Abstract
The outbreak of coronavirus disease 2019 (COVID-19) has rapidly evolved into a global pandemic. Most patients with COVID-19 have mild symptoms, but about 5% develop severe symptoms, which can include acute respiratory distress syndrome, septic shock, and multiple organ failure. Kidney involvement is frequent, with clinical presentation ranging from mild proteinuria to progressive acute kidney injury (AKI) necessitating renal replacement therapy (RRT). An understanding of the pathophysiology and mechanisms of kidney damage and AKI in the setting of critical illness and COVID-19 is emerging, although further research is needed to identify patients at risk of AKI and to guide management strategies. As no specific treatment options exist for AKI secondary to COVID-19, intensive care is largely supportive. Current approaches to prevention and management of AKI, and identification of potential indications for use of RRT and sequential extracorporeal therapies, are based mainly on clinical experience, and AKI strategies are adapted empirically to patients with COVID-19. International collaborative and cross-disciplinary research is needed to obtain adequate evidence to support current clinical approaches and to develop new approaches to management.
Introduction
As the global outbreak of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is rapidly evolving and expanding, its full spectrum of effects is becoming evident—from mild, self-limiting respiratory tract illness to severe acute respiratory distress syndrome (ARDS), multiple organ failure, and death.1 Kidney involvement is frequent in COVID-19; >40% of cases have abnormal proteinuria at hospital admission.2 Acute kidney injury (AKI) is common among critically ill patients with COVID-19, affecting approximately 20–40% of patients admitted to intensive care according to experience in Europe and the USA,3, 4 and it is considered a marker of disease severity and a negative prognostic factor for survival.1, 2 Furthermore, the overall burden of AKI in COVID-19 might be underestimated, as creatinine values at admission might not reflect true preadmission baseline kidney function, and previous serum creatinine values might not be readily available.5 Around 20% of patients admitted to an intensive care unit (ICU) with COVID-19 require renal replacement therapy (RRT) at a median of 15 days from illness onset.1 Early recognition of kidney involvement in COVID-19 and use of preventive and therapeutic measures to limit subsequent AKI or progression to more severe stages are crucial to reduce morbidity and mortality.
In this Viewpoint, we discuss current understanding of the mechanisms of kidney involvement in COVID-19 and provide a series of recommendations for clinical practice on the basis of current clinical experience, covering prevention and management of AKI and potential indications for use of RRT and sequential extracorporeal therapies, including the practicalities of their delivery. We also suggest an agenda for future research to obtain adequate evidence to support clinical approaches.
Pathophysiology of AKI in COVID-19
The cause of kidney involvement in COVID-19 is likely to be multifactorial, with cardiovascular comorbidity and predisposing factors (eg, sepsis, hypovolaemia, and nephrotoxins) as important contributors.6 Cardiorenal syndrome, particularly right ventricular failure secondary to COVID-19 pneumonia, might lead to kidney congestion and subsequent AKI. Similarly, left ventricular dysfunction might lead to low cardiac output, arterial underfilling, and kidney hypoperfusion.
