Abstract
With first cases noted towards the end of 2019 in China, COVID-19 infection was rapidly become a devastating pandemic. Even if most patients present with a mild to moderate form of the disease, the estimated prevalence of COVID-19-related severe acute respiratory failure (ARF) is 15–20% and 2–12% needed intubation and mechanical ventilation. In addition to mechanical ventilation some other techniques of respiratory support could be used in some forms of COVID-19 related ARF. This position paper of the Respiratory Support and Chronic Care Group of the French Society of Respiratory Diseases is intended to help respiratory clinicians involved in care of COVID-19 pandemic in the rational use of non-invasive techniques such as oxygen therapy, CPAP, non-invasive ventilation and high flow oxygen therapy in managing patients outside intensive care unit (ICU). The aims are: (1) to focus both on the place of each technique and in describing practical tips (types of devices and circuit assemblies) aimed to limit the risk of caregivers when using those techniques at high risk spreading of viral particles; (2) to propose a step-by-step strategy to manage ARF outside ICU.
Keywords: Non invasive ventilation, Oxygen, High flow oxygen therapy, COVID-19, Acute respiratory failure
The SARS-CoV-2 has been identified as the agent of the pandemic known as Coronavirus disease 2019 (COVID-19). The first cases were noted towards the end of 2019 in Wuhan, China.
COVID-19 infection is spontaneously resolutive in most cases. The clinical presentation can vary from mild respiratory symptoms to severe pneumonia progressing to fulminant acute respiratory failure (ARF) [1]. COVID-2019 pneumonia is characterized by bilateral infiltrates, which can progress to diffuse alveolar condensations. In less severe patients, computed tomography (CT) shows bilateral ground glass sub pleural opacities [2].
The estimated prevalence of COVID-19-related acute respiratory failure (ARF) is 15–20% [1], [3], [4]. In different published series, 41% had received O2, 4–13% of patients non-invasive ventilation (NIV) and 2–12% needed intubation and mechanical ventilation [1], [3], [5], [6], [7].
In addition to MV some other techniques of respiratory support such as non-invasive ventilation (NIV), continuous positive airway pressure (CPAP) or high flow oxygen therapy (HFOT) could be used in some forms of ARF. Those techniques are currently applied in ICU but also in a pulmonary department. But the fact that this pandemics could go beyond the capacity of the health system, may lead that those techniques might be applied in less specialized services, These position paper is intended to help respiratory clinicians involved in care of COVID-19 pandemic in managing patients outside ICU. They cannot be regarded as recommendations and reflect only authors experience during COVID-19 pandemics, their expertise in the field of respiratory support and their critical review of the literature.
1. Available tools
1.1. Oxygen therapy
Oxygen should be used in case of severe COVID-19 pneumonia probably as soon as SpO2 < 92% with a SpO2 target between 92 and 96%.
There are no randomized controlled studies concerning O2 in patients with COVID-19 but it is possible to extrapolate data from studies in severe ARF. A meta-analysis of 25 randomized controlled studies has shown that a strategy with no upper limit on O2 flow (“liberal” approach) increases the risk of in-hospital death compared to a conservative (“targeted” approach) [8]. This contrast with the results of a recent randomized controlled trial (RCT) comparing a “liberal” O2 therapy strategy (target SpO2 > 96%) to a conservative one (target SpO2 > 88% < 92%). This study showed no difference in 28-days survival but an over mortality at 90 days in the conservative group [9]. Thus, based on those data, a target of > 92 < 96% seems reasonable [10]. There are no controlled studies concerning the better interface to deliver O2. Based on clinical recommendations, a nasal cannula should be used for mild hypoxia and, if an oxygen flow >6 L min−1 is needed, a switch for a simple face mask set to deliver 5 to 10 L min−1 should be proposed. A non-rebreather mask between 10 and 15 L min−1 should be used in patients remaining hypoxemic despite using a simple mask. These latter interfaces can ensure FiO2 of 35–55% and 80–95% respectively FiO2 depending on flow and breathing pattern [11]. If available, it could be appropriate to use masks with filtered exhalation port (Filtamask™ or similar). Whatever the interface chosen, patient's comfort and decrease caregiver's risk will be prioritized when choosing the interface.
