Abstract
Cigarette smoking is increasingly concentrated among marginalized populations with limited access to evidence-based cessation treatment. This includes racial/ethnic minorities, lower income individuals, those with lower educational attainment, and residents of rural areas. To reach Healthy People 2020 objectives, successful cessation interventions must narrow these disparities. Nicotine replacement therapy (NRT) sampling is an easily translatable and scalable intervention that could enhance treatment access and thus narrow disparities. The present study examined individual-level demographic moderators of the impact of NRT sampling on cessation-related behaviors including: 1) use of a cessation medication, 2) making a 24-hour quit attempt, 3) floating abstinence, and 4) 7-day point prevalence abstinence at 6-months. Study participants included N=1,245 adult smokers enrolled in the Tobacco Intervention in Primary Care Treatment Opportunities for Providers (TIP TOP) study, a recently concluded large-scale clinical trial of NRT sampling relative to standard care within 22 primary care clinics across South Carolina. Generalized linear models examined individual-level demographic moderators of treatment effect. Results suggest that NRT sampling may be more effective among some of the most disadvantaged groups of smokers, including smokers with lower income and education, as well those who live in more rural areas. The effects of NRT sampling did not differ by race. In sum, NRT sampling is a low-cost, low-burden intervention that could be disseminated broadly to reach large numbers of smokers and potentially narrow cessation disparities.
Keywords: smoking cessation, health disparities, primary care
INTRODUCTION
Cigarette smoking is increasingly concentrated among marginalized populations with limited access to evidence-based cessation treatment1–4. This includes racial/ethnic minorities3, lower income individuals1,2,5, those with lower educational attainment6, and residents of rural areas7,8. Healthy People 2020 highlights two key priorities to increase population-level cessation rates: 1) increase smoking cessation attempts by adult smokers and 2) increase smoking cessation attempts using evidence-based strategies. Yet, racial, educational, economic, and rural/urban disparities are evident both in terms of quit attempts and use of evidence-based cessation treatments. For example, despite similar rates of past-year quit attempts between Black and White smokers (63.4% vs. 53.3%) and between smokers living below and above the poverty line (55.2% vs. 55.5%), Black smokers and lower income smokers are less likely to have used an evidence-based cessation treatment in an attempt to quit (Race: 28.9% vs. 34.3%; Income: 29.0% vs. 31.7%). In terms of education, individuals with lower educational attainment (e.g., less than a high school degree) as compared to those with higher educational attainment (e.g., an undergraduate degree) are both less likely to have made a past year quit attempt (50.4% vs. 57.6%) and are less likely to have used a cessation treatment (28.7% vs. 35.1%)9. Similar patterns are evident when comparing residents of rural vs. suburban. vs. urban areas such that individuals residing in rural areas are more likely to be current smokers10,11, have lower incidence of quit attempts12, and have limited access to cessation treatment13. To reach Healthy People 2020 objectives, successful interventions must narrow cessation-related disparities in quit attempts and use of evidence-based cessation treatments.
Nicotine replacement therapy (NRT) sampling is one candidate approach to narrow cessation-related disparities. NRT sampling refers to provision of a brief (i.e., two-week) free starter pack of NRT. We14–16 and others17,18 have shown that NRT sampling is associated with increased likelihood of cessation success across several indicators, including quit attempts and abstinence16,19. NRT sampling will likely have the greatest population-level impact on cessation outcomes if delivered within settings where smokers are likely to receive care, such as primary care20,21. As such, we recently concluded a large-scale (N=1,245) cluster randomized clinical trial within 22 primary care clinics across South Carolina to examine the impact of NRT sampling on cessation outcomes (use of a cessation medication, quit attempts, abstinence) relative to standard clinical practice22. Adult smokers were recruited via their primary care clinic, received either a two-week supply of nicotine patch + lozenge in addition to physician brief advice to quit or physician brief advice to quit alone, and then were followed for six months to assess cessation-related behaviors. Primary outcomes suggested superiority of NRT sampling relative to the sole provision of physician brief advice to quit22. More smokers in the NRT sampling group used cessation medication both immediately following their initial clinic visit (55% vs. 10%) and at six months (25% vs. 14%), NRT sampling was associated with higher rates of quit attempts during the initial month following medication receipt (24% vs. 18%), and point prevalence abstinence (PPA) rates were significantly higher among those who received NRT sampling relative to control at 1- (5% vs. 2%), 3- (10% vs. 5%), and 6-month (12% vs. 8%) follow-ups22.
