Abstract
Coronavirus disease-2019 (COVID-19) triggers a hypercoagulable state with a high incidence of thrombotic complications. We have noted a higher than expected incidence of stent thrombosis in these patients. (Level of Difficulty: Intermediate.)
Key Words: coronary artery, COVID-19, SARS-CoV-2, stent thrombosis
Abbreviations and Acronyms: ASA, acetyl salicylic acid; COVID-19, coronavirus disease-2019; DES, drug-eluting stent; LAD, left anterior descending; PCI, percutaneous coronary intervention; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; STEMI, ST-segment elevation myocardial infarction
Graphical abstract
Coronavirus disease-2019 (COVID-19) triggers a hypercoagulable state with a high incidence of thrombotic complications. We have noted a higher than…
Introduction
Advances in percutaneous coronary intervention (PCI) techniques, improvement in coronary stent designs, and more effective antithrombotic therapies have made coronary stent thrombosis a rare complication. The incidence at 30 days is <1%, whereas rates of late and very late stent thrombosis are 0.5% to 1% and 0.2% to 2% per year, respectively (1). Coronavirus disease-2019 (COVID-19) has modified the usual presentation of many diseases as we know them. This disease promotes a sustained prothrombotic state, triggered by interactions among proinflammatory cytokines, procoagulant factors, and platelets. We have recently observed an increase in stent thrombosis during the COVID-19 pandemic peak in our center, the Complejo Hospitalario Universitario de Albacete in Albacete, Spain.
Learning Objectives
-
•
COVID-19 increases both arterial and venous thrombogenicity.
-
•
The SARS-CoV-2 hypercoagulable state may lead to a stent thrombosis trigger in the presence of other mechanical and biological risk factors.
-
•
Recommendations on antithrombotic treatment and PCI for acute coronary syndromes should be maintained during COVID-19 treatment.
Case Reports
Case 1
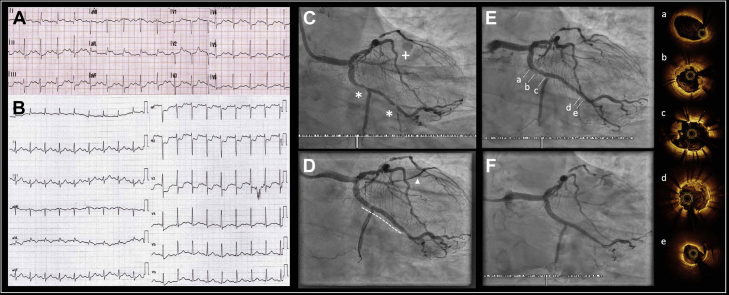
A 49-year-old man underwent primary angioplasty for a lateral ST-segment elevation myocardial infarction (STEMI) 6 h after the onset of symptoms (Figures 1A to 1F). Balloon angioplasty in a small ramus intermedius was performed. To decrease hospital length of stay, ad hoc PCI was performed in the circumflex artery with 2 overlapped drug-eluting stents (DES) (Videos 1 and 2). Thirty minutes later, there was new onset of more intense chest pain with marked ST-segment depression in the precordial leads. Acute circumflex artery stent thrombosis was confirmed by repeat angiography (Video 3). Optical coherence tomography demonstrated in-stent mixed thrombus with mild proximal stent underexpansion and a nonsignificant dissection of the distal stent edge. Intracoronary tirofiban was effective in reducing thrombus burden, and proximal overexpansion of the stent was performed (Videos 4 and 5). The patient received acetyl salicylic acid (ASA), ticagrelor, and a 24-h continuous infusion of tirofiban after the procedure. He had dry cough with a chest radiograph compatible with COVID-19 infection, but no tests were performed because we were at the early stage of the pandemic and the threshold of suspicion was high. The patient was discharged at 4 days. Serological testing 23 days later confirmed that immunoglobulin G was positive for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2).
Figure 1.
Case 1: Acute Stent Thrombosis
(A) Electrocardiogram at admission showing lateral ST-segment elevation myocardial infarction. (B) Electrocardiogram 30 min after circumflex percutaneous coronary intervention showing posterior ST-segment elevation myocardial infarction. (C) Ramus occlusion (+) and 2 critical lesions (asterisks) in the proximal circumflex artery and first marginal branch. (D) Final result after percutaneous coronary intervention of ramus (arrowhead) and circumflex arteries (dotted line indicates the position of 2 overlapped drug-eluting stents). (E) Stent thrombosis, with haziness at the (a) proximal and (d) distal edge, as well as (c) at the bifurcation. (F) Final result after stent thrombosis treatment, with complete flow restored. (a to d),optical coherence tomography imaging showing (a) no compromise of the proximal edge, (b) mild proximal underexpansion of stent with mixed thrombus, which can also be observed (c) in the origin of the second marginal branch and (d) near the distal edge, which has (e) mild dissection.