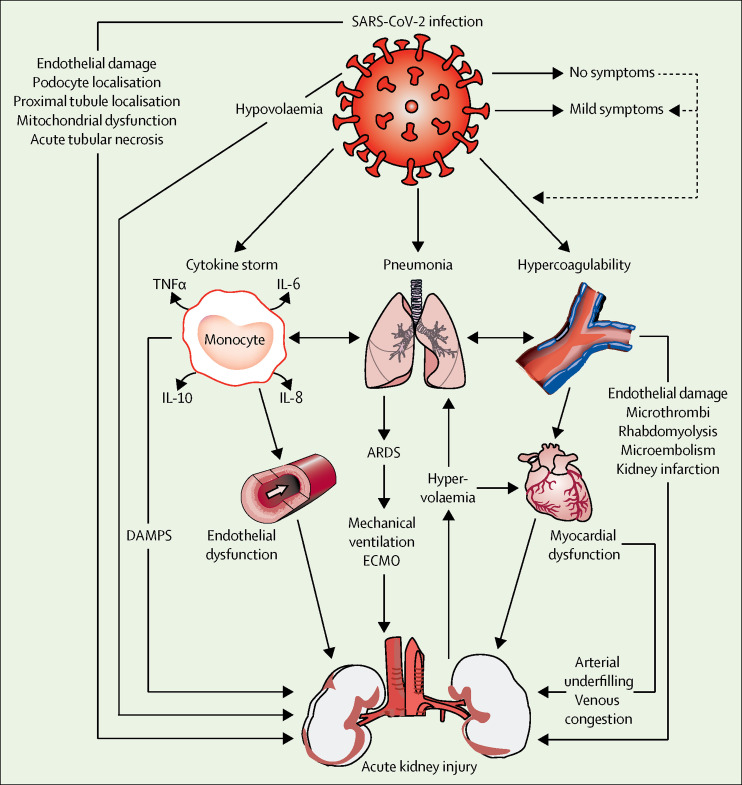
Autopsy data7 indicate that the endothelium is affected in the lung and in the kidney, where it is probably responsible for proteinuria (figure 1 ). Furthermore, virus particles were reported to be present in renal endothelial cells, indicating viraemia as a possible cause of endothelial damage in the kidney and a probable contributor to AKI.7 Additionally, SARS-CoV-2 can directly infect the renal tubular epithelium and podocytes through an angiotensin-converting enzyme 2 (ACE2)-dependent pathway and cause mitochondrial dysfunction, acute tubular necrosis, the formation of protein reabsorption vacuoles, collapsing glomerulopathy, and protein leakage in Bowman's capsule.8, 9
Figure 1.
Acute kidney injury in COVID-19
Multiple dependent pathways in the setting of COVID-19 increase the risk of acute kidney injury. The possible haemodynamic, proinflammatory, and proapoptotic consequences of lung inflammation, cytokine release syndrome, and hypercoagulability on renal function, and potential organ support options, are shown. ARDS=acute respiratory distress syndrome. COVID-19=coronavirus disease 2019. DAMPS=damage-associated molecular patterns. ECMO=extracorporeal membrane oxygenation. IL=interleukin. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. TNF=tumour necrosis factor.
Key messages.
-
•
Kidney involvement is common in patients with coronavirus disease 2019 (COVID-19); patients can present with proteinuria at hospital admission, while acute kidney injury (AKI) frequently develops at later stages in critically ill patients and is recognised as a marker of multiple organ dysfunction and disease severity
-
•
Volume depletion at admission might be a common trigger for AKI, as patients with COVID-19 typically present with fever and pre-hospital fluid resuscitation is rarely performed; lung-protective ventilation lowers the risk of new or worsening AKI by limiting ventilator-induced haemodynamic effects and the cytokine burden on the kidney
-
•
In the absence of specific treatment options for COVID-19, care is largely supportive; we recommend the implementation of the Kidney Disease: Improving Global Outcomes (KDIGO) supportive care guideline in critically ill patients at risk of AKI, the use of continuous renal replacement therapy (RRT) with specific adjustments for patients with COVID-19, and the possible use of cytokine removal strategies, ideally in the context of a clinical trial, in patients with early signs of hyperinflammation and cytokine release syndrome
-
•
We recommend that fluid balance and extracorporeal ultrafiltration be adjusted on the basis of volume responsiveness and tolerance assessment, taking into account ventilator settings and recruitment manoeuvres
-
•
For continuous RRT, we recommend cannulation of the right jugular vein and regional citrate as the preferred anticoagulation modality for RRT, as severe COVID-19 can induce a hypercoagulable state; connection of RRT to the extracorporeal membrane oxygenation circuit should be avoided to minimise clot formation in the latter
-
•
International collaborative and cross-disciplinary research is needed to rigorously test the risks and benefits of interventions for AKI in patients with COVID-19
Another potential mechanism of AKI involves SARS-CoV-2-related immune response dysregulation, as indicated by observed lymphopenia and cytokine release syndrome (cytokine storm).1, 10 Other contributors to AKI might include rhabdomyolysis, macrophage activation syndrome, and the development of microemboli and microthrombi in the context of hypercoagulability and endotheliitis.7, 11
Management of AKI in COVID-19
In the absence of specific treatment options, the care strategy for patients with COVID-19 in the ICU remains largely supportive. Given the high incidence of kidney involvement in COVID-19, it is important to consider all available treatment options to support kidney function.
