Abstract
Background and Objective
The world is currently experiencing the Coronavirus Disease-19 (COVID-19) pandemic. There is no approved drug for the definitive treatment of the disease. Various drugs are being tried for the treatment of COVID-19, including hydroxychloroquine (HCQ). This study was performed to systematically review the therapeutic role of HCQ in COVID-19 from the available literature.
Methods
PubMed, Embase, ClinicalTrials.gov, ICTRP (WHO), Cochrane Library databases, and two pre-print servers (medRxiv.org and Research Square) were searched for clinical studies that evaluated the therapeutic role of HCQ on COVID-19 until 10 May 2020. The available studies were critically analyzed and the data were extracted.
Results
A total of 663 articles were screened and 12 clinical studies (seven peer-reviewed and published studies and five non-peer-reviewed studies from pre-print servers) with a total sample size of 3543 patients were included. Some of the clinical studies demonstrated good virological and clinical outcomes with HCQ alone or in combination with azithromycin in COVID-19 patients, although the studies had major methodological limitations. Some of the other studies showed negative results with HCQ therapy along with the risk of adverse reactions.
Conclusion
The results of efficacy and safety of HCQ in COVID-19, as obtained from the clinical studies, are not satisfactory, although many of these studies had major methodological limitations. Stronger evidence from well-designed robust randomized clinical trials is required before conclusively determining the role of HCQ in the treatment of COVID-19. Clinical prudence is required in advocating HCQ as a therapeutic armamentarium in COVID-19.
Electronic supplementary material
The online version of this article (10.1007/s40261-020-00927-1) contains supplementary material, which is available to authorized users.
Key Points
| Efficacy and safety results obtained from clinical studies on the therapeutic role of HCQ in COVID-19 are not satisfactory. |
| The majority of the published studies have major methodological limitations. |
| Safety aspects associated with the use of HCQ along with azithromycin in COVID-19 warrants caution. |
| Large and robust randomized controlled trials will conclusively determine the role of HCQ in COVID-19. |
Introduction
The world is experiencing a pandemic (Coronavirus Disease-19 or COVID-19) caused by a novel strain of coronavirus (SARS-CoV-2). At the time of writing this article, more than 40 million cases of COVID 19 have been reported all over the globe [1] amounting to a mortality rate of approximately 2–3% [2]. This has resulted in an enormous health and economic burden across the world. There are intensive global efforts to try various drugs for the treatment of COVID-19 as the pandemic continues to extend. In the absence of any known effective therapy and because of the public health emergency, many drugs have been tried recently, including the 4-aminoquinoline antimalarials, chloroquine (CQ) and its derivative hydroxychloroquine (HCQ).
HCQ is most often used in chronic inflammatory diseases, including systemic lupus erythematosus and rheumatoid arthritis. Several potential mechanisms of action of HCQ against SARS-CoV-2 have been proposed. These include inhibition of virus attachment to the host cells [3], inhibition of viral release into the intracellular space by disruption of lysosome-endosome fusion [4, 5], and inhibition of the release of pro-inflammatory cytokines [5]. As observed in various in vitro and in silico studies, HCQ acts by the disruption of the interaction of the S protein of SARS-CoV-2 with the host cell membrane [6]. A study has shown that HCQ has a constant binding affinity towards the protease enzyme of the mutant variant form of SARS-CoV-2, thereby inhibiting its replication [7]. Yao et al. have shown that HCQ was more effective and potent than CQ in inhibiting the activity of SARS-CoV-2 in vitro [8]. As suggested by Liu et al., HCQ, as an anti-inflammatory agent, may inhibit a cytokine storm in SARS-CoV-2 patients leading to the reduction of the severity of infection [9]. In another study conducted by Andreani et al., the combination of HCQ and azithromycin was found to be synergistically effective in inhibiting the viral replication in vitro. It was especially found to be effective in the early stages of COVID-19 before the onset of a cytokine storm, which is related to the onset of acute respiratory distress syndrome [10].
