 13, Rapa/Alc n = 13. ###P < 0.001 versus saline-conditioned mice and *P < 0.05 versus all other groups. CPP, conditioned place preference; mTORC1, mammalian target of rapamycin complex 1
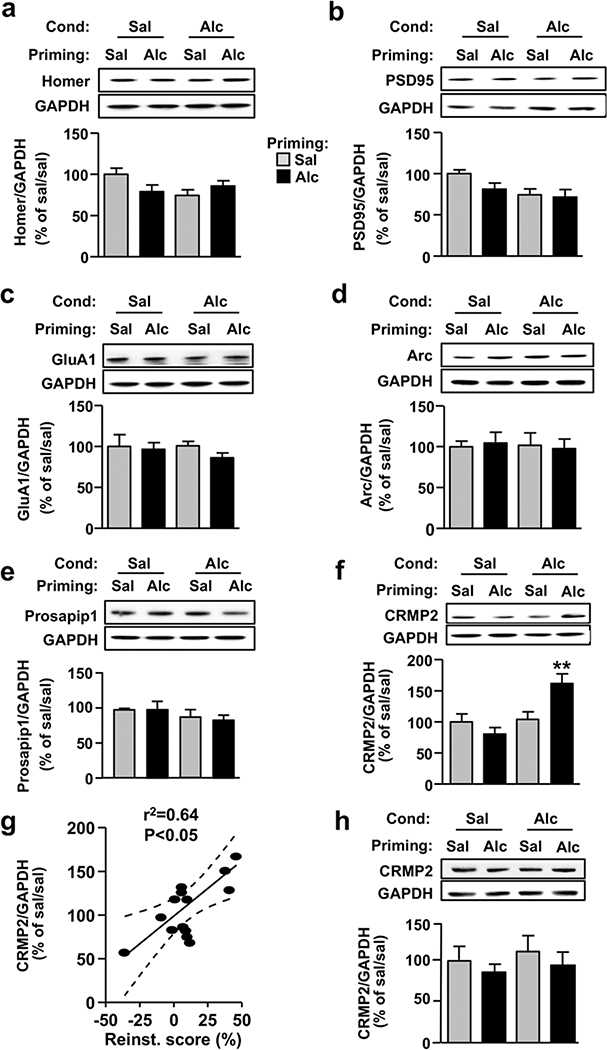
Alcohol priming-induced reinstatement of alcohol place preference increases collapsin response mediator protein-2 levels in the nucleus accumbens
We next set out to identify a possible downstream target of mTORC1, which contributes to the reinstatement of alcohol place preference. mTORC1 has been shown to promote the translation of a subset of synaptic proteins (Buffington et al. 2014; Lipton & Sahin 2014), and we generated data suggesting that excessive alcohol drinking initiates the translation of Arc, GluA1, Homer, PSD-95, Prosapip1 and CRMP2 in the NAc of rodents (Neasta et al. 2010; Beckley et al. 2016; Liu et al. 2017; Laguesse et al. 2017b). Thus, we measured the protein level of these synaptic proteins in the NAc of mice that underwent the previously described acquisition, extinc- tion and reinstatement of alcohol CPP paradigm (Fig. 1a). We observed no change in the protein levels of Homer (Fig. 5a; pConditioning = 0.23, pPriming = 0.53 and pinteraction = 0.044), PSD-95 (Fig. 5b; pConditioning = 0.03, pPriming = 0.16 and pinteraction = 0.29), GluA1 (Fig. 5c; pconditioning = 0.63, pPriming = 0.34 and pinteraction = 0.56), Arc (Fig. 5d; pConditioning = 0.87, pPriming = 0.98 and pinteraction = 0.78) and Prosapip1 (Fig. 5e; pConditioning = 0.14, pPriming = 0.81 and pinteraction = 0.8) in the NAc after reinstatement of CPP. In contrast, CRMP2 protein levels were significantly increased in the NAc of mice in response to alcohol prime-induced rein- statement of alcohol place preference (Fig. 5f). Two-way ANOVA showed a significant main effect of Conditioning (F(1, 12) = 11.03, P = 0.006), no significant effect of Priming (F(1, 12) = 2.16, P = 0.16) and significant inter- action between the two factors (F(1, 12) = 9.04, P = 0.01). Newman–Keuls post hoc tests detected a signif- icant difference between the Alc/Alc group and all the other groups (P < 0.01). As shown in Figure 5g, apositive correlation was observed between CRMP2 levels and the reinstatement scores (Pearson regression, r2 = 0.4, P = 0.0091). In contrast, we observed no change in CRMP2 levels in the DMS after CPP reinstate- ment (Fig. 5h; pConditioning = 0.29, pPriming = 0.57 and pinteraction = 0.83). Together, these results suggest that reinstatement of alcohol CPP increases the protein levels of the mTORC1 downstream target, CRMP2, specifically in the NAc.
