Abstract
Tuberous sclerosis complex (TSC) is a rare genetic syndrome that confers risk for neurodevelopmental disorders, including autism spectrum disorder and intellectual disability. Delays in social communication and early cognitive abilities are observable as early as 9 months of age in children with TSC; however, there have been no studies of early behavioral intervention in TSC. We conducted a pilot study of an evidence-based, parent-mediated behavioral intervention focused on improving early social communication and play skills in 5 children with TSC (aged 1–3 years). Participants showed maintenance and sometimes gains in developmental abilities, relative to peers, following intervention. Parents generally found the intervention to be helpful and were able to administer the intervention with fidelity. Preliminary results demonstrate initial feasibility of an early play-based, parent-mediated intervention and support the need for a large-scale, randomized clinical trial in TSC.
Keywords: autism spectrum disorder, behavioral intervention, early intervention, tuberous sclerosis complex
TUBEROUS SCLEROSIS COMPLEX (TSC) is a rare autosomal dominant disorder caused by mutations in the TSC1 or TSC2 genes. Tuberous sclerosis complex is increasingly diagnosed during infancy or even prenatally based on clinical presentation that includes the identification of cardiac or brain hamartomas (Datta, Hahn, & Sahin, 2008; Davis et al., 2017; Ebrahimi-Fakhari et al., 2018). Infants with TSC commonly first present with cardiac rhabdomyomas and/or skin lesions, with epilepsy presenting in the majority of patients within the first year of life (Davis et al., 2017). Tuberous sclerosis complex is strongly associated with neurodevelopmental disabilities, which fall under the category of “TAND,” or “TSC-associated neuropsychiatric disorders” (de Vries et al., 2018). The two most common diagnoses in this category are intellectual disability (ID) and autism spectrum disorder (ASD). Up to 80% of children with TSC experience some level of cognitive impairment, from milder learning disabilities to severe ID, and rates of ASD approach 60% (Bolton et al., 2015; Curatolo, Napolioni, &Moavero, 2010; Jeste et al., 2016). Both ID and ASD often co-occur and are sometimes difficult to disentangle. This comorbidity raises challenges not only in the diagnosis of ASD but also in the identification of appropriate intervention targets.
We recently completed the first prospective, longitudinal study of development in infants with TSC (Jeste et al., 2014). We found that infants with TSC demonstrate early delays in nonverbal cognition and social communication skills, and these delays were most prominent in those who develop ASD. In fact, by 9 months of age, social communication delays differentiated infants who were later diagnosed with ASD from those without ASD (McDonald et al., 2017). Moreover, TSC infants with ASD outcomes demonstrated a significant decline in their nonverbal cognitive abilities from 12 to 36 months of age, suggesting a greater divergence from typical development in the second and third years of life (Jeste et al., 2014).
These early behavioral markers of ASD necessitate the implementation of interventions that target early developmental skills, such as social communication and cognition, in TSC. Early intervention improves outcomes in ASD (Dawson, 2013). A growing body of work has shown that interventions targeting nonverbal communication can improve language and social interaction in toddlers at high risk for ASD (e.g., Kasari, Gulsrud, Paparella, Hellemann, & Berry, 2015). Perhaps, because of comorbid medical concerns in infancy, such as epilepsy, or limited awareness about ASD risk in TSC, there are no published studies of the effects of early behavioral intervention on development in TSC. In fact, there are few studies of behavioral intervention for ASD in genetic syndromes more broadly, despite their high penetrance for neurodevelopmental disorders. Before designing a randomized clinical trial (RCT) of early intervention in TSC, we first needed to ask whether the implementation of a targeted behavioral intervention was feasible for these families and whether we could measure stability or gain (vs. decline) in nonverbal cognitive and social communication skills in these high-risk children. Given our focus on social communication, we implemented JASPER (Joint Attention, Symbolic Play, Engagement, and Regulation), which is a Naturalistic Developmental Behavioral Intervention (Schreibman et al., 2015) that is evidence based for therapist-, teacher-, and parent-mediated models based upon results from multiple RCTs (e.g., Kasari et al., 2014, 2015). JASPER specifically targets the foundations of social communication using naturalistic behavioral strategies to increase the rate and complexity of social communication and includes parents as implementers of the intervention to promote generalization across settings and ensure maintenance of treatment gains (Kasari et al., 2015).
