Abstract
Background:
Most people with alcohol or opioid use disorders (AUD or OUD) are not diagnosed or treated for these conditions in primary care. This study takes a critical step toward quantifying service gaps and directing improvement efforts for AUD and OUD by using electronic health record (EHR) data from diverse primary care organizations to quantify the extent to which AUD and OUD are underdiagnosed and undertreated in primary care practices.
Methods:
We extracted and integrated diagnosis, medication, and behavioral health visit data from the EHRs of 21 primary care clinics within four independent healthcare organizations representing community health centers and rural hospital-associated clinics in the Pacific Northwest United States. Rates of documented AUD and OUD diagnoses, pharmacological treatments, and behavioral health visits were evaluated over a two-year period (2015–2016).
Results:
Out of 47,502 adult primary care patients, 1476 (3.1%) had documented AUD; of these, 115 (7.8%) had orders for AUD medications and 271 (18.4%) had at least one documented visit with a non-physician behavioral health specialist. Only 402 (0.8%) patients had documented OUD, and of these, 107 (26.6%) received OUD medications and 119 (29.6%) had at least one documented visit with a non-physician behavioral health specialist. Rates of AUD diagnosis and AUD and OUD medications were higher in clinics that had co-located non-physician behavioral health specialists.
Conclusions:
AUD and OUD are underdiagnosed and undertreated within a sample of independent primary care organizations serving mostly rural patients. Primary care organizations likely need service models, technologies, and workforces, including non-physician behavioral health specialists, to improve capacities to diagnose and treat AUD and OUD.
Introduction
Over 34 million US adults suffer from alcohol use disorder (AUD) or opioid use disorder (OUD) annually (Grant et al., 2015; Han et al., 2017). Together, these conditions account for 3.8% of all disability-adjusted life years, which is comparable to other chronic medical conditions including diabetes or major depression (Institute for Health Metrics and Evaluation, 2016). AUD and OUD are also largely untreated conditions; in the US adult population, only 7.6% of individuals with AUD and 14.6% of individuals with drug use disorders obtain treatment within a one-year period (Substance Abuse and Mental Health Services Administration, 2015).
There are increasing calls to improve access to AUD and OUD treatments in primary care, where treatments – especially medication-based treatments – may be more accessible, convenient, and potentially more cost efficient than traditional specialty substance use disorder treatment (Spithoff & Kahan, 2015; Bradley & Kivlahan, 2014; McLellan et al., 2013; McLellan & Woodworth, 2014). Efforts to treat AUD and OUD in primary care are further supported by improvements in coverage for substance use disorder treatment by US medical insurers and advancements in medications for AUD and OUD that can be prescribed in primary care, which, particularly for OUD, are critical components of evidence based treatment (Ducharme, Chandler, & Harris, 2016; McLellan & Woodworth, 2014; Watkins et al., 2017; Wu et al., 2017). These efforts also are aided by an increasing presence of behavioral health services co-located within primary care, which can help patients enhance their motivation for change, learn cognitive-behavioral strategies for initiating and maintaining change, maintain engagement in medication assisted treatments, and address co-occurring mental health problems (Schuckit et al., 2016).
It is well recognized that AUD and OUD are substantially underdiagnosed and undertreated within primary care (Bowman et al., 2013; Rieckmann et al., 2016; Spithoff & Kahan, 2015). This gap has been attributed to several factors, including limited clinician knowledge and training on substance use disorders, skepticism about the effectiveness of AUD and OUD treatments, lack of universal screening, and stigma around substance use (Glass et al., 2017; Kuehn, 2008; Venner et al., 2018; Wakeman & Barnett, 2018). However, the extent to which AUD and OUD are underdiagnosed is not well known, and more precisely quantifying gaps within the “cascade of care” for diagnosing and treating these conditions is a critical step for understanding service needs, directing quality improvement efforts, and monitoring improvements in care systems (Sanghavi et al., 2017; Socías, Volkow, & Wood 2015; Tai & McLellan, 2012; Williams et al., 2018, 2019).
Electronic health records (EHRs) have been identified as key tools to assist with efforts for evaluating and improving AUD and OUD care (Ghitza et al., 2011; Wu et al., 2017) and can be help characterize AUD and OUD treatment “cascades of care” (Socías et al., 2015; Williams et al., 2018, 2019). Previous studies using EHR data to quantify AUD and OUD diagnosis and treatment rates have usually taken place within large, integrated healthcare systems such as Kaiser Permanente (KP; e.g., Bahorik et al., 2017) or the Veterans Health Administration (VHA; e.g., Williams et al., 2017). For example, the KP Northern California health system estimated the one-year prevalence of documented AUD and OUD diagnoses at 6.3% and 1.4%, respectively, using data from their integrated EHR system (Bahorik et al., 2017). In the VHA, the estimated one-year prevalence of documented AUD diagnoses was similarly 6.4% and EHR records indicated that 5.1% of patients with a documented AUD diagnosis also had documented orders for an FDA-approved AUD medication (Williams et al., 2017). All of these estimates indicate substantially lower rates of diagnosis and treatment in routine care compared to the estimated prevalence of AUD and OUD within primary care clinics based on confidential, structured research interviews with a large sample of patients in urban and suburban primary care clinics in four East Coast US states (AUD: 13.9%, OUD: 4.7%; John et al., 2018; Wu et al., 2017).
Collectively, these studies have helped quantify the extent to which AUD and OUD are underdiagnosed and undertreated in large, integrated health systems that provide primary care. However, these settings may not generalize to smaller and medium sized primary care practices (e.g., clinics with ≤ 20 physicians), which constitute the majority of primary care practices in the US (Bauer et al., 2012; Liaw et al., 2016). These may also fail to generalize to other geographic regions and to clinics that serve rural and socioeconomically disadvantaged populations, which often have variable and limited integrated services to systematically diagnose and treat AUD and OUD. In one exception to this limitation, Rieckmann et al. (2016) used EHR records from a national network of 17 health centers and found lower rates documented AUD (3.0%) and OUD diagnoses (1.1%) compared to the larger integrated systems at KP and VHA. Rieckmann et al. also found low rates of prescriptions for FDA-approved medications among patients with documented AUD (3.2%) and documented OUD (29.0%). In another study using EHR data from 70 OCHIN-member community based health centers that also primarily served low-income individuals, Rieckmann et al. (2017) similarly found that 24.7% of patients with documented OUD were prescribed buprenorphine.
In the present study, we leveraged EHR data from a regional data sharing network named Data QUEST (Stephens, Andereson, Lin, & Estiri, 2016) to evaluate the rates of documented AUD and OUD diagnoses and treatments in 21 primary care clinics within four independent, community-based healthcare organizations that primarily serve rural and/or socioeconomically disadvantaged patients. We hypothesized that the prevalence of documented AUD and OUD diagnoses would be lower than the estimated prevalence of these disorders within the primary care patient population (Wu et al., 2017) and that most patients with documented AUD or OUD diagnoses would not receive co-located behavioral treatment or have documented orders for AUD or OUD medications. In exploratory analyses, we also examined the between-clinic variability in rates of AUD and OUD diagnoses and treatments and tested whether rates of diagnoses and treatments were higher among patients who were seen in clinics that had co-located behavioral health specialists.
