Summary
Background
Concerns have been raised about the possibility that inhibitors of the renin–angiotensin–aldosterone system (RAAS) could predispose individuals to severe COVID-19; however, epidemiological evidence is lacking. We report the results of a case-population study done in Madrid, Spain, since the outbreak of COVID-19.
Methods
In this case-population study, we consecutively selected patients aged 18 years or older with a PCR-confirmed diagnosis of COVID-19 requiring admission to hospital from seven hospitals in Madrid, who had been admitted between March 1 and March 24, 2020. As a reference group, we randomly sampled ten patients per case, individually matched for age, sex, region (ie, Madrid), and date of admission to hospital (month and day; index date), from Base de datos para la Investigación Farmacoepidemiológica en Atención Primaria (BIFAP), a Spanish primary health-care database, in its last available year (2018). We extracted information on comorbidities and prescriptions up to the month before index date (ie, current use) from electronic clinical records of both cases and controls. The outcome of interest was admission to hospital of patients with COVID-19. To minimise confounding by indication, the main analysis focused on assessing the association between COVID-19 requiring admission to hospital and use of RAAS inhibitors compared with use of other antihypertensive drugs. We calculated odds ratios (ORs) and 95% CIs, adjusted for age, sex, and cardiovascular comorbidities and risk factors, using conditional logistic regression. The protocol of the study was registered in the EU electronic Register of Post-Authorisation Studies, EUPAS34437.
Findings
We collected data for 1139 cases and 11 390 population controls. Among cases, 444 (39·0%) were female and the mean age was 69·1 years (SD 15·4), and despite being matched on sex and age, a significantly higher proportion of cases had pre-existing cardiovascular disease (OR 1·98, 95% CI 1·62–2·41) and risk factors (1·46, 1·23–1·73) than did controls. Compared with users of other antihypertensive drugs, users of RAAS inhibitors had an adjusted OR for COVID-19 requiring admission to hospital of 0·94 (95% CI 0·77–1·15). No increased risk was observed with either angiotensin-converting enzyme inhibitors (adjusted OR 0·80, 0·64–1·00) or angiotensin-receptor blockers (1·10, 0·88–1·37). Sex, age, and background cardiovascular risk did not modify the adjusted OR between use of RAAS inhibitors and COVID-19 requiring admission to hospital, whereas a decreased risk of COVID-19 requiring admission to hospital was found among patients with diabetes who were users of RAAS inhibitors (adjusted OR 0·53, 95% CI 0·34–0·80). The adjusted ORs were similar across severity degrees of COVID-19.
Interpretation
RAAS inhibitors do not increase the risk of COVID-19 requiring admission to hospital, including fatal cases and those admitted to intensive care units, and should not be discontinued to prevent a severe case of COVID-19.
Funding
Instituto de Salud Carlos III.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uses the angiotensin-converting enzyme 2 (ACE2) as the receptor for its spike protein to invade cells and replicate.1 ACE2 presents a high homology with ACE, a key enzyme in the regulation of blood pressure.2 In some animal studies, renin–angiotensin–aldosterone system (RAAS) inhibitors (a category that includes ACE inhibitors and angiotensin-receptor blockers) have been reported to increase expression of ACE2.3, 4, 5 These findings have led some researchers to postulate that the use of these drugs might enhance the access of SARS-CoV-2 into cells, predisposing patients to infection or increasing severity of COVID-19.6, 7, 8 This hypothesis was fuelled by results from the first case series that was published in which age, hypertension, diabetes, and coronary heart disease—conditions associated with the use of RAAS inhibitors—were identified as potential risk factors for severe cases and in-hospital deaths.9, 10, 11, 12 By contrast, other authors have proposed use of angiotensin-receptor blockers as a preventive measure, or even a therapy, for COVID-19 because of their potential to reduce lung injury caused by angiotensin II.13 RAAS inhibitors are among the most widely used drugs globally for indications such as hypertension, heart failure, kidney complications of diabetes, and myocardial infarction; hence their discontinuation because of COVID-19 could cause patients harm.14 Scientific societies and drug regulatory agencies alike have advised against their discontinuation until sound evidence is available.15
Research in context.
Evidence before this study
Inhibitors of the renin–angiotensin–aldosterone system (RAAS) have been hypothesised to predispose patients to more severe COVID-19. This hypothesis is based on two facts: these drugs have been reported to upregulate the expression of angiotensin-converting enzyme (ACE) 2, the gateway used by severe acute respiratory syndrome coronavirus 2 to enter cells, and patients with severe COVID-19 have a high prevalence of cardiovascular diseases. Many scientific societies adopted the position of not recommending the discontinuation of treatment. Yet, epidemiological evidence is lacking and the hypothesis has not been confirmed or refuted. We searched PubMed on April 15, 2020, for publications in English since Jan 1, 2020, using the search terms “COVID-19” and “ACE inhibitors OR angiotensin-converting enzyme inhibitors OR angiotensin receptor blockers OR RAAS inhibitors OR RAS inhibitors OR RAAS blockers OR RAS blockers”, filtering by “human” and “observational study”, and no result was returned.
Added value of this study
Using a case-population study design, we found no difference between the risk of COVID-19 requiring admission to hospital associated with the use of RAAS inhibitors and the risk associated with the use of other antihypertensive drugs, once fully adjusted for age, sex, and cardiovascular comorbidities and risk factors. Additionally, we found no increased risk with either ACE inhibitors or angiotensin-receptor blockers. The lack of association between RAAS inhibitors and risk of COVID-19 requiring admission to hospital was observed in both the most severe (fatal cases and those needing admission to an intensive care unit) and less severe inpatients.
Implications of all the available evidence
The available evidence supports that RAAS inhibitors are safe and should not be discontinued for fear of an increased risk of COVID-19.
To provide a solution to this urgent issue, we designed a pharmacoepidemiological study with the aim of assessing whether the odds of exposure to RAAS inhibitors relative to other antihypertensive drugs was higher among patients with COVID-19 admitted to hospital than in the general population, adjusted for age, sex, and cardiovascular comorbidities and risk factors.