Here, we describe the implementation and outcomes from a pilot intervention of JASPER in five infants with TSC (NCT02687633). We defined outcomes as (1) infant developmental milestones and social communication skills and (2) parent perception of the intervention experience. We hypothesized that there would be considerable variability in response but that infants would show stability or progression in overall development and social communication skills following the intervention and parents would describe benefits from the intervention.
METHODS
Participants
Participants described in this two-site pilot intervention study include five children with TSC who ranged in age from 13 to 45 months at the first assessment visit (Table 1). Three additional participants were not included because of incomplete participation related to difficulty visiting the treatment sites on a weekly basis. Diagnoses of TSC were based on clinical presentation (Northrup & Krueger, 2013). Families reported outside intervention hours (e.g., speech therapy) to be limited, ranging from 0.25 to 4 hr/week; no children were receiving social communication or behavioral treatments (e.g., applied behavior analysis). Seizures were controlled for all participants during the study.
Table 1.
Patient Demographics
| Age (Months) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Sex | T1 | T2 | T3 | Race/Ethnicity | Maternal Education | Genetic Diagnosis | History of Seizures? | History of Infantile Spasms? | Seizure Age at Onset (Months) | Number of Antiepileptics |
| 1 | M | 13 | – | 19 | White, not Hispanic | Graduate/professional degree | TSC2 | Y | Y | 6 | 1 |
| 2 | M | 22 | 26 | 29 | White, not Hispanic | 4-year college degree | - | Y | N | 15 | 3 |
| 3 | F | 25 | 29 | 36 | Black/African American, Hispanic | Some college | TSC2 | Y | Y | 7 | 1 |
| 4 | M | 38 | 41 | 45 | White, not Hispanic | Graduate/professional degree | TSC1 | Y | Y | 12 | 3 |
| 5 | M | 45 | 49 | - | White, not Hispanic | Associate degree | TSC1 | N | N | N/A 9.2 (4.1)a | 0 1.6 (1.3)a |
Note. F = female; M = male; N = no; N/A = not applicable; Y = yes.
Mean (SD).
Procedure
Institutional review board approval was obtained from both sites, and informed consent acquired was from all families. Children attended up to three assessment visits: a preassessment visit immediately before beginning intervention (T1), a postassessment visit within 2 weeks of completing the intervention (T2), and a follow-up approximately 6 months after initiation of intervention (T3). At T1, T2, and T3, children were administered a measure of early cognitive abilities. At T1 and T3, children were also administered a measure of ASD symptoms.
Behavioral intervention
Children engaged in a 12-week parent-mediated behavioral intervention focused on improving social communication and play skills, which included daily sessions for 2 weeks, followed by weekly sessions for 10 weeks. JASPER is an empirically supported and manualized treatment option for children, with a primary focus on improving joint engagement, joint attention, and play skills (Kasari et al., 2015). Parents are first taught to recognize the child’s current level of play and use of social communication gestures. They are then taught strategies for maintaining engagement with their child and facilitating gestures, spoken language, and play behaviors. Interventionists reached and maintained fidelity to JASPER prior to initiation of the study.
Measures
Mullen Scales of Early Learning
The Mullen Scales of Early Learning (MSEL) is a standardized measure of early cognitive abilities (Mullen, 1995) that examines visual reception, fine motor, receptive language, and expressive language skills. Each subscale yields a t-score (M = 50, SD = 10) and age equivalent. The Early Learning Composite (ELC) is calculated on the basis of these subscales, yielding a standard score (M = 100, SD = 15). Given results from Jeste et al. (2014), our primary variables of interest were changes in MSEL scores pre- to postintervention. To be included, participants had to have MSEL data at baseline and at least one outcome visit.
Autism Diagnostic Observation Schedule-Second Edition
The Autism Diagnostic Observation Schedule-Second Edition (ADOS-2) is a semistructured, play-based observational measure of social communication, play, and repetitive behaviors that is used as a diagnostic indicator of ASD (Lord, Luyster, Guthrie, & Pickles, 2012). The ADOS was performed by research-reliable examiners. Data from this measure were used as an indicator of whether the children had a clinically significant level of ASD symptoms at T1 and a secondary indicator of response to intervention. For inclusion, participants had to have data on ASD symptoms at baseline and one outcome visit. Children were considered to have ASD concern if they had a severity score of 4 or higher (Gotham, Pickles, & Lord, 2009).