Methods
Setting and Data Source
Four primary care practice organizations participated in the study. Each is a member of the WWAMI (Washington-Wyoming-Alaska-Montana-Idaho) region Practice and Research Network (WPRN), a practice-based research network that supports research and quality improvement across the WWAMI region. Each organization is a part of the Data Query Extraction Standardization Translation (Data QUEST) network, which provides technical infrastructure that supports harmonization of EHR data across disparate primary care practices for the purpose of translational research (Stephens et al., 2016; Cole, Stephens, Keppel, Estiri, & Baldwin, 2016). Prior to joining the WPRN, two of the four member organizations were affiliated with the University of Washington through residency training program sites, while the other two organizations were not affiliated with any universities or academic medical centers. The 21 primary care clinics within these organizations were community health clinics that provided primary care to patients with limited income, insurance, or other resources, and rural hospital-associated clinics that mostly served patients in small cities and rural areas. The WPRN Coordinating Center provided outreach to Data QUEST member organizations to solicit participation in the current study. Each organization was given an information sheet about the study, and all four Data QUEST member organizations with the necessary EHR elements required for the study agreed to participate. Participating organizations received $500 to cover service costs related to the study (e.g., administrator time).
EHR data from all adult patients (18 years or older) with at least one documented primary care visit between January 1, 2015 and December 31, 2016 (N=47,502) were extracted from the Data QUEST harmonized data warehouse. We included observations over this two-year period (rather than the more common one-year time frame often used when estimating prevalence rates) because a longer timeframe would include a larger sample of the primary care patient population and would allow more opportunity to detect potential AUD and OUD diagnoses and treatments due the infrequency with which many patients visit primary care.
Measures
Documented diagnoses.
Diagnoses were coded in EHRs as International Classification of Diseases (ICD) 9th or 10th edition codes that were extracted from EHR form fields associated with clinic visits and problem lists. To be included, each diagnosis had to be designated as active on the problem or associated with a clinical visit during any time within the two-year observation period. The list of ICD codes that were considered to indicate AUD and OUD diagnoses was generated based on previous research involving EHR-based AUD diagnoses (Williams et al., 2017) and manual review of ICD-9 and ICD-10 codes for OUD diagnoses and additional alcohol-attributable medical conditions. Qualifying AUD diagnoses included AUD that was not in remission (e.g., alcohol abuse, dependence), alcohol intoxication or withdrawal, and medical conditions that are 100% attributable to alcohol as classified by the US Centers for Disease Control and Prevention (CDC, 2017) and indicate a high likelihood of alcohol use disorder (e.g., alcoholic cirrhosis of liver, alcoholic fatty liver, alcoholic gastritis, etc.; see CDC, 2017). Fetal alcohol spectrum disorders were excluded. OUD diagnoses included those indicating likely OUD that was not in remission (e.g., opioid abuse, dependence, intoxication, withdrawal).
ICD codes generated through previous research with Data QUEST identified the presence of other chronic medical conditions. These conditions included hypertension (ICD-9 codes: 401–404, ICD-10 codes: I11-I13), depression (296.2, 296.3, F32, F33), diabetes (250, E10, E11), asthma (493, J45), and HIV (042, B20), which were evaluated descriptively to characterize other chronic health conditions that are commonly diagnosed and treated in primary care.
Documented treatment services in primary care.
Documented AUD and OUD treatment services were identified based on whether patients had documented orders for medications that could potentially treat their AUD or OUD or had documented visits with a co-located non-physician behavioral health specialist during the two-year observation period. Medication orders were identified using text-string searches of generic and brand-name medications, which could include orders that were originally prescribed within the participating clinics and historical orders that documented prescriptions from outside sources. For AUD, we included three FDA-approved medications (naltrexone, disulfiram, acamprosate) and one non-FDA-approved medication that demonstrated efficacy for treating AUD in a meta-analysis (topiramate; Jonas et al., 2014) which has been included in previous evaluations of AUD medications in routine care (Williams et al., 2017) and has been recommended as a treatment for AUD in some large, integrated health systems such as the VHA. For OUD, we included two FDA-approved medications (buprenorphine, naltrexone) that indicate potential treatment of OUD, given they may be used to address other issues (e.g., pain, alcohol use disorder). We did not include methadone because this medication may only be prescribed to treat OUD from federally recognized opioid treatment programs (SAMHSA, 2015). Receipt of co-located behavioral health services was identified based on documented encounters in the primary care organization with non-physician behavioral health specialists, which included psychologists, social workers, and mental health counselors. The EHR data did not indicate the type of behavioral health services delivered in these visits, but clinics typically followed a Primary Care Behavioral Health Model (Hunter et al., 2017; Sandoval et al., 2017) with services that often include warm handoffs, brief interventions, and addressing behavioral aspects of medical or psychiatric issues that may or may not be related to substance use.
Data Analysis
Primary descriptive data analyses focused on estimating the prevalence of documented AUD and OUD diagnoses and potential treatments. EHR data were analyzed as a cross-sectional dataset with binary variables reflecting the presence or absence of documented diagnoses and treatments at any point during the two-year observation period. The observed rates of documented AUD and OUD diagnoses and potential treatments were compared to the one-year estimated prevalence rates of AUD (13.9%) and OUD (4.7%) that were obtained in a previous study of US East Coast primary care patients in urban and suburban clinics via structured, confidential research interviews (Wu et al., 2017). Of note, the population studied by Wu et al. may not be representative of the population studied here due to demographic and regional differences (i.e., patients in urban and suburban areas of the East Coast US may differ from those in predominantly rural areas in the WWAMI region). Nonetheless, in our judgment, this study offered the best available comparison of AUD and OUD prevalence rates for our sample because it used structured, confidential research interviews, which are more likely to indicate the actual prevalence rates of these conditions compared to studies that rely on diagnoses made by clinicians in routine care, which greatly underestimate the actual prevalence of these conditions. In addition, Wu et al. obtained their estimates using primary care samples, where rates of substance use disorders are higher than rates obtained in nationally-representative epidemiological samples (Wu et al., 2017).
In exploratory analyses, we evaluated the variability in rates of diagnoses and treatments across the 21 participating clinics and tested whether diagnosis and treatment rates were higher among patients who were seen in clinics with co-located non-physician behavioral health specialists and among patients who were seen in larger (>20 physicians) vs. smaller or medium-sized clinics. When a given patient visited more than one clinic, they were included in analyses for each of the clinics they had visited during the study period for these clinic-level analyses1. All analyses were conducted using R software (R Core Team, 2017). Use of the data contained in the Data QUEST warehouse was determined by the University of Washington Human Subjects Division to not be considered human subjects research and was therefore exempt from IRB review.
Results
Patient Descriptive Statistics
Sample demographic information is presented in Table 1. The mean age was 47.98 years and 42.3% of patients were male. The sample was majority Caucasian (86.5%), followed by African American (2.2%), Asian (1.7%), Native American (1.2%), Native Hawaiian or Pacific Islander (0.4%), and more than one race (0.1%); 7.9% of patients had no race information. Hispanic or Latino ethnicity was reported by 13.4% of patients; 7.8% of patients did not have ethnicity information in their EHR data. Large proportions of the sample had documented chronic diseases including hypertension (31.3%), depression (19.5%), diabetes (7.6%), asthma (2.4%), and HIV (0.6%). Patients had 243,138 clinic visits2 during the two-year observation period. Visits were documented with 578 unique providers. There were 4524 patients (9.5% of sample) who visited with a behavioral health specialist during the two-year observation period; those patients had a median of 3 such visits. The majority of patients (78.3%) were only seen in one clinic; however, 18.0% of patients visited two clinics and 3.7% visited three or more clinics.
Table 1.
Demographics for patient sample
| Total N = 47,502 | ||
|---|---|---|
| N | % | |
| Age, M (SD) | 47.98 | (19.08) |
| Male | 20102 | 42.3% |
| Caucasian | 41091 | 86.5% |
| Black or African American | 1039 | 2.2% |
| Asian | 829 | 1.7% |
| American Indian or Alaska | ||
| Native | 579 | 1.2% |
| Native Hawaiian or Pacific | ||
| Islander | 181 | 0.4% |
| More than one race | 49 | 0.1% |
| No race information | 3734 | 7.9% |
| Hispanic or Latino | 6386 | 13.4% |
| Not Hispanic or Latino | 37421 | 78.8% |
| No ethnicity information | 3695 | 7.8% |
| Clinic visits (medical or | ||
| behavioral health), M (SD) | 5.18 | (5.85) |
| Any behavioral health visits | 4524 | 9.5% |
| Hypertension diagnosis | 14874 | 31.3% |
| Depression diagnosis | 9272 | 19.5% |
| Diabetes diagnosis | 3631 | 7.6% |
| Asthma diagnosis | 1138 | 2.4% |
| HIV diagnosis | 292 | 0.6% |
Clinic Descriptive Statistics
Rural-urban continuum codes (US Department of Agriculture Economic Research Service, 2013) were used to classify whether clinics were located within rural or urban counties. Nine clinics were in rural counties with populations less than 20,000; one was in a rural county with a population over 20,000; four were in urban counties with populations less than 250,000 that were known to serve rural communities, and seven were in urban counties in metro areas with populations of 250,000 to 1 million. There was a median of 8 physicians per clinic, with 5 clinics estimated3 to be small sized clinics (≤5 physicians), 11 clinics estimated to be medium sized (6 to 20 physicians), and 5 clinics that were large sized (>20 physicians; Liaw et al., 2016). One of the “small” clinics and two of the “medium” sized clinics were satellite clinics with limited hours and provider availability. There were seven clinics with fewer than 500 unique adult patients during the two-year observation period, ten clinics with 500 to 5000 unique adult patients, and four clinics with over 5000. Eight of the 21 primary care clinics (38.1%) had documented visits provided by co-located non-physician behavioral health specialists, which included 5 psychologists, 5 social workers, and 5 mental health counselors. Each of the four organizations had at least one buprenorphine prescriber.
Prevalence of Documented AUD and OUD Diagnoses
AUD diagnosis and treatment rates.
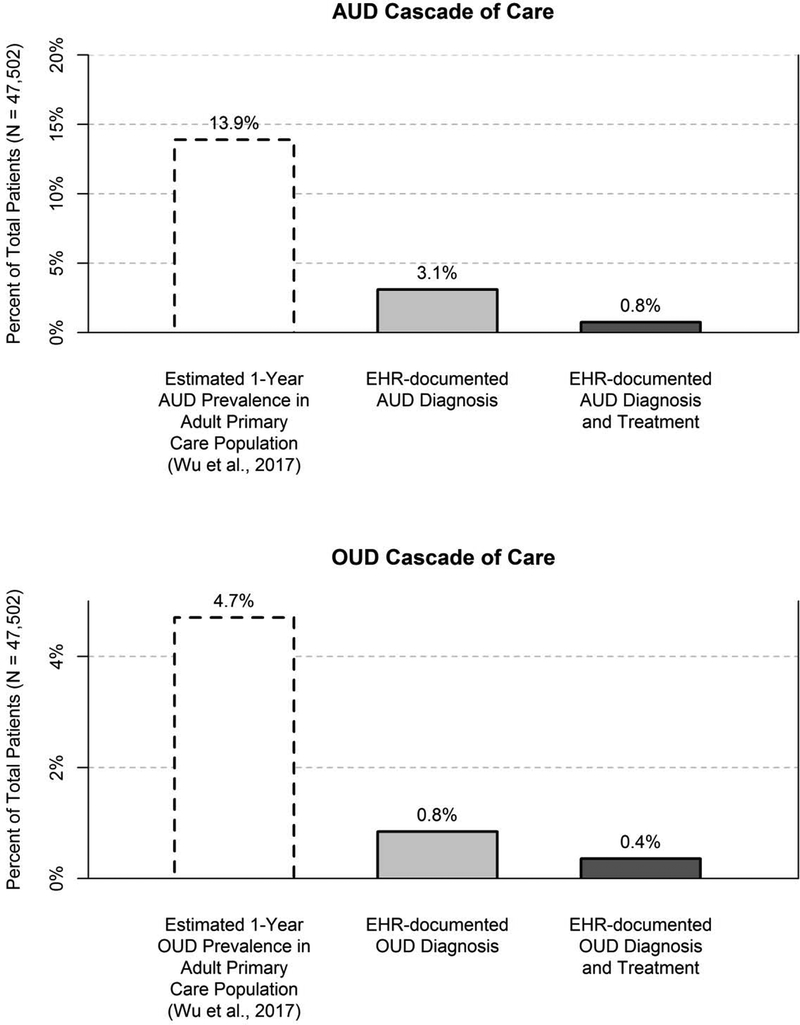
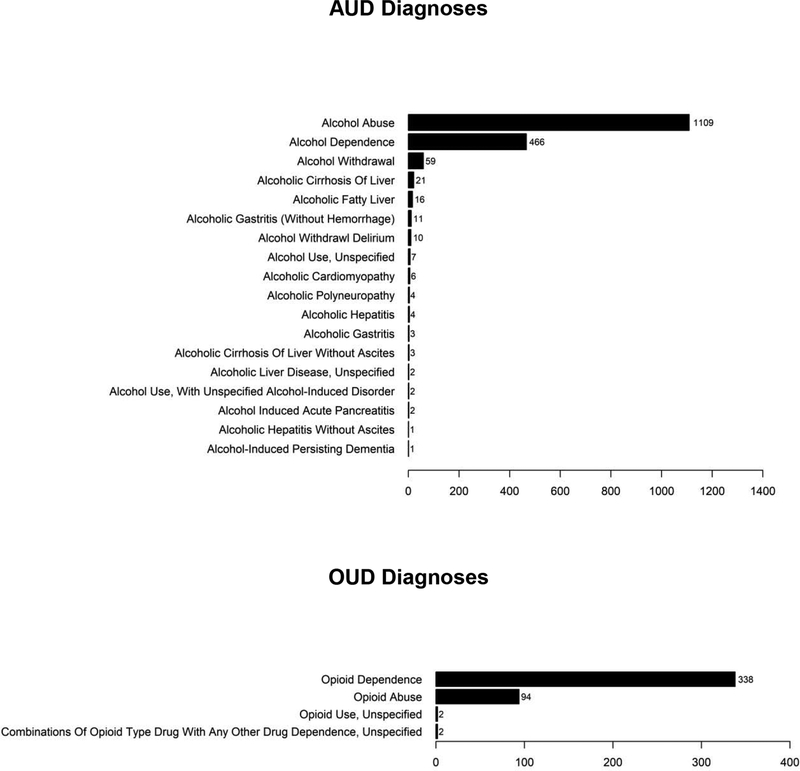
As shown in Figure 1, the estimated two-year prevalence of EHR-documented AUD was 3.1% (n=1476 patients). Of patients with documented AUD, 24.3% (n=358; 0.8% of the full sample of 47,502) had any documented potential treatment for AUD (Figure 1; specific treatments are discussed more in subsequent sections). Figure 2 displays the specific AUD diagnoses identified in the EHR, which most commonly reflected alcohol abuse and alcohol dependence rather than alcohol-attributable medical conditions.
