Abstract
Background:
Little is known about longitudinal symptom burden and its consequences for well-being, and if lifestyle moderates burden in older survivors.
Methods:
We report on 36-month data from survivors 60+ with newly diagnosed non-metastatic breast cancer and non-cancer controls recruited August 2010-June 2016. Symptom burden was a sum of self-reported symptoms/diseases: pain (yes/no), fatigue (FACT-fatigue), cognitive (FACT-cog), sleep problems (yes/no), depression (CES-D), anxiety (STAI), and cardiac problems and neuropathy (yes/no). Well-being was measured using the FACT-G, scaled from 0–100. Lifestyle included smoking, alcohol use, BMI, physical activity, and leisure activities. Mixed models assessed relationships between treatment group (chemotherapy +/− hormonal, hormonal only, control) and symptom burden, lifestyle, and covariates. Separate models tested the effects of fluctuations in symptom burden and lifestyle on function.
Results:
All groups reported high baseline symptoms, and levels remained high over time; survivor-control differences were most notable for cognitive and sleep problems, anxiety, and neuropathy. The adjusted burden score was highest among chemotherapy-exposed survivors, followed by hormonal therapy vs. controls (p<.001). Burden score was related to physical, emotional, and functional well-being (e.g., survivors with lower vs. higher burden scores had 12.4-point higher physical well-being score). The composite lifestyle score was not related to symptom burden or well-being, but physical activity was significantly associated with each outcome (<.005).
Conclusions:
Cancer and its treatments are associated with a higher level of actionable symptoms and greater loss of well-being over time in older breast cancer survivors than comparable non-cancer populations, suggesting the need for surveillance and opportunities for intervention.
Keywords: Breast cancer, symptom burden, older patients, survivorship, well-being
Precis:
Cancer and its treatments lead to a higher level of actionable symptoms and greater loss of function among older breast cancer survivors than expected based on non-cancer control experience, suggesting the need for surveillance and intervention.
Introduction
Many of the nearly four million US breast cancer survivors1 report one or more symptom commonly associated with cancer, including cardio-toxic effects, peripheral neuropathy, cognitive problems, fatigue, anxiety, depression, and sleep disturbances.2–5 Older women (age 60+) constitute the largest segment of breast cancer survivors.1 These older survivors may be especially vulnerable to a high symptom burden, and for these symptoms to affect functioning, given comorbidities6 and aging.7 We reported that pre-systemic therapy symptoms predicted 24-month function.8 However, there are little data on changes in symptom burden over time in older survivors. Additionally, recommended healthy lifestyles,9 have not been examined for their ability to moderate symptoms or improve function in older survivors.
We used data from the Thinking and Living with Cancer (TLC) cohort10 of older breast cancer survivors followed from pre-systemic treatment for 36-months. We included data from a frequency-matched non-cancer control group to test if symptom burden in older survivors exceeded those seen over 36-months the non-cancer population. Finally, we also examined whether higher symptom burden decreased physical, emotional and functional well-being, and explored whether healthy lifestyles moderated symptoms or improved well-being. These data are intended to inform discussions about survivorship care for older survivors.
Methods
This study was conducted at Georgetown University and affiliated practices (Washington, DC area), Memorial Sloan Kettering Cancer Center (New York), Moffitt Cancer Center (Tampa), City of Hope Cancer Center (Los Angeles), Hackensack University Medical Center (New Jersey), Indiana University (IU) (Indianapolis), and University of California (Los Angeles, UCLA). UCLA provides laboratory support and IU did not begin accrual until mid-2016, so data in this report are from the five other sites. All Institutional Review Boards approved the protocol.
Setting and Population
We included participants recruited between August 1, 2010 and June 1, 2016 since they had the opportunity to complete 36-month assessments; follow-up is ongoing. Eligible survivors were aged 60+, had newly diagnosed non-metastatic breast cancer, and were English-speaking. Those with stroke, head injury, major Axis I psychiatric or neurodegenerative disorders, and other recent cancer (<5 years) or past systemic therapy were ineligible. Among eligible survivors, 375 (37.2%) consented (consent rate range across sites 17.2–80.4%, median 63.5%). Consenting survivors were similar in age to non-participants. There were 375 consenting age-, race-, education- and site-frequency-matched non-cancer controls. Controls met the same exclusion criteria as survivors.
Participants were screened using the Mini-Mental State Examination (MMSE) and the Wide Range Achievement Test, 4th edition Word Reading subtest; those with scores of <24 or <3rd grade-equivalent reading level, respectively, were ineligible (1 control, 1 survivor). Data for survivors who experienced a recurrence (n=8) were excluded for the six months before recurrence; one survivor recurred close to baseline and was excluded. Eleven consenting survivors and nine controls did not complete baseline. The final sample included 362 survivors and 365 controls (Figure 1). Among participants remaining alive and eligible, 74.5% 73%, 65% of survivors and 87.8%, 79.9%, 70.2% of controls completed 12-, 24-, and 36-month assessments, respectively.
Figure 1.

