Objectives:
To determine mortality rates among adults with critical illness from coronavirus disease 2019.
Design:
Observational cohort study of patients admitted from March 6, 2020, to April 17, 2020.
Setting:
Six coronavirus disease 2019 designated ICUs at three hospitals within an academic health center network in Atlanta, Georgia, United States.
Patients:
Adults greater than or equal to 18 years old with confirmed severe acute respiratory syndrome-CoV-2 disease who were admitted to an ICU during the study period.
Interventions:
None.
Measurements and Main Results:
Among 217 critically ill patients, mortality for those who required mechanical ventilation was 35.7% (59/165), with 4.8% of patients (8/165) still on the ventilator at the time of this report. Overall mortality to date in this critically ill cohort is 30.9% (67/217) and 60.4% (131/217) patients have survived to hospital discharge. Mortality was significantly associated with older age, lower body mass index, chronic renal disease, higher Sequential Organ Failure Assessment score, lower Pao2/Fio2 ratio, higher d-dimer, higher C-reactive protein, and receipt of mechanical ventilation, vasopressors, renal replacement therapy, or vasodilator therapy.
Conclusions:
Despite multiple reports of mortality rates exceeding 50% among critically ill adults with coronavirus disease 2019, particularly among those requiring mechanical ventilation, our early experience indicates that many patients survive their critical illness.
Keywords: coronavirus, critical care, intubation, mortality, respiration, artificial, respiratory distress syndrome, adult
Coronavirus disease 2019 (COVID-19) has become one of the leading causes of death worldwide. It is estimated that 15–20% of cases require hospitalization and 3–5% require critical care. Although experience with COVID-19 continues to grow, reported mortality rates range from 50 to 97% in those requiring mechanical ventilation (1–6). These are significantly higher than the published mortality rates ranging from 35% to 46% for patients intubated with H1N1 influenza pneumonia and other causes of acute respiratory distress syndrome (ARDS) (7–10).
These high mortality rates have raised concerns as to whether invasive mechanical ventilation should be avoided in the context of COVID-19 (11–14). To help address the growing concern that critical illness, and specifically mechanical ventilation, are associated with a high risk of death, we conducted a retrospective cohort study of critically ill patients with COVID-19 across our academic health system.
MATERIALS AND METHODS
This is an observational cohort study of all patients with COVID-19 admitted to six COVID-designated ICUs at three Emory Healthcare acute-care hospitals in Atlanta, Georgia, from March 6, 2020, to April 17, 2020. COVID-19 status was based upon a positive severe acute respiratory syndrome coronavirus 2 polymerase chain reaction assay, performed either by the Georgia Department of Public Health, a referral laboratory, or the hospital-based clinical laboratory. During the study period, ICU capacity enabled the timely admission of all patients requiring critical care to a COVID-ICU. Further, all patients admitted to a COVID-ICU were cared for by a traditional ICU care team led by a critical care-trained attending physician with standard (i.e., pre-COVID) ICU staffing ratios. There were no critical shortages in medications, ventilators, dialysis machines, or other critical care equipment. Patient data, including sociodemographic information, clinical data, and laboratory data, were obtained from the electronic medical record.
Data were abstracted through May 7, 2020. Data were analyzed using a chi-square or Wilcoxon rank-sum test for categorical and continuous variables, respectively, with a two-sided p value of less than 0.05 considered statistically significant (Stata Version 12.1; StataCorp LLC, College Station, TX). This study was approved by the Emory University Institutional Review Board.
