Abstract
The outbreak of the novel coronavirus that began in late December 2019 was announced as a pandemic by the World Health Organization as the number of cases is increasing exponentially throughout the globe. We presented a patient with confirmed SARS-CoV-2 pneumonia developing symmetric polyneuropathy. To our knowledge, extrapulmonary clinical presentations of 2019 novel coronavirus disease (COVID-19) have rarely been reported. This case highlights the possible association between SARS-CoV-2 infection and nervous system involvement.
Keywords: COVID-19, SARS-CoV-2, Pneumonia, Nervous system, Polyneuropathy
Introduction
The knowledge around the ongoing pandemic of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is still evolving with the cases increasing globally. This novel pathogen is known to cause respiratory infection. A spectrum of presentations have been reported, ranging from asymptomatic infection to severe lower respiratory tract illness presenting with fever, cough, and dyspnea that may progress to acute respiratory distress syndrome (ARDS) and death [1]. However, this novel coronavirus appears to cause extrapulmonary manifestations, and here we reported the first case of symmetric polyneuropathy in a patient diagnosed with this novel infection.
Case presentation
A 68-year-old woman presented to the emergency department of a referral hospital in Tehran, Iran, complaining of persistent non-productive cough, fever, and myalgia of three-day duration. She denied any history of rhinorrhea, sore throat, dyspnea, abdominal pain, vomiting, or diarrhea. She had a medical history of poorly controlled diabetes mellitus, end-stage renal disease for which she received hemodialysis three times a week, and rheumatoid arthritis. She had undergone coronary artery bypass grafting 13 years ago. Her medications included rosuvastatin 10 mg daily, prednisolone 5 mg daily, hydroxychloroquine 200 mg daily, aspirin 80 mg daily, and losartan 50 mg twice a day. She was also on a single daily injection of 18 units of lantus (insulin glargine) for diabetes mellitus.
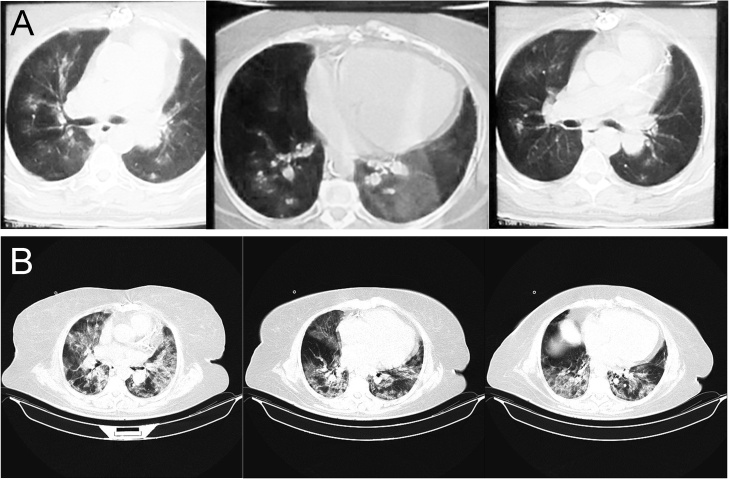
Vital signs were as follows: body temperature 39.9 °C, blood pressure 140/60 mmHg, pulse 105 beats per minute, respiratory rate 24 breaths per minute, and oxygen saturation 90% on room air. Lung auscultation revealed mild bilateral rales. The remainder of the examination was unremarkable, and no weakness or sensory disturbance was noted at that time. A chest CT scan was performed, which revealed mild bilateral patchy high-density shadows in both lungs (Fig. 1A). Considering the clinical presentation of viral pneumonia in a patient at the time of the 2019 novel coronavirus outbreak, she was admitted to the hospital with a high suspicion for SARS-CoV-2 infection. An upper respiratory tract swab tested positive for SARS-CoV-2 on real-time RT-PCR assays within 48 h. Laboratory investigation on admission reflected white blood cells 8.5 × 103/uL with lymphopenia, hemoglobin 11.5 g/dL, platelets 175 × 103/uL, potassium 5.3 mEq/L, sodium 133 mEq/L, creatinine 12 mg/dl, ESR 15 mm/hr, and CRP 121 mg/L. Her blood sugar level was 400 mg/dL at the time of admission. Hepatic transaminases and bilirubin were within the normal range.