Figure 1. Cascades of care for AUD and OUD.
OUD prevalence rates in Wu et al. were obtained from urban and suburban primary care clinics on the East Coast, which may not generalize to the current sample.
Figure 2.
Number of patients with specific documented AUD and OUD diagnoses
OUD diagnosis and treatment rates.
The two-year prevalence of EHR-documented OUD was 0.8% (n=402 patients). Of patients with documented OUD, 49.0% (n=171; 0.4% of the full sample of 47,502) had any documented potential treatment for OUD in the EHR (Figure 1). Figure 2 displays the specific OUD diagnoses that were given to patients.
Types of AUD and OUD Treatment
AUD treatments.
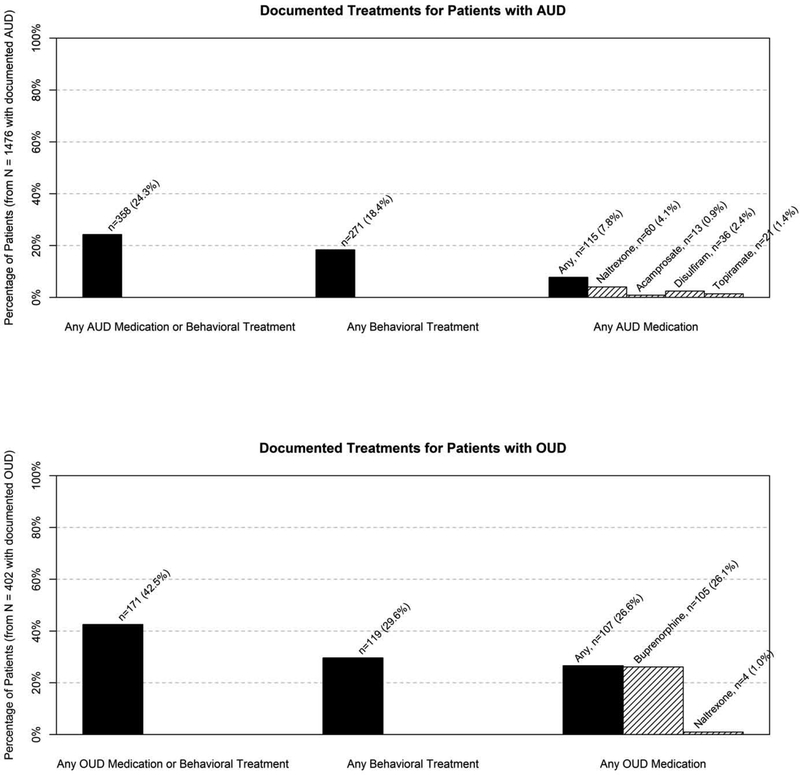
Figure 3 shows the proportions of patients with documented AUD who had documented visits with a behavioral health specialist or orders for AUD medications. Just under one in four patients (24.3%) with documented AUD had documented AUD medication orders or co-located behavioral treatment. More specifically, only 7.8% had orders for AUD medications (naltrexone, acamprosate, disulfiram, topiramate), and 18.4% had one or more documented visits with a co-located behavioral health provider. For comparison, 23.0% of patients with documented depression diagnoses had visits with a co-located behavioral health provider. Among patients with AUD who visited a co-located behavioral health provider, 54.6% had a documented depression diagnosis.
Figure 3. Specific AUD and OUD treatments among patients with documented AUD and OUD.
Among patients who received naltrexone, 10 patients with AUD and 1 patient with OUD were documented as receiving the injectable form.
OUD treatments.
Less than half of patients (42.5%) with documented OUD had documented orders for potential OUD medications or co-located behavioral treatment. More specifically, 26.6% had OUD medication orders, most commonly for buprenorphine, and 29.6% had one or more documented visits with a co-located behavioral health specialist. Among patients with OUD who had visits with a co-located behavioral health specialist, 52.1% also had a documented depression diagnosis.
Variability Across Clinics
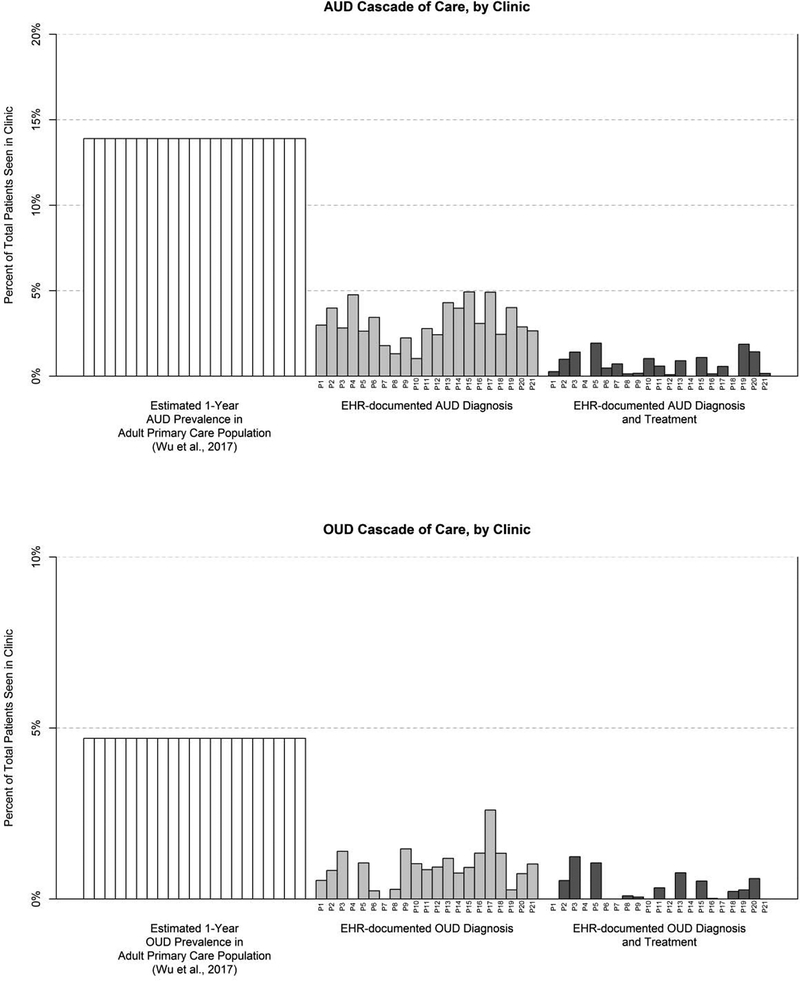
The substantial amount of variability in documented AUD and OUD diagnoses and treatments across the 21 study clinics is shown in Figure 4, where each vertical bar represents the diagnosis and treatment rate for one clinic. Documented AUD diagnoses ranged from 1.0% to 4.9% of all patients within each clinic. Documentation of potential AUD treatments ranged from 0% (in 3 clinics) to 1.9% of all patients within each clinic. Documented OUD diagnoses ranged from 0% (in 2 clinics) to 2.6%. Documentation of potential OUD treatments ranged from 0% (in 9 clinics, including the 2 clinics with no documented OUD diagnoses) up to 1.2% of all patients within each clinic.