Autism Observation Scale for Infants
The Autism Observation Scale for Infants (AOSI) is a semistructured, play-based observational measure that is used as an indicator of early risk for ASD for infants aged 6–18 months (Bryson, Zwaigenbaum, McDermott, Rombough, & Brian, 2008). The AOSI was used to determine ASD symptoms at T1 for one child who did not meet the requirements of the ADOS-2. An AOSI total score of 9 or higher was considered elevated (Bryson et al., 2008).
Caregiver Diary
Parents rated their adherence and perceived competence in the intervention on a 1–5 scale (four adherence and two competence questions, averaged across weekly sessions; Kasari et al., 2014).
Caregiver Involvement Scale
Interventionists rated parents’ fidelity to the intervention on a 1-5 scale (four items, averaged across weekly sessions; Kasari et al., 2014). Interventionists also rated parent perception of the intervention on a 1-5 scale based upon a short interview with the parent following each session. A score of 1 represented a negative perception (“didn’t like it,” “child not cooperating”) and a score of 5 a positive perception (“intervention really working”; Kasari et al., 2014).
Analysis
We took a qualitative approach to describing each child’s abilities and behavior pre- and postintervention, modeled after similar pilot studies in neurodevelopmental disorders (Hogan et al., 2017; Kolesnik et al., 2017; Steiner, Gengoux, Klin, & Chawarska, 2013). The levels of each child’s abilities are discussed relative to standardized norms (MSEL) and clinical cutoffs (ADOS-2; Figure 1) and behaviors based upon specific ADOS-2/AOSI codes. Each child is described as having “ASD concern” or “no ASD concern” based upon ADOS-2/AOSI data at T1. Interpretation of children’s development pre- to postintervention is discussed relative to expectations, given findings from our previously published longitudinal study (Jeste et al., 2014). Parent perceptions of the intervention, including qualitative feedback provided by participating parents, are also reported.
Figure 1.
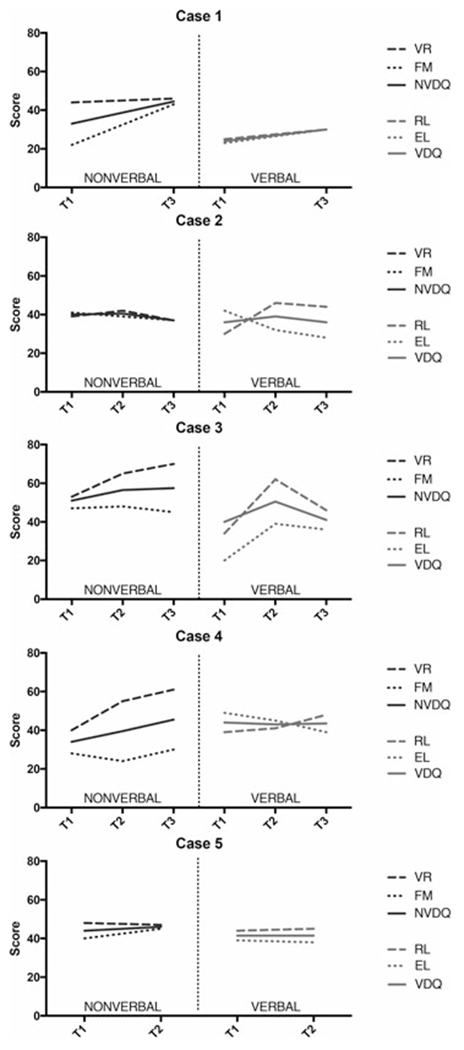
Participant cognitive abilities pre- and postintervention. EL = expressive language; FM = fine motor; NVDQ = nonverbal developmental quotient; RL = receptive language; VDQ = verbal developmental quotient; VR = visual reception.