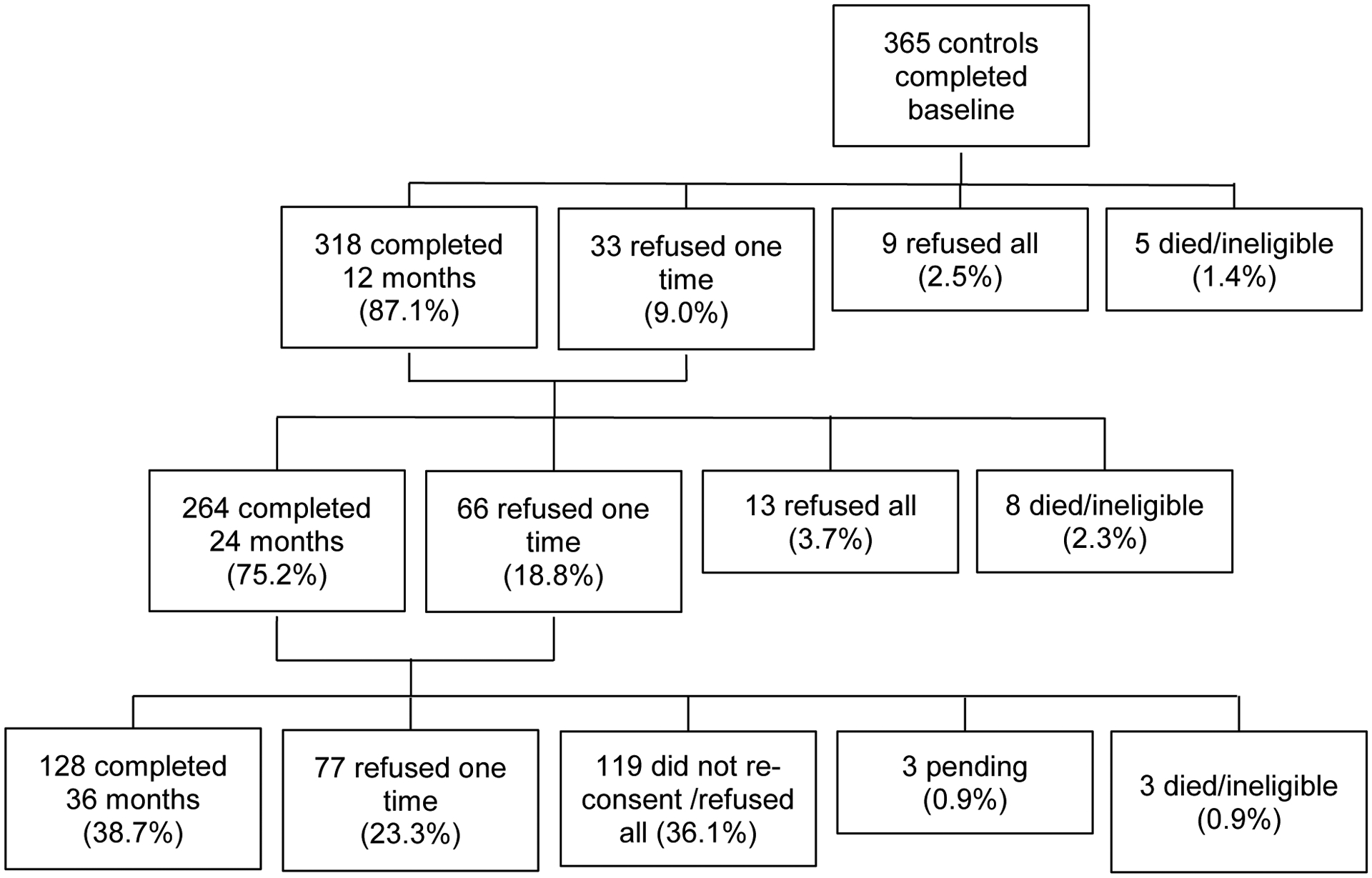
The top panel represents survivors and the bottom panel represents non-cancer controls. The percent consenting and refusing was calculated among those alive and eligible at each time point; participants become ineligible if they develop another cancer, or any cancer if a control, neurological disease, or, for survivors, have a recurrence. Numbers at 36-months drop due to administrative loss from a gap in funding. Participants may have refused one interview, but completed later interviews. Sixty-nine percent of participants completed three or four assessments, 15.2% completed two, and 16.3% completed baseline only. There were no significant differences in age, race, or education by number of completed assessments.
Data Collection
Data collection included survey (all) and medical record data (survivors) and has been described previously.10
Measures
Outcomes were symptom burden and physical, emotional, and functional well-being. Symptom burden was defined as the sum of self-reported illnesses and symptoms: cardiac disease and peripheral neuropathy, depression, anxiety, fatigue, cognitive problems, pain, and sleep problems. Symptoms were counted as yes/no or present if continuous score was >1.0 SD of the baseline control; this cut-point was based on common conventions.11 Sixteen controls with scores >3SD from the control means were excluded as outliers based on study-specific protocols.