Figure 4. Variability in documented diagnoses and treatments across clinics.
Numbers below each bar indicate different primary care clinics. OUD prevalence rates in Wu et al. were obtained from urban and suburban primary care clinics on the East Coast, which may not generalize to the current sample.
Patients who were seen in any of the eight clinics that had co-located non-physician behavioral health specialists were significantly more likely to receive an AUD diagnosis compared to patients in clinics that did not have these specialists (3.4% vs. 2.6%, relative risk [RR] = 1.32, 95% CI: 1.18 to 1.48); however, the likelihood of receiving an OUD diagnosis was nominally (but not significantly) lower in clinics with behavioral health specialists (0.8% vs. 1.0%, respectively; RR=0.82, 95% CI: 0.67 to 1.004). Patients with an AUD diagnosis were more likely to receive an AUD medication if they were seen in a clinic with co-located non-physician behavioral health specialists compared to a clinic without (8.6% vs. 5.6%, respectively, RR=1.54, 95% CI: 1.01 to 2.44). Similarly, patients with an OUD diagnosis were more likely to receive an OUD medication if they were seen in a clinic with co-located non-physician behavioral health specialists compared to a clinic without (42.3% vs. 1.3%, RR=32.60, 95% CI: 11.87 to 144.42).
Comparing “large” clinics (>20 physicians) to “small” and “medium” sized clinics (≤20 physicians), there were no differences in rates of documented AUD (mean difference=0.88% higher in large clinics, SE=0.55, p=.13) or OUD diagnoses (mean difference=0.16%, SE=0.31, p=0.61). Patients with an AUD diagnosis were not more likely to receive an AUD medication if they were seen in a large clinic (mean difference = 0.15% higher in large clinics, SE=2.3, p=.95); however, patients with OUD diagnoses were more likely to receive OUD medication if they were seen in a large clinic (mean difference = 35.86%, SE=13.58, p=.02).
Sensitivity analysis: One-year versus two-year prevalence estimates.
We intentionally used a two-year observation period in our primary analyses because many patients visit primary care infrequently; however, we also conducted sensitivity analyses to estimate AUD and OUD diagnosis and treatment rates over a one-year period (calendar year 2015) to mirror similar observation periods used in many epidemiological studies. Documented AUD diagnoses occurred at similar rates for the one-year (3.2%) and two-year (3.1%) observation periods. However, the documented prevalence for any type of AUD treatment was lower in the one-year period (18.0%) compared to the two-year period (24.3%). More specifically, the one-year observation period yielded lower rates of AUD medication orders (4.4%) compared to the two-year period (7.8%); rates of visits with a co-located non-physician behavioral health specialist were also lower in the one-year period (15.3%) compared to the two-year period (18.4%).
The one-year and two-year rates of documented OUD diagnoses were the same (0.8%). However, the one-year prevalence of documented OUD medications (20.1%) was lower than the two-year prevalence (26.6%), with a similar pattern for visits with a co-located non-physician behavioral health specialist (22.9% vs. 29.6%).
Sensitivity analyses: Problem lists versus visit diagnoses.
In additional sensitivity analyses, we compared rates of documented AUD and OUD diagnoses by deriving these diagnoses solely from problem lists (i.e., diagnoses that are associated with patients over a period of time) versus diagnoses associated with specific clinic visits (i.e., diagnoses that are manually entered by a clinician for a specific clinic visit). This analysis was motivated by patients potentially having AUD or OUD diagnoses that remain active on problem lists for long periods of time despite the diagnoses being potentially resolved or not actively addressed during visits. Out of all 1476 patients with documented AUD by either method, 1449 patients (98.2%) had the diagnosis on their problem list, whereas only 490 patients (33.2%) had the diagnosis associated with a clinical visit. Among the 402 patients with documented OUD by either method, 376 patients (93.5%) had the diagnosis on their problem list and 211 patients (52.5%) had the diagnosis associated with a visit. Thus, the estimated two-year prevalence rates of documented AUD and OUD that were reported above would be reduced substantially if we included only patients with AUD or OUD diagnosis associated with clinic visits. For patients with only visit diagnoses, rates of documented pharmacological or behavioral treatment increased to 42.9% for AUD (versus 24.3% for patients with visit and/or problem list diagnoses) and to 65.9% (versus 42.5%) for OUD, suggesting that more treatments were potentially offered when AUD or OUD diagnoses were associated with a clinic visit (versus on a problem list).
Sensitivity analyses: Generic “drug use disorder” diagnoses.
We examined rates of FDA-approved AUD and OUD medications among the 941 patients (1.9% of sample) who were not diagnosed with AUD or OUD but were diagnosed with unspecified substance use disorders (e.g., “unspecified drug dependence”). Only 22 of these patients (2.3%) were prescribed AUD medications and none were prescribed OUD medications, suggesting that our omission of generic “drug use disorder” diagnoses from our analyses was unlikely to have reduced our estimated treatment rates.
Discussion
Our study indicates the extent to which AUD and OUD appear to be underdiagnosed and undertreated in predominantly small- and medium-sized primary care clinics that primarily serve rural and socioeconomically disadvantaged populations. Our observed rates of EHR-documented AUD and OUD diagnoses were approximately four to six times lower than their estimated prevalence obtained using confidential research interviews from East Coast primary care clinics (Wu et al., 2017). Moreover, only about one in 15 patients with documented AUD had orders for efficacious AUD medications (defined as medications with FDA-approval for AUD or support in a meta-analysis; Jonas et al., 2014) and about one in five patients with documented AUD had any documented visits with a co-located non-physician behavioral health specialist. About one in four patients with documented OUD had orders for FDA-approved OUD medication, with lower rates in smaller- and medium-sized clinics compared to large clinics, even though all participating organizations had at least one buprenorphine prescriber. Combined, these low rates of diagnosis and treatment suggest that potentially only one in 17 patients with AUD and one in 14 patients with OUD is both diagnosed and provided with AUD or OUD medications or non-physician behavioral health services in primary care. Albeit, this may still be an optimistic estimate given that we could not verify how many of those medication orders went unfilled or how many medications and visits with non-physician behavioral specialists did not address AUD or OUD. These rates that suggest that underdiagnosis and undertreatment were present across the 21 clinics that were studied, with some clinics potentially having no patients that received treatment for an AUD or OUD.
Previous studies have observed low rates of documented AUD and OUD diagnoses in a national network of 17 safety-net community health centers (Rieckmann et al., 2016). However, rates of AUD diagnoses were approximately two times higher in VHA settings (Williams et al. 2017) and rates of both AUD and OUD diagnoses were also approximately two times higher in a study with KP Northern California patients (Bahorik et al., 2017). Notably, integrated services for alcohol screening, assessment, and treatment in larger integrated healthcare settings could contribute to higher rates of AUD detection and diagnosis (Bradley et al., 2011, 2019); however, the observed rates of AUD pharmacotherapy in our study were similar to those observed in the VHA by Williams et al. (2017).