RESULTS
Case 1: 13 months, male, TSC2, mild ASD concern (AOSI)
Preassessment
Case 1’s overall developmental abilities initially fell within the Very Low range. Visual reception skills were Average, whereas skills in other domains were in the Very Low range. The AOSI indicated mildly elevated concern for ASD. He did not orient to his name, engage in social babbling or social referencing, or imitate actions. Limited eye contact, inconsistent social interest, and difficulty with transitions were also observed. Basic visual attention and motor behaviors were intact, and no atypical sensory or motor behaviors were observed.
Postassessment
A notable increase in overall developmental abilities was observed at T3 (T2 missing), increasing from the Very Low to Below Average range overall. Age-appropriate gains in visual reception skills were made, whereas increases in t-scores were observed in fine motor, receptive language, and expressive language. On the ADOS-2 (Toddler Module), a clinically elevated, moderate level of early ASD symptoms was observed. He oriented to his name, used a few words and gestures, engaged in some babbling, and his imaginative play skills were emerging. Joint attention and eye contact remained limited, and unusual sensory interests were observed.
Parent ratings
The mother’s fidelity (4.28 [0.52]) and perception (4.40 [0.53]) of the intervention were rated highly on the Caregiver Involvement Scale. A moderate level of adherence (3.83 [0.88]) and a moderately high level of competence (4.11 [0.80]) were reported on the Caregiver Diary. She shared that the intervention “has taught me to play more age-appropriate games with my child” and “to simplify objects, labels, and play.”
Case 2: 22 months, male, moderate ASD concern (ADOS)
Preassessment
Overall developmental abilities on the MSEL fell in the Below Average range at T1. His visual reception skills were Below Average and fine motor skills were Average. His expressive language skills were also Average, but his receptive language was in the Below Average range. The ADOS (Toddler Module) indicated a clinically significant, moderate level of ASD symptoms. His babbling and word usage during the ADOS were limited, although vocalizations were directed to others frequently. Gestures were limited and eye contact inconsistent. Occasional repetitive behaviors were observed. However, he shared his enjoyment with others and showed some emerging joint attention and play skills.
Postassessment
Case 2’s overall developmental abilities were stable at T2 and T3. Visual reception t-scores were steady, whereas fine motor skills fell slightly relative to peers. A large increase was observed in his receptive language t-score; however, he did not gain expressive language skills, leading to a decrease in his t-score. On the ADOS (Toddler Module) at T3, his score no longer fell within the clinical range. He communicated using several recognizable single words, pointed and gestured, and engaged in various joint attention behaviors. Eye contact and the quality of his social overtures remained inconsistent.
Parent ratings
The Caregiver Involvement Scale indicated high levels of parent fidelity (4.52 [0.34]) and perception (4.67 [0.49]) of the intervention. The Caregiver Diary showed a moderate level of adherence (3.64 [0.38]) and a moderately high level of competence (4.03 [0.48]) with the intervention. The mother shared that “every time we meet I get more ways to help him or have a better understanding of the strategies”; “the training has improved my ability to keep him engaged during play.”
Case 3: 25 months, female, TSC2, no ASD concern (ADOS)
Preassessment
At T1, Case 3’s overall developmental abilities were within the Average range. Visual reception, fine motor, and receptive language skills were Average prior to treatment, whereas expressive language fell Below Average. Her ADOS (Toddler Module) score indicated little to no concern for ASD. She used some single words to communicate, directed vocalizations to others frequently, and initiated joint attention. No repetitive behaviors were observed. Her eye contact was inconsistent, and she did not imitate the examiner’s actions.
Postassessment
At T2, a notable increase in overall developmental abilities was observed, although the standard score fell somewhat by T3. Her visual reception t-score improved significantly. She did not achieve age-appropriate gains in fine motor skills, although they were Average across time points. An increase in her receptive language t-score was observed from T1 to T2; this growth did not continue to T3. Slow growth was observed in expressive language, with her score falling Below Average across time points. On the ADOS (Module 1) at T3, her score again indicated little concern for ASD. She used a number of words and short phrases, pointed, used appropriate eye contact, initiated joint attention, and demonstrated some functional play skills.