Although these results suggest that NRT sampling offers general promise for increasing medication use, quit attempts, and abstinence, it is less clear if NRT sampling has any differential effect across subgroups of smokers. Understanding these effects is critical to determine whether NRT sampling is an appropriate intervention to address cessation-related disparities. NRT sampling could potentially work to narrow disparities by increasing access to medications for groups that otherwise may have limited access, but could also widen disparities if less effective among more marginalized groups. As such, the purpose of the present study was to examine individual-level demographic moderators of the impact of NRT sampling on cessation-related behaviors including: 1) use of a cessation medication, 2) making a 24-hour quit attempt, 3) floating abstinence (any 7-day period of abstinence during the trial), and 4) 7-day PPA at 6-months. Demographic moderators were selected based on prior literature indicative of associations with cessation-related disparities and include: 1) race3, 2) income1,2,5, 3) education6, and 4) rurality7,8.
METHODS
Description of the Parent Trial
The present study is a secondary data analysis of the Tobacco Intervention in Primary Care Treatment Opportunities for Providers (TIP TOP) study, a large, recently completed comparative effectiveness trial (Clinical Trials Registration Number NCT02096029) of NRT sampling within primary care. Details of the study design23 and primary outcomes22 have been previously reported. Briefly, adult smokers were recruited across 22 South Carolina primary care clinics, wherein all study procedures (screening, consent, baseline assessment, and intervention delivery) were administered by clinic staff during routine visits. To standardize information providers received prior to initiation of recruitment, cessation-certified research staff gave a one-time, 60–90 minute in-person training of all study procedures to each clinic.
Inclusion/exclusion criteria were kept broad to increase results generalizability. Participants were required to be: a) age 18+, b) a smoker of at least five cigarettes per day on ≥25 days out of the last 30 days, c) English speaking, and d) recruited through a primary care site active in the study. Exclusion criteria included FDA contraindications for NRT use (pregnancy/breastfeeding; recent cardiovascular trauma). Randomization (standard care vs. standard care + NRT sampling) was at the clinic level, and was stratified by rural (vs. urban) and small (vs. large) clinics. Participants in all clinics received a take-home bag that included basic information on smoking cessation as well as a brochure with referral to the state quitline. Participants within clinics randomized to the standard care + NRT sampling condition also received within their take-home bags two-week supplies of nicotine patches and lozenges in uniform doses (14mg patch, 4mg lozenge). Following baseline consent and treatment delivery, all participants were followed for six months via phone to collect study outcomes. Analyses in the present study are based on the full sample (N=1,245) of participants enrolled in TIP TOP. See Table 1 for participant demographics.
Table 1.
Participant Demographics
| Full Sample (N=1245) | Standard Care (SC) (n=652) | SC + NRT Sampling (n=593) | |
|---|---|---|---|
| Age (M(SD)) in years | 50.7 (13.5) | 51.0 (13.6) | 50.4 (13.4) |
| Gender (% Female) | 757 (61%) | 368 (56%) | 389 (66%) |
| Race (%) | |||
| White | 776 (62%) | 345 (53%) | 431 (73%) |
| Black | 446 (36%) | 292 (45%) | 154 (26%) |
| Other | 23 (2%) | 15 (2%) | 8 (1%) |
| Education (%) | |||
| ≤ High School diploma | 796 (64%) | 420 (64%) | 376 (63%) |
| > High School diploma | 449 (36%) | 232 (36%) | 217 (37%) |
| Annual Household Income (%)* | |||
| < $50k | 782 (80%) | 434 (84%) | 348 (75%) |
| ≥$50k | 197 (20%) | 82 (16%) | 115 (25%) |
| Rurality (M(SD)) | 5.8 (1.8) | 6.0 (1.9) | 5.6 (1.8) |
| Baseline cigarettes per day (M(SD)) | 15.2 (9.0) | 15.0 (9.3) | 15.3 (8.6) |
266 are missing data on income, %’s are from non-missing data
Cessation Outcomes
All cessation outcomes were captured via participant self-report at one-, three-, and six-months post study enrollment. Primary outcomes for this analysis include: 1) cessation medication utilization, defined as use of any FDA-approved cessation medication (NRT, varenicline, bupropion) at any point during the follow-up period, 2) any quit attempt lasting at least 24-hours during the follow-up period, 3) floating abstinence, defined as any 7-day period of non-smoking at any point during the study, and 4) 7-day PPA at the 6-month follow-up assessment.
Data Analytic Plan
Potential demographic moderators including race, income, education level, and rurality were examined to determine their relationships with outcomes of interest (any medication use, any 24-hour quit attempt, 7-day floating abstinence, and PPA at 6-month follow-up) in combination with treatment (NRT sampling + standard care vs. standard care alone). For data analytic purposes, race, income, and education were dichotomized as follows: 1) Race: White or Black (98% of the sample identified as either White or Black), 2) Total household income: less than or greater than/equal to $50,000 per year, 3) Education: high school diploma, GED, less than a high school diploma (collapsed), or more than a high school diploma. The decision to dichotomize education as less than or equal to a high school diploma vs. more than a high school diploma was based on prior literature demonstrating that the annual cessation rate for smokers with less than or equal to 12 years of education is two-thirds that of smokers with more than 12 years of education25. To assess rurality, participant zip code was matched to a continuous indicator of rurality via a publicly available isolation index26. This isolation index captures the trade-off between access to resource rich, high population density areas and the cost to travel to those areas, with higher values indicative of more limited access to resources. Prior research indicates that the isolation index is at least as good as, if not better than, other commonly used rural classification systems for explaining health-related measures26.