RESULTS
Patient Characteristics
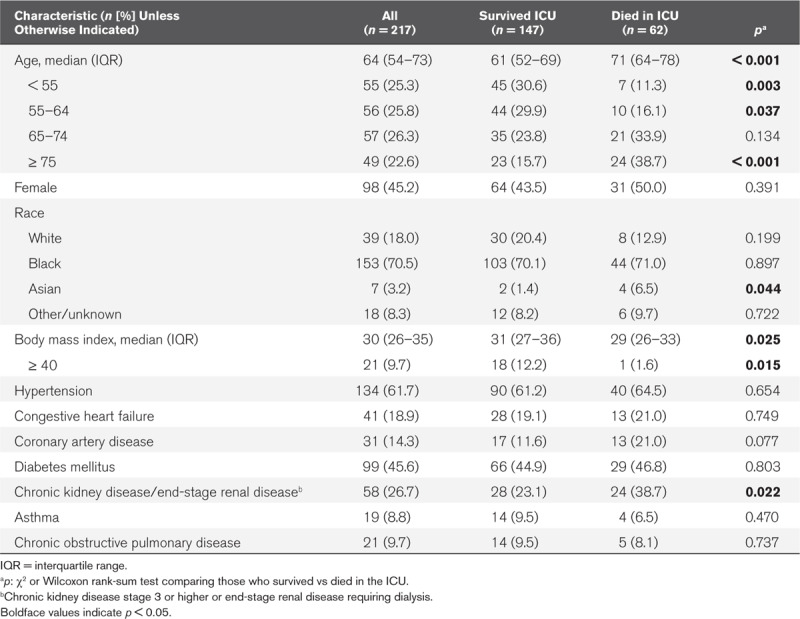
From March 6, 2020, to April 17, 2020, 217 critically ill adults with COVID-19 infection were admitted to the ICU (Fig. 1A). The median patient age was 64 (interquartile range [IQR], 54–73), with 49 patients (22.6%) who were 75 years or older (Table 1). There were 98 females (45.2%) and the majority of patients were black (153 [70.5%]). Hypertension was the most common comorbid condition (134 [61.7%]), followed by diabetes (99 [45.6%]). Twenty-one patients (9.7%) had morbid obesity, with a body mass index of 40 or greater.
Figure 1.
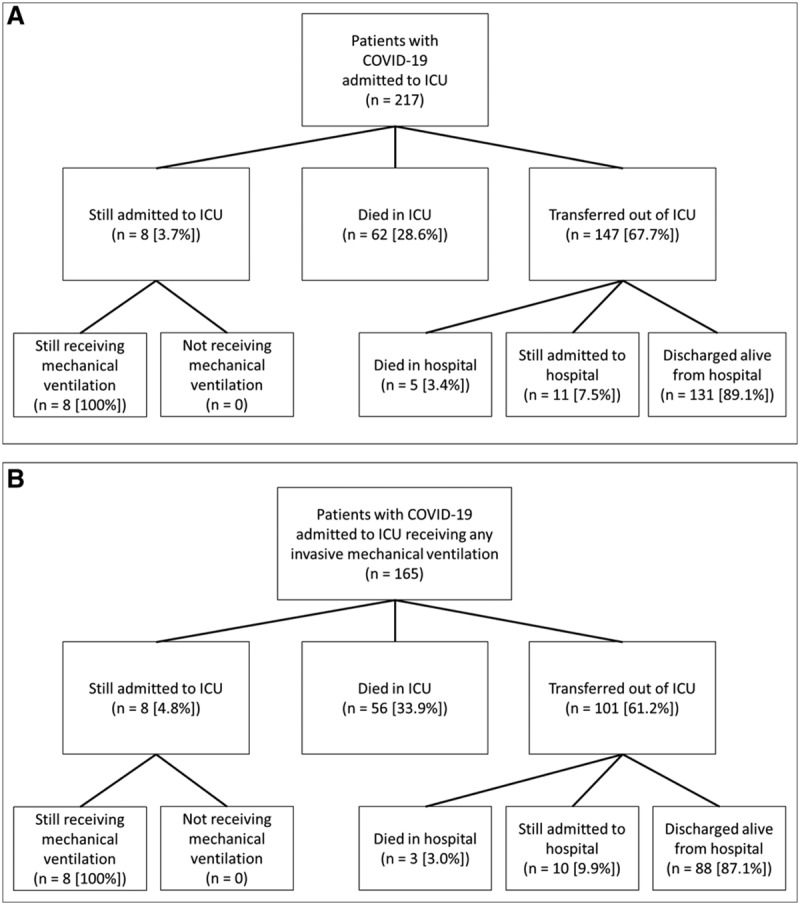
Flow diagram for study patients who were admitted to a coronavirus disease-ICU (COVID-ICU) (A), and received invasive mechanical ventilation (B). COVID-19 = coronavirus disease 2019.
TABLE 1.
Sociodemographic and Baseline Clinical Characteristics of Patients Admitted to a Coronavirus Disease-ICU
ICU Admission and Interventions
Initial ICU clinical findings, critical care interventions, and outcomes are summarized in Table 2. At admission to the ICU, the median Sequential Organ Failure Assessment (SOFA) score was 7 (IQR, 5–11), the median d-dimer was 1,731 ng/mL (IQR, 934–6,948 ng/mL; upper limit of normal 298 ng/mL), and the median C-reactive protein was 190 mg/L (IQR, 126–262 mg/L; upper limit of normal 10 mg/L). The median initial Pao2/Fio2 ratio was 132 (IQR, 100–178).
TABLE 2.
Patient Characteristics on ICU Admission, ICU Clinical Interventions, and Outcomes
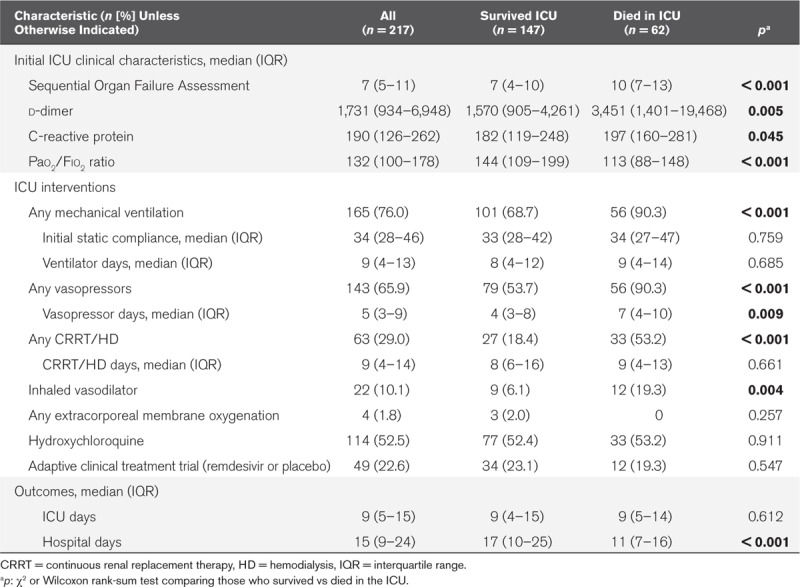
There were 165 patients (76.0%) who received invasive mechanical ventilation. The median static lung compliance on the first day of intubation was 34 mL/cm H2O (IQR, 28–46 mL/cm H2O). A total of 143 patients (65.9%) required vasopressor support for shock and 63 (29.0%) required renal replacement therapy, either in the form of continuous renal replacement therapy or intermittent hemodialysis. Use of inhaled pulmonary vasodilators was relatively uncommon (22 [10.1%]) and four patients (1.8%) received extracorporeal membrane oxygenation. Overall, 114 patients (52.5%) received at least one dose of hydroxychloroquine and 49 patients (22.6%) were enrolled in the National Institutes of Health-funded adaptive clinical treatment trial (ACTT) of remdesivir (ClinicalTrials.gov NCT04280705).
ICU Outcomes
Among the 217 patients in the cohort, 147 (67.7%) have been transferred alive from the ICU, 62 died (28.6%) in the ICU (five additional deaths occurred among patients transferred to the floor), and 8 (3.7%) remain in the ICU, all of whom are still mechanically ventilated (Fig. 1A). Three of the patients transferred from the ICU went directly to a long-term acute care facility while still receiving mechanical ventilation through a tracheostomy. The median ICU length of stay for patients still in the ICU is 32 days (IQR, 28–41 d). Among patients who received invasive mechanical ventilation, ICU mortality is 33.9% (56/165) and hospital mortality is 35.7% (59/165) (Fig. 1B).
The median age of patients who died was significantly older than for those who survived (71 yr [IQR, 64–78 yr] vs 61 yr [IQR, 52–69 yr]; p < 0.001) (Table 1). There was no difference in survival between sexes, but patients who died were less likely to be morbidly obese. Although patients of Asian race did have higher mortality, there were only seven Asian patients in the cohort. Patients with either chronic kidney disease (stage 3 or higher) or end-stage renal disease requiring hemodialysis also had higher mortality.