Online Video 1.
Case 1: Midocclusion of the Ramus Artery
The ramus artery occlusion was the index lesion. Critical stenoses in circumflex artery is also present.
Online Video 2.
Case 1: Final Result After Percutaneous Coronary Intervention of the Circumflex and Ramus Arteries
Online Video 3.
Case 1: Angiogram of the Stent Thrombosis
No occlusive thrombosis was present, but haziness is seen proximal to the edges of the stent. Haziness is also seen at the bifurcation with the second marginal branch, with distal occlusion of this branch.
Online Video 4.
Case 1: Optical Coherence Tomography of the Stent Thrombosis
Mixed thrombus is seen mainly at the proximal segment, with mild underexpansion. Thrombus at the origin of the bifurcation with second marginal branch, mild dissection (<60º and < 2 mm length) at distal edge.
Online Video 5.
Case 1: Angiogram Showing Final Result After Glycoprotein IIb-IIIa Inhibitor Use and Overexpansion of the Proximal Segment of the Stent
Case 2
A 71-year-old man was admitted in 2007 for an inferior STEMI that was treated with right coronary artery DES. He presented with a high-risk non-STEMI resulting from very late right coronary artery stent thrombosis. Thrombectomy, tirofiban, and 2 DESs restored flow. The patient reported fever and cough some days before admission; blood testing and chest radiograph showed COVID-19–compatible findings. The patient remained asymptomatic, and no confirmatory tests were performed for the same reason as in the first patient.
Case 3
An 86-year-old man with history of non-STEMI in 2018 that was treated with left anterior descending (LAD) artery DES underwent primary angioplasty for 6 h of chest pain and an anterior STEMI. Very late LAD artery stent thrombosis was found, and a new DES was implanted. The patient was asymptomatic, but because the COVID-19 pandemic had reached its peak, a pre-admission polymerase chain reaction test was performed, with a positive result. The patient had a favorable course and was discharged 5 days later.
Case 4
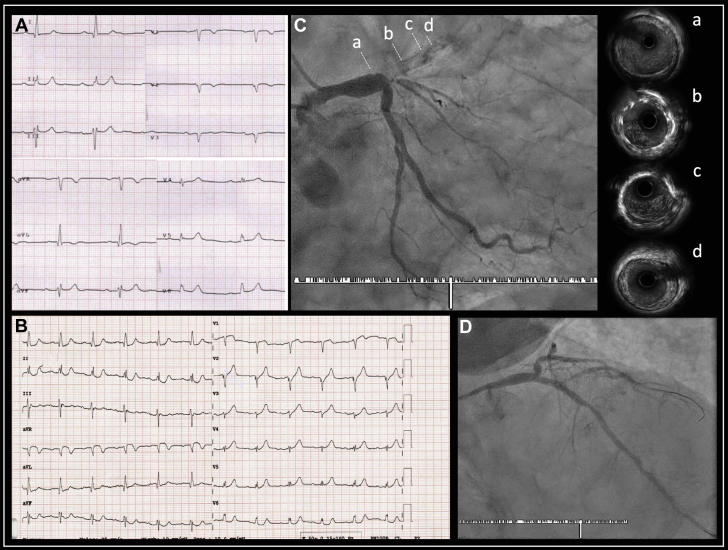
An 85-year old man underwent PCI with ostial LAD artery DES implantation in 2005. He presented at the pandemic peak with chest pain, a left ventricular ejection fraction of 30%, and anterior ST-segment elevation with prior Q waves (Figures 2A to 2D). The result of immunoglobulin M serological testing was positive despite the absence of respiratory symptoms. Angiography demonstrated very late LAD artery stent thrombosis (Video 6), which was treated with balloon angioplasty, thrombectomy, and tirofiban (Video 7). Intravascular ultrasound was performed and showed in-stent thrombus, appropriate stent expansion, and nonsignificant neoatherosclerosis (Video 8). The patient was treated with combined lopinavir and ritonavir, and despite his age, received ASA and prasugrel as antiplatelet therapy. Ten days later, prasugrel was replaced by clopidogrel (after antiviral treatment was completed), and the patient was discharged.