Figure 5. CRMP2 levels are increased in the NAc following priming-induced reinstatement of alcohol place preference.
Homer (a), PSD95 (b), GluA1 (c), Arc (d), Prosapip1 (e) and CRMP2 (f) levels in the NAc of saline- (Sal) or alcohol- (Alc) conditioned and primed mice was determined by western blot analysis. NAc samples are identical to the tissue samples used in Figure 2c–d. (g) Scatter plot showing the relationship between CPP score on the reinstatement test and CRMP2 levels in the NAc. Centerline is the linear regression and dashed lines are the 95% confidence interval. (h) CRMP2 levels in the DMS were determined by western blot analysis. ImageJ was used for optical density quantification. Data are represented as the mean ratio ± SEM of protein to GAPDH and are expressed as percentage of the control sal/sal group. n = 4, **P < 0.01 versus all other groups. CPP, conditioned place preference; CRMP2, collapsin response mediator protein-2; DMS, dorsomedial striatum; NAc, nucleus accumbens
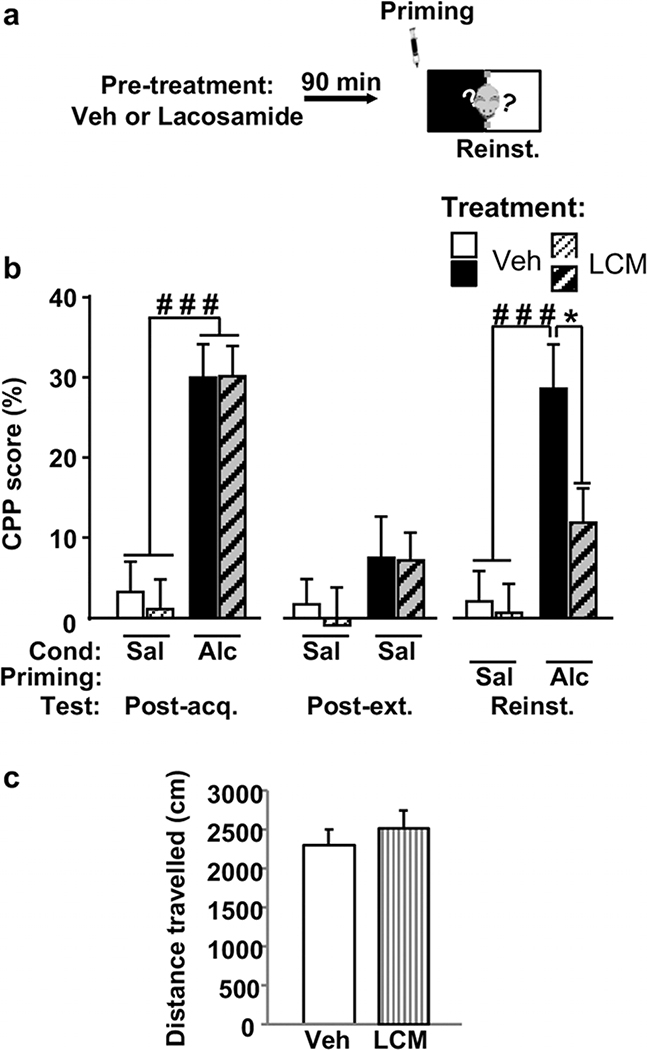
Systemic administration of lacosamide reduces alcohol priming-induced reinstatement of alcohol place preference
Collapsin response mediator protein-2 is a microtubule- binding protein implicated in the regulation of dendritic morphology (Ip et al. 2014; Quach et al. 2015). Abnormal CRMP2 function has been associated with the development of several neurological disorders (Ip et al. 2014; Quach et al. 2015). We previously found that excessive alcohol drinking increases CRMP2 translation in the NAc (Liu et al. 2017). We further found that pharmacological inhibition of CRMP2 as well as shRNA-mediated knockdown of CRMP2 in the NAc decrease excessive alcohol consumption (Liu et al. 2017). As CRMP2 protein levels are elevated as a result of alcohol-induced reinstatement of CPP, we hypothesized that CRMP2 plays a role in mechanisms underlying rein- statement of alcohol CPP. To test this possibility, mice underwent the acquisition and extinction of alcohol CPP protocol as shown in Figure 1a, and on the reinstate- ment test day, animals were i.p. administered with saline or 10 mg/kg of lacosamide (Beyreuther et al. 2007), a specific inhibitor of CRMP2 that acts in part by preventing CRMP2 binding to microtubules (Wilson et al. 2012; Wilson & Khanna 2015). Ninety minutes