Parent ratings
On the Caregiver Involvement Scale, the parent’s fidelity (4.25 [0.20]) and perception (4.50 [0.58]) of the intervention were rated highly. Her mother reported high levels of adherence (4.50 [0.76]) and competence (4.38 [1.41]) with the intervention on the Caregiver Diary. Qualitative comments included the following: “It gave me concrete ways to help my child communicate, improve her vocabulary, and interact with her family and language”; “it has improved my child’s communication skills and my ability to help her with her communication”; “it has also given me skills for more beneficial interaction between my child and the adults in her life.”
Case 4: 37 months, male, TSC1, high ASD concern (ADOS)
Preassessment
Case 4’s overall developmental abilities were Below Average at T1. His visual reception skills were within the Average range, whereas his fine motor skills were in the Very Low range. His expressive language was Average and receptive language Below Average. The ADOS (Module 2) indicated a clinically significant and high level of ASD symptoms. He used phrase speech with an odd intonation and regularly used immediate echolalia and stereotyped utterances. Gestures and eye contact were inconsistent, and the quality of his social behaviors was limited. Unusual sensory interests and repetitive behaviors were observed. He did, however, show some nice joint attention abilities.
Postassessment
His overall ELC score improved from T1 to T2 and T3; this was mainly due to large increases in his visual reception abilities at T2 and T3 and more modest increases in his receptive language. Age-appropriate gains were made in fine motor skills over time. He did not make gains in expressive language, which fell from the Average to Below Average range. On the ADOS at T3, his score continued to exceed the clinical cutoff, although at a moderate versus high level. Phrases were spoken at a higher level than at T1 and did not include repetitive language. No unusual sensory behaviors and minimal repetitive play were observed. Eye contact and social reciprocity continued to be inconsistent.
Parent ratings
The Caregiver Involvement Scale indicated high ratings of caregiver fidelity (4.43 [0.49]) and perception (4.54 [0.69]) of the intervention. Caregiver Diary ratings revealed a moderate level of adherence (3.96 [0.30]) and moderately high competence (4.08 [0.37]) with the intervention. His mother reported that “repeating what he says to help him be ‘heard’—think it has built his confidence”; “working on his play skills, pointing, and using language was beneficial”; “it’s been most helpful in providing me with strategies to use at home.”
Case 5: 44 months, male, TSC1, moderate ASD concern (ADOS)
Preassessment
Case 5’s overall developmental abilities were in the Average range at T1. His visual reception, fine motor, and receptive language skills were Average, whereas his expressive language fell Below Average. The ADOS (Module 2) indicated a clinically significant and moderate level of ASD symptoms at T1. He used phrase speech characterized by unusual intonation and occasional stereotyped language. He had difficulty engaging in reciprocal conversation and used gestures inconsistently. Socially, he skillfully used eye contact and engaged in some joint attention behaviors, but his social overtures were infrequent and of varying quality. Repetitive play and motor mannerisms were observed. Imaginative and functional play behaviors were present.
Postassessment
At T2 (T3 missing), his overall developmental abilities remained steady. He made age-appropriate gains in visual reception, receptive language, and expressive language, whereas his fine motor score increased. Although his ADOS score indicated a slightly higher level of ASD symptoms at T2, he was administered a Module 3 versus Module 2, suggesting increased functional language postintervention. He used complex speech with occasional grammatical errors (vs. phrase speech at T1 ) and continued to engage in repetitive and stereotyped language at times. He asked some questions and shared some information about himself but still did not engage in reciprocal conversation. Social overtures continued to be of variable quality, with reduced quality of eye contact but regular use of gestures. Repetitive behaviors were not observed.
Parent ratings
On the Caregiver Involvement Scale, parent fidelity (4.69 [0.24]) and perception (4.83 [0.30]) of the intervention were rated highly. On the Caregiver Diary, his mother reported high levels of adherence (4.63 [0.52]) and competence (4.77 [0.59]) with the intervention. Qualitatively, she shared: “Knowing the best way to communicate with my child has been most beneficial”; “being part of the play sessions was useful”; “my child has more confidence in telling me things.”