Figure 2.
Case 4: Very Late Stent Thrombosis
(A) Baseline electrocardiogram showing anterior Q waves with negative T waves in the precordial leads. (B) Electrocardiogram on admission, with ST-segment elevation in the precordial leads and reciprocal changes in the inferior leads. (C) Complete ostial left anterior descending artery stent thrombosis; (a to d) indicate the level of the images obtained with intravascular ultrasound. (D) Left anterior descending artery flow restored after percutaneous coronary intervention. Intravascular ultrasound images: (a), left main coronary artery immediately proximal to stent; (b) and (c), correct expansion without malapposition or neoatherosclerosis and with thrombus adhered to the stent; (d), distal edge of the stent, without complications.
Online Video 6.
Case 4: Angiogram Showing Occlusive Ostial Left Anterior Descending Artery Stent Thrombosis
Online Video 7.
Case 4: Angiogram Showing Final Thrombolysis In Myocardial Infarction III Flow Result After Glycoprotein IIb-IIIa Inhibitor Use, Thrombectomy, and Balloon Angioplasty of the Left Anterior Descending Artery Thrombosis
Online Video 8.
Case 4: Intravascular ultrasound Showing In-stent Fresh Thrombus With Correct Expansion and No or Mild Neoatherosclerosis
Proximal and distal edges are not damaged.
Discussion
The COVID-19 pandemic has significantly decreased worldwide interventional cardiology activity. In Spain, cardiac catheterization procedures have been reduced by 48%, with a reduction of 40% for primary angioplasty (2). Similar data have been reported in the United States (3). Compared with the immediate period before the pandemic peak (February 1 to 23, 2020), we experienced a 38% decrease in PCIs at our center between March 15, 2020 and April 5, 2020 (31 vs. 50). Moreover, we had an increase in the incidence of stent thrombosis (4 vs. 0; 13% of PCIs performed during this period). In 2019, we performed 899 PCIs, with 11 (1.2%) cases of stent thrombosis. Given the perception of a high rate of stent thrombosis among COVID-19 cases in other centers in Spain, a prospective registry is being conducted.
A greater thrombogenic predisposition, both arterial and venous, during COVID-19 has been established. Pathophysiologically, the cytokine storm that occurs 5 to 7 days after the onset of symptoms promotes the coagulation cascade, as well as platelet activation mediated by interleukin-6 and tissue factor. The latter induces an increase in thrombin and fibrin synthesis, as well as platelet production. Thrombocytosis can occur, as can high levels of D-dimer and fibrinogen, with intravascular disseminated coagulation criteria often fulfilled (4,5). Additionally, endothelial damage, which could be caused by the virus binding to the angiotensin-converting enzyme receptor and the stasis promoted by the permanent inflammation, would complete the Virchow triad criteria (6).
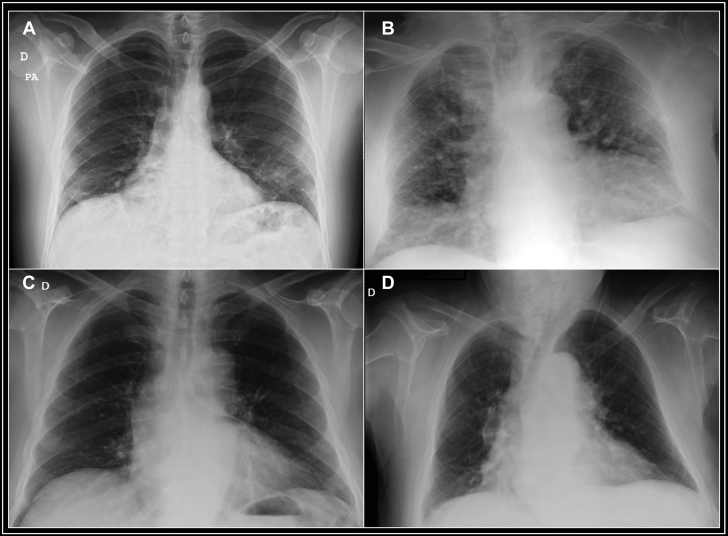
We present 1 case of acute stent thrombosis and 3 very late stent thrombosis cases (Table 1). Despite no initial COVID-19 testing in 2 cases, symptoms and subsequent testing (Figures 3A to 3D) supported that the patients were infected at the time of stent thrombosis (Table 2).