All outcomes of interest were treated as binary (either having the event of interest or no). Generalized linear mixed models, using a logit link for binary data and including a random intercept component for site to account for clustering within the primary care clinics, were used for hypothesis testing. Each model included main effects for treatment and demographic, as well as a treatment by demographic interaction. Due to a small amount of missing data across demographic variables, sample size varied across models (Race: n=1,222; Income: n=979; Education: n=1,245; Rurality: n=1,226). All analyses were based on intent-to-treat principles and missing data were conservatively assumed to be in the direction of no use/still smoking. Regarding missing data, rates were similar between treatment groups, with slightly higher retention rates in the standard care alone group (Month 1: NRT 72% retention vs. 76% in standard care alone; Month 3: NRT 61% vs. 67%; Month 6: NRT 58% vs. 60%). For income status, data were missing for 266 participants. As such, further exploratory analyses were done within this group to detect noticeable patterns across outcomes. Demographically, this group was similar to (age) or landed between (race, education) the other income groups. Those not reporting income resided in more rural areas and had a higher percentage of females than the other groups. For most cessation outcomes (medication utilization, floating abstinence, and PPA at six months), the missing income group was similar to the higher income group; however, for 24-hour quit attempts, those with missing income data were more similar to the lower income group. Given the lack of consistency across demographics/outcomes and the similarity to outcomes observed in other groups, it is difficult to assess whether there is or is not potential response bias related to reporting of income.
Based on the secondary nature of this study, significance level for interactions was determined to be α=0.10. The decision to set α=0.10 was made to increase sensitivity within this exploratory analysis, though it also should be noted that it increases the possibility of finding an interaction significant by chance. For models where the interaction was statistically significant (p≤0.1), effects of treatment and demographic were interpreted through the interactions; for models where the interaction was not statistically significant, only main effects of treatment and demographic were interpreted. For all models, regardless of significance of the interaction, contrast statements were used to calculate the odds ratio and 95% confidence interval (CI) for NRT sampling + standard care vs. standard care alone for each level of the demographic variable in the context of the interaction. These effects were plotted in forest plots (Figure 1) for visualization. As rurality indicated by the isolation index was continuous, the effects of treatment on outcomes of interest were examined at the 25th (low rurality), 50th (average rurality), and 75th (high rurality) percentiles for rurality to understand the nature of the treatment by rurality interactions. The 25th percentile for rurality for the study sample was an isolation index score of 4.35, the 50th percentile was 6.08, and the 75th percentile was 7.33. These values map fairly well onto lower (4.0), median (4.8), and upper (6.1) quartiles determined in the original isolation index scale validation study26, with the present study sample (all based within South Carolina) residing in somewhat more rural areas in general than the geographic distribution of the country as a whole. For each outcome, odds ratios and 95% CIs are reported. Data analysis for this paper was generated using SAS software Version 9.4.
Figure 1. The Impact of Treatment on Cessation Outcomes as a Function of Group.