Although CQ and HCQ both have the potential to act against SARS-CoV-2, CQ, particularly at a higher dose, is associated with a higher risk of toxicity and should not be recommended for critically ill patients with COVID-19 [11]. As a result, HCQ has been vouched as a better therapeutic option in COVID-19 [12]. However, there is insufficient good-quality data to support the unmitigated efficacy of HCQ in COVID-19. Furthermore, while generally considered safe, there are potential risks associated with HCQ, including QT prolongation, myopathy, retinal toxicity, and rhabdomyolysis [13], especially at higher doses. The other adverse effects of HCQ include nausea, diarrhea, and abnormal liver functions. Across the world, there have been several reports of overdoses in people self-medicating with HCQ during the current pandemic [14, 15]. Furthermore, considering the recent finding that SARS-CoV-2 can itself show significant cardiac involvement [16], it is imperative to determine the efficacy and safety of HCQ in treating COVID-19 to assist in developing treatment protocols. Hence, we aimed to systematically review the literature and generate evidence of the therapeutic role of HCQ in patients diagnosed with COVID-19.
Methods
Study Design
We aimed to include published clinical studies that evaluated the therapeutic role of HCQ on COVID-19. Pre-clinical studies, case reports, case series, reviews, commentaries, viewpoints, or opinions were excluded. Studies that evaluated the prophylactic role of HCQ were also excluded.
Search Strategy
PubMed, Embase, ClinicalTrials.gov, ICTRP (WHO), Cochrane Library databases [Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL) and Cochrane Methodology Register], and two pre-print servers (medRxiv.org and Research Square) were searched from inception until 10 May 2020. The search terms used in various combinations were: “Coronavirus Disease-19”, “COVID-19”, “2019-nCoV”, “coronavirus”, “coronavirus disease”, “SARS-CoV-2”, “severe acute respiratory syndrome”, “treatment”, “therapy”, “anthraquinone”, “hydroxychloroquine”, and “HCQ”. These search terms were adapted for use with different bibliographic databases in combination with database-specific filters for studies, if available. The search strategy was used to obtain the titles and the abstracts of the relevant studies in English, and they were independently screened by two authors, who subsequently retrieved abstracts, and if necessary, the full text of articles to determine the suitability. The systematic review protocol could not be pre-registered as the current pandemic is an ongoing public health emergency, thereby resulting in a paucity of time to permit pre-registration.
Analysis of the Selected Articles
All the studies were critically analyzed. The risks of bias of each study were analyzed by the Cochrane risk of bias tool [17] for the randomized controlled trials and Newcastle–Ottawa scale [18] for the observational studies. Data related to the key efficacy and safety outcomes related to the use of HCQ from the included studies were noted. No assumptions or simplifications were made during the process. Disagreement resolution was performed with a third author.
Results
Search Results
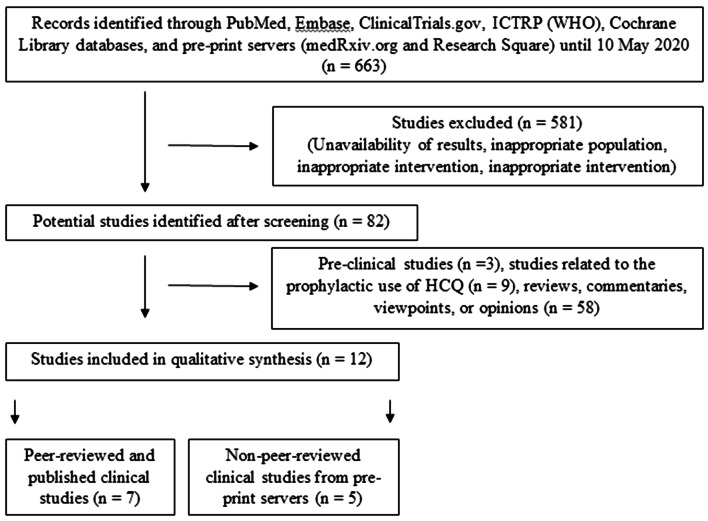
A total of 663 articles were screened and 12 clinical studies (seven peer-reviewed and published studies [19–25] and five non-peer-reviewed studies from pre-print servers [26–30]) were included (Fig. 1). The summary of the clinical studies is highlighted in Tables 1 and 2, and the quality of the included studies is depicted in Tables s1 and s2 (Supplementary Material).
Fig. 1.
Flowchart depicting the steps of qualitative synthesis of evidence from the literature. HCQ hydroxychloroquine, ICTRP (WHO) international clinical trials registry platform of the world health organization
Table 1.
Summary of the peer-reviewed and published clinical studies on the therapeutic role of HCQ in COVID-19
| Author (country) | Study design | Sample size (treatment/control) and male (%) | Age in years (mean ± SD or range) | Inclusion criteria | Study arms | Primary outcome | Results of the primary outcomes | Key adverse events with HCQ use |
|---|---|---|---|---|---|---|---|---|
| Gautret et al. [20] (France) | Open-label non-randomized clinical trial | 36 (20/16) (41.7% male) |
51.2 ± 18.7 (treatment) 37.3 ± 24.0 (control) |
SARS-CoV-2 carriage in nasopharyngeal sample |
Treatment: 200 mg of HCQ thrice daily for 10 days; six patients also received azithromycin (500 mg on day 1, 250 mg on days 2–5) Control: did not receive HCQ Symptomatic treatment and antibiotics were provided |
Outcome of a nasopharyngeal swab on day 6 | 70.0% (treatment) vs. 12.5% (control) virologically cured (P < 0.001) | Not reported |
| Gautret et al. [19] (France) | Prospective observational study | 80 (53.8% male) | 20–88 | SARS-CoV-2 carriage in nasopharyngeal sample | 200 mg of HCQ thrice daily for 10 days; azithromycin (500 mg on day 1, 250 mg on days 2–5) | Clinical outcome, outcome of a nasopharyngeal swab, and length of stay in IDU | 97.5% improved clinically, 93% virologically cured by day 8, and mean length of stay in IDU was 5 days | Nausea, vomiting, diarrhea, and blurred vision |
| Chen et al. [21] (China) | Randomized controlled trial | 30 (15/15) (70% male) |
50.5 ± 3.8 (treatment) 46.7 ± 3.6 (control) |
Tested positive for COVID-19 |
Treatment: 400 mg of HCQ daily for 5 days plus conventional treatment Control: conventional treatment |
Outcome of a nasopharyngeal swab on day 7 | 86.7% (treatment) vs. 93.3% (control) virologically cured (P > 0.05) | Transient diarrhea, and abnormal liver functions |
| Molina et al. [22] (France) | Prospective observational study | 11 (63.6% male) | 20–77 | Tested positive for COVID-19 | 200 mg of HCQ thrice daily for 10 days; azithromycin (500 mg on day 1, 250 mg on days 2–5) | Outcome of a nasopharyngeal swab on days 5–6 | 20% virologically cured | QT prolongation, death, and ICU transfers |
| Mercuro et al. [24] (USA) | Retrospective observational study | 90 (51.1% male) | 60.1 ± 16.7 | Tested positive for COVID-19 | Treatment with HCQ alone (41.1%) or in combination with azithromycin (58.9%) | Change in QT interval and other adverse drug vents | HCQ and azithromycin prolonged the QTc interval significantly | Intractable nausea, premature ventricular contractions, right bundle branch block, and hypoglycemia |
| Geleris et al. [23] (USA) |
Prospective observational study |
1376 (56.8% male) | ≥ 18 | Tested positive for COVID-19 in nasopharyngeal or oropharyngeal sample |
Treatment: 600 mg of HCQ twice daily on day 1; 400 mg of HCQ daily for 4 days; optional azithromycin (500 mg on day 1, 250 mg on days 2–5) Control: did not receive HCQ Symptomatic treatment and antibiotics were provided |
Composite of time to intubation or death (time-to-event analysis) | No significant association between HCQ and intubation or death (hazard ratio, 1.04; 95% CI: 0.82–1.32) | Not reported |
| Million et al. [25] (France) | Uncontrolled non-comparative observational study | 1061 (46.4% male) | 43.6 ± 15.6 | Tested positive for COVID-19 | Treated for at least 3 days with HCQ and azithromycin and followed-up for 9 days | Worsening, viral shedding persistence, and death | 91.7% had good clinical outcome and virological cure, 4.4% had viral shedding persistence, 0.47% died | 1.5% case fatality rate among patients who received HCQ and azithromycin |
All studies involved hospitalized (non-ICU) patients with conformed SARS-CoV-2 infection
COVID-19 Coronavirus Disease-19; HCQ hydroxychloroquine, ICU intensive care unit, IDU infectious disease unit
Table 2.
Summary of the non-peer-reviewed (at the time of preparing this manuscript) clinical studies from pre-print servers on the therapeutic role of HCQ in COVID-19
| Author (country) | Study design | Sample size (treatment/control) and male (%) | Age in years (mean ± SD or range) | Inclusion criteria | Study arms | Primary outcome | Results of the primary outcomes | Key adverse events with HCQ use |
|---|---|---|---|---|---|---|---|---|
| Chen et al. [26] (China) | Randomized controlled trial | 62 (31/31) (46.8% male) | 44.7 ± 15.3 | Tested positive for COVID-19 |
Treatment: 200 mg of HCQ twice daily for 5 days plus standard treatment Control: standard treatment |
Time to clinical recovery | The time to clinical recovery was significantly shortened with HCQ treatment | Rash and headache |
| Mahévas et al. [27] (France) | Hospital record-based observational study | 181 (84/97) (71.1% male) | 18–80 | Tested positive for COVID-19 | HCQ group: 600 mg of HCQ within 48 h of hospitalization Non-HCQ group: no HCQ | Transfer to the ICU within 7 days of inclusion and/or death from any cause | 20.2% of patients in the HCQ group were transferred to the ICU or died within 7 days vs. 22.1% in the non-HCQ group | QT prolongation, first-degree atrioventricular block, right bundle branch block, ICU transfer |
| Magagnoli et al. [28] (USA) | Retrospective analysis of hospital records (observational study) | 368 (100% male) | > 65 years | Patients hospitalized with COVID-19 | HCQ alone or in combination with azithromycin | Death and the need for mechanical ventilation | Increased overall mortality with HCQ monotherapy, and HCQ alone or in combination with azithromycin did not reduce the risk of mechanical ventilation | Increased mortality following HCQ monotherapy |
| Tang et al. [29] (China) | Randomized controlled trial | 150 (75/75) (55% male) | 46.1 ± 14.7 | Tested positive for COVID-19 |
HCQ group: 1200 mg daily for three days followed by 800 mg daily for 2 (mild/moderate patients) or 3 (severe patients) weeks plus standard treatment Control: plus standard treatment |
28-day negative conversion rate of SARS-CoV-2 | The overall 28-day negative conversion rate was similar between the two groups | Upper respiratory tract infection, diarrhea, and blurred vision |
| Ramireddy et al. [30] (USA) | Retrospective analysis of hospital records (observational study) | 98 (61% male) | 62 ± 17 | Tested positive for COVID-19, treated with HCQ alone or in combination with azithromycin, and with two electrocardiograms performed | HCQ alone or in combination with azithromycin | Baseline QTc and post-medication critical QTc prolongation | With the drug combination, the QTc prolongation was several-fold | QT prolongation |
All studies involved hospitalized (non-ICU) patients with conformed SARS-CoV-2 infection
COVID-19 Coronavirus Disease-19, HCQ hydroxychloroquine, ICU intensive care unit
Efficacy of HCQ in COVID-19
Among the published clinical studies, Gauret et al. [19, 20] and Chen et al. [21] have demonstrated very good virological and clinical outcomes with HCQ therapy alone or in combination with azithromycin. Million et al. [25] have also demonstrated good virological and clinical outcomes with HCQ therapy. Molina et al. however, have shown negative results with HCQ treatment [22]. Among the non-peer-reviewed studies included from pre-print servers, Chen et al. [26] have demonstrated good virological and clinical outcomes with HCQ treatment. The results of Mahévas et al. [27], Magagnoli et al. [28], Tang et al. [29], and Ramireddy et al. [30] were negative or equivocal. Likewise, Geleris et al. [23] reported no significant effect of HCQ on intubation or death in COVID-19 patients.
Safety of HCQ in COVID-19
In the studies of Gauret et al. [19, 20], Chen et al. [21], and Million et al. [25], HCQ was found to be safe with mild adverse reactions, such as nausea, vomiting, and transient abnormal liver functions. Molina et al. [22] and Mercuro et al. [24] have reported QT prolongation associated with HCQ treatment. HCQ therapy was associated with serious adverse reactions, such as death, QT prolongation, first-degree atrioventricular block, diarrhea, and blurred vision in the non-peer-reviewed studies included from pre-print servers [26–30].
Critical Appraisal of the Included Studies
It is relevant to mention that there were several major methodological limitations to these studies as evident from the high risks of bias in the majority of the included studies. The randomized controlled trials had mostly selection, performance, and detection biases, while the observational studies had predominantly comparability, exposure, and outcome biases. The studies of Chen et al. [21] and Gautret et al. [31] were underpowered. Chen et al. [21] included patients with mild symptoms only and they were concomitantly treated with other antivirals. In the first study, Gautret et al. [31] did not randomize the patients or include drop-outs in the final analysis. There were heterogeneities in terms of the viral load between the two groups at baseline and the investigators deviated from the registered protocol in terms of the outcome measures. Clinical outcomes, although extremely important, were not reported. In the second study, Gautret et al. [19] neither included a control arm nor mentioned the eligibility criteria precisely. Likewise in the study of Geleris et al. [23], the HCQ-treated patients were more severely ill at baseline. In the study of Chen et al., there was a small improvement in body temperature and cough with a higher dose of HCQ. However, the endpoints specified in the published protocol differed from those reported, the results of the low-dose HCQ group were not reported, and the trial was prematurely terminated [26]. The largest observational study of Million et al., with a sample size of 1061 patients, also did not have a control arm [25]. Further, no clinically relevant medium- or long-term follow-up data are reported in any of these studies. Another major factor to be considered is that very few studies have focused on the safety aspect of HCQ in the treatment of COVID-19.
Discussion
We systematically reviewed the literature and compiled the available evidence of the therapeutic role of HCQ in COVID-19 from clinical studies. In silico studies have shown the disruption of the interaction of the S protein of SARS-CoV-2 with the host cell membrane following the application of HCQ, as well as the role of HCQ that can complement an evolving SARS-CoV2 main protease [6]. A good in vitro efficacy of HCQ alone or in combination with azithromycin against SARS-CoV-2 was shown in vitro pre-clinical studies as well [6, 8, 9]. Some other authors have also demonstrated the in vitro efficacy of HCQ against SARS-CoV in Vero cells and Crandell-Reese feline kidney (CRFK) cells [32]. However, the translational value of the pre-clinical studies to clinical ones is of concern. Despite showing good in vitro efficacy, CQ showed poor in vivo efficacy in earlier studies with Zika virus [33], Ebola virus [34, 35], and Chikungunya virus [36], as well as poor clinical outcomes in dengue fever [37, 38] and influenza [39]. This in vitro-in vivo disparity may be partly because of the complex pharmacokinetics of 4-aminoquinolines [40], and hence, the same applies to HCQ. This warrants further clarification about COVID-19 pathogenesis before using HCQ despite promising in vitro results [41].
Likewise, although some of the clinical studies have shown a good efficacy of HCQ alone or in combination with azithromycin in achieving virological as well as clinical endpoints in patients with COVID-19, the studies had major methodological limitations. A majority of the included studies had high risks of bias. Some studies showed negative results with HCQ along with serious concerns of HCQ-related toxicities. None of the studies included critically ill COVID-19 patients with multiple co-morbidities and the treatment period was very short. Hence, the real clinical benefits of HCQ in COVID-19 are still elusive. It is important to mention that although viral clearance is important, medium- and long-term clinical outcomes are much more relevant, and these need to be studied.
The daily divided dose of HCQ studied in COVID-19 was between 400 mg and 1200 mg for 5–10 days. The dosing recommendations for HCQ in the special population, such as pregnant women, obese patients, and pediatric population or patients with systemic co-morbidities diagnosed with COVID-19 are unavailable. Based on the 50% maximal effective concentration (EC50), the therapeutic dose of HCQ can be calculated. A physiologically based pharmacokinetic modeling study recommended a loading dose of HCQ of 400 mg twice daily for 1 day followed by 200 mg twice daily for the treatment of COVID-19 [8]. Through simulation, it was found that a loading dose of 800 mg of HCQ followed by 600 mg in 6 h and then 600 mg daily for 4 days achieved a daily trough concentration above EC50 in > 50% of the subjects [42].
From the safety point of view, short-term HCQ treatment has been considered safe, even in pregnancy [43]. However, the addition of azithromycin may lead to QT prolongation [44], as well as bundle branch block [45]. Nonetheless, these issues could be tackled with the inpatient use of ambulatory telemetry monitors [46]. The use of HCQ was found to be safe in some of the included clinical studies, while in the other it led to serious adverse reactions, including death. HCQ treatment might warrant monitoring of blood counts, serum electrolytes, blood glucose, and liver and renal functions [47]. In an earlier systematic review, the authors recommended that HCQ is worthy of treatment as an experimental drug in COVID-19 [47]. However, because of the lack of robust data on the efficacy and safety of HCQ, other authors have vouched for clinically relevant medium- and long-term follow-up results and safety data from well-designed robust studies before advocating the routine use of HCQ in COVID-19 [48]. The need for a robust antimicrobial stewardship program to fight the COVID-19 pandemic has also been stressed [49]. Two recent editorials in the British Medical Journal [13] and New England Journal of Medicine [50] have highlighted the need for well-designed, adequately powered, randomized controlled trials of CQ or HCQ, and a recent article in the Lancet has also raised concern on the use of HCQ in critically ill COVID-19 patients [51]. Likewise, a recent systematic review in JAMA has stressed the efficacy of HCQ alone or in combination with azithromycin in COVID-19, although the authors have highlighted the importance of randomized clinical trials before the widespread use of these drugs [52].
It is pertinent to mention here that HCQ has been also advocated to be used as a prophylactic agent against COVID-19 for some specific high-risk population like asymptomatic healthcare workers involved in the care of suspected or confirmed cases of COVID-19 and asymptomatic household contacts of laboratory-confirmed cases [53]. In a previous systematic review, it was shown that although pre-clinical results with HCQ are promising, there is a dearth of evidence to support the clinical efficacy of HCQ in preventing COVID-19 [54]. Similar views were published in other journals [55, 56]. Several ongoing clinical trials are evaluating the prophylactic and therapeutic role of HCQ in COVID-19. The results, including the interim ones, of these trials are awaited.
However, in this ongoing challenging scenario, considering the absence of any other definitive therapy in COVID-19, the mixed efficacy, and the safety profile of HCQ, we feel that clinicians should carefully weigh risks and benefits of HCQ alone or in combination with azithromycin. