Abstract
Objectives:
Spain has been one of the countries most severely affected by the coronavirus disease 2019. This study aims to describe a series of children admitted to a PICU due to coronavirus disease 2019 infection.
Design:
Prospective observational study.
Setting:
Tertiary hospital in Madrid, Spain.
Patients:
Children admitted to the PICU with severe acute respiratory syndrome coronavirus 2 (severe acute respiratory syndrome coronavirus 2) infection, from March 1, 2020, to April 15, 2020.
Interventions:
Observational study.
Measurements and Main Results:
Epidemiologic data, previous clinical characteristics, support therapy needed, imaging tests, laboratory observations on admission, and pharmacologic therapy. Eleven children were admitted to the PICU, with suspected coronavirus disease 2019; the polymerase chain reaction test was positive in seven. The median age was 100.7 months (range, 0.5–162). Five were admitted from the emergency department and two from the ward. The Pediatric Sequential Organ Failure Assessment score was 3 (range, 0–9), and Pediatric Risk of Mortality II score was 4 (range, 0–16). All children were previously healthy except one (allogeneic hematopoietic stem cell transplantation). Respiratory symptoms and fever were prevalent. A chest radiograph led to a pneumonia diagnosis. Not all patients presented with lymphopenia on admission. d-Dimer and ferritin were elevated. All patients needed oxygen therapy through a nasal cannula; five patients received high-flow nasal cannula therapy, which was later substituted with noninvasive ventilation in four. Mechanical ventilation was necessary in two patients on the first day of PICU admission. Two children required mechanical ventilation and inotropic support. Tocilizumab was applied in two intubated children. Also, four children received heparin. No patients died.
Conclusions:
On the whole, the children were previously healthy and are more than 1 year old. Respiratory symptoms were the leading cause of PICU admission, making respiratory support the principal therapy. Patients requiring mechanical ventilation showed deterioration on the first day of admission. These children seemed to require close monitoring, and multicenter studies are necessary.
Keywords: children, coronavirus disease 2019, mechanical ventilation, noninvasive ventilation, pediatric critical care, severe acute respiratory syndrome coronavirus 2
In January 2020, a novel coronavirus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was described in Wuhan, China. This virus causes the coronavirus disease 2019 (COVID-19), and its rapid spread has led to the declaration of a global health emergency and pandemic by the World Health Organization (1). Spain is one of the most severely affected countries by this disease, and Madrid, its capital, has seen the highest rate of infection and mortality in the country (2). At the time of writing, more than 170,000 cases have been detected in Spain, causing 17,500 deaths. In Madrid, over 47,000 cases and 6,400 deaths have been confirmed (3).
In previous epidemics caused by coronaviruses, such as severe acute respiratory syndrome and the Middle East respiratory syndrome, scant data on pediatric patients were published. Similarly, COVID-19 seems to affect children to a lesser degree. This absence of data is even more pronounced in children who require PICU admission (1, 4).
The Madrid regional health authority decided to centralize pediatric care to optimize care for adults with COVID-19. Under this measure, the pediatric emergency departments of all hospitals remained in operation, although all children requiring admission were transferred to one of the two tertiary hospitals. In this brief report, we describe the epidemiologic and clinical features of children admitted to one such PICU during the first month and a half of this situation. The report’s main objective is to describe the characteristics of these patients so as to increase the knowledge of children critically ill with COVID-19.
We performed a prospective observational study based on data from patient medical records. Patients were included into the study based on two criteria. First, patients were required to have a confirmed SARS-CoV-2 infection based on nasopharyngeal swab specimens, using real-time reverse-transcriptase polymerase chain reaction (PCR). In case of suspected COVID-19, PCR testing was repeated. Second, all the patients studied had been admitted to the PICU. The study was carried out from March 1, 2020, to April 15, 2020.
The following data were collected: epidemiologic features, history, support therapy needed, imaging tests, laboratory tests on admission, and pharmacologic therapy. All data were obtained after acquiring informed consent from the parents or caregivers of the patients. The hospital ethics committee approved this study. A descriptive analysis of the results was conducted using the SPSS 16.0 software package for Windows (IBM Company, New York, NY). Median and range were used for quantitative data.