later, animals were injected with a priming dose of alco- hol or saline (Fig. 6a) and tested for alcohol reinstate- ment. As shown in Figure 6b (left panel), in the Post- acquisition test, two-way ANOVA showed a significant main effect of Conditioning (F(1, 42) = 49.71, P < 0.0001), no effect of Groups (F(1, 42) = 0.06, P = 0.81) and no interaction between the two factors (F(1, 42) = 0.09, P = 0.76). Following analysis using method of contrast indicated that alcohol-conditioned animals exhibited a significantly higher CPP scores compared with saline-treated animals (Ps < 0.001). The acquired alcohol CPP was then extinguished during the extinction phase. As shown in Figure 6b (middle panel), in the Post-extinction test, no significant difference was observed in the CPP scores between mice condi- tioned with saline and those conditioned with alcohol. Two-way ANOVA showed no effects of Conditioning (F(1, 42) = 2.85, P = 0.09), Groups (F(1, 42) = 0.13,P = 0.72) and interaction between the two factors (F(1, 42) = 0.07, P = 0.78). Importantly, lacosamide treatment significantly reduced reinstatement of CPP after subse- quent alcohol exposure compared with vehicle treated group (Fig. 6b (right panel). Two-way ANOVA showed significant main effects of Priming (F(1, 42) = 17.84, P < 0.001), significant main effects of Treatment(F(1, 42) = 4.1, P = 0.049) but no significant interaction between both factors (F(1, 42) = 2.95, P = 0.093)). Following analysis using method of contrast revealed a significant difference between vehicle and lacosamide treatment in alcohol-primed mice (P < 0.05). Finally, as shown in Figure 6c, systemic administration of 10 mg/kg of lacosamide did not alter mice locomotion (t(8) = 0.69, P = 0.51), and we previously showed that even a higher dose of lacosamide (20 mg/kg) does not al- ter spontaneous locomotor activity in open field test (Liu et al. 2017). Together, these data suggest that inhibition of CRMP2 function prior to alcohol priming is sufficient to reduce the re-emergence of alcohol-seeking behavior. It also indicates that CRMP2 is critical for the recall of previously extinguished alcohol place preference.
Figure 6. Systemic administration of the CRMP2 inhibitor lacosamide prevents reinstatement of alcohol place preference and does not affect spontaneous locomotor activity.