Patients who died had a higher SOFA score on ICU admission (median, 10; IQR, 7–13) than those who survived (median, 7; IQR, 4–10; p < 0.001) (Table 2). Median d-dimer values were more than two times higher in those who died than those who survived (3,451 [IQR, 1,401–19,468] vs 1,570 [IQR, 905–4,261]; p 0.005). Similarly, the initial C-reactive protein and Pao2/Fio2 ratios were significantly worse in patients who died in the ICU (p 0.045 and < 0.001, respectively). Compared to those who survived, patients who died in the ICU were more likely to have respiratory failure requiring invasive mechanical ventilation (90.3% vs 68.7%; p < 0.001), shock requiring vasopressors (90.3% vs 53.7%; p <0.001), renal failure requiring renal replacement therapy (53.2% vs 18.4%; p <0.001), and receive inhaled vasodilators (19.3% vs 6.1%; p < 0.001).
Overall, mortality among patients who received vasopressors was 39.2% (56/143), while 33 of 63 patients (52.4%) with renal failure requiring renal replacement therapy died. Twelve of the 22 patients (54.5%) who received inhaled vasodilator for refractory hypoxemia died. Among the 62 patients who died in the ICU, including three patients who had an advance directive not to be intubated, the median time from ICU admission to death was 9 days (IQR, 5–14 d).
DISCUSSION
Our early experience with this large cohort of critically ill patients with COVID-19 demonstrates a mortality rate of 30.9% overall, which is substantially lower than the 50–97% reported in the published literature to date (1–6). Additionally, the 35.7% mortality for the approximately three-quarters of patients in our cohort who required mechanical ventilation is also markedly lower than previous reports. These data indicate that a majority of critically ill patients with COVID-19 can have good clinical outcomes and support the ongoing use of mechanical ventilation for patients with acute respiratory failure.
In some of the earliest reports of COVID-19 from Wuhan, mortality rates among those admitted to ICUs ranged from 52% to 62% and increased to 86–97% among those requiring invasive mechanical ventilation (5, 6, 15, 16). In more recent data from the United Kingdom, 67% of those who had received mechanical ventilation died, as compared with 22% of patients intubated with viral pneumonia in the preceding 3 years (3). Early reports of smaller cohorts from Seattle, where some of the first COVID-19 outbreaks occurred in the United States, indicated that 50–67% of patients admitted to the ICU and 71–75% of those receiving invasive mechanical ventilation died (1, 2). A recently published report from New York found 24.5% mortality among those who required mechanical ventilation, but with 72.2% of patients still admitted to the hospital (4).
Taken together, these reports have raised concerns that survival among those receiving mechanical ventilation is exceedingly poor (11–14). In contrast to the majority of prior reports, our data provide evidence that mortality rates in COVID-19 can be comparable to those seen with ARDS and other infectious pneumonias (7–10).
In our cohort, mortality was associated with older age, with 42.5% mortality in those age 65 and above as compared with 11.3% in those under age 55. Mortality was also associated with severity of illness on arrival to the ICU and need for ICU interventions including mechanical ventilation, vasopressor support, renal replacement therapy, and inhaled vasodilators. Approximately half of our cohort received at least one dose of hydroxychloroquine and nearly one-quarter received at least one dose of a study drug as part of the ACTT trial (remdesivir vs placebo), but there was no difference in survival for either of those groups. Finally, although eight patients (3.7%) are still admitted to the ICU at the time of this report, 131 (60.4%) have been discharged from the hospital.
Several local and regional considerations may have influenced the observed outcomes. First, the arrival and peak of the COVID-19 pandemic in Georgia were later than in many of the regions from earlier reports. This delay provided time to establish organizational structures, acquire equipment, prepare personnel, create consensus-driven clinical protocols, and align resources across a large healthcare system. In addition, while patient volumes did merit the redesignation of several specialty ICUs as COVID-ICUs, all critically ill patients with COVID-19 were admitted to preexisting ICUs and cared for by critical care teams with experience managing acute respiratory failure and at standard patient-to-provider ratios. Further, while an analysis of the impact of clinical interventions on survival is beyond the scope of this brief report, our internal guidelines emphasized early intubation and standard lung-protective ventilation strategies. Future studies are needed to better understand the impact of individual patient risk factors, clinical interventions and treatments, and health system factors that can improve mortality in the face of this global pandemic.
CONCLUSIONS
In a cohort of critically ill adults with COVID-19, we report an early mortality rate of 30.9% overall and 35.7% for patients who received mechanical ventilation. Although there may be a several factors underlying these findings, these results suggest that most patients with acute respiratory failure from COVID-19 may recover, even with severe disease requiring intubation and mechanical ventilation.