1.2. Continuous Positive Airway Pressure (CPAP)
CPAP with added O 2 could be used to improve oxygenation, if conventional O 2 failed and there is no urgent indication for intubation or as a surrogate while waiting for intubation.
This solution is simpler, less expensive and possibly less harmful than NIV.
CPAP must be applied under a strict supervision of a trained physician. ICU team must be prevented and readily available.
The interfaces used to deliver CPAP must be those available or those more familiar for the team. A helmet could be an alternative to limit exposition to droplets dispersion, and remains an option for expert teams Circuits and masks must be adapted to reduce caregiver's risks (Fig. 1 ).
Fig. 1.
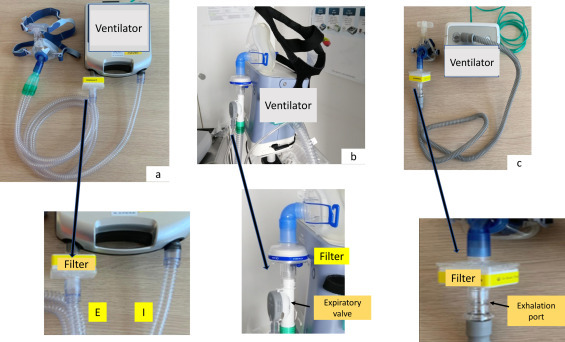
Different ventilator circuit assemblies to reduce viral spreading. (a) Ventilator using double circuit with an integrated expiratory valve. The filter* must be interposed between the expiratory arm and the ventilator. (b) Ventilator using single-limb circuit with an active expiratory valve: the filter* must be interposed between the mask and the expiratory valve. (c) Ventilator using single limb circuit with intentional leaks. Not-vented mask must be preferred if available. In this case a deported exhalation port (Whisper Swivel or similar) must be added. The filter* must be interposed between the mask and the deported exhalation port. If a non-vented mask is not available an alternative is to seal the intentional leak of the vented mask. In the last case caution must be taken not to block anti-asphyxia valve. I: inspiratory arm; E: expiratory arm. * There are not published studies comparing the efficacy of different filters.
In hypoxemic ARF, applying an extrinsic positive end expiratory pressure (PEEP) increases alveolar recruitment and improves oxygenation. Furthermore, there is high level of evidence about the efficacy of adding PEEP during invasive ventilation in ARDS patients. Nevertheless, we lack information about the effectiveness of applying non-invasive PEEP. Experiences from Italian and Chinese teams are shared but not published.
As CPAP is easier to use than NIV, more available and need less expertise, this technique could be offered as a first line therapy in selected patients, as a gap to invasive MV, in particular when resources are limited or if there is no immediate access to invasive ventilation. A detail of CPAP-delivering devices, their limits and advantages is showed in Fig. 2.
Fig. 2.
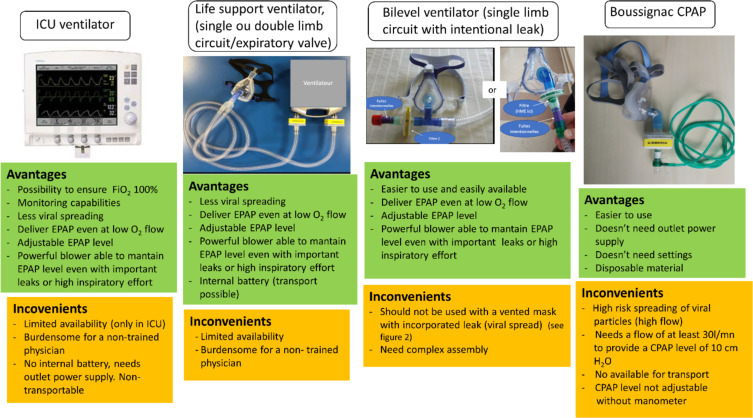
Different devices providing non-invasive CPAP therapy.