Methods
Study design and participants
In this case-population study, we used an epidemiological approach, conceived as a surveillance method to assess adverse drug effects,16 to provide a rapid answer to our study question. Briefly, this method uses data from a series of patients with an illness from a specific region (cases) and data from patients randomly sampled from a primary health-care database in the same region (population controls). Assuming that the primary health-care database represents the source population of the cases, a random sample of controls from that database would provide a valid estimate of the prevalence of the exposure and covariates in the source population, approaching the primary base paradigm of case-control studies.17
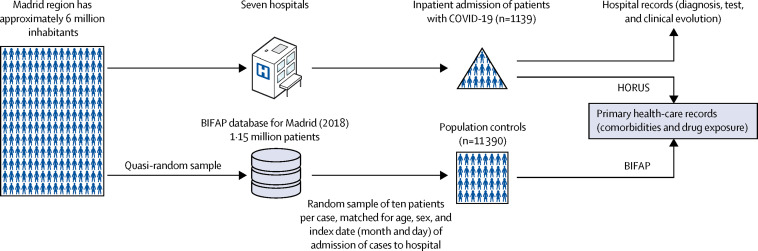
Here, we collected data on patients (cases) aged 18 years or older with a PCR-confirmed diagnosis of COVID-19 who were admitted to seven hospitals in Madrid, Spain, between March 1 and March 24, 2020. During the study period, the Madrid region was in the upward part of the outbreak, with an average of 465 inpatient admissions, 48 intensive care unit (ICU) admissions, and 76 deaths per day associated with COVID-19 (official data are available online). The seven participating hospitals belong to the National Health System (NHS) and are distributed all over the Autonomous Community of Madrid (appendix p 2). Cases were selected consecutively according to the date of admission to hospital until the planned sample size was met in a competitive manner. For our population controls, we used a random sample of ten individuals per case, individually matched to our cases by sex, age (exact), region (ie, Madrid), and date (day and month), extracted from the last available year (2018) from the primary health-care database Base de datos para la Investigación Farmacoepidemiológica en Atención Primaria (BIFAP; figure 1 ).
Figure 1.
Case-population study design
COVID-19 cases requiring admission to hospital were selected consecutively from seven hospitals in Madrid, Spain. Data were collected for ten individuals per case who were matched for age, sex, and index date (day and month) of hospital admission of cases (matched controls) from the 2018 Madrid region database of BIFAP, a national primary health-care database. Drug exposure and comorbidities before the index date (2020 for cases and 2018 for controls) were collected from primary health-care records of the NHS in Madrid: for cases through HORUS (an online platform to access primary-care clinical records from any health-care centre of the NHS in Madrid) and for controls through BIFAP. BIFAP=Base de datos para la Investigación Farmacoepidemiológica en Atención Primaria. NHS=National Health System.
The Ethics Research Committee of the University Hospital Príncipe de Asturias (the coordinating centre) assessed the study protocol and granted a favourable opinion on March 18, 2020, including a waiver for the informed consent of patients taking part in the study. The data extracted were fully anonymised and no attempt was made to interview patients or their relatives. The study complied with the provisions of the Spanish legislation and the Declaration of Helsinki 2013. The Scientific Committee of BIFAP granted access to pseudonymised data for controls on March 26, 2020. The study protocol is registered in the EU electronic Register of Post-Authorisation Studies, EUPAS34437, and is available online.
Data sources and collection
Information on disease and the clinical disease course of cases was retrieved from hospital medical records, while information on drug exposure and comorbidities before admission were mainly obtained from electronic primary health-care records that can be accessed through the NHS's primary health-care data platform, HORUS, by authorised health-care workers in Madrid. All electronic case report forms were sent on a weekly basis from each hospital to the coordinating centre, where data quality control was done. This quality control involved the selection of a random sample of clinical records from each hospital and double-checking the information collected on drug exposure and comorbidities through HORUS. Information on drug exposure and comorbidities of population controls was obtained from the database BIFAP, owned by the Spanish Agency for Medicines and Medical Devices, which extracts information from the electronic primary health-care records of the NHS from participating regions. This database contains information from 1·15 million patients from Madrid, and the distribution of age and sex among patients is comparable to the population census of the region (appendix pp 3–4). The HORUS and BIFAP databases access the same primary health-care data of patients in the Madrid NHS, and the catchment population of the seven hospitals taking part in the study and the population attending primary care were the same.
Outcomes
The main outcome variable was admission to hospital of patients with COVID-19 confirmed by a positive PCR test. Hospitals posted in-house protocols for clinical management and hospital admission based on criteria issued by the Ministry of Health, including, but not limited to, respiratory failure (oxygen saturation <90%, severe hypoxaemia [partial pressure of oxygen <60 mm Hg], or breathing rate >30 breaths per min, while breathing ambient air); abnormal chest x-ray compatible with COVID-19-associated pneumonia (bilateral pneumonia or unilateral pneumonia with damage in different lung lobes); and relevant clinical alterations including haemodynamic, hepatic, renal, or haematological derangements, together with clinically significant laboratory abnormalities (such as abnormal increase in D-dimer, ferritin, lactate dehydrogenase, or C-reactive protein), or severe lymphocytopenia. The date of admission was considered the index date. Then, we followed up patients and identified those who had been admitted to the ICU or who died in hospital. When considering a severity analysis, these patients were considered to be the most severe cases.
The antihypertensive drugs examined were ACE inhibitors, angiotensin-receptor blockers, renin inhibitors, aldosterone antagonists, calcium-channel blockers, diuretics, β-blockers, and α-blockers for cardiovascular indications (appendix p 8). Also, we grouped ACE inhibitors, angiotensin-receptor blockers, renin inhibitors, and aldosterone antagonists (alone or combined with any drug) in a variable called RAAS inhibitors; and calcium-channel blockers, β-blockers, diuretics, and α-blockers (alone or combined with other drugs different from RAAS inhibitors) in a variable called other antihypertensive drugs.
We defined exposure to the drug or drugs of interest as current use when an individual had a prescription lasting until the month before the index date; otherwise exposure was defined as non-use. For the main analysis, we generated a variable with the following mutually exclusive categories: non-use of any antihypertensive drug, current use of RAAS inhibitors, and current use of other antihypertensive drugs and we used current use of other antihypertensive drugs as the reference category (unless otherwise specified). When a patient used a RAAS inhibitor concomitantly with any other antihypertensive drug, they were always assigned to the RAAS inhibitor category. Subsequently, we disaggregated the category current use of RAAS inhibitors into their different pharmacological subgroups: ACE inhibitors, angiotensin-receptor blockers (excluding current users of ACE inhibitors), aldosterone antagonists (excluding current users of ACE inhibitors or angiotensin-receptor blockers), and renin inhibitors (excluding current users of any other RAAS inhibitor). For some analyses, we also disaggregated the category current use of other antihypertensive drugs into its respective components (calcium-channel blockers, β-blockers, diuretics, and α-blockers), and used current use of calcium-channel blockers as the reference category. Among current users of RAAS inhibitors, we distinguished when they used the drugs in monotherapy or combined with other antihypertensive drug (either in fixed-dose combinations or concomitant use as separate medicinal products). The date of first prescription of the current treatment episode was also extracted to estimate the duration of treatment (categorised as up to 1 year and longer than 1 year).