During the study period, 512 patients were admitted to our hospital as inpatients. Twenty-four children with COVID-19 were hospitalized in the pediatric ward; of these, two children were transferred to the PICU. Eleven children were admitted to the PICU with suspected COVID-19; seven had positive PCR test results (1.4% of total admissions and 5% of children admitted to the ward with COVID-19). We included seven children; three patients with a clinical presentation compatible with SARS-CoV-2 infection were excluded due to two negative PCR test results. Four of the seven patients were male. The median age was 100.7 months (range, 0.5–16). Five children were admitted from the emergency department and two from the ward. The only patient with a relevant history was a boy who had received an allogeneic hematopoietic stem cell transplantation. One patient was admitted to the PICU because of ketoacidosis; he underwent SARS-CoV-2 PCR testing due to the risk of community transmission and the need to remain isolated in the event of a positive result. Almost all cases presented with respiratory symptoms and fever on PICU admission (Table 1). The Pediatric Sequential Organ Failure Assessment score was 3 (range, 0–9), and the Pediatric Risk of Mortality II score was 4 (range, 0–16). All patients developed lymphopenia, with two during the first 24 hours of admission. Regarding classical biomarkers, C-reactive protein was 19.7 mg/dL (range, 40.1–0.11) and procalcitonin was 5.3 ng/mL (range, 37.7–0.07). Imaging tests evidenced bilateral pneumonia or infiltrates in three of eight cases in the first 24 hours of admission (Fig. 1). One child presented with a predominance of neurologic symptoms. A brain CT scan was performed, revealing cerebral thrombosis (Table 1, case 2). All patients needed oxygen therapy through a nasal cannula. Five received oxygen through a high-flow nasal cannula (HFNC); this therapy was later changed to noninvasive ventilation (NIV) in four patients. Two patients also required mechanical ventilation (MV) on the first day of PICU admission. The two children with MV needed additional vasoactive support. None of the children required renal replacement therapy. Only the patient admitted with symptoms not compatible with COVID-19 did not receive empirical pharmacologic treatment. The drugs administered were azithromycin (6/7), lopinavir/ritonavir (6/7), corticosteroids (5/7), and hydroxychloroquine (6/7). Remdesivir, immunoglobulins, and tocilizumab were given to the patient with previous hematopoietic stem cell transplantation, Also, prophylactic low-weight heparin was administered in two of the seven patients. The girl with cranial thrombosis also received continuous unfractionated heparin, which was later substituted with therapeutic low-weight heparin. At the end of this preliminary study, five of seven had been discharged from our PICU (Table 1).
TABLE 1.