The low rates of documented AUD and OUD diagnoses and treatments observed here are likely attributable to many factors. Patients may not prioritize or voice concerns about alcohol or opioids with their providers or may lack opportunities to discuss treatment options. Co-occurring medical and psychiatric conditions are common and may compete for clinical attention during primary care visits. Providers often lack training and confidence in assessing and treating substance use (Elwy et al., 2013; Ozer et al., 2004), and may subsequently avoid addressing these conditions with patients. Inconsistent practices in screening, assessment, and treatment across settings may further decrease diagnosis and treatment rates (Williams et al., 2015), explaining some variability across clinics. Prescribing OUD medications has particular hurdles in primary care, with physicians and administrators citing regulatory prohibitions, limited workforce training, and lack of institutional support as perceived barriers to prescribing buprenorphine (Hutchinson et al., 2014; Knudsen, Abraham, & Oser, 2011). Qualitative research by Hutchinson et al. (2014) also found that primary care physicians cited a lack of integrated behavioral healthcare specialists as the most common reason for not prescribing buprenorphine for patients with OUD, and our study provides quantitative evidence that aligns with these reported limitations. We found that patients with OUD diagnoses were more likely to receive OUD medications if they were seen in in clinics with co-located non-physician behavioral health specialists than if they were seen in clinics without co-located behavioral health specialists. Moreover, this finding also extended to AUD, as patients in clinics with co-located behavioral health specialists were more likely to receive an AUD diagnosis and, once diagnosed, more likely to receive AUD medications. Although the AUD and OUD medications studied here can be prescribed by primary care providers without the presence of co-located non-physician behavioral health specialists, it is possible that having such specialists co-located within primary care contributes to these higher rates of diagnosis and treatment that are observed in the current study. Importantly, however, the associations between co-located behavioral health and increased diagnosis and treatment rates in our study were correlational, and it is also possible that a host of other variables could account for the relationships that were observed. For example, clinics can vary considerably in terms of their clinical approaches, policies, cultures, and resources related to diagnosing and treating behavioral health conditions and substance use disorders. These factors could simultaneously affect the likelihood of having co-located behavioral health specialists and the extent to which AUD and OUD are addressed, even if the availability of co-located behavioral health did not directly cause AUD and OUD to be diagnosed or treated more frequently.
Improving rates of AUD and OUD diagnosis and treatment in primary care may require implementation of workflows to routinely screen for unhealthy levels of drinking or opioid use, which if present, could be followed by more detailed assessments of AUD and OUD symptoms (Bradley et al., 2019; Bobb et al., 2017; Marsden et al., 2019; National Council for Behavioral Health, 2018). For example, electronic clinical reminders designed to facilitate screening and brief intervention for alcohol have been shown to increase rates of screening for heavy drinking at the VHA (Bradley et al., 2006) and at Kaiser-Permanente Washington (Bobb et al., 2017), although implementation of these reminders has often been challenging (Williams et al., 2011) and is not always associated with reductions in patient drinking (Williams et al., 2009, 2010, 2017).
There is increasing support for implementing brief screens for problem opioid use (e.g., McNeely et al., 2016; US Preventive Services Task Force, 2019). Other EHR-based clinical support tools could guide primary care clinicians through decision-making protocols for assessing, treating, and monitoring AUD and OUD (Bobb et al., 2017; Hallgren, Bauer, & Atkins, 2017; Lapham, et al., 2012) or facilitate remote delivery of AUD and OUD treatment (Ramsey, 2015), particularly for patients in rural areas or with limited treatment access, but these also require particular attention to addressing patient buy-in and acceptability, provider adoption and training, and system-level policies and reimbursement (Ramsey, 2015; Williams et al., 2016).
Integrated service delivery models that incorporate specialty providers with expertise in AUD and OUD can also be implemented to ensure that AUD and OUD services are prioritized in primary care (Chou et al., 2016). These models often delegate care integration, coordination, and routine patient contact to non-physician specialists, who may have a greater capacity to address AUD- and OUD-related needs and can incorporate higher-fidelity AUD- and OUD-focused psychotherapeutic techniques in their clinical interactions (Aharonovich et al., 2017; Mullin et al., 2015; Watkins et al., 2017). Even though many clinics (including many of those studied here) have co-located behavioral health specialists, they may not be utilizing service models that systematically prioritize assessment or treatment for AUD and OUD as opposed to mental health conditions like depression, which was much more commonly diagnosed in our sample (19.5% of patients) than AUD and OUD. Nonetheless, behavioral specialists who do not specialize in AUD or OUD treatment could still potentially help patients with AUD and OUD by helping them manage stress and comorbid mental health conditions, both of which are highly prevalent in patients with AUD and OUD and are associated with worse treatment outcomes (McLean et al., 2019). Integrating care for substance use disorders within primary care could also reduce stigma associated with AUD and OUD by emphasizing these conditions as equally important to health as other chronic conditions, such as depression, diabetes, or asthma (Shim & Rust, 2013). Reducing the perception of these conditions as only being treatable in specialty care settings may particularly benefit patients who perceive psychiatric diagnostic labels as more stigmatizing, and, in turn, are less likely to obtain care in specialty treatment settings due to stigma (Campbell et al., 2016).
Limitations
There are several limitations to our study. AUD and OUD diagnoses on problem lists and visit diagnoses may not reflect whether these diagnoses were actually discussed during clinical visits. Further, with our EHR data it was not possible to know which types of behavioral interventions were delivered, or for what conditions medications or behavioral interventions were used. For example, buprenorphine could have been used to treat pain-related conditions, topiramate could have addressed other psychiatric or neurological conditions, and behavioral health interventions could have addressed a comorbid mental health condition, such as depression, which was diagnosed in over half of patients with AUD or OUD who visited co-located behavioral health specialists. We also could not directly address whether diagnoses remained on problem lists despite being resolved. Providers could have documented AUD and OUD in unstructured data fields (e.g., patient history notes) which could not be detected in our analyses. We could not obtain pharmacy data to detect whether medications orders were filled by patients. Some patients may have received AUD or OUD treatments from outside clinics through referrals made in primary care, which could not be captured with our dataset unless they were entered by clinicians as historical medication orders. We could not evaluate clinical practices within the participating clinics to validate the rates of AUD and OUD among patients using gold-standard methods (e.g., confidential structured research interviews), which would help contextualize the current study findings. Although Wu et al. (2017) provide high-quality prevalence estimates of AUD and OUD diagnoses in primary care using confidential structured research interviews, their estimated prevalence rates are based on patients in cities and suburbs of the US East Coast that may differ from patients in small cities and rural areas of the Pacific Northwest of the United States.
Conclusions
This study helps quantify the potential extent of diagnostic and treatment service gaps in primary care organizations serving rural and socioeconomically disadvantaged patients. Rates of AUD and OUD diagnoses in these settings are lower than what has been established in previous studies of larger integrated health systems (Bahorik et al., 2017; Williams et al., 2017). Primary care clinics with co-located behavioral health specialists may be more likely to diagnose AUD and more likely to treat AUD and OUD with effective medications.
Highlights.
Rates of AUD and OUD diagnosis and treatment in primary care are not well known
We used electronic health record data to study AUD & OUD diagnoses and treatments
AUD & OUD were diagnosed less frequently than their estimated population prevalence
Few diagnosed patients had orders for FDA-approved AUD or OUD medications
Efforts are needed to increase AUD and OUD detection and treatment in primary care
Acknowledgments
This study was funded by National Institute on Alcoholism and Alcohol Abuse (NIAAA) award K01AA024796, National Center for Advancing Translational Sciences award UL1TR002319, and National Institute on Drug Abuse award 3UG1DA013714. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAAA, NCATS, NIDA, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
However, for the primary analyses that focus on the full sample, each patient was counted only once regardless of how many clinics they visited.