Statistical analysis
We estimated the sample size needed for different scenarios of effect size (ie, odds ratio [OR]) and prevalence of use of antihypertensive drugs in controls, assuming an α error of 0·05 and a power of 80%. According to this calculation, a sample size of 1000 cases and 10 000 controls would allow us to detect an OR of 1·5 or greater if the prevalence of use of RAAS inhibitors among controls was at least 5%.
We express quantitative variables as mean (SD) and qualitative variables as frequencies and percentages. Differences in means were assessed using the Student's t test and differences in percentages were assessed using the χ2 test. We describe the distribution of comorbidities among cases and controls and their association with COVID-19 requiring admission to hospital was assessed through univariable conditional logistic regression to calculate crude ORs) and 95% CIs (adjusted for age and sex due to matching).
When assessing potential confounding factors (covariates), we considered the presence of the following comorbidities at the index date: history of hypertension, diabetes, dyslipidaemia (defined as use of lipid-lowering drugs), ischaemic heart disease, atrial fibrillation, heart failure, thromboembolic disease, cerebrovascular accident, asthma, chronic obstructive pulmonary disease, chronic renal failure, and cancer. With the cardiovascular risk factors and comorbidities, we constructed a composite variable of background cardiovascular risk with the following three categories: patients with history of any of ischaemic heart disease, cerebrovascular accident, heart failure, atrial fibrillation, or thromboembolic disease; patients with any of the following cardiovascular risk factors (and no cardiovascular disease): hypertension, dyslipidaemia, diabetes, or chronic renal failure; and the remainder of patients without any cardiovascular diseases or risk factors.
We assessed the association between current use of RAAS inhibitors and risk of COVID-19 requiring admission to hospital, compared with either current use of other antihypertensive drugs (main analysis) or non-use of any antihypertensive drug, through univariable and multivariable conditional logistic regressions. In the multivariable model, we included all covariates described as potential confounding factors (with the exception of hypertension) to calculate adjusted ORs and 95% CIs. Current users of RAAS inhibitors had a prevalence of hypertension over 90% (appendix p 13); for this reason, we did not include hypertension in the multivariable model because the potential for confounding was minimal.
We assessed potential effect modification by age (<70 vs ≥70 years), sex, hypertension, diabetes, and background cardiovascular risk through a stratified analysis. We assessed the statistical interaction by comparing the adjusted ORs across different strata using the Altman and Bland test of interaction.18 Statistical significance was set at a p value of less than 0·05. However, for stratified analyses we applied Bonferroni correction to allow for multiple testing, for which we set the statistical significance level at a p value of less than 0·008 (0·05 divided by 6—ie, the number of stratified analyses done).
According to COVID-19 severity we differentiated two groups of cases: fatal cases and those requiring admission to an ICU (ie, most severe), and less severe cases. In each group, we analysed the association with RAAS inhibitors as described earlier and compared the adjusted OR across the strata using the Altman and Bland test of interaction.18
We planned four sensitivity analyses. First, an analysis for the correction for secular trends. The control series was sourced from a 2018 population, the last available year in the BIFAP database, while cases were from 2020. Thus, in case the prevalence of use of RAAS inhibitors increased over time, the exposure among controls would be an underestimate of 2020 data and, as a result, the adjusted ORs could be overestimated. To assess the magnitude of this potential problem, we examined trends of use of different antihypertensive drug subgroups in the Madrid population in BIFAP over the period 2012–18 and forecast the result corresponding to 2020 via a linear regression model. Then, we divided the prevalence of use estimate for 2020 by the prevalence observed in 2018 for every antihypertensive drug class and took this ratio as an indicator of the overestimation of adjusted ORs. For RAAS inhibitors, the ratio was 1·04 overall (1·05 for ACE inhibitors and 1·01 for angiotensin-receptor blockers), whereas for other antihypertensive drugs, including calcium-channel blockers, the ratio was close to 1·00 (appendix pp 5–7). In the sensitivity analysis, we corrected for these patterns of use, dividing the adjusted OR of RAAS inhibitors, ACE inhibitors, and angiotensin-receptor blockers by their respective estimated ratios 2020:2018. Second, we assessed the potential effect of media alerts. Concerns about the association between RAAS inhibitors and severe COVID-19 were highlighted in several letters to medical journals8, 9, 10 and news began to appear in the Spanish press and social media networks after March 16, 2020, when the Spanish Agency for Medicines and Medical Devices published a note recommending not to discontinue these drugs.15 Because this news might have had an influence on adherence to recorded treatment, we stratified the results into two study periods: March 1–16, and March 17–24. Third, we excluded aldosterone antagonists and renin inhibitors from the RAAS inhibitor group. For the main analysis we included aldosterone antagonists in the RAAS inhibitor group because these drugs have also been reported to upregulate ACE2 expression;19 but we did a sensitivity analysis to estimate the adjusted ORs among RAAS inhibitors excluding aldosterone antagonists and renin inhibitors. Finally, we included hypertension in our multivariable model.
We did all statistical analyses using STATA/SE (version 15).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We collected data on 1139 cases and 11 390 matched controls. Despite being matched by age and sex, cases had a greater prevalence of diverse comorbidities than did controls (table 1 ).
Table 1.