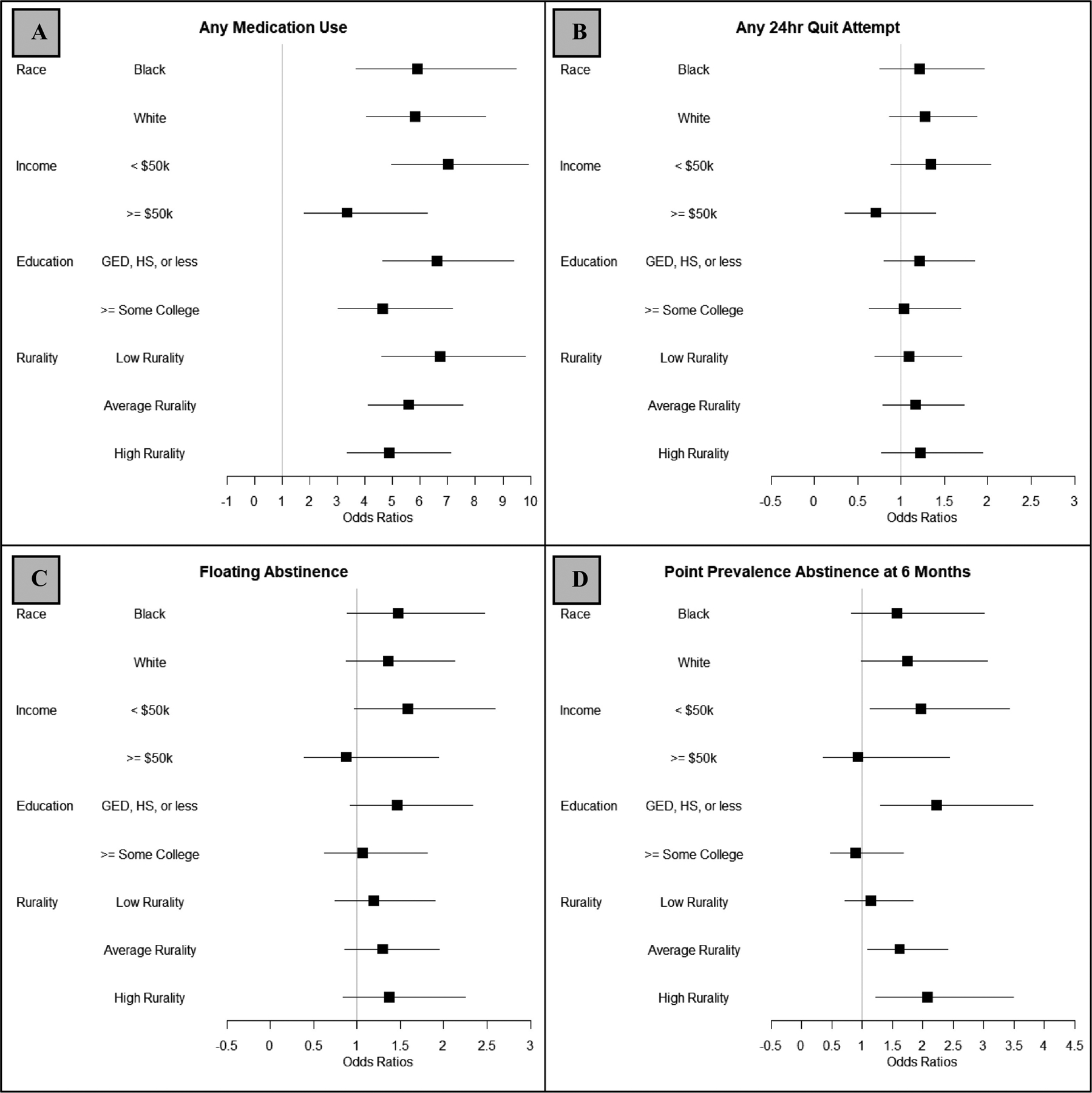
Note: Results are paneled for each cessation outcome: A) any medication use, B) any 24-hour quit attempt, C) floating abstinence, and D) 7-day point prevalence abstinence at 6-months. Odds ratios and accompanying 95% confidence intervals are presented within each demographic group as a function of NRT sampling vs. control (referent). Rurality was examined at the median rurality (average rurality) for the study sample as well as at the 75th (high rurality) and 25th (low rurality) percentiles.
RESULTS
Results are presented below in the case of either significant interactions (i.e., where treatment is differentially associated with cessation outcomes among various sub-groups) or significant main effects, when the interaction was non-significant. Effect sizes for NRT + standard care vs. standard care alone within each group (e.g., within each race group), regardless of the significance of the interaction, are plotted in Figure 1 as forest plots. Raw counts [n(%)] and modeling results [odds ratios (ORs) and 95% confidence intervals (95% CIs)] are presented in Table 2 for all outcomes, split by treatment and demographics.
Table 2.