Defined as an EHR-documented “visit” entry, which included visits for medical and/or behavioral health but excluded visits that were documented solely as chart reviews or orders. This analysis excluded repeated visits within the same day.
The number of unique physicians per clinic was estimated using visit data from adult patients only. Therefore, these estimates would exclude any physicians who exclusively saw children under 18 years old.
References
- Aharonovich E, Sarvet A, Stohl M, DesJarlais D, Tross S, Hurst T, … & Hasin D (2017). Reducing non-injection drug use in HIV primary care: a randomized trial of brief motivational interviewing, with and without HealthCall, a technology-based enhancement. Journal of Substance Abuse Treatment, 74, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahorik AL, Satre DD, Kline-Simon AH, Weisner CM, & Campbell CI (2017). Alcohol, Cannabis, and Opioid Use Disorders, and Disease Burden in an Integrated Health Care System. Journal of addiction medicine, 11(1), 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer MS, Deane Leader HU, Lai Z, & Kilbourne AM (2012). Primary care and behavioral health practice size: the challenge for healthcare reform. Medical Care, 50(10), 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb J, Lee A, Lapham G, Oliver M, Ludman E, Achtmeyer C, … & Bradley K (2017). Evaluation of a pilot implementation to integrate alcohol-related care within primary care. International Journal of Environmental Research and Public Health, 14(9), 1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman S, Eiserman J, Beletsky L, Stancliff S, & Bruce RD (2013). Reducing the health consequences of opioid addiction in primary care. The American journal of medicine, 126(7), 565–571. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Caldeiro RM, Hallgren KA, & Kivlahan DR (2019). Making measurement-based care for addictions a reality in primary care. Addiction, 114(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, & Kivlahan DR (2014). Bringing patient-centered care to patients with alcohol use disorders. JAMA, 311(18), 1861–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, & Kivlahan DR (2006). Implementation of evidence-based alcohol screening in the Veterans Health Administration. American Journal of Managed Care, 12(10), 597–606. [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, & Bradley KA (1998). The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Archives of Internal Medicine, 158(16), 1789–1795. [DOI] [PubMed] [Google Scholar]
- Campbell DG, Bonner LM, Bolkan CR, Lanto AB, Zivin K, Waltz TJ, … & Chaney EF (2016). Stigma predicts treatment preferences and care engagement among Veterans Affairs primary care patients with depression. Annals of Behavioral Medicine, 50 (4), 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2017). Alcohol-Related Disease Impact (ARDI): Methods. Alcohol and Public Health: Alcohol-Related Disease Impact https://nccd.cdc.gov/DPH_ARDI/Info/Methods.aspx. Accessed February 5, 2019.
- Chou R, Korthuis PT, Weimer M, Bougatsos C, Blazina I, Zakher B, … & McCarty D (2016). Medication-assisted treatment models of care for opioid use disorder in primary care settings. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK402352/ [PubMed]
- Cole AM, Stephens KA, Keppel GA, Estiri H, & Baldwin L (2016). Extracting Electronic Health Record Data in a Practice-Based Research Network: Lessons Learned from Collaborations with Translational Researchers. eGEMS, 4(2), 1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme LJ, Chandler RK, & Harris AH (2016). Implementing effective substance abuse treatments in general medical settings: Mapping the research terrain. Journal of Substance Abuse Treatment, 60, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwy AR, Horton NJ, & Saitz R (2013). Physicians’ attitudes toward unhealthy alcohol use and self-efficacy for screening and counseling as predictors of their counseling and primary care patients’ drinking outcomes. Substance Abuse Treatment, Prevention, and Policy, 8 (1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Sparenborg S, & Tai B (2011). Improving drug abuse treatment delivery through adoption of harmonized electronic health record systems. Substance abuse and rehabilitation, 2, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JE, Andréasson S, Bradley KA, Finn SW, Williams EC, Bakshi AS, … & Saitz R (2017). Rethinking alcohol interventions in health care: a thematic meeting of the International Network on Brief Interventions for Alcohol & Other Drugs (INEBRIA). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, … & Hasin DS (2015). Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry, 72(8), 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren KA, Bauer AM, & Atkins DC (2017). Digital technology and clinical decision making in depression treatment: Current findings and future opportunities. Depression and Anxiety, 34(6), 494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, & Jones CM (2017). Prescription opioid use, misuse, and use disorders in US adults: 2015 National Survey on Drug Use and Health. Annals of Internal Medicine, 167(5), 293–301. [DOI] [PubMed] [Google Scholar]
- Hunter CL, Funderburk JS, Polaha J, Bauman D, Goodie JL, & Hunter CM (2018). Primary care behavioral health (PCBH) model research: Current state of the science and a call to action. Journal of Clinical Psychology in Medical Settings, 25(2), 127–156. [DOI] [PubMed] [Google Scholar]
- Hutchinson E, Catlin M, Andrilla CHA, Baldwin LM, & Rosenblatt RA (2014). Barriers to primary care physicians prescribing buprenorphine. The Annals of Family Medicine, 12(2), 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Health Metrics and Evaluation. (2016). GBD Compare. Accessed August 24, 2018 from http://vizhub.healthdata.org/gbd-compare.
- John WS, Zhu H, Mannelli P, Schwartz RP, Subramaniam GA, & Wu LT (2018). Prevalence, patterns, and correlates of multiple substance use disorders among adult primary care patients. Drug and Alcohol Dependence, 187, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, … & Garbutt JC (2014). Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA, 311(18), 1889–1900. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. Despite benefit, physicians slow to offer brief advice on harmful alcohol use. JAMA 2008;299:751–3. [DOI] [PubMed] [Google Scholar]
- Lapham GT, Achtmeyer CE, Williams EC, Hawkins EJ, Kivlahan DR, & Bradley KA (2012). Increased documented brief alcohol interventions with a performance measure and electronic decision support. Medical Care, 50(2), 179–187. [DOI] [PubMed] [Google Scholar]
- Liaw WR, Jetty A, Petterson SM, Peterson LE, & Bazemore AW (2016). Solo and small practices: A vital, diverse part of primary care. The Annals of Family Medicine, 14(1), 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean RR, Armstrong JL, & Sofuoglu M (2019). Stress and opioid use disorder: A systematic review. Addictive Behaviors, 98. [DOI] [PubMed] [Google Scholar]
- Marsden J, Tai B, Ali R, Hu L, Rush AJ, & Volkow N (2019). Measurement-based care using DSM-5 for opioid use disorder: Can we make opioid medication treatment more effective? Addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Starrels JL, Tai B, Gordon AJ, Brown R, Ghitza U, … & Lindblad R (2013). Can substance use disorders be managed using the chronic care model? Review and recommendations from a NIDA consensus group. Public Health Reviews, 35(2), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, & Woodworth AM (2014). The Affordable Care Cct and treatment for “substance use disorders:” Implications of ending segregated behavioral healthcare. Journal of Substance Abuse Treatment, 46(5), 541–545. [DOI] [PubMed] [Google Scholar]
- McNeely J, Wu LT, Subramaniam G, Sharma G, Cathers LA, Svikis D, … & O’grady KE (2016). Performance of the tobacco, alcohol, prescription medication, and other substance use (TAPS) tool for substance use screening in primary care patients. Annals of Internal Medicine, 165(10), 690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin DJ, Forsberg L, Savageau JA, & Saver B (2015). Challenges in developing primary care physicians’ motivational interviewing skills. Families, Systems, & Health, 33(4), 330. [DOI] [PubMed] [Google Scholar]
- National Council for Behavioral Health. (2018). Implementing Care for Alcohol & Other Drug Use in Medical Setting: An Extension of SBIRT. https://www.thenationalcouncil.org/wp-content/uploads/2018/03/021518_NCBH_ASPTReport-FINAL.pdf.