Demographic and clinical characteristics of cases with COVID-19 and population controls
| Cases (n=1139) | Controls (n=11 390) | Crude odds ratio* | |||
|---|---|---|---|---|---|
| Sex | |||||
| Male | 695 (61·0%) | 6950 (61·0%) | .. | ||
| Female | 444 (39·0%) | 4440 (39·0%) | .. | ||
| Age, years | 69·1 (15·4) | 69·1 (15·4) | .. | ||
| Comorbidities | |||||
| Hypertension | 617 (54·2%) | 5644 (49·6%) | 1·27 (1·10–1·46) | ||
| Diabetes | 310 (27·2%) | 2311 (20·3%) | 1·50 (1·30–1·73) | ||
| Dyslipidaemia | 444 (39·0%) | 3530 (31·0%) | 1·49 (1·30–1·70) | ||
| Ischaemic heart disease | 119 (10·5%) | 862 (7·6%) | 1·46 (1·19–1·80) | ||
| Heart failure | 80 (7·0%) | 400 (3·5%) | 2·18 (1·68–2·82) | ||
| Atrial fibrillation | 138 (12·1%) | 970 (8·5%) | 1·54 (1·26–1·88) | ||
| Thromboembolic disease | 44 (3·9%) | 290 (2·6%) | 1·55 (1·12–2·14) | ||
| Cerebrovascular accident | 73 (6·4%) | 569 (5·0%) | 1·32 (1·02–1·70) | ||
| COPD | 119 (10·5%) | 923 (8·1%) | 1·35 (1·10–1·66) | ||
| Asthma | 78 (6·9%) | 630 (5·5%) | 1·26 (0·99–1·61) | ||
| Cancer | 200 (17·6%) | 1573 (13·8%) | 1·35 (1·14–1·60) | ||
| Chronic renal failure | 89 (7·8%) | 573 (5·0%) | 1·65 (1·29–2·09) | ||
| Background cardiovascular risk | |||||
| Cardiovascular diseases† | 312 (27·4) | 2403 (21·1%) | 1·98 (1·62–2·41) | ||
| Cardiovascular risk factors‡ | 504 (44·3) | 4983 (43·8%) | 1·46 (1·23–1·73) | ||
| No cardiovascular disease or risk factors | 323 (28·4) | 4004 (35·2%) | 1 (ref) | ||
| Current use§ | |||||
| RAAS inhibitors | 497 (43·6%) | 3822 (33·6%) | 1·63 (1·43–1·87) | ||
| ACE inhibitors | 240 (21·1%) | 2192 (19·2%) | 1·13 (0·97–1·31) | ||
| Angiotensin-receptor blockers | 244 (21·4%) | 1616 (14·2%) | 1·70 (1·45–1·98) | ||
| Aldosterone antagonists | 38 (3·3%) | 218 (1·9%) | 1·78 (1·25–2·53) | ||
| Renin inhibitors | 1 (0·1%) | 8 (0·1%) | 1·25 (0·16–9·99) | ||
| Other antihypertensive drugs | 529 (46·4%) | 3844 (33·8%) | 1·90 (1·66–2·18) | ||
| Calcium-channel blockers | 212 (18·6%) | 1459 (12·8%) | 1·59 (1·35–1·87) | ||
| Diuretics | 347 (30·5%) | 2579 (22·6%) | 1·58 (1·37–1·83) | ||
| β-blockers | 200 (17·6%) | 1303 (11·4%) | 1·69 (1·43–1·99) | ||
| α-blockers | 40 (3·5%) | 183 (1·6%) | 2·24 (1·58–3·18) | ||
| Participating hospitals | |||||
| Hospital Universitario Príncipe de Asturias | 315 (27·7%) | 3150 (27·7%) | .. | ||
| Hospital Universitario de La Princesa | 200 (17·6%) | 2000 (17·6%) | .. | ||
| Hospital Universitario Ramón y Cajal | 176 (15·5%) | 1760 (15·5%) | .. | ||
| Hospital Clínico San Carlos | 127 (11·2%) | 1270 (11·2%) | .. | ||
| Hospital Central de la Defensa Gómez Ulla | 123 (10·8%) | 1230 (10·8%) | .. | ||
| Hospital Universitario Puerta de Hierro-Majadahonda | 99 (8·7%) | 990 (8·7%) | .. | ||
| Hospital Universitario de Getafe | 99 (8·7%) | 990 (8·7%) | .. | ||
Data are n (%), mean (SD), or odds ratio with 95% CI in parentheses. ACE=Angiotensin-converting enzyme. COPD=chronic obstructive pulmonary disease. RAAS=renin–angiotensin–aldosterone system.
The exposure to the specific comorbidity or drug was compared with non-exposure of that specific comorbidity or drug; crude odds ratios are adjusted for sex and age.
Includes ischaemic heart disease, cerebrovascular accident, heart failure, atrial fibrillation, and thromboembolic disease.
Includes hypertension, dyslipidaemia, diabetes, and chronic renal failure.
Patients can be counted several times if they were current users of two or more antihypertensive drugs belonging to different pharmacological classes, hence total exceeds 100%.
The prevalence of use of different subgroups of antihypertensive drugs was higher in cases than in controls, which yielded positive associations with risk of COVID-19 requiring admission to hospital in both crude and fully adjusted analyses compared with non-use of any antihypertensive drug (adjusted OR for RAAS inhibitors 1·71, 95% CI 1·46–2·01; and for other antihypertensive drugs 1·82, 1·47–2·26; appendix p 9). When use of other antihypertensive drugs was set as the reference category, the adjusted OR associated with the current use of RAAS inhibitors was 0·94 (0·77–1·15; table 2 ). No significant increase in the risk of COVID-19 requiring admission to hospital was observed with either ACE inhibitors or angiotensin-receptor blockers, nor did we find any difference when these drugs were used in monotherapy or in combination with other drugs (table 2).
Table 2.
Risk of COVID-19 requiring admission to hospital and current use of RAAS inhibitors compared with current use of other antihypertensive drugs (main analysis)
| Cases (n=1139) | Matched controls (n=11 390) | Crude oddsratio* | Adjusted odds ratio† | ||
|---|---|---|---|---|---|
| Current use of other antihypertensive drugs | 155 (13·6%) | 1129 (9·9%) | 1 (ref) | 1 (ref) | |
| Current use of RAAS inhibitors | 497 (43·6%) | 3822 (33·6%) | 0·94 (0·77–1·14) | 0·94 (0·77–1·15) | |
| ACE inhibitors | 240 (21·1%) | 2192 (19·2%) | 0·78 (0·63–0·97) | 0·80 (0·64–1·00) | |
| Monotherapy | 82 (7·2%) | 757 (6·7%) | 0·75 (0·57–1·00) | 0·83 (0·62–1·12) | |
| Combinations | 158 (13·9%) | 1435 (12·6%) | 0·80 (0·63–1·01) | 0·78 (0·62–0·99) | |
| Angiotensin-receptor blockers | 237 (20·8%) | 1552 (13·6%) | 1·11 (0·89–1·38) | 1·10 (0·88–1·37) | |
| Monotherapy | 38 (3·3%) | 328 (2·9%) | 0·82 (0·56–1·20) | 0·87 (0·60–1·28) | |
| Combinations | 199 (17·5%) | 1224 (10·8%) | 1·18 (0·94–1·48) | 1·15 (0·92–1·45) | |
| Aldosterone antagonists | 19 (1·7%) | 71 (0·6%) | 2·05 (1·20–3·49) | 1·68 (0·97–2·91) | |
| Renin inhibitors | 1 (0·1%) | 7 (0·1%) | 1·08 (0·13–8·86) | 1·04 (0·13–8·62) | |
| Non-use | 487 (42·8%) | 6439 (56·5%) | 0·47 (0·38–0·58) | 0·55 (0·44–0·68) | |
Data are n (%) or odds ratio with 95% CI in parentheses. The different pharmacological classes examined are mutually exclusive categories, so that patients who used combinations are counted only once, applying the following criteria: ACE inhibitors include current users of any ACE inhibitors alone or combined with any other antihypertensive drug (in fixed-dose combinations or in different medicinal products); angiotensin-receptor blockers include current users of any angiotensin-receptor blocker, alone or combined with any other antihypertensive drug that is not an ACE inhibitor (in fixed-dose combinations or in different medicinal products); aldosterone antagonists include current users of any antagonist of aldosterone, alone or combined with any other antihypertensive drug that is not an ACE inhibitor or angiotensin-receptor blocker (in fixed-dose combinations or in different medicinal products); other antihypertensive drugs include calcium-channel blockers, diuretics, β-blockers, and α-blockers, alone or combined (excluding RAAS inhibitors; either in fixed-dose combinations or in different medicinal products). ACE=angiotensin-converting enzyme. RAAS=renin–angiotensin–aldosterone system.