DISCUSSION
As we gain a deeper understanding of the earliest developmental manifestations of TSC, in particular social communication and cognitive delays that precede ASD or ID diagnoses, it is necessary to test models of early intervention that may improve developmental trajectories and outcomes. We studied a small but clinically representative sample of young children with TSC, as evidenced by the high rate of epilepsy, developmental delays, and ASD symptoms. The high education levels of our families, however, may reflect a selection bias toward families with enough resources to participate in a rather demanding study. Our results can be summarized along three key themes: (1) Delivery of weekly behavioral intervention was feasible in infants with TSC, but the requirement that sessions occur in person may pose challenges for recruitment and retention of young children with this rare condition; (2) infants receiving JASPER exhibited variable but clinically meaningful gains in a variety of developmental domains; and (3) parent and interventionist reports suggested the acceptability and fidelity of JASPER in this population. These pilot data have guided the development of an ongoing RCT of JASPER in TSC and can inform future studies of behavioral intervention in rare genetic syndromes.
Despite having a genetic diagnosis that clearly confers a high risk for ASD and ID, these toddlers were receiving relatively few early intervention services before enrolling in this study and no services focused on building social communication skills. Although it is possible that families receiving fewer services may more actively seek out an early intervention study, leading to recruitment bias, it is likely that this disparity in early intervention results from factors intrinsic to the clinical realities of having a child with TSC. These toddlers have other medical comorbidities, such as epilepsy, that require immediate attention and may limit parents’ ability to seek out or participate in interventions. There also may be limited awareness by clinicians and caregivers about the early neurodevelopmental manifestations of TSC.
Although the administration of JASPER proved feasible, poor recruitment and attrition were factors that require further consideration and have guided the design of the ongoing RCT. Of the eight children enrolled, three were unable to complete the required assessments or the entire intervention protocol. Recruitment occurred across a large geographic area, requiring families to travel to attend sessions. Such challenges are inherent in studies of rare and/or medically complex disorders. The development of alternative strategies, such as remote assessment and intervention delivery, is needed to maximize participation, inclusion of all interested families, and generalizability of findings. The inclusion of parents as mediators of treatment, as in the current behavioral intervention, may allow for increased accessibility through remote intervention approaches.
To examine response to intervention, we assessed both child developmental outcomes and the parent experience. Most studies of JASPER have examined more discrete nonverbal communication behaviors (e.g., joint engagement) as the primary outcome. Here, we focused on clinically relevant developmental/cognitive domains because of the prior natural history study that provided us with context around expected developmental changes in TSC. That study showed that infants with TSC/ASD experienced declines in their cognitive scores, particularly in the non-verbal domain, from 12 to 36 months, reflecting their inability to keep up with peers. This slow but steady decrease in development has also been reported in a subset of infants with a familial risk for ASD through a latent class analysis (Landa, Gross, Stuart, & Bauman, 2012). Another more recent study also described a decline in adaptive skills in young children with ASD and low cognitive ability (Farmer, Swineford, Swedo, & Thurm, 2018). These data underscore the importance of defining natural developmental trajectories in children with developmental disabilities, as the change exacted with intervention must be placed in the context of expected change rather than against typically developing norms. JASPER’s apparent beneficial effect on development in the current study may also be mediated by other key factors, such as improved parental understanding of their child’s abilities and needs, introduction to techniques in interacting with their child to boost overall skills, and close monitoring of their child’s development that in itself promotes healthy development. These mediating factors can be quantified and examined in a larger scale trial.
As a whole, children in this pilot study showed a trajectory of stability and, in some cases, gains in their standardized developmental scores with intervention. For example, Case 2 demonstrated ASD concern based on the ADOS at entry. After intervention, he was below the clinical cutoff for ASD on this measure, with qualitative analysis suggesting gains in functional language use (i.e., babbling to single-word usage) and nonverbal communication skills such as pointing and gesturing. His nonverbal cognitive scores remained steady, with notable increases in receptive language skills observed. Without a natural history study to provide some developmental context for this population, a lack of change in MSEL t-scores might be interpreted as a lack of response to an intervention, rather than a meaningful improvement from the expected change in development. As rare disorder patient alliances develop patient registries and facilitate the collection of natural history data, we will have a more robust framework in which to understand subtle (yet perhaps clinically meaningful) change with interventions.