Cessation Outcomes by Treatment and Demographic Group
| Any Medication Use | Any 24hr Quit Attempt | Floating Abstinence | PPA at 6 Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRT n (%) | SC n (%) | NRT:SC* OR (95% CI) | NRT n (%) | SC n (%) | NRT:SC* OR (95% CI) | NRT n (%) | SC n (%) | NRT:SC* OR (95% CI) | NRT n (%) | SC n (%) | NRT:SC* OR (95% CI) | |
| Race | ||||||||||||
| Black | 104 (68%) | 76 (26%) | 5.9 (3.7, 9.5) | 79 (51%) | 138 (47%) | 1.2 (0.8, 2.0) | 52 (34%) | 81 (28%) | 1.5 (0.9, 2.5) | 21 (14%) | 28 (10%) | 1.6 (0.8, 3.0) |
| White | 276 (64%) | 82 (24%) | 5.8(4.1, 8.4) | 165 (38%) | 113 (33%) | 1.3 (0.9, 1.9) | 96 (22%) | 58 (17%) | 1.4 (0.8, 2.1) | 47 (11%) | 22 (6%) | 1.7 (1.0, 3.1) |
| Income | ||||||||||||
| < $50k | 243 (70%) | 107 (25%) | 7.0 (5.0, 9.9) | 161 (46%) | 174 (40%) | 1.3 (0.9, 2.0) | 99 (28%) | 93 (21%) | 1.6 (1.0, 2.6) | 45 (13%) | 31 (7%) | 2.0 (1.1, 3.4) |
| ≥ $50k | 66 (57%) | 24 (29%) | 3.4 (1.8, 6.3) | 34 (30%) | 32 (39%) | 0.7 (0.4, 1.4) | 23 (20%) | 19 (23%) | 0.9 (0.4, 1.9) | 12 (10%) | 9 (11%) | 0.9 (0.4, 2.4) |
| Education | ||||||||||||
| ≤ HS | 252 (67%) | 100 (28%) | 6.6 (4.7, 9.4) | 162 (43%) | 165 (39%) | 1.2 (0.8, 1.8) | 97 (26%) | 83 (20%) | 1.5 (0.9, 2.3) | 48 (13%) | 26 (6%) | 2.2 (1.3, 3.8) |
| > HS | 134 (62%) | 60 (26%) | 4.7 (3.0, 7.2) | 87 (40%) | 94 (41%) | 1.0 (0.6, 1.7) | 55 (25%) | 59 (25%) | 1.1 (0.6, 1.8) | 22 (10%) | 26 (11%) | 0.9 (0.5, 1.7) |
| Low | 140 (67%) | 48 (24%) | 6.4 (3.9, 10.7) | 88 (42%) | 94 (48%) | 1.1 (0.7, 1.7) | 58 (28%) | 59 (30%) | 1.2 (0.7, 19) | 24 (12%) | 26 (13%) | 1.1 (0.7, 1.8) |
| Average | 144 (66%) | 49 (25%) | 5.7 (3.5, 9.4) | 93 (43%) | 64 (33%) | 1.2 (0.8, 1.7) | 53 (24%) | 36 (19%) | 1.3 (0.9, 2.0) | 24 (11%) | 9 (5%) | 1.6 (1.1, 2.4) |
| High | 98 (61%) | 62 (25%) | 5.0 (3.0, 8.1) | 66 (41%) | 97 (39%) | 1.2 (0.8, 1.9) | 40 (25%) | 46 (18%) | 1.4 (0.8, 2.2) | 21 (13%) | 17 (7%) | 2.1 (1.2, 3.5) |
Note
From generalized linear mixed model with random intercept effect for site;
n (%) values are from tertiles for raw values; Odds ratios and 95% CIs are from models where rurality is treated as a continuous measure looking at estimate statements for low (4.35), average (6.08), or high (7.33) values of rurality
Medication Utilization
Race.
The interaction between race and treatment was a non-significant predictor of medication utilization (p>0.9). Among both Black (OR=5.92, 95% CI: 3.69, 9.49) and White (OR=5.82, 95% CI: 4.05, 8.36) smokers, NRT sampling led to increased medication utilization.
Income.
The interaction between income and treatment significantly predicted likelihood of medication utilization (p=0.04). Across both low- and high-income smokers, NRT sampling led to increased use of medication, but this effect was significantly stronger among low income smokers. Whereas among high income smokers, those randomized to the NRT sampling condition were 3.36 (95% CI: 1.80, 6.26) times more likely to use a cessation medication during the follow-up period than those randomized to control, among low income smokers, those randomized to the NRT sampling condition were 7.03 times (95% CI: 4.98, 9.91) more likely to use medication relative to control.
Education.
The interaction between education and treatment was a non-significant predictor of medication utilization (p=0.2). NRT sampling increased medication utilization among both smokers with lower (OR=6.61, 95% CI: 4.65, 9.39) and higher (OR=4.66, 95% CI: 3.02, 7.18) educational attainment.
Rurality.
The interaction between rurality and treatment was a non-significant predictor of medication utilization (p=0.2). NRT sampling relative to control led to increased utilization of cessation medication among smokers living in less rural areas (OR=6.73, 95% CI: 4.62, 9.80), areas average in rurality (OR=5.58, 95% CI: 4.11, 7.56), and more rural areas (OR=4.87, 95% CI: 3.34, 7.10).
Incidence of 24-Hour Quit Attempts
Income.
The interaction between income and treatment significantly predicted incidence of a 24-hour quit attempt (p=0.07). Among lower income smokers, likelihood of making a 24-hour quit attempt was 1.34 times (95% CI: 0.89, 2.04) higher among those randomized to the NRT sampling vs. control condition. This relationship was inverted among higher income smokers (OR=0.71, 95% CI: 0.36–1.40), such that likelihood of making a 24-hour quit attempt was higher among those randomized to control vs. NRT sampling.