Adjusted for sex and age.
Adjusted for the matching variables plus history of diabetes, dyslipidaemia, ischaemic heart disease, heart failure, atrial fibrillation, thromboembolic disease, cerebrovascular accident, chronic obstructive pulmonary disease, asthma, cancer, and chronic renal failure.
The adjusted ORs associated with other antihypertensive drugs compared with non-use by different pharmacological subgroups were 1·96 (95% CI 1·43–2·69) for calcium-channel blockers, 1·79 (1·32–2·43) for diuretics, 1·68 (1·16–2·43) for β-blockers, and 1·96 (0·57–6·71) for α-blockers (appendix p 9). When the current use of calcium-channel blockers was set as the reference category, the adjusted OR was 0·87 (0·65–1·18) for RAAS inhibitors, 0·74 (0·54–1·02) for ACE inhibitors, and 1·02 (0·74–1·40) for angiotensin-receptor blockers (appendix p 10).
Compared with long-term (longer than 1 year) users of other antihypertensive drugs, long-term users of RAAS inhibitors had an adjusted OR of 0·96 (95% CI 0·76–1·21) for risk of COVID-19 requiring admission to hospital. Likewise, compared with short-term users (up to 1 year) of other antihypertensive drugs, short-term users of RAAS inhibitors had an adjusted OR of 1·39 (95% CI 0·92–2·10).
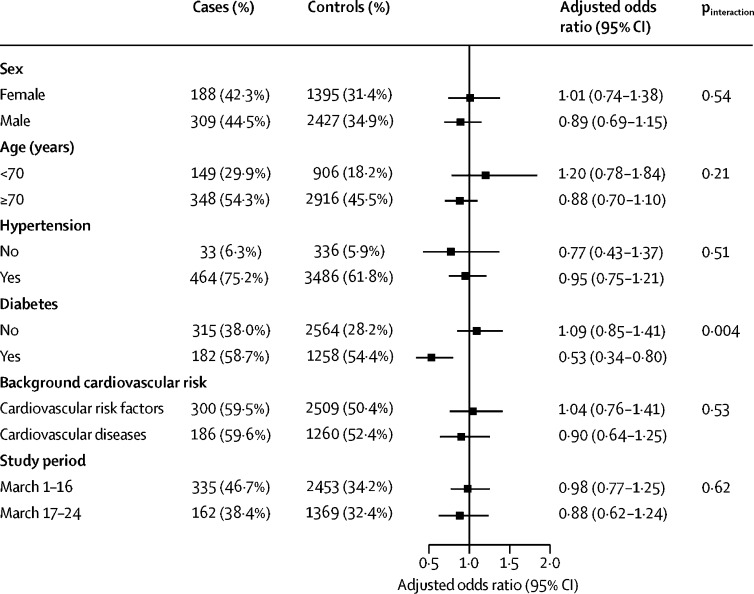
The results of the assessment of the potential effect modification of RAAS inhibitors by age (<70 and ≥70 years), sex, diabetes, hypertension, and background cardiovascular risk are shown in figure 2 . No significant interaction was observed with any variable, except for diabetes, for which a significantly reduced risk of COVID-19 requiring admission to hospital associated with RAAS inhibitors was observed (adjusted OR 0·53; 0·34–0·80; test of interaction, p=0·004; still significant after applying the Bonferroni correction for multiple testing [corrected significance level fixed at 0·008]).
Figure 2.
Association between current use of RAAS inhibitors and risk of COVID-19 requiring admission to hospital compared with current use of other antihypertensive drugs, stratified by different variables
Number (%) of cases and controls given is the number exposed to RAAS inhibitors in each stratum and the resulting odds ratio, adjusted for the matching variables and other comorbidities different from the one examined. RAAS=renin–angiotensin–aldosterone system.
393 (34·5%) of 1139 cases had the most severe form of COVID-19: 283 (24·8%) died in hospital and 110 (9·7%) survived but required admission to an ICU. The mean duration of hospital stay was 13 days (SD 8) among survivors and 11 days (SD 7) among non-survivors. Broadly, compared with patients with less severe COVID-19 in our study, patients with the most severe COVID-19 were older (mean age 75·3 years [SD 12·3] vs 65·8 years [SD 15·9]; p<0·0001) and a higher proportion were male (264 [67·2%] of 393 with severe disease vs 431 [57·8%] of 746 with less severe disease; p=0·001; table 3 ). Compared with current use of other antihypertensive drugs, the adjusted OR associated with the current use of RAAS inhibitors was 1·08 (95% CI 0·80–1·47) among the most severe cases and 0·86 (0·66–1·11) among the less severe cases (table 3). In both severity groups, the 95% CIs for the adjusted ORs of ACE inhibitors and angiotensin-receptor blockers compared with other antihypertensive drugs overlapped with each other, indicating no significant differences (table 3).
Table 3.