We selected these eight symptoms/illnesses since they tend to cluster8 and/or include known treatment effects (e.g., neuropathy).2 We included myocardial infarction, congestive heart failure, arrhythmia, and angina as possible treatment-toxicity related. Scores ≥16 on the Center for Epidemiologic Studies Depression (CES-D) Scale defined clinical depression (alpha=.86).12 The State-Trait Anxiety Inventory (STAI) measured state anxiety (Cronbach’s alpha=.86).13 Fatigue was assessed using the FACT-fatigue scale (alpha=.90).14 Cognitive problems were assessed using the FACT-cog (alpha=.90).15
Well-being was measured with FACT-G scales for physical (alpha= .77), and emotional (alpha= .77) and functional well-being (alpha= .82)16 We used the FACT-G rather than FACT-B to examine survivors in relation to a non-cancer control group. Scores were rescaled from 0–100, with higher scores representing better well-being. Minimum clinically important differences on the 0–100 scale were 8.3–12.5.17
Covariates
The main predictor of symptom burden was treatment group (chemotherapy +/−hormonal treatment, hormonal only, non-cancer control). Lifestyle was based on American Cancer Society recommendations scored from 0 to 5, where 5 is the healthiest:9 physical activity (600+ mets/week), alcohol (0–1 vs. >1 serving per day), BMI (<30 vs. 30+), past or never smoking s vs. currently smoking, and having more vs. less leisure activities.
Potential covariates included race (white vs. non-white), education (years), and marital status, comorbid illnesses not considered cancer-related (e.g., hypertension, diabetes), and surgery and breast radiotherapy (for cases). Site was included to capture unmeasured setting-specific variability.
Statistical Analysis
ANOVA, chi-squared tests, and Exact tests were used to compare characteristics by treatment-group and evaluate potential confounders.
Random-effects fluctuation mixed models tested the effect of treatment-group and lifestyle on symptom burden using data from up to four observation points (baseline, 12, 24, and 36-months). Lifestyle was included as a between-person (having an average lifestyle that differed from the average of other participants) and a within-person predictor (having healthier lifestyle compared to one’s own average).18 Covariates included age, race, site, and other comorbidities not included as symptoms.
Separate random-effects fluctuation models examined how treatment-group and symptom burden were related to physical, emotional, and functional well-being. Surgery type and radiation were not related to outcomes, so were not included in the treatment groups. Covariates included lifestyle, age, race, site, and other comorbidities. Since some of the well-being scales included 1–2 items about symptoms, we repeated analyses excluding those items from the well-being scale, and the relationship of symptoms and well-being were unchanged; we present data with the full well-being scales for comparability to other studies.
Since drop-out or death can lead to informative missing data respect to outcomes, we used baseline covariates for inverse probability weighting to reduce bias and boost efficiency.19 Results without weighting were similar to weighted results.
Finally, to explore how each symptom affected the relationship between treatment and well-being, we built a series of step-wise models progressively adding each individual symptom one at a time and examining the change in the model goodness-of-fit (Akaike Information Criterion [AIC]); we repeated this process to evaluate the individual components of the composite lifestyle measure.
In all models, estimates reaching two-sided p<0.05 were considered statistically significant. When multiple (K) comparisons were performed for a set of analyses, we used the conservative Bonferroni adjusted type I error (0.05/K). Analyses were conducted using SAS Version 9.4.b (SAS Institute Inc., Cary, NC, USA).
Results
Participants were 60 to 98 years old (Table 1). There was a high rate of all symptoms at baseline before systemic therapy. Over time, survivors treated with chemotherapy (+/−hormonal treatment) tended to have the highest levels of peripheral neuropathy, depression, and pain. Survivors exposed to either chemotherapy (+/−hormonal treatment) or hormonal therapy exhibited a pattern of elevated fatigue, sleep disturbance, and cardiovascular problems compared to controls over time. (Figure 2).
Table 1.