(a) Mice underwent acquisition and extinction of alcohol place preference as depicted in Figure 1. On day 14, mice were pre-treated with vehicle (Veh) or lacosamide (LCM, 10 mg/kg, i.p.). Ninety minutes later, mice received a priming injection of saline or alcohol (0.9 g/kg, i.p.) and underwent the reinstatement test. (b) CPP score on the post-acquisition (Post-acq.), post-extinction (Post-ext.) and re- instatement (Reinst.) tests. Data are represented as mean percentage ± SEM of time spent in the drug-paired compartment during the tests mi- nus time spent in the same compartment on the pre-acquisition session. (c) Mice were injected with either Veh or LCM (10 mg/kg, i.p.) 90 minutes prior to the beginning of the locomotion test. Data are represented as mean percentage ± SEM of spontaneous locomotor activity measured during 30 minutes. (b) Veh/Sal n = 11, LCM/Sal n = 10, Veh/Alc n = 12, LCM/Alc n = 14, (c) n = 5. (b) ###P < 0.001 versus saline- conditioned mice and *P < 0.05 versus Veh/Alc group. CPP, conditioned place preference
DISCUSSION
Here, we present data to suggest that reinstatement of alcohol reward memory activates mTORC1 in the NAc shell resulting in the translation of its downstream targets CRMP2. Our data further reveal that pharmaco- logic inhibition of either mTORC1 or CRMP2 with the FDA approved drugs rapamycin (Dowling et al. 2010) and lacosamide (Wilson & Khanna 2015), respectively, attenuates alcohol-reward associated memories.
Reinstatement of reward-seeking behaviors classically refers to the renewal of an extinguished drug-seeking or drug-taking behavior in response to exposure to the drug itself, drug-associated cue or context, or acute stress (Epstein et al. 2006; Bossert et al. 2013). Here, in order to examine the involvement of mTORC1 in the relapse to alcohol seeking, we used a CPP paradigm widely used to explore the association between the rewarding effects of a drug and specific environmental cues (Tzschentke 2007; Napier et al. 2013). We confirmed previous results (Bhutada et al. 2012; Roger-Sanchez et al. 2012; Al-Hasani et al. 2013) indicating that an extinguished alcohol-induced CPP could be reinstated by the non-contingent administration of a low priming dose of alcohol. The level of the reinstated response is considered as an index of level of motivation for environmental contexts previously paired with drug treatments (Bossert et al. 2013), and the alcohol prime is likely to enable reinstatement of alcohol place preference by facilitating the retrieval of a complex association between context (alcohol-associated compartment) and the rewarding properties of alcohol.
The induction, expression, extinction and reinstate- ment of alcohol seeking are thought to depend on drug-induced neuroadaptations in several brain areas Al-Hasani et al. 2013) indicating that an extinguished alcohol-induced CPP could be reinstated by the non-contingent administration of a low priming dose of alcohol. The level of the reinstated response is considered as an index of level of motivation for environmental contexts previously paired with drug treatments (Bossert et al. 2013), and the alcohol prime is likely to enable reinstatement of alcohol place preference by facilitating the retrieval of a complex association between context (alcohol-associated compartment) and the rewarding properties of alcohol.
The induction, expression, extinction and reinstate- ment of alcohol seeking are thought to depend on drug-induced neuroadaptations in several brain areas that regulate drug rewarding and motivation effects (Ron & Barak 2016) including the NAc (Salgado & Kaplitt 2015). Interestingly, the priming dose of alcohol used in this study 0.9 g/kg produces a small but detectable CPP, but a single systemic administration of 0.9 g/kg alcohol did not affect mTORC1 activity. It is highly plausible that repeated systemic administration of alcohol sensitizes mTORC1 signaling pathway, leading to an increased activity of mTORC1. It is also highly plau- sible that mTORC1 is activated during the retrieval of alcohol-associated memories. This result is in line with our previous work showing that reactivation of alcohol- associated memories using context, alcohol odor and taste activates mTORC1 in several brain regions in rats, resulting in relapse to alcohol seeking and drinking (Barak et al. 2013). Our data further show that there is a tight correlation between the level of mTORC1 activa- tion in the NAc and the CPP score. Furthermore, we ob- served that mTORC1 is activated in the NAc shell but not in the Nac core, the DLS or DMS of mice following alcohol priming-induced reinstatement of alcohol CPP. Together, data indicate that alcohol exposure induces distinct neuroadaptations in different striatal regions. These find- ings are in line with previous data showing that different signaling cascades are induced by alcohol in discrete brain regions and subpopulations of neurons (Ron & Barak 2016). Thesefindingsarealso in line with previ- ous studies showing that the NAc shell is critical for cue- (Richard & Fields 2016) and context- (Perry & McNally 2013) induced reinstatement of extinguished drug-seeking behavior. Additionally, context-induced reinstatement of operant responding for a beer solution was shown to be correlated with increased expression of c-Fos in the ventral part of the NAc shell (Hamlin et al. 2007).
Importantly, we found that the systemic administra- tion of rapamycin abolished reinstatement of alcohol CPP. This result could not be due to a nonspecific effects of rapamycin such as alteration in locomotor activity or motor coordination as both behaviors are intact in response to a single systemic or subchronic administra- tion of the drug (Blundell et al. 2008; Cleary et al. 2008; Neasta et al. 2010; Hadamitzky et al. 2018).
We previously reported that alcohol-related memories could be reactivated after exposure to sensory properties of alcohol itself (odor and taste), which specifically activates mTORC1 in select amygdalar and cortical regions in rats tested in operant conditioning paradigms (Barak et al. 2013). Furthermore, we (Barak et al. 2013) and others (Lin et al. 2014) demonstrated that rapamycin adminis- tration immediately following memory reactivation disrupts the alcohol consolidated memories. Interestingly, the retrieval of alcohol-associated memories during the reconsolidation window is not driven by mTORC1 path- way in the NAc (Barak et al. 2013). There are a few but critical differences in the methods used herein and in Barak et al. (2013). The main one is that Barak et al. assessed the effects of cues/context-induced recall of alcohol-associated memories in rats that underwent long-term exposure to alcohol in operant self- administration task whereas the mice in the current study were not exposed to alcohol prior to the acquisition of alcohol place preference phase. Moreover, unlike the CPP paradigm, the operant self-administration is an appetitive learning task where animals escalate their voluntary alcohol intake driven by progressive increase of the motivation for the drug. This possibility is consistent with several lines of evidence suggesting that neurobiological mechanisms underlying CPP and self- administration are distinct (Bardo & Bevins 2000). Another difference between the two studies is the choice of reactivation parameters [pharmacologically priming herein versus context and odor-taste cue in (Barak et al. 2013)]. Therefore, although the activation of mTORC1 in specific brain regions probably depends on the length of alcohol exposure and the behavioral paradigm, it is clear that the mTORC1 in the mesocorticolimbic system is critical for relapse of alcohol-seeking behaviors.
We previously showed that long-term excessive alcohol consumption induces the translation of GluA1, Arc, CamKII, PSD-95, CRMP2, Prosapip1 and Homer in the NAc (Liu et al. 2017; Laguesse et al. 2017b) and that a single alcohol drinking session initiates the translation of Homer and GluA1 but not PSD-95 (Beckley et al. 2016). Here, we report that the reinstatement of alcohol CPP is associated with increased protein levels CRMP2 (Liu et al. 2017) but not Homer, PSD-95, GluA1, Arc and Prosapip1 in the NAc. These data suggest that although mTORC1 is activated in the NAc in response to long-term excessive drinking of alcohol as well as a single administration of a subthreshold dose of alcohol, the molecular transducers of mTORC1 are not the same in both situations.
CRMP2 is a microtubule-binding protein that regu- lates microtubule assembly (Ip et al. 2014; Quach et al. 2015; Nagai et al. 2017). We previously reported that ex- cessive alcohol consumption promotes the mTORC1- dependent translation of CRMP2 in the NAc, resulting in increased microtubule assembly (Liu et al. 2017). We further showed that downregulation of CRMP2 levels greatly reduced alcohol consumption and that disrupting CRMP2 function using lacosamide also reduced alcohol drinking (Liu et al. 2017). Although we herein measured the protein levels rather than the direct translation of CRMP2 mRNA, it is likely that the increase in the protein levels of CRMP2 by a priming dose of alcohol is the result of the mTORC1-dependent translation of the mRNA. Microtubules infiltrations into dendritic spines have been correlated with spine enlargement and synaptic strength- ening, which are considered to be the basis of memory formation (Shirao & Gonzalez-Billault 2013; Lamprecht 2014). Interestingly, an increased dendritic spine diame- ter in the Nac core was found in response to context- induced reinstatement of drug seeking (Stankeviciute et al. 2014). It is therefore tempting to speculate that reinstatement of alcohol-seeking is mediated by CRMP2- dependent structural plasticity in the NAc.