1.3. Non Invasive Ventilation (NIV)
NIV with added O 2 could be used to improve oxygenation and/for providing ventilatory support, if conventional O 2 failed and there is no urgent indication for intubation or as a surrogate while waiting for intubation.
In those cases NIV must be applied under a strict supervision of a trained physician. ICU team must be prevented and readily available.
NIV should be considered in patients who will not be admitted to ICU for intubation.
The interfaces used to deliver NIV must be those available or those more familiar for the team A helmet could be an alternative to limit exposition to droplets dispersion, and remains an option for expert teams.
Circuits and masks must be adapted to reduce caregiver's risks (Fig. 2).
There are no published data concerning NIV use outside ICU in patients with COVID-19. NIV indications in ICU patients with COVID-19-related ARF range from 11 to 34% [12]. Meta-analysis of RCT conducted in severe hypoxemic ARF showed that NIV could reduce the rate of intubation and mortality. However, they included patients with immunosuppression, acute heart failure (AHF) and postoperative ARF [7], [13], [14], [15]. Moreover, in ARF etiologies other than AHF, NIV has shown a high level of failure with higher mortality (28%) than those treated with O2 (23%) or HFOT (13%) [16],
In a cohort of patients with Middle East respiratory syndrome, NIV was associated with better survival and a shorter length of stay (LOS) compared patients who were intubated without previous NIV. However, NIV showed a high failure rate (92.4%) requiring intubation [17]. Finally, associating NIV and prone position reduced intubation in patients with moderate ARDS related to viral pneumonia [18].
On the other side, NIV could be harmful in those patients as it could worsen lung damage due to high pressures and high VT and could delay intubation [19], [20]. Moreover, NIV as CPAP is at high risk spreading of viral particles. In this field, WHO guidelines for the management of ARF in COVID-19 advocate the use of CPAP or NIV, provided that appropriate personal protective equipment is worn [21].
Hence NIV could be considered as a first line therapy outside the ICU in particular when resources are limited or if there is no immediate access to invasive ventilation. In those cases NIV must be applied under a strict supervision of a trained physician to detect early and rapid worsening, a frequent condition in COVID-19 pneumonia. ICU team must be prevented and readily available. When a patient is treated with NIV, monitoring should be intensified. If hypoxemia worsens the patient must be transferred to ICU if eligible for intubation. This decision will integrate not only the clinical severity, but also underlying pathologies and the “living wills” or end-of-life decisions (if available) of the patient and his family. This requires previous discussion on a case-by-case basis.
NIV should also be considered in patients no eligible to be admitted to ICU or in those with do not intubate (DNI) decision. In those cases, NIV must be continued only if it is well tolerated and a benefit is obtained. Otherwise, comfort measures only, including pharmacological measures, may be applied to relieve dyspnea [22].
Some patients with underlying respiratory disease (COPD, obesity hypoventilation, restrictive disease) complicated with COVID-19 pneumonia could benefit from de novo NIV.
In patients on long term NIV, treatment must be adapted during hospitalization, possibly by temporarily lowering the pressures if excessive leaks are present. Circuits and masks must be adapted (Fig. 2).
When available a helmet interface could be applied. A randomized controlled study conducted in ARDS has shown that NIV when delivered by a helmet decrease intubation rate and mortality [23]. Moreover helmet limits viral spreading [24].
It is imperative not to insist with NIV or O2 in patients that worsen. This can lead to a delay in intubation, which can be fatal. Increased vigilance is necessary since muscle exhaustion is expressed late in those patients. In all cases, the intubation must be anticipated, carried out according to rigorous procedures and under conditions limiting the risk of caregivers.