Baseline Characteristics of Older Breast Cancer Survivors and Non-Cancer Controls
| Non-Cancer Controls1 N=349 | Survivors N=3621 | |||
|---|---|---|---|---|
| Chemo +/−Hormonal N=99 | Hormonal Only N=249 | p-value | ||
| %(n) or mean(SD) | ||||
| Socio-demographic | ||||
| Age, Mean SD (range) | 68.0(7.0) (60 – 91) | 66.2(4.8) (60 – 84) | 68.6(6.3) (60 – 98) | 0.007 |
| 0.908 | ||||
| 78.4(273) | 78.8(78) | 79.9(199) | ||
| Non-White | 21.6(75) | 21.2(21) | 20.1(50) | |
| Married vs. not | 48.5(166) | 59.2(58) | 61.3(144) | 0.006 |
| Education, years | 15.4(2.3) | 15.3(2.2) | 15.1(2.1) | 0.270 |
| Clinical (cases only) | ||||
| <.001 | ||||
| - | 0.0(0) | 15.7(39) | ||
| - | 40.4(40) | 61.0(152) | ||
| - | 44.4(44) | 22.1(55) | ||
| Stage 3 | - | 15.2(15) | 1.2(3) | |
| <.001 | ||||
| - | 69.7(69) | 99.6(248) | ||
| Negative | - | 30.3(30) | 0.4(1) | |
| 0.110 | ||||
| - | 50.5(50) | 59.9(148) | ||
| Mastectomy | - | 49.5(49) | 40.1(99) | |
| Radiotherapy (BCS only) | - | 45.5(45) | 59.0(147) | 0.022 |
| Lifestyle Factors | ||||
| 0.073 | ||||
| 3.6(12) | 9.4(9) | 5.6(13) | ||
| Former/never | 96.4(321) | 90.6(87) | 94.4(219) | |
| 0.252 | ||||
| 78.4(240) | 85.5(71) | 76.9(163) | ||
| > 1 | 21.6(66) | 14.5(12) | 23.1(49) | |
| <.001 | ||||
| 20.5(66) | 36.6(30) | 35.5(70) | ||
| >/= 600 | 79.5(256) | 63.4(52) | 64.5(127) | |
| 0.026 | ||||
| 24.6(82) | 36.1(35) | 33.1(79) | ||
| <30 | 75.4(251) | 63.9(62) | 66.9(160) | |
| Leisure Activities3 | 6.6(2.0) | 6.9(2.1) | 6.4(2.1) | 0.129 |
| Baseline Well-being4 | ||||
| Physical, mean(SD) | 92.1(9.0) | 82.7(15.8) | 82.8(16.1) | <.001 |
| Emotional, mean(SD) | 91.4(9.4) | 74.2(20.1) | 84.3(15.3) | <.001 |
| Functional, mean(SD) | 84.6(14.6) | 71.9(21.3) | 73.4(20.4) | <.001 |
| Baseline Symptoms | ||||
| Pain | 53.8(182) | 68.5(63) | 61.6(138) | 0.021 |
| Fatigue5 | 15.4(52) | 32.3(30) | 36.2(81) | <.001 |
| Self-reported cognition | 130.5(14.0) | 129.0(17.4) | 128.4(18.7) | 0.317 |
| Anxiety7 | 15.3(51) | 37.6(35) | 28.7(64) | <.001 |
| Sleep problems | 24.6(83) | 35.5(33) | 37.6(85) | 0.003 |
| Depression8 | 3.6(12) | 23.3(21) | 10.5(23) | <.001 |
| Cardiac disease | 7.7(26) | 5.4(5) | 10.7(24) | 0.245 |
| Peripheral neuropathy | 0.0(0) | 1.0(1) | 0.0(0) | 0.049 |
ER=estrogen receptor
Numbers may not add to 100% due to missing data; 14 survivors missing therapy. Non-white includes Black, Hispanic, and AAPI; one control missing race. P- values for differences between the three groups based on chi-square, Anova, or Fisher’s exact.
Mets are calculated from the IPAQ.
There were 11 leisure activities reported as yes/no.
The well-being based on the FACT-G.16 Scores were normalized from 0–100. Higher scores=greater well-being.
Fatigue scores based on the FACT-fatigue.14 Higher scores=less fatigue.
Self-reported cognition was based on the FACT-Cog.15 Higher scores= indicating cognition.
Based on the STAI State Anxiety Scale.13 Higher scores=more anxiety.
Depression defined by score above 16 on the CES-D.12
Figure 2.
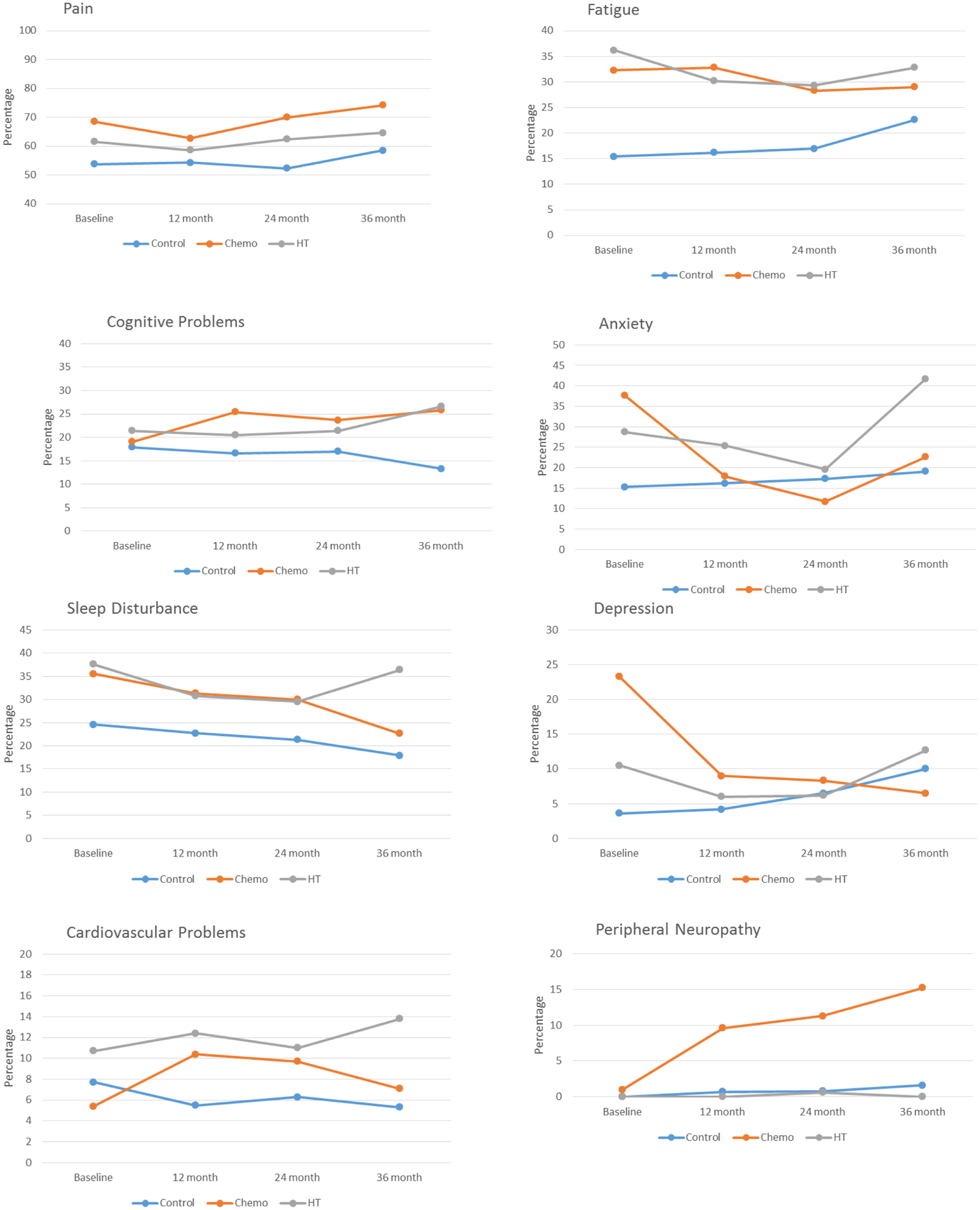
Percent of Older Breast Cancer Survivors and Non-Cancer Controls Reporting Specific Symptoms by Treatment and Time
Difference significant for cognitive problems (p=.01), anxiety (p=.01), sleep (p=.02), and neuropathy (p=.014) (note Bonferroni corrected p value =.05/8, or p=.00625).
Symptom Burden