We show that systemic administration of the CRMP2 inhibitor, lacosamide, attenuates alcohol priming- induced reinstatement of CPP. This effect is not related to a nonspecific effect of lacosamide because spontaneous locomotion [Fig. 6c and (Liu et al. 2017)] and basal anxiety-like behavior (Liu et al. 2017) are preserved in animals treated with this drug. Thus, our study also suggests that lacosamide initially developed for the treat- ment of epilepsy (Stohr et al. 2007) could represent a promising new strategy for the treatment of relapse of alcohol-seeking behavior. It is important to note however, that lacosamide was shown to block neuronal firing by interfering with sodium channel slow inactivation pro- cesses (Rogawski et al. 2015). Thus, we cannot exclude the possibility that lacosamide abolishes alcohol rein- statement, at least in part, through CRMP-2independent mechanisms.
In summary, our work shows that reactivation of alcohol-associated memories using alcohol priming in a CPP task recruits the mTORC1/CRMP2 signaling and that pharmacological inhibition of this pathway disrupts priming-induced reinstatement of alcohol place preference. Our results provide a molecular mechanism of how re-exposure to alcohol after abstinence leads to reactivation of alcohol-related memories. Importantly, our study also provides new evidence to suggest that targeting mTORC1 signaling could be an effective relapse-prevention strategy in alcoholics.
Supplementary Material
Acknowledgement
This study was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA), R01 AA027474 (DR).
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Data S1. Supporting Information
References
- Akbar M, Egli M, Cho YE, Song BJ, Noronha A (2018) Medica- tions for alcohol use disorders: an overview. Pharmacol Ther 185:64–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Foshage AM, Bruchas MR (2013) Locus coeruleus kappa-opioid receptors modulate reinstatement of cocaine place preference through a noradrenergic mecha- nism. Neuropsychopharmacology 38:2484–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Liu F, Ben Hamida S, Yowell QV, Neasta J, Kharazia V, Janak PH, Ron D (2013) Disruption of alcohol-related memo- ries by mTORC1 inhibition prevents relapse. Nat Neurosci 16:1111–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA (2000) Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 153:31–43. [DOI] [PubMed] [Google Scholar]
- Beckley JT, Laguesse S, Phamluong K, Morisot N, Wegner SA, Ron D (2016) The first alcohol drink triggers mTORC1- dependent synaptic plasticity in nucleus accumbens dopamine D1 receptor neurons. J Neurosci 36:701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Hamida S, Neasta J, Lasek AW, Kharazia V, Zou M, Carnicella S, Janak PH, Ron D (2012) The small G protein H-Ras in the mesolimbic system is a molecular gateway to alcohol-seeking and excessive drinking behaviors. J Neurosci 32:15849–15858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyreuther BK, Freitag J, Heers C, Krebsfanger N, Scharfenecker U, Stohr T (2007) Lacosamide: a review of preclinical proper- ties. CNS Drug Rev 13:21–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutada P, Mundhada Y, Ghodki Y, Dixit P, Umathe S, Jain K (2012) Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of exposure to stress and modulation by mecamylamine. J Psychopharmacol 26:315–323. [DOI] [PubMed] [Google Scholar]
- Blundell J, Kouser M, Powell CM (2008) Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem 90:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y (2013) The rein- statement model of drug relapse: recent neurobiological find- ings, emerging research topics, and translational research. Psychopharmacology (Berl) 229:453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington SA, Huang W, Costa-Mattioli M (2014) Translational control in synaptic plasticity and cognitive dysfunction. Annu Rev Neurosci 37:17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary C, Linde JA, Hiscock KM, Hadas I, Belmaker RH, Agam G, Flaisher-Grinberg S, Einat H (2008) Antidepressive-like effects of rapamycin in animal models: implications for mTOR inhibi- tion as a new target for treatment of affective disorders. Brain Res Bull 76:469–473. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Fischbach SC, Janak PH (2016) Nucleus accumbens core and shell are differentially involved in general and outcome-specific forms of Pavlovian-instrumental transfer with alcohol and sucrose rewards. Eur J Neurosci 43:1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N (2010) Dis- secting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta 1804:433–439. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y (2006) Toward a model of drug relapse: an assessment of the validity of the rein- statement procedure. Psychopharmacology (Berl) 189:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ (2014) Neural and psychological mechanisms under- lying compulsive drug seeking habits and drug memories—indications for novel treatments of addiction. Eur J Neurosci 40:2163–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadamitzky M, Herring A, Kirchhof J, Bendix I, Haight MJ, Keyvani K, Luckemann L, Unteroberdorster M, Schedlowski M (2018) Repeated systemic treatment with rapamycin affects behavior and amygdala protein expression in rats. Int J Neuropsychopharmacol 10.1093/ijnp/pyy017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP (2007) The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience 146:525–536. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M (2006) Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther 111(3):855–876. [DOI] [PubMed] [Google Scholar]
- Ip JP, Fu AK, Ip NY (2014) CRMP2: functional roles in neural development and therapeutic potential in neurological diseases. Neuroscientist 20:589–598. [DOI] [PubMed] [Google Scholar]
- James MH, Quinn RK, Ong LK, Levi EM, Charnley JL, Smith DW, Dickson PW, Dayas CV (2014) mTORC1 inhibition in the nu- cleus accumbens ‘protects’ against the expression of drug seek- ing and ‘relapse’ and is associated with reductions in GluA1 AMPAR and CAMKIIalpha levels. Neuropsychopharmacology 39:1694–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Chaudhri N (2010) The potent effect of environmen- tal context on relapse to alcohol-seeking after extinction. Open Addict J 3:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguesse S, Morisot N, Phamluong K, Ron D (2017a) Region specific activation of the AKT and mTORC1 pathway in re- sponse to excessive alcohol intake in rodents. Addict Biol 22:1856–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguesse S, Morisot N, Shin JH, Liu F, Adrover MF, Sakhai SA, Lopez MF, Phamluong K, Griffin WC 3rd, Becker HC, Bender KJ, Alvarez VA, Ron D (2017b) Prosapip1-dependent synaptic adaptations in the nucleus accumbens drive alcohol intake, seeking, and reward. Neuron 96:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R (2014) The actin cytoskeleton in memory forma- tion. Prog Neurobiol 117:1–19. [DOI] [PubMed] [Google Scholar]
- Lin J, Liu L, Wen Q, Zheng C, Gao Y, Peng S, Tan Y, Li Y (2014) Rapamycin prevents drug seeking via disrupting reconsolidation of reward memory in rats. Int J Neuropsychopharmacol 17:127–136. [DOI] [PubMed] [Google Scholar]
- Lipton JO, Sahin M (2014) The neurology of mTOR. Neuron 84:275–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Laguesse S, Legastelois R, Morisot N, Ben Hamida S, Ron D (2017) mTORC1-dependent translation of collapsin response mediator proteins-2 drives neuroadaptations underlying excessive alcohol-drinking behaviors. Mol Psychiatry 22:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JL, Salling MC, Almli LM, Ratanatharathorn A, Uddin M, Galea S, Wildman DE, Aiello AE, Bradley B, Ressler K, Koenen KC (2015) Frequency of alcohol con- sumption in humans; the role of metabotropic glutamate receptors and downstream signaling pathways. Transl Psy- chiatry 5:e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Moos BS (2006) Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Ad- diction 101:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai J, Baba R, Ohshima T (2017) CRMPs function in neu- rons and glial cells: potential therapeutic targets for neuro- degenerative diseases and CNS injury. Mol Neurobiol 54:4243–4256. [DOI] [PubMed] [Google Scholar]