As NIV is at high risk spreading of viral particles circuits and masks must be adapted to reduce caregiver's risks [14], [25], [26] (Fig. 2). Moreover, caution may be taken to ensure a good interface fitting for CPAP or NIV systems, to minimize widespread dispersion of exhaled air and reduce risk of airborne transmission.
1.4. High Flow Oxygen Therapy (HFOT)
HFOT could be used to improve oxygenation, if conventional O 2 failed and there is no urgent indication for intubation or as a surrogate while waiting for intubation.
As HFOT is at high risk spreading of viral particles, protective measures must be applied to reduce caregiver's exposition.
HFOT delivers a high-flow gas mixture (up to 70 L/min) with variable FiO2 (up to 100%) administered by a nasal cannula. Compared to conventional oxygen HFOT can ensure a constant and known FiO2. Other advantages are dead space reduction and generation of a low PEEP level allowing alveolar recruitment [27].
There are no published data concerning HFOT in patients with COVID-19 pneumonia.
In a RCT conducted in severe hypoxemic ARF, HFOT reduced 90-days mortality but not intubation rate compared to conventional oxygen [17]. Otherwise, a meta-analysis of 9 RCT shows a decrease in intubation rate but without improving survival or length of stay [28], [29], [30] an important issue in the field of COVID-19 pandemic considering an expected scarcity of ventilators.
As with NIV, adding prone position to HFOT may help to avoid intubation in patients with moderate ARDS related to viral pneumonia [18].
The same precautions cited above for NIV should be taken if HFOT is used as a first line therapy in patients qualifying for intubation.
Even if published studies did not demonstrate an increased risk for caregivers when comparing HFOT to conventional O2 [31], [32] to limit the risk of contamination, the following measures are suggested when using HFOT:
-
•
ensure maximum sealing of the interface;
-
•
limit the flow rate to the minimum necessary. Prefer higher FiO2 instead of higher flow (i.e.: start at 30 L/min and increase FiO2 to ensure target SaO2);
-
•
patient must wear a surgical mask during care.
A detail of HFOT-delivering devices, their limits and advantages is showed in Fig. 3 .
Fig. 3.
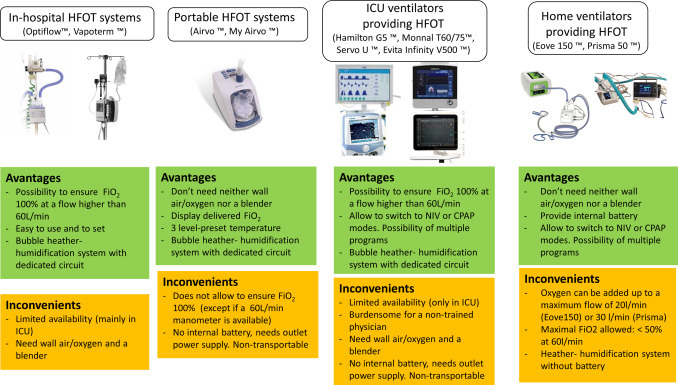
Different devices providing HFOT therapy.
1.5. Nebulized treatments
As nebulization is at high risk spreading of viral particles, nebulized treatments should be limited as much as possible.
Spray and powders should be preferred to provide inhaled therapy, preferably by using a personal inhalation chamber. If not possible, minimal distance of 1 m must be respected and the room must aerate during nebulization.
1.6. Tracheal aspirations
In the case of tracheostomized ventilator-dependent patients a “closed suction system” must be used.
2. Patient management
The proposed flow chart strategy to manage ARF outside ICU in COVID-19 patients is showed in Fig. 4 .
Fig. 4.