Table 1.
Summary of Cases of Stent Thrombosis Presented During the COVID-19 Pandemic
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age, yrs | 49 | 71 | 86 | 85 |
| Sex | Male | Male | Male | Male |
| COVID-19 status | IgG + | Suspicion | PCR + | IgM + |
| COVID-19 presentation | Cough, bilateral ground-glass infiltrates | Cough, fever, bilateral ground-glass infiltrates | Asymptomatic, bilateral ground-glass infiltrates | Asymptomatic, bilateral ground-glass infiltrates. |
| COVID19 treatment | No | No | Hydroxychloroquine Acetylcysteine |
Hydroxychloroquine Azithromycin Lopinavir-ritonavir Ceftriaxone |
| Presentation | STEMI | NSTEMI | STEMI | STEMI |
| Physical examination | Rales up to ½ lung fields | Normal | Normal | Peripheral edema |
| Heart rate (beats/min) | 110 | 53 | 75 | 80 |
| Blood pressure (mm Hg) | 150/86 | 150/85 | 160/80 | 110/60 |
| So2 (%) | 90 | 96 | 95 | 95 |
| Temperature (ºC) | 36.5 | 37.5 | 36 | 35.6 |
| Vessel responsible | Proximal circumflex – OM (90% stenosis) | Mid-RCA (occluded) | Proximal LAD (occluded) | Proximal LAD (occluded) |
| Stent thrombosed | DES ×2 (Ultimaster 3 × 15 mm) | BMS (Driver 3.5 × 18 mm) | DES (Cypher 3 × 18 mm) | DES (Synergy 3.5 × 32 mm) |
| Timing | Acute (30 min) | Very late (13 yrs) | Very late (2 yrs) | Very late (4 yrs) |
| APT before admission | None | ASA | ASA | ASA |
| APT during PCI | ASA + clopidogrel | ASA+ clopidogrel | ASA + clopidogrel | ASA+ prasugrel |
| Anticoagulation during PCI | UFH 8,000 IU | UFH 5,000 IU | UFH 8,000 IU | UFH 7,000 IU |
| Vascular approach | Right radial | Left radial | Left radial | Left radial |
| PCI technique | GPI BA OCT guidance |
Thrombectomy DES (Synergy 4 × 28 mm) GPI |
DES (Ultimaster 3 × 15 mm) | Thrombectomy BA IVUS guidance |
| APT discharge | ASA + ticagrelor | ASA+ ticagrelor | ASA + clopidogrel | ASA + clopidogrel |
| LVEF (%) at discharge | 45% | 55% | 45% | 30% |
| Risk factors for stent thrombosis | ||||
| Patient | DM LVD ACS |
CKD ACS |
Age DM LVD CKD ACS PAD |
Age LVD ACS |
| Lesion | Bifurcation | No | No | Ostial lesion |
| Procedural | Primary PCI Multivessel PCI Malapposition Underexpansion Dissection |
No | First-generation DES Overlapped stents |
Long stent |
ACS = acute coronary syndrome; APT = antiplatelet therapy; ASA = acetyl salicylic acid; BA = balloon angioplasty; BMS = bare metal stent; CKD = chronic kidney disease; COVID-19 = coronavirus disease-2019; DES = drug-eluting stent; DM = diabetes mellitus; GPI = glycoprotein IIb-IIIa inhibitor; IU = international units; IVUS = intravascular ultrasound; NSTEMI = non–ST-segment elevation myocardial infarction; OCT = optical coherence tomography; OM = obtuse marginal branch; RCA = right coronary artery; LAD = left anterior descending; LVEF = left ventricular ejection fraction; LVD = left ventricular dysfunction; PAD = peripheral artery disease; PCI = percutaneous coronary intervention; So2 = oxygen saturation; STEMI = ST-segment elevation myocardial infarction; UFH = unfractionated heparin.
Figure 3.
Chest Radiographs of Patients
(A) Case 1, peripheral interstitial infiltrates, mainly affecting the left base and subpleural regions. (B) Case 2, both central and peripheral alveolointerstitial infiltrates, probably from mixed heart failure. (C) (Case 3) and (D) (Case 4), mild infiltrates, mainly central and resulting from mild heart failure. D = right; PA = posteroanterior.