Risk of COVID-19 requiring admission to hospital and current use of RAAS inhibitors compared with current use of other antihypertensive drugs, by severity of disease
| Cases (n=1139) | Matched controls (n=11 390) | Adjusted odds ratio* | ||
|---|---|---|---|---|
| Most severe cases | ||||
| n | 393 | 3930 | .. | |
| Sex | ||||
| Male | 264/393 (67·2%) | 2640/3930 (67·2%) | .. | |
| Female | 129/393 (32·8%) | 1290/3930 (32·8%) | .. | |
| Age, years | 75·3 (12·3) | 75·3 (12·3) | .. | |
| Current use | ||||
| Other antihypertensive drugs | 64/393 (16·3%) | 484/3930 (12·3%) | 1 (ref) | |
| RAAS inhibitors† | 215/393 (54·7%) | 1592/3930 (40·5%) | 1·08 (0·80–1·47) | |
| ACE inhibitors | 101/393 (25·7%) | 905/3930 (23·0%) | 0·92 (0·65–1·29) | |
| Angiotensin-receptor blockers | 105/393 (26·7%) | 655/3930 (16·7%) | 1·25 (0·89–1·77) | |
| Non-use | 114/393 (29·0%) | 1854/3930 (47·2%) | 0·48 (0·34–0·69) | |
| Less severe cases | ||||
| n | 746 | 7460 | .. | |
| Sex | ||||
| Male | 431/746 (57·8%) | 4310/7460 (57·8%) | .. | |
| Female | 315/746 (42·2%) | 3150/7460 (42·2%) | .. | |
| Age, years | 65·8 (15·9) | 65·8 (15·9) | .. | |
| Current use | ||||
| Other antihypertensive drugs | 91/746 (12·2%) | 645/7460 (8·7%) | 1 (ref) | |
| RAAS inhibitors† | 282/746 (37·8%) | 2230/7460 (29·9%) | 0·86 (0·66–1·11) | |
| ACE inhibitors | 139/746 (18·6%) | 1287/7460 (17·3%) | 0·74 (0·56–0·99) | |
| Angiotensin-receptor blockers | 132/746 (17·7%) | 897/7460 (12·0%) | 0·99 (0·74–1·33) | |
| Non-use | 373/746 (50·0%) | 4585/7460 (61·5%) | 0·57 (0·43–0·75) | |
Data are n (%), mean (SD), and odds ratio with 95% CI in parentheses. Most severe cases are those who died and those admitted to an intensive care unit. Less severe cases are all other inpatients. The different pharmacological classes examined are mutually exclusive categories, so that patients who used combinations are counted only once, applying the following criteria: ACE inhibitors include current users of any ACE inhibitors alone or combined with any other antihypertensive drug (in fixed-dose combinations or in different medicinal products); angiotensin-receptor blockers include current users of any angiotensin-receptor blocker, alone or combined with any other antihypertensive drug that is not an ACE inhibitor (in fixed-dose combinations or in different medicinal products); and other antihypertensive drugs include calcium-channel blockers, diuretics, β-blockers, and α-blockers, alone or combined (excluding RAAS inhibitors; either in fixed-dose combinations or in different medicinal products). ACE=angiotensin-converting enzyme. RAAS=renin–angiotensin–aldosterone system.
Adjusted for the matching variables plus history of diabetes, dyslipidaemia, ischaemic heart disease, heart failure, atrial fibrillation, thromboembolic disease, cerebrovascular accident, chronic obstructive pulmonary disease, asthma, cancer, and chronic renal failure.
Including ACE inhibitors, angiotensin-receptor blockers, aldosterone antagonists, and renin inhibitors.
In our first sensitivity analysis, the adjusted OR of current use of RAAS inhibitors compared with current use of other antihypertensive drugs corrected for secular trends was 0·90 (95% CI 0·74–1·11), and 0·76 (0·61–0·95) for ACE inhibitors and 1·09 (0·87–1·36) for angiotensin-receptor blockers. In our second sensitivity analysis, up to March 16, the adjusted OR associated with current use of RAAS inhibitors compared with current use of other antihypertensive drugs was 0·98 (95% CI 0·77–1·25) and for March 17 onwards was 0·88 (0·62–1·24; figure 2). In our third sensitivity analysis, the exclusion of aldosterone antagonists and renin inhibitors from the RAAS inhibitor group hardly had any effect (overall adjusted OR 0·92, 95% CI 0·76–1·12; among the most severe cases, 1·06, 0·78–1·44; and among the less severe cases, 0·84, 0·65–1·09; appendix pp 11–12). And in our final sensitivity analysis, the inclusion of hypertension in the multivariable model had little effect on the adjusted ORs for RAAS inhibitors (1·00, 0·82–1·23), ACE inhibitors (0·85, 0·68–1·06), or angiotensin-receptor blockers (1·17, 0·93–1·47).
Discussion
Here we show that the current use of RAAS inhibitors is not associated with an increased risk of COVID-19 requiring admission to hospital (including fatal cases and those admitted to an ICU) compared with other antihypertensive drugs. No substantial difference was observed between ACE inhibitors and angiotensin-receptor blockers, nor among short-term and long-term users. Sex, age, and background cardiovascular risk did not significantly affect the results, although use of RAAS inhibitors was associated with a reduced risk of COVID-19 requiring admission to hospital in patients with diabetes.
Our results do not support the hypothesis that previous intake of RAAS inhibitors facilitates or increases the severity of COVID-19. This hypothesis emerged when two facts were linked: the finding that RAAS inhibitors upregulate the expression of ACE2,3, 4, 5, 19 and the observation that hypertension, diabetes, and ischaemic heart disease were highly prevalent among patients with severe COVID-19,9, 10, 11, 12 conditions for which RAAS inhibitors are widely used. However, these facts are only two pieces of a more complex puzzle. Evidence to support the idea that ACE2 might have a dual role in COVID-19 is increasing.20 On the one hand, ACE2 overexpression might increase the susceptibility of cells to SARS-CoV-2, but, on the other hand, its downregulation associated with older age, male sex, and cardiovascular comorbidities, and further heightened by SARS-CoV-2 binding and internalisation21, 22 could increase the unopposed action of angiotensin II and have a key role in the subsequent organ injury.23 ACE2 counteracts the deleterious effect of RAAS axis. In patients with COVID-19, Liu and colleagues24 reported that serum concentrations of angiotensin II were significantly higher in infected individuals than non-infected individuals and was linearly associated with viral load and lung damage. ACE2 inactivates angiotensin II and increases the generation of angiotensin 1–7, a peptide that, acting on the Mas receptor, exerts a vasodilatory effect and anti-inflammatory and antioxidative actions.20 In patients with acute respiratory distress syndrome (ARDS), Reddy and colleagues25 observed a higher ratio of angiotensin 1–7:angiotensin I among survivors than non-survivors, which adds evidence to the idea that the counter-regulation exerted by the axis of ACE2–angiotensin 1–7–Mas receptor benefits patients with ARDS. In line with this theory, recombinant soluble ACE2 has been shown in animal models of ARDS to protect subjects from lung injury,26, 27, 28 and clinical trials are underway in patients with COVID-19 (NCT04287686). Finally, to our knowledge, the case series of patients with COVID-19 published to date has not been adjusted for important potential confounding factors, such as sex, age, and cardiovascular comorbidities.29 In our study, we used controls who were matched to cases for sex and age, and we also adjusted for a number of comorbidities.