The adjusted symptom burden was greatest for survivors who received chemotherapy +/−hormonal therapy, followed by survivors who received hormonal therapy, then controls, considering covariates (p<.001, Table 2). Lifestyle was not related to symptoms and did not change the treatment-group effect, (Table 2) but higher physical activity reduced symptoms (p=.04). Interactions between lifestyle and treatment were not significant, so were not included in the final symptom model.
Table 2.
Factors Associated with Symptoms Burden among Older Breast Cancer Survivors and Non-cancer Controls
| Treatment Model n=653 | Treatment and Lifestyle Model N=653 | |||
|---|---|---|---|---|
| Beta (SE) | p-value | Beta (SE) | p-value | |
| Treatment | <.001 | <.001 | ||
| Chemotherapy vs. control | 0.77(0.15) | 0.78(0.15) | ||
| Hormonal vs. control | 0.48(0.11) | 0.50(0.11) | ||
| Lifestyle | ||||
| Between-person lifestyle | 0.00(0.06) | 0.982 | ||
| Within-person lifestyle | 0.16(0.04) | <.001 | ||
| AIC | 5728.0 | 5653.3 | ||
Random-effects mixed fluctuation models; controlling for other comorbidities at baseline, age, race, site. Considers inverse probability of dropping out or dying.
Well-being
Treatment-group was associated with physical, functional and emotional well-being scales. When a woman’s symptom burden was higher than other women or than the woman’s usual level, her well-being score was worse (p. 001)(Table 3). The magnitude of effect of symptoms on each well-being scale was clinically meaningful. For instance, when a woman had a greater vs. lower symptom burden, her adjusted physical well-being score was 12.4 points lower (p <.001). Survivors had higher symptom burden than controls, but the impact of symptom burden on well-being did not differ by group. Lifestyle was not related to well-being and did not change the impact of treatment or symptoms on well-being, (Table 3) but greater physical activity was associated with better physical and functional well-being (p<.004). Interaction terms There was no significant interaction between symptoms and lifestyle in effect on well-being and were not retained in the final models.
Table 3.
Associations of Symptom Burden and Well-Being Outcomes over 36-Months among Older Breast Cancer Survivors and Non-cancer Controls
| Physical Well-being1 N=653 | Emotional Well-being1 N=653 | Functional Well-being1 N=653 | |||||
|---|---|---|---|---|---|---|---|
| Beta (SE) | P-value2 | Beta (SE) | P-value2 | Beta (SE) | P-value2 | ||
| Other Comorbidities | −0.65(0.18) | 0.0004 | −0.38(0.26) | 0.1353 | −0.26(0.28) | 0.3382 | |
| Treatment Group | |||||||
| Chemo vs. control | −2.34(0.92) | 0.0018 | −5.87(1.28) | <0.0001 | −2.18(1.38) | 0.2753 | |
| HT vs. control | −2.06(0.65) | −0.98(0.91) | −0.75(0.98) | ||||
| Symptom Burden | |||||||
| Between-person effect | −4.95(0.25) | <0.0001 | −4.88(0.35) | <0.0001 | −8.04(0.38) | <0.0001 | |
| Within-person effect | −4.15(0.25) | <0.0001 | −3.63(0.29) | <0.0001 | −5.73(0.35) | <0.0001 | |
| Lifestyle | |||||||
| Between-person lifestyle | 0.72(0.36) | 0.0449 | −0.27(0.50) | 0.5874 | 0.76(0.54) | 0.1603 | |
| Within-person lifestyle | 0.71(0.39) | 0.0671 | −0.93(0.46) | 0.0446 | 1.07(0.55) | 0.0521 | |
| AIC | 11833.5 | 12480.1 | 12979.5 | ||||
Random-effects mixed fluctuation models, controlling for age, race, site, considering probability of dropping out or dying, predicting FACT-G scale scores.16
The Bonferroni corrected significance level is p= .05/3, or p=.0167.