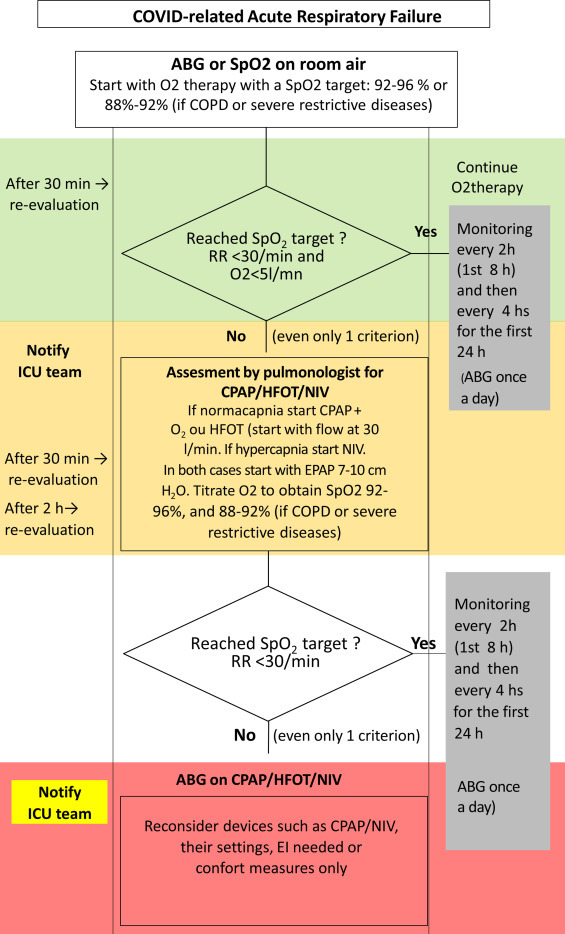
Flow chart strategy for managing ARF in COVID-19 patients (modified from [33] ABG: Arterial Blood Gases; COPD: chronic obstructive pulmonary disease; RR: respiratory rate; HFOT: high flow oxygen therapy; CPAP: continuous positive airway pressure; NIV: non-invasive ventilation; EPAP: expiratory positive airway pressure; EI: endotracheal intubation).
It is crucial to ensure an appropriate triage of patients at the admission. As those patients are at risk of early and rapid worsening, it seems reasonable that those with a rapidly progressive form (i.e presenting a SpO2 < 94% and a RR > 30 while receiving > 6 L O2) must be promptly assessed by the ICU staff to decide proper allocation.
2.1. Step by step approach
-
•
Check and treat comorbidities.
-
•Begin conventional oxygen therapy by using nasal prongs to obtain the target RR and SpO2:
-
∘if predominant oral breathing or intolerance to high flow, an oxygen mask could be proposed,
-
∘masks with filtered exhalation port could be used if available,
-
∘there is no limit in terms of O2 flow rate but if > 6 L/min or a mask with reservoir bag is needed, ICU team must be prevented and promptly available,
-
∘venturi masks should be precluded;
-
∘
-
•
then, if oxygen therapy failed, non-invasive respiratory assistance should be proposed with CPAP and/or HFOT as a first choice. NIV should be used as a second line therapy in case of CPAP or HFOT failure, and mainly if hypercapnia develops;
-
•
HFOT could be an alternative in the absence of CPAP/NIV or as a therapeutic ceiling option (HFOT allows higher FiO2 but there is hypothetically a greater risk of drops diffusion and low PEEP levels are generated) [33].
-
•Close monitoring is needed during at least the first 48–72 hours, including SpO2, RR and clinical assessment (ventilatory mechanics/use of accessory muscles). Three issues highlighted by different teams managing those patients requires particular attention:
-
∘an initially stable patient may suddenly become unstable (with refractory hypoxemia and high fever),
-
∘a delayed re-aggravation was noted in a significant percentage of patients (stability then rapid worsening after 48 h, up to 7 days),
-
∘it was described in some of these patients an impaired perception of dyspnea despite severe hypoxemia. A possible explanation is a neuroinvasive potential of COVID-19 that could spread in brainstem affecting respiratory center and/or mechano-/chemoreceptors [34].