To our knowledge, two epidemiological studies have been published to date that aimed to explore the association between RAAS inhibitors and COVID-19 comparing severe cases with less severe cases among inpatients.30, 31 Neither of these studies found an increased risk of severe outcomes associated with these drugs, and in one study30 a substantially reduced risk of death and transfer to a critical care unit within 7 days of admission to hospital was observed among users of ACE inhibitors (OR 0·29, 95% CI 0·10–0·75). Notably, in both studies, the authors considered not only exposure to ACE inhibitors before (7 days) or at admission to hospital, but also during hospital stay. In our study we used population controls, not just a case series, and only considered drugs used before admission.
Several cardiovascular comorbidities are clearly linked to severe COVID-19, and RAAS inhibitors are often used to treat such cardiovascular conditions; thus substantial confounding by indication can be expected.32 In our view, this bias explains the positive association we found between current use of any antihypertensive drug and risk of COVID-19 requiring admission to hospital compared with non-use of such drugs, which did not disappear after full adjustment for comorbidities. This problem can be mitigated if current users of RAAS inhibitors are compared with current users of other drugs that share, at least partially, their indications, but not their safety issues.32 For instance, some researchers have recommended calcium-channel blockers as a suitable alternative treatment to RAAS inhibitors, because calcium-channel blockers have not been reported to increase the expression of ACE2.8 Following this reasoning, other antihypertensive drugs in general, and calcium-channel blockers in particular, can be considered a more valid comparator group than non-use of such drugs. We followed this approach and reassuringly we found no increased risk when using either of these drug groups as the reference group. Nevertheless, RAAS inhibitors do not share all indications with other antihypertensive drugs; hence, the comorbidity pattern associated with use of these drugs might not overlap and some residual confounding by indication could remain. To explore the extent of this possibility, we examined the comorbidity pattern associated with various types of antihypertensive drugs among controls and found much fewer differences among the types of drug than when they were compared with non-users, which reinforces the validity of our approach (appendix p 13).
The risk reduction associated with RAAS inhibitors among individuals with diabetes who had been admitted to hospital for COVID-19 (not explained by chance) deserves a comment. Diabetes has been reported as a risk factor for severe COVID-19.33 The biological underpinning for this clinical observation is not yet known, but experimental models of diabetes in mice have shown that ACE activity is high in the lungs.34 If this activity also occurs in humans, patients with diabetes who have COVID-19 might present a great imbalance in the ACE:ACE2 ratio (ACE activity would be high due to diabetes and ACE2 would be low due to downregulation induced by SARS-CoV-2) that could explain the increased severity of COVID-19 among patients with diabetes and also the protective effect of RAAS inhibitors suggested by our data.
The lack of an increased risk of COVID-19 requiring admission to hospital associated with RAAS inhibitors (compared with other antihypertensive drugs) was found in both patients with most severe and less severe disease, suggesting that the outpatient use of these drugs neither facilitates nor aggravates the infection.
Our study has several limitations. First, we used different data sources to extract information from cases (hospital records and primary health-care records through HORUS, consulted case by case by local researchers) and controls (BIFAP database, which automatically extracted data from primary health-care records); however, both HORUS and BIFAP access the same primary health-care data, which was the main source for determining comorbidity and drug exposure in our study, making major information bias unlikely. Second, cases were recorded as of March, 2020, whereas controls were sourced from records in March, 2018, which might have affected the results due to secular trends in use of antihypertensive drugs; however, when we corrected for secular trends we saw little change in the estimated adjusted OR. Third, we did not collect data on smoking and other lifestyle habits (eg, exercise, diet, alcohol intake), which might have affected the association between RAAS inhibitors and COVID-19 requiring admission to hospital; however, these habits are correlated with several comorbidities that were included in the regression model. Fourth, we obtained information on drug prescriptions, but adherence to treatment cannot be guaranteed. Hence, when we have used the term drug use, we should say drug prescribed. The effect of non-adherence in our results is difficult to measure but would most probably cause a non-differential misclassification of exposure that, if relevant, would distort the estimates towards the null value. Fifth, we did not collect information on clinical and analytical covariates during patients' stay in hospital, nor did we collect information on in-hospital treatment (eg, if RAAS inhibitors were continued or withheld in hospital), which might have been interesting for the assessment of the association of antihypertensive drugs with severity of and fatality due to COVID-19. Sixth, we did not analyse any dose effects. Finally, as in any other observational study, residual confounding due to unmeasured or unknown confounders cannot be ruled out.
In summary, the results of the present study suggest that the outpatient use of RAAS inhibitors does not increase the risk of COVID-19 requiring admission to hospital, including its most severe forms. This finding should be confirmed using other data sources, study designs, and populations. Meanwhile, the data available, along with the important role of ACE inhibitors and angiotensin-receptor blockers in the management of several cardiovascular diseases, do not support their discontinuation as a preventive measure against COVID-19.
Data sharing
After publication, study data will be made available on reasonable request to the corresponding author. A proposal with a detailed description of study objectives and statistical analysis plan will be needed for assessment of requests. Additional materials might also be required during the process of assessment. De-identified participant data will be provided after approval from the principal researchers of the participating hospitals.
Acknowledgments
Acknowledgments
This study was funded by a research grant from the Institute of Health Carlos III (COV20/00027). We thank the health professionals, patients, and administrative staff from the hospitals that participated in this study, and the primary care practitioners and staff of BIFAP. We also thank Arturo Álvarez, for his diligence and skill in extracting the information from population controls, and Dolores Montero and Miguel A Maciá. We also thank María José Bleda, Luis A García Rodríguez, and Alberto García-Lledó who provided helpful suggestions and comments during preparation of the protocol, statistical analysis plan, and manuscript. This Article is dedicated to all researchers' relatives and friends who have died due to COVID-19; they gave us moral strength to complete this research. The results, discussion, and conclusions in this Article are those of the authors and do not necessarily represent the position of their Institutions or the Spanish Agency for Medicines and Medical Devices.