Clinical management
Implementation of the Kidney Disease: Improving Global Outcomes (KDIGO) supportive care guideline (eg, avoidance of nephrotoxins, regular monitoring of serum creatinine and urine output, consideration of haemodynamic monitoring) in critically ill patients with kidney involvement is likely to reduce the occurrence and severity of AKI in COVID-19, but requires validation.12 Mitigation of volutrauma and barotrauma through the application of lung-protective ventilation lowers the risk of new or worsening AKI by limiting ventilation-induced haemodynamic effects and the cytokine burden on the kidney.13 Novel tubular damage biomarkers should be incorporated in future randomised clinical trials to investigate their value in AKI prediction and management.6
Another important option is to adjust fluid balance according to volume responsiveness and tolerance assessment. This strategy aims to restore normal volume status to avoid volume overload and reduce the risk of pulmonary oedema, right ventricular overload, congestion, and subsequent AKI. Volume depletion at admission might be common in patients with COVID-19, as they typically present with fever and pre-hospital fluid resuscitation is rarely performed. In these cases, hypovolaemia should be corrected to prevent AKI. Relatively high positive end-expiratory pressure strategies and recruitment manoeuvres have been used in ARDS secondary to COVID-19;14 these strategies could further compromise cardiac output in the setting of relative hypovolaemia.
RRT and extracorporeal support
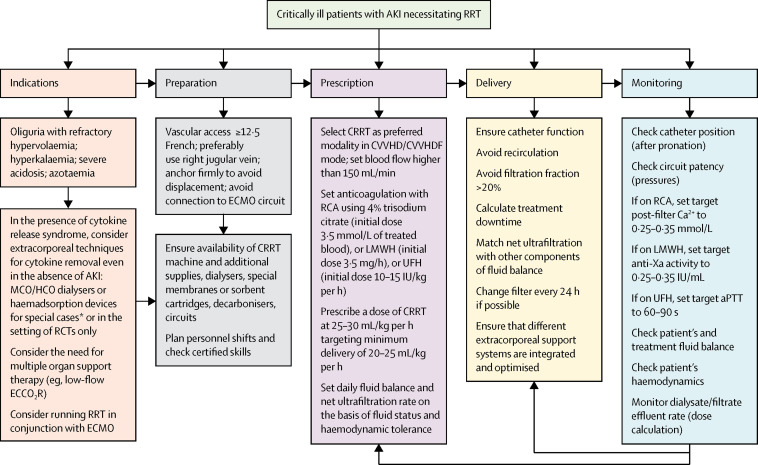
If conservative management fails, RRT should be considered in patients with volume overload, especially those with refractory hypoxaemia. In patients with COVID-19 and AKI, early initiation of RRT and sequential extracorporeal organ support (ECOS)15 seem to provide adequate organ support and prevent progression of disease severity (figure 2 ). This approach, however, should be tested in future clinical trials. Continuous RRT (CRRT) is the preferred modality in haemodynamically unstable patients with COVID-19.
Figure 2.
Management of acute kidney injury necessitating renal replacement therapy in patients with COVID-19
Management recommendations focus on AKI in COVID-19 rather than usual practice for AKI, and are based largely on our clinical experience. All therapeutic options need to be tested in rigorous studies and ideally randomised trials in the context of COVID-19. In the absence of specific therapies, all options should be considered according to each patient's needs. The extracorporeal therapies included in the figure for consideration can be complementary to pharmacological support. The activity targets for anticoagulant therapy are indicative only and should be tailored to each patient's characteristics and clinical condition. The general principle is that maximal anticoagulation should be achieved in the extracorporeal circuit with minimal systemic effects; if systemic anticoagulation is indicated, an integrated prescription should be considered. *These treatments might be indicated in special cases in which immunodysregulation is evident, inflammatory parameters or cytokines are elevated, and other supportive therapies are failing or insufficient; haemadsorption can be achieved with haemoperfusion or CRRT with membranes with high adsorption properties. AKI=acute kidney injury. Anti-Xa=anti-factor Xa. aPTT=activated partial thromboplastin time. Ca2+=ionised calcium. COVID-19=coronavirus disease 2019. CRRT=continuous renal replacement therapy. CVVHD=continuous veno-venous haemodialysis. CVVHDF=continuous veno-venous haemodiafiltration. ECCO2R=extracorporeal carbon dioxide removal. ECMO=extracorporeal membrane oxygenation. HCO=high cutoff. LMWH=low-molecular-weight heparin. MCO=medium cutoff. RCA=regional citrate anticoagulation. RCTs=randomised controlled trials. RRT=renal replacement therapy. UFH=unfractionated heparin.
During the turning of patients into the prone position, the dialysis catheter needs to be secured and monitored to avoid dislocation or kinking. The right jugular vein is the preferred insertion site, as the catheter exit site and anchoring remain visible after prone positioning. In an Italian study involving 1591 ICU patients with COVID-19, 27% required prone positioning.16 Extracorporeal membrane oxygenation (ECMO) was performed in 1% of these patients. When RRT is carried out in conjunction with ECMO, RRT should be performed through venous access independent of the ECMO circuit to minimise clot formation in the latter.15 Connection of RRT to ECMO, however, might be the only option in some patients because of the paucity of sites available for direct cannulation. In this case, the RRT outflow should be connected to the pre-oxygenator limb of the ECMO circuit, as the oxygenator can serve as a protective barrier and minimise risk of systemic gas embolism in the lungs; frequent surveillance of the system connections, CRRT circuit, and oxygenator pressures can minimise clot formation. Greater systemic anticoagulation could also alleviate this problem, but would increase the risk of haemorrhage.
A hypercoagulable state is often observed in severely ill patients with COVID-19.1 As such, anticoagulation protocols for the extracorporeal circuit must be tailored to the needs of individual patients. In CRRT, regional citrate anticoagulation is more efficacious than other anticoagulation methods in terms of prolonging the extracorporeal circuit lifespan and reducing the risk of bleeding. From our experience, lowering the post-filter ionised calcium concentration to approximately 0·25–0·35 mmol/L (usual concentration 0·30–0·45 mmol/L) in patients with COVID-19 is a viable option to prolong filter patency; this target is usually achieved with a starting dose of citrate 3·5 mmol/L of treated blood. If using low-molecular-weight heparin (LMWH), we recommend an initial dose of 3·5 mg/h and systemic anti-factor Xa activity of 0·25–0·35 IU/mL. Lastly, if using unfractionated heparin (UFH), we recommend an initial dose of 10–15 IU/kg per h and an activated partial thromboplastin time of 60–90 s. The targets for ionised calcium, anti-factor Xa activity, and activated partial thromboplastin time are indicators only, based on recent clinical experience with patients with COVID-19 rather than usual practice with AKI, and should be tailored to the needs of individual patients.
Evidence suggests that pre-filter anticoagulation with LMWH provides a higher circuit lifespan than with UFH.17 Additionally, continuous veno-venous haemodialysis modality (CVVHD) provides a longer filter lifespan with less internal haemoconcentration in the filter. CRRT should be delivered with a minimum dose of 20–25 mL/kg per h.12 This will usually require a higher dose prescription because of variable therapy downtime.