Table 2.
Summary of Laboratory Testing
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| COVID-19 tests | IgM−/IgG + | Not performed | PCR + | IgM+/IgG− |
| D-dimer (45–500 μg/l) | 630 | 539 | 662 | 1,251 |
| Fibrinogen (150–450 mg/dl) | 443 | 271 | 263 | 263 |
| Partial thromboplastin time (25–39 seg) | 32.7 | 28.3 | 29.4 | 31.1 |
| Prothrombin time (70%–120%) | 67 | 93 | 93 | 55 |
| Platelets (140–400 × 103/μl) | 175 | 127 | 167 | 165 |
| C-reactive protein (0–5 mg/l) | 12 | 24 | 9.6 | 40.5 |
| Ferritin (30–400 ng/ml) | 1,233 | 1,010 | 72 | 2,411 |
| Lymphocyte count (1–4 × 103/μl) | 1,590 | 930 | 2,240 | 790 |
| High-sensitivity T troponin peak (0–14 pg/ml) | 2,404 | 3,324 | 2,406 | 7,782 |
| Creatine kinase peak (38–174 U/l) | 874 | 634 | 523 | 1,276 |
| GFRe (ml/min) | 110 | 56 | 68 | 44 |
COVID-19 = coronavirus disease-2019; GFRe = estimated glomerular filtration rate; IgG = immunoglobulin G; IgM = immunoglobulin M; PCR = polymerase chain reaction; seg = segmented.
Very late stent thrombosis with a first-generation DES occurs in the presence of a sustained inflammatory response. Other factors such as delayed endothelialization, late malapposition, or neoatherosclerosis plaque rupture can sometimes be implicated (7). Two of our patients had chronic kidney disease, which can induce a permanent inflammatory response, and 1 patient had a first-generation DES. The patient with acute stent thrombosis had mild proximal stent underexpansion detected by optical coherence tomography. All patients were receiving appropriate antiplatelet therapy at the time of stent thrombosis; the patient with acute thrombosis was following an ASA-clopidogrel regimen, whereas the 3 patients with very late stent thrombosis were taking ASA therapy. We think that SARS-CoV-2 infection triggered stent thrombosis in these patients.
Finally, the latest official statements for COVID-19 patients recommend following current PCI guidelines (8). Expert recommendations have been published on antithrombotic therapy management, with special consideration given to possible interactions with the drugs used for COVID-19 (9). This is especially important with drugs metabolized through CYP3A4, such as clopidogrel or ticagrelor, which could interact with antiviral agents such as lopinavir combined with ritonavir or darunavir combined with cobicistat. Therefore, prasugrel could be the drug of choice in patients without contraindications (prior stroke) but who are >75 years of age or have a weight <60 kg if antiviral agents are considered indispensable. Because of the low evidence on the effectiveness of these antiviral agents against SARS-CoV-2, antiplatelet therapy should be prioritized for COVID-19 patients in the setting of acute coronary syndromes.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Gori T., Polimeni A., Indolfi C. Predictors of stent thrombosis and their implications for clinical practice. Nat Rev Cardiol. 2019;16:243–256. doi: 10.1038/s41569-018-0118-5. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez-Leor O., Cid-Álvarez B., Ojeda S. Impacto de la pandemia de COVID-19 sobre la actividad asistencial en cardiología intervencionista en España. REC Interv Cardiol. 2020;2:82–89. [Google Scholar]
- 3.Garcia S., Albaghdadi M.S., Meraj P.M. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranucci M., Ballotta A., Di Dedda U. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020 Apr 17 doi: 10.1111/jth.14854. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atri D., Siddiqi H.K., Lang J. COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. J Am Coll Cardiol Basic Transl Sci. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vivas D., Roldán V., Esteve-Pastor M.A. Recomendaciones sobre el tratamiento antitrombótico durante la pandemia COVID-19. Posicionamiento del Grupo de Trabajo de Trombosis Cardiovascular de la Sociedad Española de Cardiología. Rev Esp Cardiol. 2020 Apr 22 doi: 10.1016/j.recesp.2020.04.006. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torrado J., Buckley L., Durán A. Restenosis, stent thrombosis, and bleeding complications: navigating between Scylla and Charybdis. J Am Coll Cardiol. 2018;71:1676–1695. doi: 10.1016/j.jacc.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Hendren N.S., Drazner M.H., Bozkurt B. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]