Effects of Specific Symptoms on Well-being
Each individual symptom was significantly related to physical well-being, with the largest effects seen for depression, pain, and sleep disturbance (Table 4). Similar results were seen for emotional and functional well-being (not shown).
Table 4.
Impact of Individual Symptoms on Physical Well-Being among Older Breast Cancer Survivors and Non-cancer Controls
| Base Model 1 N=653 | Cognitive Problems Model 1 N=648 | Pain Model 1 N=648 | Sleep Problems Model 1 N=648 | Fatigue Model 1 N=648 | Depression and Anxiety Model 1 N=645 | Neuropathy and Cardiac Disease Model 1 N=645 | |
|---|---|---|---|---|---|---|---|
| Beta (SE) | |||||||
| Comorbidity | −1.78(0.23)** | −1.62(0.21)** | −0.73(0.20)** | −0.71(0.19)** | −0.17(0.17) | −0.18(0.16) | −0.20(0.16) |
| Treatment | |||||||
| Chemo v. control | −6.55(1.17)** | −5.74(1.09)** | −4.13(0.96)** | −3.55(0.94)** | −2.40(0.80)** | −2.04(0.77)** | −1.82(0.81)** |
| HT v. control | −4.74(0.83)** | −4.32(0.78)** | −3.62(0.68)** | −3.05(0.67)** | −1.72(0.58)** | −1.79(0.55)** | −1.90(0.55)** |
| Cognition | |||||||
| Within person | −2.78(1.07)** | −1.91(1.00) | −1.82(0.98) | −0.72(0.92) | 0.25 0.91) | 0.66(0.92) | |
| Between person | −6.67(1.51)** | −6.33(1.38)** | −5.49(1.36)** | −2.10(1.25) | −2.43(1.25) | −2.77(1.26)* | |
| Pain | |||||||
| Within person | −7.76(0.69)** | −7.70(0.68)** | −6.57(0.64)** | −6.92(0.63)** | −6.83(0.63)** | ||
| Between person | −4.06(1.12)** | −3.17(1.10)** | −2.15(0.99)* | −2.11(0.96)* | −2.10(0.96)* | ||
| Sleep | |||||||
| Within person | −4.05(0.75)** | −2.63(0.71)** | −1.67(0.70)* | −1.62(0.70)* | |||
| Between person | −1.65(1.18) | −0.73(1.06) | −0.82(1.05) | −0.95(1.06) | |||
| Fatigue | |||||||
| Within person | −9.68(0.80)** | −8.71(0.79)** | −8.67(0.79)** | ||||
| Between person | −4.38(1.24)** | −4.34(1.22)** | −4.42(1.22)** | ||||
| Anxiety | |||||||
| Within person | −0.71(0.81) | −0.64(0.81) | |||||
| Between person | 3.55(1.22)** | 3.63(1.22)** | |||||
| Depression | |||||||
| Within person | −10.6(1.29)** | −10.9(1.29)** | |||||
| Between person | 1.20(1.92) | 1.38(1.93) | |||||
| Peripheral neuropathy | |||||||
| Within person | −1.07(2.18) | ||||||
| Between person | −0.42(3.65) | ||||||
| Cardiovascular | |||||||
| Within person | −0.55(1.33) | ||||||
| Between person | 1.94(1.73) | ||||||
| AIC | 12381.1 | 12153.4 | 11850.6 | 11779.0 | 11431.7 | 11208.4 | 11152.4 |
Random-effects fluctuation models; base model includes other baseline comorbidity, age, race, site, treatment group, and considers inverse probability of dropping out or dying.
p values <0.05
p-value of <0.001
DISCUSSION
This study illustrates that over the 36-months after diagnosis older breast cancer survivors have a higher symptom burden than seen in similar older women without cancer. The highest magnitude of effect of treatment on symptom burden was seen for those exposed to chemotherapy (+/− hormonal therapy), but those on hormonal therapy alone also had a significantly greater symptom burden than women without cancer. Higher symptom burden was significantly associated with clinically meaningful declines in well-being. Composite lifestyle did not moderate treatment effects, independently ameliorate symptoms, or improve function, but the individual component of physical activity did improve outcomes.