- Napier TC, Herrold AA, de Wit H (2013) Using conditioned place preference to identify relapse prevention medications. Neurosci Biobehav Rev 37:2081–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Barak S, Hamida SB, Ron D (2014) mTOR complex 1: a key player in neuroadaptations induced by drugs of abuse. J Neurochem 130:172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D (2010) Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci U S A 107:20093–20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CJ, McNally GP (2013) mu-Opioid receptors in the nucleus accumbens shell mediate context-induced reinstatement (re- newal) but not primed reinstatement of extinguished alcohol seeking. Behav Neurosci 127:535–543. [DOI] [PubMed] [Google Scholar]
- Quach TT, Honnorat J, Kolattukudy PE, Khanna R, Duchemin AM (2015) CRMPs: critical molecules for neurite morpho- genesis and neuropsychiatric diseases. Mol Psychiatry 20:1037–1045. [DOI] [PubMed] [Google Scholar]
- Richard JM, Fields HL (2016) Mu-opioid receptor activation in the medial shell of nucleus accumbens promotes alcohol con- sumption, self-administration and cue-induced reinstatement. Neuropharmacology 108:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Tofighy A, White HS, Matagne A, Wolff C (2015) Current understanding of the mechanism of action of the an- tiepileptic drug lacosamide. Epilepsy Res 110:189–205. [DOI] [PubMed] [Google Scholar]
- Roger-Sanchez C, Aguilar MA, Rodriguez-Arias M, Aragon CM, Minarro J (2012) Age- and sex-related differences in the acquisition and reinstatement of ethanol CPP in mice. Neurotoxicol Teratol 34:108–115. [DOI] [PubMed] [Google Scholar]
- Ron D, Barak S (2016) Molecular mechanisms underlying alcohol-drinking behaviours. Nat Rev Neurosci 17:576–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ (2010) The addicted synapse: mechanisms of syn- aptic and structural plasticity in nucleus accumbens. Trends Neurosci 33:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado S, Kaplitt MG (2015) The nucleus accumbens: a com- prehensive review. Stereotact Funct Neurosurg 93:75–93. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R (2006) Behavioural assessment of drug reinforcement and addictive features in rodents: an over- view. Addict Biol 11:2–38. [DOI] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell 168:960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirao T, Gonzalez-Billault C (2013) Actin filaments and micro- tubules in dendritic spines. J Neurochem 126:155–164. [DOI] [PubMed] [Google Scholar]
- Stankeviciute NM, Scofield MD, Kalivas PW, Gipson CD (2014) Rapid, transient potentiation of dendritic spines in context- induced relapse to cocaine seeking. Addict Biol 19:972–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr T, Kupferberg HJ, Stables JP, Choi D, Harris RH, Kohn H, Walton N, White HS (2007) Lacosamide, a novel anti- convulsant drug, shows efficacy with a wide safety marginin rodent models for epilepsy. Epilepsy Res 74:147–154. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Corlett PR, Taylor JR (2011) Aberrant learning and memory in addiction. Neurobiol Learn Mem 96:609–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12:227–462. [DOI] [PubMed] [Google Scholar]
- Wang X, Luo YX, He YY, Li FQ, Shi HS, Xue LF, Xue YX, Lu L (2010) Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci 30:12632–12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2014) Global status report on Alcohol and health.
- Wilson SM, Khanna R (2015) Specific binding of lacosamide to collapsin response mediator protein 2 (CRMP2) and direct im- pairment of its canonical function: implications for the thera- peutic potential of lacosamide. Mol Neurobiol 51:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Xiong W, Wang Y, Ping X, Head JD, Brittain JM, Gagare PD, Ramachandran PV, Jin X, Khanna R (2012) Pre- vention of posttraumatic axon sprouting by blocking collapsin response mediator protein 2-mediated neurite outgrowth and tubulin polymerization. Neuroscience 210:451–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.