The emergent 21st century coronaviruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), are associated with high mortality rates due to severe lung involvement with diffuse alveolar damage and pulmonary tissue destruction. A feature of the coronavirus disease 2019 (COVID-19) pandemic has been an increased mortality in Black, Asian, and Minority Ethnic groups in the UK, which has resulted in the UK Government setting up an emergency investigative task force. In the USA, mortality from COVID-19 has been particularly high in African American communities in large cities. Mortality in the earlier 2012 Middle East respiratory syndrome coronavirus was strongly linked to diabetes, hypertension, and pre-existent heart disease; and similar epidemiological trends emerged from Wuhan, China during the current COVID-19 pandemic.1, 2
SARS-CoV-2 viral cellular entry is via the angiotensin-converting enzyme 2 (ACE2) receptor, which contributes to blood pressure regulation, cardiac tissue remodelling, and immunity.3 Cardiac myocytes and endothelium express the ACE2 receptor, which is known to regulate cardiac development in the experimental setting.3 The ACE2 tropism of the virus has also focused epidemiological research on ACE and angiotensin II receptor blocker drugs as potential culprits for infection. Furthermore, ACE2 expression on endothelial cells has led to the concept of a virally mediated endotheliitis that might be linked to mortality.3
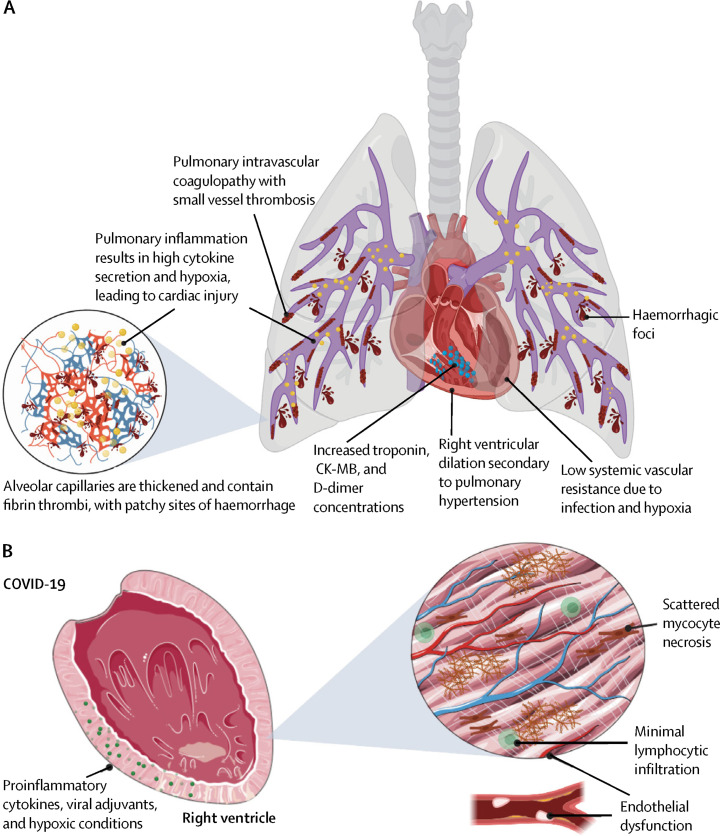
In the Lancet Respiratory Medicine, Sharon Fox and colleagues4 report on post-mortem findings in ten African American patients from New Orleans, LA, USA. In these ten patients, they affirm the findings of diffuse alveolar damage and pulmonary disease evolution towards adult respiratory distress syndrome. They also report extensive pulmonary thrombosis, microhaemorrhage, vessel wall damage, and inflammation. Similar pulmonary vascular changes were reported in COVID-19 cases from Europe.5 Other viral infections are known to trigger inflammation related thrombosis, which functions to constrain infection dissemination.6 In our own work,6, 7 we have pointed out how the anatomical juxtaposition of the infected alveolar ACE2+ cells with extensive pulmonary vasculature leads to development of pulmonary hypertension due to a pulmonary intravascular coagulopathy (PIC; figure ).
Figure.