The rates of symptoms in this study are similar to other reports,20–22 except for less peripheral neuropathy.23 By including a non-cancer group, we were able to demonstrate that older breast cancer survivors experienced a higher burden of symptoms and decrement in function than controls. These findings could inform long-term clinical care to address the persistent effects of treatment, since symptoms could affect completion of hormonal therapy.
It has been more than a decade since the Institute of Medicine highlighted the unmet needs of cancer survivors,24 but 50% of survivors still report not getting help to address symptoms.25 These data, together with our findings, suggest that survivorship care should emphasize screening for and discussion of symptoms including sleep difficulties, depression, anxiety, pain, and fatigue,26 especially since these symptoms are actionable. System-level interventions like chart reminders might increase symptom screening, since oncologists with training about cancer-related symptoms or who use electronic records with prompts are more likely to talk to survivors about care needs.27 Professional guidelines could also place greater emphasis on symptom recognition and management. Addressing symptom burden is especially salient for older survivors, since our results demonstrate that symptom burden was associated with clinically meaningful decrements in well-being.
We did not find benefits for healthy lifestyles, perhaps since we had limited sensitivity and variability in this measure. We did find that being more physically active did reduce symptoms and improve well-being. Lifestyle interventions including exercise,28,29 reductions in sedentary time,30 yoga,31 cognitive re-training,32 and weight loss have been shown to increase well-being in other studies,33–36 so this remains an important topic for survivorship care visits.37
Our study has many strengths, including a large sample, a non-cancer control group, and data over 36-months. There are also several caveats that should be noted in considering our results. First, it is difficult to attribute symptoms to cancer, but having a control group allowed valid inference regarding differences in matched cancer vs. non-cancer populations. Use of an additively-scored symptom checklist approach like ours has been used in similar studies with good concurrent validity.38,39 Second, we did not measure all possible symptoms, such as lymphedema, post-tramautic stress disorder, sexual dysfunction, or financial stress; these are important to consider in future research. Third, it is difficult to show indivudal changes in symptoms over time, but our fluctuation models tested the effects of having a different symptom burden at each time point. Fourth, we did not include social well-being ,since we this varied based on need, rather than QOL. Fifth, we had limited variabilty in lifestyle; this remains an important area for more research. Finally, our cohort was well-educated, and may not represent all older survivors. However, given the strong association of socioeconomic status and health,40 our rates of symptoms and impact on function may underestimate those in broader populations.
Overall, this study moves the field forward by demonstrating that cancer and its treatments lead to a higher level of actionable symptom burden, and greater loss of well-being over the first 36-months than expected based on the experience of matched non-cancer controls. Future research is needed to understand factors that contribute to resilience or vulnerability to a high symptom burden and functional decline. Until then, survivorship care guidelines9,41 should include clear recommendations for surveillance and treatment of symptoms among older survivors.
Acknowledgments
This work was conducted while Dr. Jacobsen was at Moffitt Cancer Center. The views expressed are those of the authors and do not necessarily represent the official views of the National Cancer Institute.
Supported by National Cancer Institute (NCI) grants R01CA129769 and R35 CA197289 to JM. Also supported in part by NCI grant P30CA51008 to LK, and support of the Biostatistics and Bioinformatics and Non-Therapeutic Shared Resources. DBT was supported in part by NCI grants T32CA117865 and F31CA220964. JC was supported in part by an ACS Research Scholars grant 128660-RSG-15-187-01-PCSM. TAA and JCR were supported in part by NCI grants R01CA172119 and P30 CA008748. SKP was supported in part by American Cancer Society Research Scholars grant 130336-RSG-17-023-01-CPPB, and HSLJ was supported in part by R01 CA164109, R01 CA214647, and R01 CA219389.
Footnotes
ClinicalTrials.gov Identifier: NCT03451383
There are no relevant conflicts of interest.
References
- 1.Bluethmann SM, Mariotto AB, Rowland JH: Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev 25:1029–36, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carver JR, Shapiro CL, Ng A, et al. : American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 25:3991–4008, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Mandelblatt JS, Jacobsen PB, Ahles T: Cognitive Effects of Cancer Systemic Therapy: Implications for the Care of Older Patients and Survivors. Journal of Clinical Oncology 32:2617–2626, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanoff HK, Deal AM, Krishnamurthy J, et al. : Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst 106:dju057, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gullett JM, Cohen RA, Yang GS, et al. : Relationship of fatigue with cognitive performance in women with early-stage breast cancer over 2 years. Psychooncology 28:997–1003, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandelblatt JS, Stern RA, Luta G, et al. : Cognitive Impairment in Older Patients With Breast Cancer Before Systemic Therapy: Is There an Interaction Between Cancer and Comorbidity? Journal of Clinical Oncology 32:1909–1918, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. : Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–56, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Tometich DB, Small BJ, Carroll JE, et al. : Pretreatment Psychoneurological Symptoms and Their Association With Longitudinal Cognitive Function and Quality of Life in Older Breast Cancer Survivors. J Pain Symptom Manage 57:596–606, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Runowicz CD, Leach CR, Henry NL, et al. : American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol 34:611–35, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Mandelblatt JS, Small BJ, Luta G, et al. : Cancer-Related Cognitive Outcomes Among Older Breast Cancer Survivors in the Thinking and Living With Cancer Study. Journal of Clinical Oncology 36:3211–3222, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wefel JS, Vardy J, Ahles T, et al. : International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol 12:703–8, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Radloff LS: The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement 1:385–401, 1977 [Google Scholar]
- 13.Spielberger C: Manual for the State-Trait Anxiety Inventory (STAI: Form Y). Consulting Psychologists Press; Palo Alto, CA, 1983 [Google Scholar]
- 14.Yellen SB, Cella DF, Webster K, et al. : Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 13:63–74, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Wagner LI, Sweet J, Butt Z, et al. : Measuring patient self-reported cognitive function: Development of the Functional Assessment of Cancer Therapy–Cognitive Function Instrument, 2009 [Google Scholar]
- 16.Cella DF, Tulsky DS, Gray G, et al. : The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. Journal of Clinical Oncology 11:570–579, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Webster K, Cella D, Yost K: The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes 1:79, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loh KP, Janelsins MC, Mohile SG, et al. : Chemotherapy-related cognitive impairment in older patients with cancer. J Geriatr Oncol 7:270–80, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perkins NJ, Cole SR, Harel O, et al. : Principled Approaches to Missing Data in Epidemiologic Studies. Am J Epidemiol 187:568–575, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juhl AA, Christiansen P, Damsgaard TE: Persistent Pain after Breast Cancer Treatment: A Questionnaire-Based Study on the Prevalence, Associated Treatment Variables, and Pain Type. J Breast Cancer 19:447–454, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamood R, Hamood H, Merhasin I, et al. : Chronic pain and other symptoms among breast cancer survivors: prevalence, predictors, and effects on quality of life. Breast Cancer Res Treat 167:157–169, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Schmidt ME, Wiskemann J, Steindorf K: Quality of life, problems, and needs of disease-free breast cancer survivors 5 years after diagnosis. Qual Life Res 27:2077–2086, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Mustafa Ali M, Moeller M, Rybicki L, et al. : Long-term peripheral neuropathy symptoms in breast cancer survivors. Breast Cancer Res Treat 166:519–526, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Medicine Io: From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC, The National Academies Press, 2006 [Google Scholar]
- 25.Smith TG, Troeschel AN, Castro KM, et al. : Perceptions of Patients With Breast and Colon Cancer of the Management of Cancer-Related Pain, Fatigue, and Emotional Distress in Community Oncology. J Clin Oncol 37:1666–1676, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brauer ER, Long EF, Melnikow J, et al. : Communicating Risks of Adjuvant Chemotherapy for Breast Cancer: Getting Beyond the Laundry List. J Oncol Pract 15:e98–e109, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forsythe LP, Parry C, Alfano CM, et al. : Use of survivorship care plans in the United States: associations with survivorship care. J Natl Cancer Inst 105:1579–87, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soares Falcetta F, de Araujo Vianna Trasel H, de Almeida FK, et al. : Effects of physical exercise after treatment of early breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 170:455–476, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Penttinen H, Utriainen M, Kellokumpu-Lehtinen PL, et al. : Effectiveness of a 12-month Exercise Intervention on Physical Activity and Quality of Life of Breast Cancer Survivors; Five-year Results of the BREX-study. In Vivo 33:881–888, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welch WA, Ehlers D, Gavin KL, et al. : Effects of reallocating sedentary time with physical activity on quality of life indicators in breast cancer survivors. Psychooncology 28:1430–1437, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danhauer SC, Addington EL, Cohen L, et al. : Yoga for symptom management in oncology: A review of the evidence base and future directions for research. Cancer 125:1979–1989, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferguson RJ, McDonald BC, Rocque MA, et al. : Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psychooncology 21:176–86, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salerno EA, Rowland K, Kramer AF, et al. : Acute aerobic exercise effects on cognitive function in breast cancer survivors: a randomized crossover trial. BMC Cancer 19:371, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnsson A, Broberg P, Kruger U, et al. : Physical activity and survival following breast cancer. Eur J Cancer Care (Engl) 28:e13037, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Irwin ML, Smith AW, McTiernan A, et al. : Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol 26:3958–64, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palesh O, Scheiber C, Kesler S, et al. : Management of side effects during and post-treatment in breast cancer survivors. Breast J 24:167–175, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Kelly DL, Yang GS, Starkweather AR, et al. : Relationships Among Fatigue, Anxiety, Depression, and Pain and Health-Promoting Lifestyle Behaviors in Women With Early-Stage Breast Cancer. Cancer Nurs, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Portz JD, Kutner JS, Blatchford PJ, et al. : High Symptom Burden and Low Functional Status in the Setting of Multimorbidity. J Am Geriatr Soc 65:2285–2289, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gierk B, Kohlmann S, Kroenke K, et al. : The somatic symptom scale-8 (SSS-8): a brief measure of somatic symptom burden. JAMA Intern Med 174:399–407, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Phelan JC, Link BG, Tehranifar P: Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav 51 Suppl:S28–40, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Network NCC: Breast cancer treatment guidelines. https://subscriptions.nccn.org/gl_login.aspx?ReturnURL=http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf, 2013