Epidemiologic and Clinical Features, Radiologic Findings, and Management of Children Admitted for Critical Care Due to Severe Acute Respiratory Syndrome Coronavirus 2 Infection
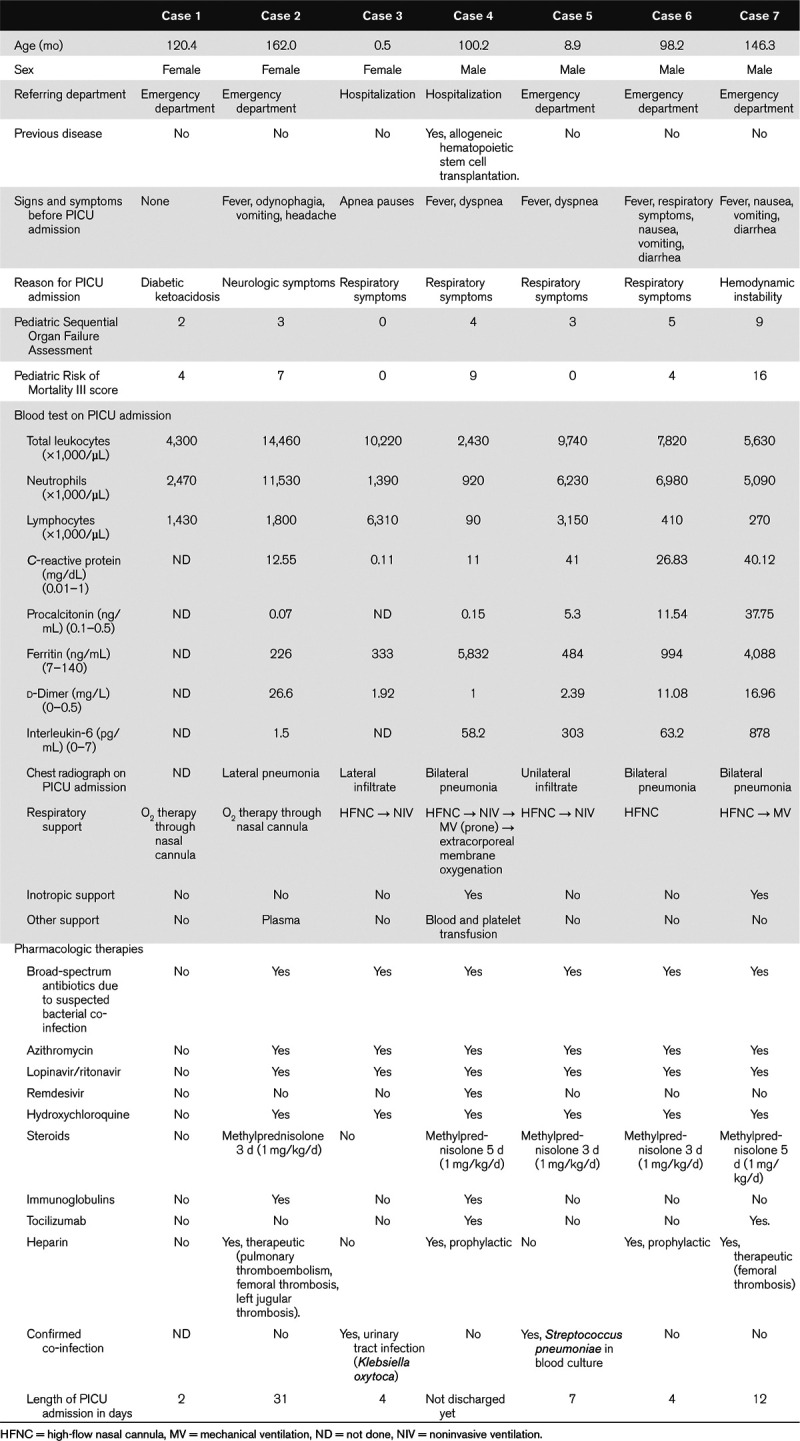
Figure 1.
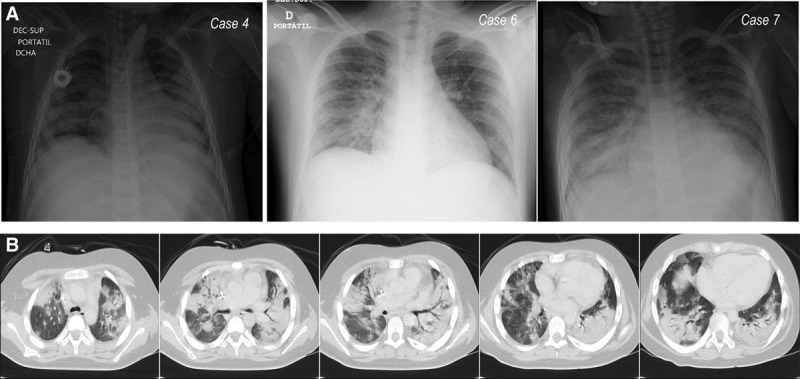
Image tests from three children admitted to the PICU because of severe acute respiratory syndrome coronavirus 2 infection. A, Chest radiograph of three cases. B, Chest CT of case 4 performed before PICU admission.
This report describes a large single-center series studies of children critically ill due to COVID-19. Nearly all the children admitted to our PICU were previously healthy. Only two of our admitted cases were children less than 1 year old. Respiratory symptoms were prevalent, and many presented with fever. Regarding complementary tests, chest radiograph imaging was of assistance in diagnosing pneumonia. The blood tests performed on these patients showed that lymphopenia was not always present on admission, but rather developed later. Also, d-dimer and ferritin were usually elevated. As for therapy, respiratory support was necessary in almost all cases. The children included did not complain of dyspnea, but all presented with increased respiratory work of some degree. We started HFNC therapy in five children, and for almost all of them, it was necessary to substitute this therapy with NIV. The two patients receiving MV experienced deterioration on the first day of admission despite close monitoring and rapid change to noninvasive oxygen support. All cases (except case 1) received the drugs empirically recommended for COVID-19; we used tocilizumab only in intubated children. Finally, heparin was administered to four children; this indication was based on the presence of thrombosis (therapeutic) or d-dimer levels (prophylactic).
Our data do not lend themselves to broad conclusions, though certain patterns do emerge. The fact that almost all cases studied were previously healthy children is logical, given that children are mostly a healthy population (5) with substantially fewer comorbidities than adults. We attribute the relatively high number of PICU admissions (i.e., seven children in 45 d) to selection bias (6, 7). In light of previously published reports, it appears that this rate of PICU admission is higher than in other countries with fewer hospital admissions. As we explained in the Introduction, in Madrid, there were only two active PICUs during the pandemic; so we received a higher number of children, including COVID-19 cases (2). We also observed that only two cases required inotropic support, and four needed respiratory support beyond nasal cannula or HFNC. The patients described here presented with mild to severe illness and responded favorably to supportive management. In some cases, PICU admission may have been premature, driven by uncertainty as to the course of the disease in these children.