Model for high mortality in COVID-19 in at-risk groups
(A) SARS-CoV-2 driven lung inflammation is associated with PIC, including extensive thrombosis micro-haemorrhaging. (B) Fibrinolytic mechanisms fail to keep abreast of the immune driven thrombosis with subacute pulmonary hypertension development with elevations in troponin and other markers reflecting diffuse myocardial mechanical stressing and ischaemia, especially in the right ventricle. Factors including obesity and diabetes might also contribute to pulmonary inflammation and PIC seen in COVID-19. Cardiac factors (including pre-existing macrovascular or microvascular arterial disease, cardiomyocyte hypertrophy, increased extracellular matrix deposition, and fibrosis with diastolic dysfunction) associated with systemic hypertension, type 2 diabetes, obesity, metabolic syndrome, and other factors, might become clinically relevant in the most at-risk groups including African Americans. Other contributory factors including hypercytokinemia from pulmonary MAS-like activation that could perturb cardiac function in addition to causing peripheral vasodilation contributing to lower systemic vascular resistance. Finally, RNAaemia, viral proteins without actual viraemia, or actual viral myocarditis, could also contribute to dysregulation cardiac endothelial or myocyte function. PIC=pulmonary intravascular coagulopathy. CK-MB=creatine kinase myocardial band.
Employing classical pathology techniques, Fox and colleagues4 carefully inspected hearts and noted right ventricular dilatation indicative of acute pulmonary hypertension. Histological examination of the hearts failed to show compelling evidence for a viral myocarditis or small vessel cardiac vasculitis. Histological examination also showed limited cardiac fibre necrosis and evident macrophages, which might be explained by the patients' cardiac risk factors and severe perimortem hypoxaemia.4 There was no marked myocardial lymphocyte infiltration and little pulmonary lymphocytic infiltration. Peripheral blood lymphopenia with poor prognosis has been repeatedly shown, and another study8 noted substantial pulmonary lymphocyte accumulation, possibly pointing towards sequestration as an explanation for lymphopenia.
In the study by Fox and colleagues,4 and in limited other data,2 elevated D-dimer, B-type natriuretic peptide, and troponin concentrations at hospital presentation were inversely associated with clinical outcome, supporting the generalisability of the study. However, severe thrombotic complications are not restricted to the lung microvasculature, and include deep vein thrombosis in up to a third of cases, pulmonary embolism, and ischaemic stroke, none of which were noted in the report by Fox and colleagues.4
Severe COVID-19 cases have substantial elevation in proinflammatory cytokines concentrations, likened to a cytokine storm. Cytokine storms associated with chimeric antigen receptor (CAR)-T cell therapy might trigger a cardiomyopathy that responds to corticosteroids or anticytokine therapy, including antagonism of interleukin-6,9 but the immune mechanism in COVID-19 is probably very different.7 The role of cytokines in heart injury has been extensively researched, and sepsis-associated inflammation without direct cardiac infection might lead to left ventricular dysfunction and remodelling.10 However, the immune reaction associated with COVID-19, unlike immune activation with CAR T-cell therapy, might be taking place in the face of persistent pulmonary viral replication, so the potential disutility of inflammation suppression awaits clinical trial results of anticytokine blockers.7
Some adult and paediatric cases, with and without lung involvement or active COVID-19, have reported pathology suggestive of cardiac Kawasaki Disease,11 which points to cardiac pathology independent of viral alveolitis and PIC, and could represent maladaptive adaptive immune system responses.
Studies including electron microscopy, PCR data for cardiac viral RNA, or SARS-CoV-2-related protein distribution data were not reported. A prior study12 of SARS-CoV-1 pathology identified viral RNA in the heart in seven (35%) of 20 cases, and macrophage infiltration but no clear-cut viral myocarditis in the SARS-CoV-2 positive hearts.
The integrated pulmonary and cardiac pathology from African Americans with severe COVID-19 strongly supports bipartite cardiopulmonary pathology in populations with increased cardiac risk factors that could explain the increased mortality. These findings have wide implications beyond pathology and to selective isolation strategies to protect individuals at high risk of cardiovascular events.
© 2020 CDC/Science Photo Library
Acknowledgments
We declare no competing interests. DMcG is supported by the National Institute for Health Research (NIHR) at the Leeds Biomedical Research Centre, Leeds Teaching Hospitals, Leeds, UK.
References
- 1.Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C. Clinical Characteristics of 138 Hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intens Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox SE, Akmatbekov A, Harbert JL, Li G, Brown LQ, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30243-5. published online May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wichmann D, Sperhake J-P, Lütgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Int Med. 2020 doi: 10.7326/M20-2003. published online May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGonagle D, O'Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30121-1. published online May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vabret N, Britton GJ, Gruber C. Immunology of COVID-19: current state of the science. Immunity. 2020 doi: 10.1016/j.immuni.2020.05.002. published online May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimabukuro-Vornhagen A, Gödel P, Subklewe M. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakihana Y, Ito T, Nakahara M, Yamaguchi K, Yasuda T. Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care. 2016;4:22. doi: 10.1186/s40560-016-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020 doi: 10.1016/S0140-6736(20)31094-1. published online May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oudit GY, Kassiri Z, Jiang C. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]