-
∘
-
•
In cases with DNI decision, it is recommended to dispose an end-of-life sedation protocol to be applied if patient's condition worsens (http://www.sfap.org/actualite/outils-et-ressources-soins-palliatifs-et-covid-19).
In summary: non- invasive respiratory support could be useful in treating COVID-19-related ARF. A rational use of different techniques (oxygen therapy, CPAP, NIV or HFOT) by a trained pulmonologist could allow to prevent clinical aggravation and reduce the risk of ICU admission. Then, those techniques should be considered as a first line therapy outside the ICU in particular when resources are limited or if there is no immediate access to invasive ventilation. A step-by step approach, under a strict supervision of a trained physician, must be applied to detect early and rapid aggravation. In patients eligible for intubation. ICU team must be prevented and readily available to an immediate transfer to ICU as soon as the patient's condition impairs.
Disclosure of interest
The authors have not supplied their declaration of competing interest.
Acknowledgements
Stefano Nava (Italy), Javiers Sayas (Spain), Michelle Chatwin (UK), Manel Lujan (Spain), Annalisa Carlucci (Italy) for sharing information.
Special acknowledgement to the colleagues of the Italian Thoracic Society (AIPO–ITS) and Italian Respiratory Society (SIP/IRS) for their original idea of the flow chart proposed in this paper.
A French version of this document is available online in open format (http://www.splf.fr/wp-content/uploads/2020/04/RespiPreREA-SPLF-GAVO2avril2020.pdf).
Contributor Information
GAVO2 collaborators:
M. Mercy, L. Grassion, S. Pontier, M. Patout, R. Luque, C. Delafosse, C. Raherison-Semjen, B. Maître, L. Duthoit, A. Mendoza, L. Jacquin, J.C. Borel, P. Cervantes, J.-P. Janssens, J.-F. Chabot, C. Morelot-Panzini, and D. Jaffuel
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y., Dong C., Hu Y., Li C., Ren Q., Zhang X., et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020:200843. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu D.K., Kim L.H.-Y., Young P.J., Zamiri N., Almenawer S.A., Jaeschke R., et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693–1705. doi: 10.1016/S0140-6736(18)30479-3. [DOI] [PubMed] [Google Scholar]
- 9.Barrot L., Asfar P., Mauny F., Winiszewski H., Montini F., Badie J., et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382:999–1008. doi: 10.1056/NEJMoa1916431. [DOI] [PubMed] [Google Scholar]
- 10.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E., et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004363. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Driscoll B., Howard L.S., Earis J., Mak V. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72:i1–i90. doi: 10.1136/thoraxjnl-2016-209729. [DOI] [PubMed] [Google Scholar]
- 12.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020 doi: 10.1001/jama.2020.4031. [Epub 2020/03/14] [DOI] [PubMed] [Google Scholar]
- 13.Wang T., Zhang L., Luo K., He J., Ma Y., Li Z., et al. Noninvasive versus invasive mechanical ventilation for immunocompromised patients with acute respiratory failure: a systematic review and meta-analysis. BMC Pulmon Med. 2016;16:129. doi: 10.1186/s12890-016-0289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X.-P., Zhang X.-C., Hu S.-L., Xu J.-Y., Xie J.-F., Liu S.-Q., et al. Noninvasive ventilation in acute hypoxemic nonhypercapnic respiratory failure: a systematic review and meta-analysis. Crit Care Med. 2017;45:e727–e733. doi: 10.1097/CCM.0000000000002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zayed Y., Banifadel M., Barbarawi M., Kheiri B., Chahine A., Rashdan L., et al. Noninvasive oxygenation strategies in immunocompromised patients with acute hypoxemic respiratory failure: a pairwise and network meta-analysis of randomized controlled trials. J Intensive Care Med. 2019 doi: 10.1177/0885066619844713. [885066619844713] [DOI] [PubMed] [Google Scholar]
- 16.Frat J.-P., Thille A.W., Mercat A., Girault C., Ragot S., Perbet S., et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 17.Alraddadi B.M., Qushmaq I., Al-Hameed F.M., Mandourah Y., Almekhlafi G.A., Jose J., et al. Noninvasive ventilation in critically ill patients with the Middle East respiratory syndrome. Influenza Other Respir Viruses. 2019;13:382–390. doi: 10.1111/irv.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding L., Wang L., Ma W., He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24:28. doi: 10.1186/s13054-020-2738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brochard L., Lefebvre J.-C., Cordioli R.L., Akoumianaki E., Richard J-C.M. Noninvasive ventilation for patients with hypoxemic acute respiratory failure. Semin Respir Crit Care Med. 2014;35:492–500. doi: 10.1055/s-0034-1383863. [DOI] [PubMed] [Google Scholar]
- 20.Slutsky A.S., Ranieri V.M. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 21.2020. WHO Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance.https://www.apps.who.int/iris/handle/10665/330893 [Google Scholar]
- 22.Tripodoro V.A., Rabec C.A., De Vito E.L. Withdrawing noninvasive ventilation at end-of-life care: is there a right time? Curr Opin Support Palliat Care. 2019;13:344–350. doi: 10.1097/SPC.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 23.Patel B.K., Wolfe K.S., Pohlman A.S., Hall J.B., Kress J.P. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315:2435–2441. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui D.S., Chow B.K., Lo T., Ng S.S., Ko F.W., Gin T., et al. Exhaled air dispersion during noninvasive ventilation via helmets and a total facemask. Chest. 2015;147:1336–1343. doi: 10.1378/chest.14-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowler R.A., Guest C.B., Lapinsky S.E., Sibbald W.J., Louie M., Tang P., et al. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med. 2004;169:1192–1202. doi: 10.1164/rccm.200305-715OC. [DOI] [PubMed] [Google Scholar]
- 26.Hui D.S.C., Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin N Am. 2019;33:869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drake M.G. High-flow nasal cannula oxygen in adults: an evidence-based assessment. Ann Am Thorac Soc. 2018;15:145–155. doi: 10.1513/AnnalsATS.201707-548FR. [DOI] [PubMed] [Google Scholar]
- 28.Ni Y.-N., Luo J., Yu H., Liu D., Liang B.-M., Liang Z.-A. The effect of high-flow nasal cannula in reducing the mortality and the rate of endotracheal intubation when used before mechanical ventilation compared with conventional oxygen therapy and noninvasive positive pressure ventilation. A systematic review and meta-analysis. Am J Emerg Med. 2018;36:226–233. doi: 10.1016/j.ajem.2017.07.083. [DOI] [PubMed] [Google Scholar]
- 29.Ou X., Hua Y., Liu J., Gong C., Zhao W. Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ. 2017;189:E260–E267. doi: 10.1503/cmaj.160570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rochwerg B., Granton D., Wang D.X., Helviz Y., Einav S., Frat J.P., et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45:563–572. doi: 10.1007/s00134-019-05590-5. [DOI] [PubMed] [Google Scholar]
- 31.Leung C.C.H., Joynt G.M., Gomersall C.D., Wong W.T., Lee A., Ling L., et al. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect. 2019;101:84–87. doi: 10.1016/j.jhin.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Raboud J., Shigayeva A., McGeer A., Bontovics E., Chapman M., Gravel D., et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PloS One. 2010;5:e10717. doi: 10.1371/journal.pone.0010717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.http://www.aiponet.it/news/speciale-covid-19/2426-managing-the-respiratory-care-of-patients-with-covid-19-english-version.html [updated 2020-04-01 21:35:40].
- 34.Li Y.-C., Bai W.-Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]