ACKNOWLEDGMENTS
We would like to extend our most profound thanks and gratitude to our colleagues in the Emory Critical Care Center and Emory Healthcare who have worked so hard to provide excellent clinical care during this global pandemic.
Emory COVID-19 Quality and Clinical Research Collaborative Members (in alphabetical order): Max W. Adelman, Scott Arno, Sara C. Auld, Theresa Barnes, William Bender, James M. Blum, Gaurav Budhrani, Stephanie Busby, Laurence Busse, Mark Caridi-Scheible, David Carpenter, Nikulkumar Chaudhari, Craig M. Coopersmith, Lisa Daniels, Johnathan A. Edwards, Jane Fazio, Babar Fiza, Eliana Gonzalez, Ria Gripaldo, Charles Grodzin, Robert Groff, Alfonso C. Hernandez-Romieu, Max Hockstein, Dan Hunt, Craig S. Jabaley, Jesse T. Jacob, Colleen Kraft, Greg S. Martin, Samer Melham, Nirja Mehta, Chelsea Modlin, David J. Murphy, Jung Park, Deepa Patel, Cindy Powell, Amit Prabhaker, Jeeyon Rim, Ramzy Rimawi, Chad Robichaux, Nicholas Scanlon, Milad Sharifpour, Bashar Staitieh, Michael Sterling, Jonathan Suarez, Colin Swenson, Nancy Thakkar, Alexander Truong, Hima Veeramachaneni, Alvaro Velasquez, Michael Waldmann, Max Weinmann, Thanushi Wynn, and Joel Zivot.
Footnotes
Drs. Auld and Caridi-Scheible contributed equally to this article.
This work was supported by the following grants: National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases K23 AI134182 (to Dr. Auld), NIH/Clinical and Translational Science Awards UL1TR002378.
Drs. Auld and Blum received support for article research from the National Institutes of Health (NIH). Dr. Blum’s institution received funding from the NIH received funding from Clew Medical. Dr. Jacob received funding from UptoDate. Dr. Martin received funding from Grifols. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid -19 in critically ill patients in the Seattle region — case series. New Engl J Med 2020 Mar 30. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020; 323:1612-1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Intensive Care National Audit & Research Centre: ICNARC Report on COVID-19 in Critical Care. 2020. Available at: https://www.icnarc.org/DataServices/Attachments/Download/cbcb6217-f698-ea11-9125-00505601089b. Accessed May 21, 2020
- 4.Richardson S, Hirsch JS, Narasimhan M, et al. and the Northwell COVID-19 Research Consortium: Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020 Apr 22. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2015; 30:994–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estenssoro E, Ríos FG, Apezteguía C, et al. Pandemic 2009 influenza A in Argentina. Am J Respir Crit Care Med 2010; 182:41–48 [DOI] [PubMed] [Google Scholar]
- 8.Domínguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 2009; 302:1880–1887 [DOI] [PubMed] [Google Scholar]
- 9.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 10.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 2008; 133:1120–1127 [DOI] [PubMed] [Google Scholar]
- 11.Dondorp AM, Hayat M, Aryal D, et al. Respiratory support in novel coronavirus disease (COVID-19) patients, with a focus on resource-limited settings. Am J Trop Med Hyg 2020 Apr 21. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Begley S. With Ventilators Running Out, Doctors Say the Machines Are Overused for Covid-19. STATnewscom. 2020 Available at: https://www.statnews.com/2020/04/08/doctors-say-ventilators-overused-for-covid-19/. Accessed April 20, 2020.
- 13.Ravani S, Allday E. Some Doctors Question Wisdom of Using Ventilators on Coronavirus Patients. San Francisco Chronicle. 2020 Available at: https://www.sfchronicle.com/bayarea/article/Some-doctors-question-the-wisdom-of-using-15190978.php. Accessed April 20, 2020.
- 14.Hamilton J. Ventilators Are No Panacea for Critically Ill COVID-19 Patients. National Public Radio. 2020 Available at: https://www.npr.org/sections/health-shots/2020/04/02/826105278/ventilators-are-no-panacea-for-critically-ill-covid-19-patients. Accessed April 20, 2020.
- 15.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]