- Ozer EM, Adams SH, Gardner LR, Mailloux DE, Wibbelsman CJ, & Irwin CE Jr (2004). Provider self-efficacy and the screening of adolescents for risky health behaviors. Journal of Adolescent Health, 35(2), 101–107. [DOI] [PubMed] [Google Scholar]
- Ramsey C, Dziura J, Justice AC, Altalib HH, Bathulapalli H, Burg M, … & Kulas J (2017). Incidence of mental health diagnoses in veterans of operations Iraqi freedom, enduring freedom, and New Dawn, 2001–2014. American journal of public health, 107(2), 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann TR, Gideonse N, Risser A, DeVoe JE, & Abraham AJ (2017). Treating opioid dependence with buprenorphine in the safety net: Critical learning from clinical data. The Journal of Behavioral Health Services & Research, 44(3), 351–363. [DOI] [PubMed] [Google Scholar]
- Rieckmann T, Muench J, McBurnie MA, Leo MC, Crawford P, Ford D, … & Wright N (2016). Medication-assisted treatment for substance use disorders within a national community health center research network. Substance Abuse, 37(4), 625–634. [DOI] [PubMed] [Google Scholar]
- Rural–urban continuum codes. Washington (DC): US Department of Agriculture, Economic Research Service. (2013). http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx. [Google Scholar]
- Sandoval BE, Bell J, Khatri P, & Robinson PJ (2018). Toward a unified integration approach: Uniting diverse primary care strategies under the Primary Care Behavioral Health (PCBH) Model. Journal of Clinical Psychology in Medical Settings, 25(2), 187–196. [DOI] [PubMed] [Google Scholar]
- Sanghavi D, Altan A, Hane C, & Bleicher P (2017, December 18). To address the opioid crisis, build a comprehensive national framework. Health Affairs Blog. doi: 10.1377/hblog20171215.681297 [DOI] [Google Scholar]
- Schuckit MA (2016). Treatment of opioid-use disorders. New England Journal of Medicine, 375(4), 357–368. [DOI] [PubMed] [Google Scholar]
- Shim R, & Rust G (2013). Primary care, behavioral health, and public health: Partners in reducing mental health stigma. American Journal of Public Health, 103(5), 774–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socías ME, Volkow N, & Wood E (2016). Adopting the ‘cascade of care’framework: An opportunity to close the implementation gap in addiction care? Addiction, 111(12), 2079–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spithoff S, & Kahan M (2015). Paradigm shift: Moving the management of alcohol use disorders from specialized care to primary care. Canadian Family Physician, 61(6), 491–493. [PMC free article] [PubMed] [Google Scholar]
- Stephens KA, Anderson N, Lin C, & Estiri H (2016). Implementing Partnership-driven Clinical Federated Electronic Health Record Data-sharing Networks. International Journal of Medical Informatics, 93, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2015). Mental Health Services Administration. Behavioral Health Barometer: United States, 2014 HHS Publication No (p. 4). SMA-15-4895 Rockville: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2015). Federal guidelines for opioid treatment programs. Accessed August 20, 2019 at https://store.samhsa.gov/system/files/pep15-fedguideotp.pdf
- Tai B, & McLellan AT (2012). Integrating information on substance use disorders into electronic health record systems. Journal of substance abuse treatment, 43(1), 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Preventive Services Task Force. (2019). Draft recommendation statement: Illicit drug use, including nonmedical use of prescription drugs: Screening. Accessed August 20, 2019 at https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/drug-use-in-adolescents-and-adults-including-pregnant-women-screening
- Venner KL, Sánchez V, Garcia J, Williams RL, & Sussman AL (2018). Moving Away from the Tip of the Pyramid: Screening and Brief Intervention for Risky Alcohol and Opioid Use in Underserved Patients. The Journal of the American Board of Family Medicine, 31(2), 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman SE, & Barnett ML (2018). Primary care and the opioid-overdose crisis – Buprenorphine myths and realities. New England Journal of Medicine, 379, 1–4. doi: 10.1056/NEJMp1802741 [DOI] [PubMed] [Google Scholar]
- Watkins KE, Ober AJ, Lamp K, Lind M, Setodji C, Osilla KC, … & Diamant A (2017). Collaborative care for opioid and alcohol use disorders in primary care: The SUMMIT randomized clinical trial. JAMA Internal Medicine, 177(10), 1480–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Achtmeyer CE, Kivlahan DR, Greenberg D, Merrill JO, Wickizer TM, … & Bradley KA (2010). Evaluation of an electronic clinical reminder to facilitate brief alcohol-counseling interventions in primary care. Journal of studies on alcohol and drugs, 71(5), 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Achtmeyer CE, Thomas RM, Grossbard JR, Lapham GT, Chavez LJ, … & Bradley KA (2015). Factors underlying quality problems with alcohol screening prompted by a clinical reminder in primary care: a multi-site qualitative study. Journal of General Internal Medicine, 30(8), 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Achtmeyer CE, Young JP, Rittmueller SE, Ludman EJ, Lapham GT, … & Bradley KA (2016). Local implementation of alcohol screening and brief intervention at five Veterans Health Administration primary care clinics: Perspectives of clinical and administrative staff. Journal of Substance Abuse Treatment, 60, 27–35. [DOI] [PubMed] [Google Scholar]
- Williams EC, Gupta S, Rubinsky AD, Glass JE, Jones-Webb R, Bensley KM, & Harris AH (2017). Variation in receipt of pharmacotherapy for alcohol use disorders across racial/ethnic groups: A national study in the US Veterans Health Administration. Drug & Alcohol Dependence, 178, 527–533. [DOI] [PubMed] [Google Scholar]
- Williams EC, Johnson ML, Lapham GT, Caldeiro RM, Chew L, Fletcher GS, … & Bradley KA (2011). Strategies to implement alcohol screening and brief intervention in primary care settings: A structured literature review. Psychology of Addictive Behaviors, 25(2), 206. [DOI] [PubMed] [Google Scholar]
- Williams EC, Lapham G, Achtmeyer CE, Volpp B, Kivlahan DR, & Bradley KA (2010). Use of an electronic clinical reminder for brief alcohol counseling is associated with resolution of unhealthy alcohol use at follow-up screening. Journal of General Internal Medicine, 25(1), 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Nunes EV, Bisaga A, Levin FR, & Olfson M (2019). Development of a Cascade of Care for responding to the opioid epidemic. The American Journal of Drug and Alcohol Abuse, 45(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Nunes EV, Bisaga A, Pincus HA, Johnson KA, Campbell AN, … & Olfson M (2018). Developing an opioid use disorder treatment cascade: A review of quality measures. Journal of Substance Abuse Treatment, 91, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, McNeely J, Subramaniam GA, Brady KT, Sharma G, VanVeldhuisen P, … & Schwartz RP (2017). DSM-5 substance use disorders among adult primary care patients: Results from a multisite study. Drug and Alcohol Dependence, 179, 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]