Contributors
FJdA, DR-P, and SR-M contributed to conceptualisation and study design. FJdA, SR-M, MG, and AR-M contributed to the methods. VL, SR-M, GM-A, MA, AG-L, LL, OL, GAC-S, MAG, MP, EG-R, LP, IdP, FA-S, LR-M, MG, and DR-P contributed to data collection. SR-M, FJdA, AR-M, MG, and AT contributed to data analysis. All authors contributed to data interpretation. FJdA, SR-M, and AR-M wrote the first draft of the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content and gave final approval. VL contributed to revision of English language. FJdA applied for funding. All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
MED-ACE2-COVID19 study group
Spain: F J de Abajo, S Rodríguez-Martín, V Lerma, A Rodríguez-Miguel, D Rodríguez-Puyol, D Barreira-Hernández (Hospital Universitario Príncipe de Asturias, Madrid); G Mejía-Abril, F Abad-Santos, P Zubiaur, E Santos-Molina, E Pintos-Sánchez, M Navares-Gómez (Hospital Universitario de La Princesa, Madrid); A García-Luque, M Puerro, R M Aparicio, V García-Rosado, C Gutiérrez-Ortega (Hospital Central de la Defensa Gómez Ulla, Madrid); L Laredo, E González-Rojano, C Pérez, A Ascaso, C Elvira (Hospital Clínico San Carlos, Madrid); M Aguilar, M A Gálvez, I de Pablo (Hospital Universitario Ramón y Cajal, Madrid); O Laosa, L Pedraza, L Rodríguez-Mañas (Hospital Universitario de Getafe, Madrid); G A Centeno-Soto, B Ruiz-Antorán (Hospital Universitario Puerta de Hierro Majadahonda, Madrid); M Gil (Agencia Española de Medicamentos y Productos Sanitarios, Madrid); and A Tobías (Instituto de Diagnóstico Ambiental y Estudios del Agua, CSIC Barcelona).
Declaration of interests
We declare no competing interests.
Contributor Information
Francisco J de Abajo, Email: francisco.abajo@uah.es.
MED-ACE2-COVID19 study group:
D Barreira-Hernandez, P Zubiaur, E Santos-Molina, E Pintos-Sánchez, M Navares-Gómez, R M Aparicio, V García-Rosado, C Gutiérrez-Ortega, C Pérez, A Ascaso, and C Elvira
Supplementary Material
References
- 1.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127–e00128. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li XC, Zhang J, Zhuo JL. The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res. 2017;125(PtA):21–38. doi: 10.1016/j.phrs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrario CM, Jessup J, Chappell MC. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 4.Ocaranza MP, Godoy I, Jalil JE. Enalapril attenuates downregulation of angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rats. Hypertension. 2006;48:572–578. doi: 10.1161/01.HYP.0000237862.94083.45. [DOI] [PubMed] [Google Scholar]
- 5.Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296:F398–F405. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- 6.O'Mara G. Could ACE inhibitors and particularly ARBs increase susceptibility to COVID-19 infection? BMJ. 2020;368:m406. [Google Scholar]
- 7.Sommerstein R. Preventing a covid-19 pandemic. BMJ. 2020;368:m810. doi: 10.1136/bmj.m810. [DOI] [PubMed] [Google Scholar]
- 8.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Yu Y, Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. published online Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W, Ni Z, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang JJ, Dong X, Cao YY. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. published online Feb 19. [DOI] [PubMed] [Google Scholar]
- 12.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurwitz D. Angiotensin receptor blockers as tentative SARS-COV-2 therapeutics. Drug Dev Res. 2020 doi: 10.1002/ddr.21656. published online March 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agencia Española de Medicamentos y Productos Sanitarios; March 16, 2020. Medicamentos antihipertensivos que actúan sobre el sistema renina-angiotensina e infeccción por COVID-19.https://www.aemps.gob.es/informa/notasinformativas/medicamentosusohumano-3/seguridad-1/2020-seguridad-1/medicamentos-antihipertensivos-que-actuan-sobre-el-sistema-renina-angiotensina-e-infeccion-por-covid-19/ [Google Scholar]
- 16.Rodríguez-Martín S, Martín-Merino E, Lerma V. Active surveillance of severe cutaneous adverse reactions: a case-population approach using a registry and a health care database. Pharmacoepidemiol Drug Saf. 2018;27:1042–1050. doi: 10.1002/pds.4622. [DOI] [PubMed] [Google Scholar]
- 17.Rothman KJ, Greenland S, Lash TL. Case-control studies. In: Rothman KJ, Greenland S, Lash TL, editors. Modern epidemiology. 3rd edn. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia, PA: 2008. pp. 111–127. [Google Scholar]
- 18.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keidar S, Gamliel-Lazarovich A, Kaplan M. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res. 2005;97:946–953. doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- 20.Guo J, Huang Z, Lin L, Jv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuba K, Imai Y, Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie X, Chen J, Wang X, Zhang F, Liu Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78:2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25785. published online March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Yang Y, Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy R, Asante I, Liu S. Circulating angiotensin peptides levels in acute respiratory distress syndrome correlate with clinical outcomes: a pilot study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai Y, Kuba K, Rao S. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou Z, Yan Y, Shu Y. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;5 doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang P, Gu H, Zhao Z. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep. 2014;4 doi: 10.1038/srep07027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sommerstein R, Kochen MM, Messerli FH, Gräni C. Coronavirus disease 2019 (COVID-19): do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bean D, Kraljevic Z, Searle T. Treatment with ACE-inhibitors is associated with less severe disease with SARS-Covid-19 infection in a multi-site UK acute hospital trust. medRxiv. 2020 doi: 10.1101/2020.04.07.20056788. published online April 11. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Wang Z, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiology. 2020 doi: 10.1001/jamacardio.2020.1624. published online April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Psaty BM, Koepsell TD, Lin D. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47:749–754. doi: 10.1111/j.1532-5415.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 33.Guo W, Li M, Dong Y. Diabetes as a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3319. published online March 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roca-Ho H, Riera M, Palau V, Pascual J, Soler MJ. Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int J Mol Sci. 2017;18:E563. doi: 10.3390/ijms18030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
After publication, study data will be made available on reasonable request to the corresponding author. A proposal with a detailed description of study objectives and statistical analysis plan will be needed for assessment of requests. Additional materials might also be required during the process of assessment. De-identified participant data will be provided after approval from the principal researchers of the participating hospitals.