Floating Abstinence
Income.
The interaction between income and treatment significantly predicted incidence of floating abstinence (p=0.1). Among lower income smokers, likelihood of floating abstinence was 1.59 times higher among those randomized to NRT sampling vs. control (95% CI: 0.97, 2.59). This relationship was inverted among higher income smokers (OR=0.87, 95% CI: 0.39–1.94), such that likelihood of floating abstinence was higher among those randomized to control vs. NRT sampling.
7-Day PPA at 6-Months
Income.
Although the interaction between income and treatment was a nonsignificant predictor of 7-day PPA at 6-months (p=0.2), among lower income smokers, NRT sampling was associated with significantly higher odds of 7-day PPA relative to control (OR=1.97, 95% CI: 1.13, 3.42). Among higher income smokers, the odds of 7-day PPA did not significantly differ as a function of treatment (OR=0.93, 95% CI: 0.35, 2.44).
Education.
The interaction between education and treatment was a significant predictor of 7-day PPA at 6-months (p=0.02). Among smokers with lower educational attainment, those who received NRT sampling were 2.23 times (95% CI: 1.30, 3.82) more likely to report 7-day PPA. Among smokers with higher educational attainment, likelihood of 7-day PPA at 6-months did not significantly differ as a function of treatment condition (OR=0.89, 95% CI: 0.47, 1.68).
Rurality.
The interaction between rurality and treatment was a significant predictor of 7-day PPA at 6-months (p=0.06). Among smokers residing in less rural; i.e., more urban areas, likelihood of 7-day PPA did not significantly differ as a function of receiving NRT sampling vs. control (OR=1.15, 95% CI: 0.72, 1.84). In contrast, among smokers residing in areas at the median for rurality and in more rural areas, NRT sampling was associated with increased likelihood of 7-day PPA relative to control (Median OR=1.62, 95% CI: 1.09, 2.41; 75th percentile OR=2.07, 95% CI: 1.23, 3.50).
DISCUSSION
Overall, these results suggest that the effects of NRT sampling may be more robust among the most disadvantaged groups of smokers. This includes lower income smokers, smokers with lower educational attainment, and smokers who live in more rural areas. Regarding income, the effect of NRT sampling on cessation medication utilization was significantly stronger among lower income (i.e., < $50k per year annual household income) smokers as compared to higher income (i.e., ≥ $50k per year annual household income) smokers. For other cessation outcomes, including making a 24-hour quit attempt, floating abstinence, and 7-day PPA at six months, among lower income smokers, NRT sampling led to increases in all. These results are consistent with prior research indicating that cessation programs for low income smokers that include free medication are associated with improved cessation outcomes27,28. As such, free access to medication, such as is provided via NRT sampling, may play a key role in promoting cessation among lower income smokers.
Regarding education, the effect of NRT sampling on 7-day PPA at six months was strongest among smokers with lower educational attainment. Whereas 6% of smokers with a high school diploma or less who were randomized to standard care reported PPA at six months, 13% of those randomized to NRT sampling reported PPA. As such, NRT sampling may help to bolster cessation rates among smokers with lower educational attainment.
Regarding rurality, the effect of NRT sampling on 7-day PPA at six months was strongest among smokers living in more rural areas. Among smokers living in the most urban areas, 7-day PPA was similar when comparing those randomized to NRT sampling vs. control (12% vs. 13%). In contrast, among those residing in the most rural areas, 7-day PPA was nearly doubled as a function of receiving NRT sampling vs. control (13% vs. 7%). Thus, NRT sampling may similarly be a promising approach to improve cessation rates relative to standard care for residents of more rural areas.
If replicated, these results together suggest that NRT sampling could narrow income-, education-, and rurality-related demographic disparities in smoking cessation. There are several possibilities as to the mechanism underlying the generally stronger effects of NRT sampling among smokers of lower income, lower educational attainment, who reside in more rural areas. Conceptual and empirical models suggest that smokers from more disadvantaged groups generally receive less smoking cessation treatment content and face unique individual-level and environmental barriers to treatment receipt1,29. Regarding environmental barriers to accessing NRT specifically, prior research indicates that NRT is less available and, when available, more expensive in poorer neighborhoods30. These barriers can be addressed through dissemination of concrete, evidence-based cessation treatment strategies to all smokers, regardless of demographic characteristics1. Because NRT sampling is provided for free during already occurring medical visits to all smokers regardless of motivation to quit, financial, structural, and attitudinal barriers to receipt of cessation treatment may be reduced.