Previous series of studies have described that severe COVID-19 was more prevalent in infants. However, in our experience, older children developed more serious disease (6, 7). This finding should be confirmed by multicenter studies (8). As for complimentary testing, not all cases presented with lymphopenia on admission, and in some cases, this outcome appeared later. Our patients showed elevated d-dimer levels. Other markers (such as ferritin and interleukin-6) were not present in all patients. These indicators were elevated in patients with a more severe disease course.
Turning now to respiratory management, we observed that the use of nasal cannula was insufficient, even where no other instabilities were observed. Patients who received HFNC therapy were later moved to NIV. In cases where MV was needed, deterioration took place during the first day of admission, possibly indicating a need for close monitoring of children diagnosed with bilateral pneumonia and COVID-19. One child with an underlying condition suffered a bilateral pneumothorax after 4 weeks of MV. After this complication, he developed refractory hypoxemia and hypercapnia. It was impossible to successfully modify the ventilator settings without increasing air leak. Because of the clinical deterioration and after discussion with the oncologist, we decided to initiate extracorporeal membrane oxygenation (ECMO). He currently remains on ECMO support.
As for pharmacologic therapy, Table 1 shows that almost all cases were treated empirically with the drugs proposed to treat COVID-19 (9). Antimicrobial treatment was added based on clinical suspicion. Case 2 received antimicrobial treatment due to previous odynophagia. Case 3 underwent antibiotic therapy for a urinary tract infection. All other cases received broad-spectrum antibiotics. Inotropic support was applied only in children with MV. We used corticosteroids in five cases. We chose to start them, given the presence of findings consistent with macrophage activation syndrome (MAS) or immune dysregulation (Table 1). Despite the presence of an active viral infection, we chose to administer methylprednisolone, which was subsequently withdrawn over 3 or 5 days based on clinical progression (Table 1). In one case, methylprednisolone was used despite no suspicion of MAS. In that patient, because of the presence of massive thrombosis (brain, lungs, and lower extremities), we empirically started methylprednisolone to treat the suspected inflammation related to SARS-CoV-2 binding to endothelial receptors. Tocilizumab was administered to both children who later received MV. This monoclonal antibody was administered as a second-level therapy in our treatment protocol, which was given in the absence of a favorable response to supportive therapies and methylprednisolone. We cannot draw any conclusions on the effects of drug therapy due to the lack of a control group and because one of our main objectives was to avoid any adverse effects produced by these measures.
This study has several limitations. It is based on data from a single center and as such must be interpreted with caution. Also, this article shows preliminary data. The pandemic is not ended, and we may observe new PICU admissions. An external validation of our results or management approaches should be undertaken for purposes of reproducibility. We applied pharmacologic therapy that is not evidence based, thus preventing us from drawing conclusions as to their utility.
In conclusion, this manuscript presents a series of children requiring critical care due to COVID-19. All were treated in a tertiary PICU setting during the pandemic. The patients described were previously healthy for the most part and are over 1 year old. Respiratory symptoms were the leading cause of admission, and as a result, respiratory support was the principal therapy. Patients receiving MV showed deterioration on the first day of admission. It appears that close monitoring is needed to effectively treat these children. Multicenter studies are necessary for comparison and to contribute new knowledge about treating children critically ill with COVID-19.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Peeri NC, Shrestha N, Rahman MS, et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. 2020 Feb 22; doi: 10.1093/ije/dyaa033. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 2020 Apr 8; doi: 10.1001/jamapediatrics.2020.1346. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Situación de COVID-19 en España, Ministerio de Sanidad. 2020 Available at: https://covid19.isciii.es/. Accessed April 5, 2020.
- 4.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 2020; 38:1–9 [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: An overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J 2020; 39:355–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: Clinical and epidemiological features. Clin Infect Dis. 2020 Feb 28; doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W, Zhang Q, Chen J, et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med 2020; 382:1370–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics 2020; 145:e2020070232179660 [Google Scholar]
- 9.Cunningham AC, Goh HP, Koh D. Treatment of COVID-19: Old tricks for new challenges. Crit Care 2020; 24:91. [DOI] [PMC free article] [PubMed] [Google Scholar]