Parent delivery of intervention promotes scalability and engages parents directly in monitoring and advocating for their child’s developmental progress. For this pilot study, parents rated their experience and interventionists rated parents’ fidelity with the intervention, both of which were rated highly. Qualitatively, key themes that emerged include enhanced proficiency in playing with their child in a developmentally appropriate way, improved communication with their child, and an introduction to specific strategies to engage with their child. It is likely that parent perception of the intervention and their proficiency in intervention delivery play key modifier roles in children’s outcome, requiring more targeted measurement in future clinical trials. Parents’ improved awareness about their children’s development and methods to interact with them points to a potential information gap and opportunity to enhance awareness about the early neurodevelopmental manifestations of TSC.
Infant development represents a complex interplay of genetics and environment. Mutations in TSC genes cause profound aberrations in cortical development, from hamartomas (tubers) to more distributed disruptions in neural connectivity (Tsai & Sahin, 2011). Although these structural abnormalities are static, they do not preclude the potential of an enriched early environment to enhance further cortical development and, as a result, neurodevelopment and behavior. Despite several studies of early behavioral intervention in infants with early signs of ASD, this study represents the first attempt to systematically evaluate the feasibility and effects of targeted early behavioral intervention in TSC. Our data show initial feasibility and acceptability of a parent-mediated behavior intervention in a clinically representative sample of infants with TSC and support the need for a large-scale RCT of early behavioral intervention that will examine intervention effects on brain development through direct assessments of neural function and behavior in these high-risk infants.
Acknowledgments
The research reported in this article was supported by a Pilot Clinical Trial Award from the Department of Defense (DOD CDMRP TSCRP: W81XMH-15–1-0183). The aid hots acknowledge the contributions of Scott Huberty and the broader TSC study team in recruitment, assessment, intervention, and data management. The authors thank the families who participated in this research.
Footnotes
S.S.J. serves as a consultant for Roche Pharmaceuticals and on the professional advisory board for the Tuberous Sclerosis Alliance. The remaining authors (N.M.M., C.H., A.B.C., A.G., C.K., and C.A.N.) do not have any conflicts of interest associated with this study.
REFERENCES
- Bolton PF, Clifford M, Tye C, Maclean C, Humphrey A, le Marechal K, … Yates JR (2015). Intellectual abilities in tuberous sclerosis complex: Risk factors and correlates from the Tuberous Sclerosis 2000 Study. Psychological Medicine, 45, 2321–2331. doi: 10.1017/s0033291715000264 [DOI] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, & Brian J (2008). The Autism Observation Scale for Infants: Scale development and reliability data. Journal of Autism and Developmental Disorders, 38, 731–738. [DOI] [PubMed] [Google Scholar]
- Curatolo P, Napolioni V, & Moavero R (2010). Autism spectrum disorders in tuberous sclerosis: Pathogenetic pathways and implications for treatment. Journal of Child Neurology, 25, 873–880. doi: 10.1177/0883073810361789 [DOI] [PubMed] [Google Scholar]
- Datta AN, Hahn CD, & Sahin M (2008). Clinical presentation and diagnosis of tuberous sclerosis complex in infancy. Journal of Child Neurology, 23, 268–273. doi: 10.1177/0883073807309250 [DOI] [PubMed] [Google Scholar]
- Davis PE, Filip-Dhima R, Sideridis G, Peters JM, Au KS, Northrup H, … Tuberous Sclerosis Complex Autism Center of Excellence Research Network. (2017). Presentation and diagnosis of tuberous sclerosis complex in infants. Pediatrics, 140(6). doi: 10.1542/peds.2016-4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G (2013). Early intensive behavioral intervention appears beneficial for young children with autism spectrum disorders. Journal of Pediatrics, 162, 1080–1081. doi: 10.1016/j.jpeds.2013.02.049 [DOI] [PubMed] [Google Scholar]