In the absence of established treatment options for COVID-19, the pathophysiological rationale might support the use of high cutoff or medium cutoff membranes in CVVHD to increase cytokine removal.10 Also, in the early phases of cytokine storm, the application of haemoperfusion with sorbent cartridges might prevent cytokine-induced kidney damage. These treatments might be indicated in special cases in which immunodysregulation is evident, inflammatory parameters or cytokines are elevated, and other supportive therapies are failing or insufficient. Although encouraging results have been reported, evidence for these treatments is limited at present, so they should otherwise be applied only in the context of a clinical trial to determine their safety and efficacy.10 Extracorporeal treatments do not compromise the experimental antibody-based therapies used in COVID-19, such as tocilizumab, intravenous immunoglobulins, and convalescent plasma administration. Neither haemodialysis filters nor haemadsorption cartridges remove antibodies, as their size (eg, 150 kDa for IgG) far exceeds the upper size of molecules that can be removed with RRT or haemadsorption (around 60 kDa).18
Lung-protective ventilation with tidal volume at 6 mL/kg predicted body weight might lead to hypercapnia, respiratory acidosis, increased need for vasopressors, and AKI. In these patients, extracorporeal carbon dioxide removal (ECCO2R) might help to avoid progression of clinical severity.19 If respiratory exchanges further deteriorate, ECMO might be indicated. However, limitations to the provision of ECMO during the COVID-19 outbreak include a lack of recruitable ECMO consoles or disposable equipment, suitably trained staff, and rooms with the requisite infrastructure. In centres with available dialysis, low-flow ECCO2R (<500 mL/min) using CRRT platforms could be carried out by dialysis specialists at any time of the day and might be an option for patients with hypercapnia as the main indication. This approach, however, should be tested in future clinical trials. When CRRT is coupled with ECCO2R, clinicians should maintain a blood flow of >400 mL/min to ensure adequate carbon dioxide removal.13
In some patients, bacterial infection co-occurs with SARS-CoV-2 infection and a sepsis-like syndrome can develop. In such patients, the use of sequential extracorporeal therapies10, 11, 12, 13, 14, 15, 20, 21 (eg, endotoxin removal, cytokine removal and immunomodulation, ECOS for various organs) should be considered according to current evidence or pathophysiological rationale, as clinical progression can be rapid and changes in pathophysiology over the disease course might indicate different treatment approaches during the ICU stay.
Conclusions and future perspectives
In the absence of specific anti-SARS-CoV-2 treatments, supportive care and use of sequential extracorporeal therapies for critically ill patients with evidence of kidney involvement provide a life support bridge to recovery and enhance the probability of a favourable outcome. The decision to use sequential extracorporeal therapies should take into account the technical effort and dedicated skills of the multidisciplinary staff that are needed for safe and effective therapy delivery. Careful patient selection for sequential extracorporeal therapies is necessary because age and comorbidities seem to influence outcomes in critically ill patients with COVID-19.22
Further research is needed to improve understanding of AKI secondary to COVID-19, to obtain adequate evidence to support the clinical approaches discussed here, and to develop new approaches to monitoring and management (panel ). Fostering an international collaborative and cross-disciplinary research culture will be crucial to rigorously test therapies in clinical trials and to rapidly identify patients with COVID-19 who are at risk of AKI and who stand to benefit from established and emerging therapeutic approaches.
Panel. Research priorities to improve management of acute kidney injury in COVID-19.
-
•
Research is needed to establish the value of novel tubular damage biomarkers in prediction and management of acute kidney injury (AKI) in patients with coronavirus disease 2019 (COVID-19); studies should also focus on their potential to guide optimal fluid management, ventilation strategies, and recruitment manoeuvres in COVID-19
-
•
Clinical trials should investigate the early initiation of renal replacement therapy (RRT) and sequential extracorporeal therapies as means to provide adequate organ support and to prevent progression of COVID-19 severity
-
•
Trials are needed to clarify the role of haemadsorption and other extracorporeal systems for cytokine removal in cytokine storm scenarios in patients with COVID-19
-
•
Studies should aim to clarify the safety and feasibility of low-flow extracorporeal carbon dioxide removal using RRT platforms in hypercapnic patients with COVID-19, acute respiratory distress syndrome, and AKI
-
•
Studies should establish the proportion of patients with a superimposed bacterial sepsis and the role of sequential extracorporeal therapy (endotoxin removal, cytokine removal and immunomodulation, extracorporeal organ support) in their management
Search strategy and selection criteria
We searched PubMed for original research papers, reviews, editorials, and commentaries published between Jan 1, 2020, and May 2, 2020, using the following terms: (“renal” OR “kidney”) AND/OR (“coronavirus disease 2019” OR “severe acute respiratory syndrome coronavirus 2”). Only English language manuscripts were selected. Relevant guidelines for management of suspected COVID-19 published by WHO were also reviewed.