Across cessation milestones, NRT sampling was not differentially effective as a function of race. This result is somewhat in contrast to prior research, which highlights that Black smokers are less likely than White smokers to successfully quit smoking3,4, despite having higher motivation to quit31 and being more likely to make a quit attempt32. One key reason for this racial disparity in smoking cessation may be differential access to and utilization of evidence-based cessation medications including NRT33,34. Additionally, our prior research and that of others indicates that Black smokers in particular may hold negative misperceptions about NRT that could undermine usage35–37. As observed here, an intervention such as NRT sampling, which can be applied uniformly across all groups of smokers, could help to increase treatment receipt among Black smokers and subsequently narrow race-related cessation disparities. The sampling experience in itself may then help to combat negative misperceptions about NRT that would deter future medication use. Future research that assesses the impact of NRT sampling on medication misperception among Black smokers may help to disentangle the effects of NRT sampling among this group.
This study is not without limitations. First, this was a secondary data analysis of a recently completed clinical trial that was neither designed nor powered for the subgroup analyses examined herein. As such, results should be interpreted with caution, and future research designed specifically to examine the impact of NRT sampling on cessation-related disparities is warranted. Second, cessation outcomes for this trial were based on self-report and were not biochemically verified. Third, each demographic moderator was examined independently rather than examining additional interactions within each demographic group. It is possible that these effects could be bolstered or diminished when considering additional subgroup analyses (e.g., among high rurality smokers, examining income as a predictor of cessation). Finally, all study participants were recruited from primary care clinics within South Carolina. As such, results may not generalize to smokers outside of South Carolina or who are not engaged with primary care.
In conclusion, NRT sampling is a low cost, low burden intervention that could be disseminated broadly to reach large numbers of smokers. If specifically targeted toward groups of smokers that tend to have lower rates of successful cessation and lower rates of evidence-based treatment utilization, including smokers with low income, low educational attainment, who live in rural areas, and/or who are racial minorities, NRT sampling could narrow cessation disparities.
HIGHLIGHTS.
NRT sampling may be more effective among the most disadvantaged smokers.
This includes smokers with lower income and education and who live in rural areas.
NRT sampling could potentially narrow cessation disparities.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (R01 DA021619, K23 DA045766, and K01 DA047433). The authors have no conflicts of interest to declare. The sponsor had no role in the design and conduct of the study; or in the preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sheffer CE, Stitzer M, Landes R, Brackman SL, Munn T, Moore P. Socioeconomic disparities in community-based treatment of tobacco dependence. Am J Public Health. 2012;102(3):e8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiscock R, Bauld L, Amos A, Fidler JA, Munafo M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci. 2012;1248(1):107–123. [DOI] [PubMed] [Google Scholar]
- 3.Trinidad DR, Perez-Stable EJ, White MM, Emery SL, Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health. 2011;101(4):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piper ME, Cook JW, Schlam TR, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;12(6):647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill S, Amos A, Clifford D, Platt S. Impact of tobacco control interventions on socioeconomic inequalities in smoking: review of the evidence. Tob Control. 2014;23(e2):e89–97. [DOI] [PubMed] [Google Scholar]
- 6.Wetter DW, Cofta-Gunn L, Irvin JE, et al. What accounts for the association of education and smoking cessation? Prev Med. 2005;40(4):452–460. [DOI] [PubMed] [Google Scholar]
- 7.Doescher MP, Jackson JE, Jerant A, Gary Hart L. Prevalence and trends in smoking: a national rural study. J Rural Health. 2006;22(2):112–118. [DOI] [PubMed] [Google Scholar]
- 8.Doogan NJ, Roberts ME, Wewers ME, et al. A growing geographic disparity: Rural and urban cigarette smoking trends in the United States. Prev Med. 2017;104:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting Smoking Among Adults—United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457–1464. [DOI] [PubMed] [Google Scholar]
- 10.Vander Weg MW, Cunningham CL, Howren MB, Cai X. Tobacco use and exposure in rural areas: Findings from the Behavioral Risk Factor Surveillance System. Addict Behav. 2011;36(3):231–236. [DOI] [PubMed] [Google Scholar]