- de Vries PJ, Belousova E, Benedik MP, Carter T, Cottin V, Curatolo P, … Jansen AC. (2018). TSC-associated neuropsychiatric disorders (TAND): Findings from the TOSCA natural history study. Orphanet Journal of Rare Diseases, 13, 157. doi: 10.1186/s13023-018-0901-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Mann LL, Poryo M, Graf N, von Kries R, Heinrich B, … Meyer S (2018). Incidence of tuberous sclerosis and age at first diagnosis: New data and emerging trends from a national, prospective surveillance study. Orphanet Journal of Rare Disorders, 13, 117. doi: 10.1186/s13023-018-0870-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer C, Swineford L, Swedo SE, & Thurm A (2018). Classifying and characterizing the development of adaptive behavior in a naturalistic longitudinal study of young children with autism. Journal of Neurodevelopmental Disorders, 10, 1–9. doi: 10.1186/s11689-017-9222-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, & Lord C (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39,693–705. doi: 10.1007/s10803-008-0674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan AL, Caravella KE, Ezell J, Rague L, Hills K, & Roberts JE (2017). Autism spectrum disorder symptoms in infants with fragile X syndrome: A prospective case series. Journal ofAutism and Developmental Disorders, 47, 1628–1644. doi: 10.1007/s10803-017-3081-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste SS, Varcin KJ, Hellemann GS, Gulsrud AC, Bhatt R, Kasari C, … Nelson CA III. (2016). Symptom profiles of autism spectrum disorder in tuberous sclerosis complex. Neurology, 87, 766772. doi: 10.1212/wnl.0000000000003002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste SS, Wu JY, Senturk D, Varcin K, Ko J, McCarthy B, … Nelson CA III. (2014). Early developmental trajectories associated with ASD in infants with tuberous sclerosis complex. Neurology, 83, 160–168. doi: 10.1212/wnl.0000000000000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasari C, Gulsrud A, Paparella T, Hellemann G, & Berry K (2015). Randomized comparative efficacy study of parent-mediated interventions for toddlers with autism. Journal of Consulting and Clinical Psychology, 83(3), 554–563. doi: 10.1037/a0039080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasari C, Lawton K, Shih W, Barker TV, Landa R, Lord C, … Senturk D (2014). Caregiver-mediated intervention for low-resourced preschoolers with autism: An RCT. Pediatrics, 134, e72–e79. doi: 10.1542/peds.2013-3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnik AM, Jones EJH, Garg S, Green J, Charman T, Johnson MH, & Team E-B (2017). Early development of infants with neurofibromatosis Type 1: A case series. Molecular Autism, 8, 62. doi: 10.1186/s13229-017-0178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, & Bauman M (2012). Latent class analysis of early developmental trajectory in baby siblings of children with autism. Journal of Child Psychology and Psychiatry, 53, 986–996. doi: 10.1111/j.1469-7610.2012.02558.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Luyster R, Guthrie W, &Pickles A (2012). Patterns of developmental trajectories in toddlers with autism spectrum disorder. Journal of Consulting and Clinical Psychology, 80, 477–489. doi: 10.1037/a0027214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald NM, Varcin KJ, Bhatt R, Wu JY, Sahin M, Nelson CA III, & Jeste SS (2017). Early autism symptoms in infants with tuberous sclerosis complex. Autism Research, 10, 1981–1990. doi: 10.1002/aur.1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E (1995). Mullen Scales of Early Learning Circle Pines, MN: American Guidance Service. [Google Scholar]
- Northrup H., Krueger DA.,& International Tuberous Sclerosis Complex Consensus Group. (2013). Tuberous sclerosis complex diagnostic criteria update: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatric Neurology, 49, 243–254. doi: 10.1016/j.pediatrneurol.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibman L, Dawson G, Stahmer AC, Landa R, Rogers SJ, McGee GG, … Halladay A (2015). Naturalistic developmental behavioral interventions:Empirically validated treatments for autism spectrum disorder. Journal of Autism and Developmental Disorders, 45, 2411–2428. doi: 10.1007/s10803-015-2407-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AM, Gengoux GW, Klin A, & Chawarska K (2013). Pivotal response treatment for infants at-risk for autism spectrum disorders: A pilot study. Journal of Autism and Developmental Disorders, 43, 91102. doi: 10.1007/s10803-012-1542-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P, & Sahin M (2011). Mechanisms of neurocognitive dysfunction and therapeutic considerations in tuberous sclerosis complex. Current Opinion in Neurology, 24, 106–113. doi: 10.1097/WCO.0b013e32834451c4 [DOI] [PMC free article] [PubMed] [Google Scholar]