Contributors
All authors contributed to the writing of the manuscript. CR conceived the outline, designed the figures, and reviewed several drafts. TR did the literature search and wrote part of the manuscript. FH-S wrote part of the manuscript and revised it in its final form in conjunction with all authors.
Declaration of interests
CR has received support for acting as an advisory board member for ASAHI, Baxter, GE, Jafron, and Medtronic, and speaker's fees from Astute, bioMérieux, B. Braun, Cytosorbents, ESTOR, FMC, and Toray, all unrelated to this manuscript. TR and FH-S declare no competing interests.
References
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acute kidney injury in COVID-19 patients. ESICMtv Webinar. Posted April 17, 2020. https://www.esicm.org/blog/?p=2789
- 4.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 doi: 10.1001/jama.2020.6775. published online April 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pei G, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020 doi: 10.1681/ASN.2020030276. published online April 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394:1949–1964. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- 7.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020 doi: 10.1016/j.kint.2020.04.003. published online April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19) Kidney Int Rep. 2020 doi: 10.1016/j.ekir.2020.04.002. published online April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020 doi: 10.1038/s41581-020-0284-7. published online April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 13.Joannidis M, Forni LG, Klein SJ, et al. Lung-kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med. 2020;46:654–672. doi: 10.1007/s00134-019-05869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med. 2020;8:433–434. doi: 10.1016/S2213-2600(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Husain-Syed F, Ricci Z, Brodie D, et al. Extracorporeal organ support (ECOS) in critical illness and acute kidney injury: from native to artificial organ crosstalk. Intensive Care Med. 2018;44:1447–1459. doi: 10.1007/s00134-018-5329-z. [DOI] [PubMed] [Google Scholar]
- 16.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. published online April 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joannidis M, Kountchev J, Rauchenzauner M, et al. Enoxaparin vs unfractionated heparin for anticoagulation during continuous veno-venous hemofiltration: a randomized controlled crossover study. Intensive Care Med. 2007;33:1571–1579. doi: 10.1007/s00134-007-0719-7. [DOI] [PubMed] [Google Scholar]
- 18.Honore PM, Hoste E, Molnar Z, et al. Cytokine removal in human septic shock: where are we and where are we going? Ann Intensive Care. 2019;9:56. doi: 10.1186/s13613-019-0530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanelli V, Cantaluppi V, Alessandri F, et al. Extracorporeal CO2 removal may improve renal function of patients with acute respiratory distress syndrome and acute kidney injury: an open-label, interventional clinical trial. Am J Respir Crit Care Med. 2018;198:687–690. doi: 10.1164/rccm.201712-2575LE. [DOI] [PubMed] [Google Scholar]
- 20.Klein DJ, Foster D, Walker PM, Bagshaw SM, Mekonnen H, Antonelli M. Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: a post hoc analysis of the EUPHRATES trial. Intensive Care Med. 2018;44:2205–2212. doi: 10.1007/s00134-018-5463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Rosa S, Samoni S, Ronco C. Sequential extracorporeal therapy collaborative device and timely support for endotoxic, septic, and cardiac shock: a case report. Blood Purif. 2019 doi: 10.1159/000505146. published online Dec 19. [DOI] [PubMed] [Google Scholar]
- 22.Ronco C, Navalesi P, Vincent JL. Coronavirus epidemic: preparing for extracorporeal organ support in intensive care. Lancet Respir Med. 2020;8:240–241. doi: 10.1016/S2213-2600(20)30060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]