- 11.Roberts ME, Doogan NJ, Kurti AN, et al. Rural tobacco use across the United States: how rural and urban areas differ, broken down by census regions and divisions. Health Place. 2016;39:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brasky TM, Hinton A, Doogan NJ, et al. Characteristics of the Tobacco User Adult Cohort in Urban and Rural Ohio. Tob Regul Sci. 2018;4(1):614–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberhardt MS, Pamuk ER. The importance of place of residence: examining health in rural and nonrural areas. Am J Public Health. 2004;94(10):1682–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burris JL, Heckman BW, Mathew AR, Carpenter MJ. A mechanistic test of nicotine replacement therapy sampling for smoking cessation induction. Psychol Addict Behav. 2015;29(2):392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpenter MJ, Alberg AJ, Gray KM, Saladin ME. Motivating the unmotivated for health behavior change: a randomized trial of cessation induction for smokers. Clin Trials. 2010;7(2):157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpenter MJ, Hughes JR, Gray KM, Wahlquist AE, Saladin ME, Alberg AJ. Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: a randomized clinical trial. Arch Intern Med. 2011;171(21):1901–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham JA, Kushnir V, Selby P, Tyndale RF, Zawertailo L, Leatherdale ST. Effect of mailing nicotine patches on tobacco cessation among adult smokers: A randomized clinical trial. JAMA Intern Med. 2016;176(2):184–190. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham JA, Kushnir V, Selby P, Tyndale RF, Zawertailo L, Leatherdale ST. Beyond quitting: Any additional impact of mailing free nicotine patches to current smokers? Nicotine Tob Res. 2018;20(5):654–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jardin BF, Cropsey KL, Wahlquist AE, et al. Evaluating the effect of access to free medication to quit smoking: a clinical trial testing the role of motivation. Nicotine Tob Res. 2014;16(7):992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verbiest M, Brakema E, van der Kleij R, et al. National guidelines for smoking cessation in primary care: a literature review and evidence analysis. NPJ Prim Care Respir Med. 2017;27(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papadakis S, McDonald P, Mullen KA, Reid R, Skulsky K, Pipe A. Strategies to increase the delivery of smoking cessation treatments in primary care settings: a systematic review and meta-analysis. Prev Med. 2010;51(3–4):199–213. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter MJ, Wahlquist AE, Dahne J, et al. Nicotine replacement therapy sampling for smoking cessation within primary care: Results from a pragmatic cluster randomized clinical trial. Addiction. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahne J, Wahlquist AE, Boatright AS, et al. Nicotine replacement therapy sampling via primary care: Methods from a pragmatic cluster randomized clinical trial. Contemp Clin Trials. 2018;72:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiore MC, Jaen CR, Baker T, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 25.Zhuang Y-L, Gamst AC, Cummins SE, Wolfson T, Zhu S-H. Comparison of smoking cessation between education groups: findings from 2 US National Surveys over 2 decades. Am J Public Health. 2015;105(2):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doogan NJ, Roberts ME, Wewers ME, Tanenbaum ER, Mumford EA, Stillman FA. Validation of a new continuous geographic isolation scale: A tool for rural health disparities research. Soc Sci Med. 2018;215:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu SS, van Ryn M, Nelson D, et al. Proactive tobacco treatment offering free nicotine replacement therapy and telephone counselling for socioeconomically disadvantaged smokers: a randomised clinical trial. Thorax. 2016;71(5):446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koma JW, Donohue J, Barry CL, Huskamp HA, Jarlenski M. Medicaid coverage expansions and cigarette smoking cessation among low-income adults. Med Care. 2017;55(12):1023–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houston TK, Scarinci IC, Person SD, Greene PG. Patient smoking cessation advice by health care providers: the role of ethnicity, socioeconomic status, and health. Am J Public Health. 2005;95(6):1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernstein SL, Cabral L, Maantay J, et al. Disparities in access to over-the-counter nicotine replacement products in New York City pharmacies. Am J Public Health. 2009;99(9):1699–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendricks PS, Westmaas JL, Park T, et al. Smoking abstinence-related expectancies among American Indians, African Americans, and women: Potential mechanisms of tobacco-related disparities. Psychol Addict Behav. 2014;28(1):193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahende JW, Malarcher AM, Teplinskaya A, Asman KJ. Quit attempt correlates among smokers by race/ethnicity. Int J Environ Res Public Health. 2011;8(10):3871–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu SS, Kodl MM, Joseph AM, et al. Racial/ethnic disparities in the use of nicotine replacement therapy and quit ratios in lifetime smokers ages 25 to 44 years. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1640–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu SS, Sherman SE, Yano EM, Van Ryn M, Lanto AB, Joseph AM. Ethnic disparities in the use of nicotine replacement therapy for smoking cessation in an equal access health care system. Am J Health Promot. 2005;20(2):108–116. [DOI] [PubMed] [Google Scholar]
- 35.Carpenter MJ, Ford ME, Cartmell K, Alberg AJ. Misperceptions of nicotine replacement therapy within racially and ethnically diverse smokers. J Natl Med Assoc. 2011;103(9–10):885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan KK, Garrett-Mayer E, Alberg AJ, Cartmell KB, Carpenter MJ. Predictors of cessation pharmacotherapy use among black and non-Hispanic white smokers. Nicotine Tob Res. 2011;13(8):646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yerger VB, Wertz M, McGruder C, Froelicher ES, Malone RE. Nicotine replacement therapy: perceptions of African-American smokers seeking to quit. J Natl Med Assoc. 2008;100(2):230–236. [DOI] [PubMed] [Google Scholar]