Fig. 1.
(A) chest CT scan performed on day 1, which shows mild bilateral patchy high-density shadows in both lungs. (B) chest CT scan taken on day 6 demonstrates severe bilateral ground-glass opacities consistent with acute respiratory distress syndrome (ARDS).
After admission, the patient received supportive care and management of symptoms, including oxygen supplementation, and compassionate therapy consisting of lopinavir/ritonavir 400/100 twice a day, and oseltamivir 30 mg daily as per the Iranian interim guidelines for the management of COVID-19 at that time [2]. Other than her routine medications, novaRapid (insulin aspart) 4 units three times a day was added to her regimen.
On day 3 of hospitalization, while the patient’s vital signs remained stable and O2 saturation was 94% breathing room air, she started to develop generalized hypotonia in lower extremities. There was bilateral weakness on motor examinations, and deep tendon reflexes were absent. Her cranial nerves were normal, and other neurological examinations were unremarkable. Lumbar puncture was performed by a neurologist, which failed to yield any CSF in two repeated attempts. MRI of the whole spine, nerve conduction velocity (NCV), and electromyographic (EMG) studies were requested. After discussion, the neurologist decided to proceed with the administration of methylprednisolone 250 mg IV for possible virus-related immune reaction provided that her blood sugar and electrolytes were controlled.
On day 6, she started having difficulty breathing, and her O2 saturation dropped to 78% as her respiratory status deteriorated gradually. A chest CT scan taken at the time demonstrated severe bilateral ground-glass opacities consistent with acute respiratory distress syndrome (ARDS, Fig. 1B). She was intubated and mechanically ventilated in response to respiratory distress. However, she developed cardiac arrest and, unfortunately, died on the same day after multiple resuscitations attempts.
Discussion
Several human coronaviruses have been identified, and the recent emergence of the SARS-CoV-2 was announced as being responsible for an ongoing pandemic of COVID-19 [3]. Evidence shows that many respiratory viruses including human coronaviruses exhibit extrapulmonary symptoms, and remarkably, may involve the nervous system and consequently cause a variety of neurological signs and symptoms. Damage to the nervous system may be the result of direct neural invasion or replication by the virus or indirect virus-induced host immune responses [4].
The neurotropic tendency of the common human coronavirus has been confirmed with the presence of the virus in CSF samples of patients with multiple sclerosis (MS) through several methods in different studies [[5], [6], [7], [8]]. In addition, there have been reports of neurologic manifestations as polyneuropathies caused by emerging human coronaviruses of the twenty-first century. A case series presented data of four Severe Acute Respiratory Syndrome (SARS) patients who developed polyneuropathy, myopathy, or both approximately three weeks after the onset of SARS with a probable diagnosis of critical-illness polyneuropathy (CIP) and/or critical-illness myopathy (CIM) [9]. A case of the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) was reported with developing weakness and numbness in lower limbs and inability to walk with a likely diagnosis of critical illness polyneuropathy (CIP) [10]. Another report of four patients with MERS diagnosis exhibited neurological manifestations of encephalitis overlapping with Guillain–Barré syndrome (GBS), weakness resulting from extended ICU stay or GBS, and acute polyneuropathy that resulted from a toxin or infection [11].
Here we presented the case of a patient with pneumonia due to SARS-CoV-2 complicated by symmetric polyneuropathy. In such a situation, the diagnosis could be suspected by a judicious combination of the clinical and paraclinical findings. Unfortunately, the patient died before a detailed neurologic investigation could be performed, and given the lack of such information in our case, we considered a few differential diagnoses. Since no symptoms were present before the development of COVID-19 and toxic neuropathy was excluded after a review of all her medications, critical illness polyneuropathy (CIP) and Guillain–Barré syndrome (GBS) were considered to be the most likely diagnosis. CIP develops as an acute complication of severe illness and presents with limb and respiratory muscle weakness [12]. Due to the progressive symmetric muscle weakness and the absence of deep tendon reflexes, GBS was also a possible diagnosis. Although there was a lack of upper limbs involvement, it would have developed if the patient lived longer [13].
A recent review suggested SARS-CoV-2 neuroinvasive properties to be partially accountable for respiratory failure development [14]. Further investigation needs to be done regarding the polyneuropathies associated with human coronaviruses, and whether the virus directly damages peripheral nerves or factors mediating inflammatory response are responsible for the neural damage. Awareness of this complication will have an impact on the treatment of the SARS-CoV-2 related respiratory failure presenting with neuropathy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Sources of funding
The authors received no specific funding for this work.
Consent
Written informed consent was obtained from the patient’s next of kin (her husband) for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
-
1
Fereshteh Ghiasvand: Conceptualization, Data collection, Writing- Original draft preparation
-
2
Maryam Ghadimi: Conceptualization, Writing – Original draft preparation, Writing- Reviewing and Editing
-
3
Fatemeh Ghadimi: Conceptualization, Writing – Original draft preparation, Writing – Reviewing and Editing
-
4
Samin Safarpour: Conceptualization, Data collection
-
5
Roghieh Hosseinzadeh: Conceptualization, Data collection
-
6
SeyedAhmad SeyedAlinaghi: Conceptualization, Data collection, Writing- Original draft preparation, Writing- Reviewing and Editing, Supervision
Ethics committee approval
Ethics approval was obtained from the ethics committee of Tehran University of Medical Sciences.
CRediT authorship contribution statement
Fereshteh Ghiasvand: Conceptualization, Data curation, Writing - original draft. Maryam Ghadimi: Conceptualization, Writing - original draft, Writing - review & editing. Fatemeh Ghadimi: Conceptualization, Writing - original draft, Writing - review & editing. Samin Safarpour: Conceptualization, Data curation. Roghieh Hosseinzadeh: Conceptualization, Data curation. SeyedAhmad SeyedAlinaghi: Conceptualization, Data curation, Writing - original draft, Writing - review & editing, Supervision.
References
- 1.McIntosh K. 2020. Coronavirus disease 2019 (COVID-19) UptoDate; 2020 [Available from: https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19#H1354847215] [Google Scholar]
- 2.Azadmanesh K. first edition. 2020. National interim guidelines for the management of COVID-19 2020. [Available from: https://irimc.org/Portals/0/NewsAttachment/%20%20%20%20%20%20%20.pdf] [Google Scholar]
- 3.WHO . World Health Organization; 2020. WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020. [Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020] [Google Scholar]
- 4.Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses. 2019;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbour N., Day R., Newcombe J., Talbot P.J. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74(19):8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burks J.S., DeVald B.L., Jankovsky L.D., Gerdes J.C. Two coronaviruses isolated from central nervous system tissue of two multiple sclerosis patients. Science. 1980;209(4459):933–934. doi: 10.1126/science.7403860. [DOI] [PubMed] [Google Scholar]
- 7.Murray R.S., Brown B., Brian D., Cabirac G.F. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann Neurol. 1992;31(5):525–533. doi: 10.1002/ana.410310511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart J.N., Mounir S., Talbot P.J. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 1992;191(1):502–505. doi: 10.1016/0042-6822(92)90220-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai L.-K., Hsieh S.-T., Chao C.-C., Chen Y.-C., Lin Y.-H., Chang S.-C. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004;61(11):1669–1673. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- 10.Algahtani H., Subahi A., Shirah B. Neurological complications of middle east respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep Neurol Med. 2016;2016 doi: 10.1155/2016/3502683. 3502683- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. 2017;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou C., Wu L., Ni F., Ji W., Wu J., Zhang H. Critical illness polyneuropathy and myopathy: a systematic review. Neural Regen Res. 2014;9(1):101–110. doi: 10.4103/1673-5374.125337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vriesendorp F.M. 2020. Guillain-Barré syndrome in adults: clinical features and diagnosis. UptoDate; 2018 [Available from: https://www.uptodate.com/contents/guillain-barre-syndrome-in-adults-clinical-features-and-diagnosis] [Google Scholar]
- 14.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may be at least partially responsible for the respiratory failure of COVID‐19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]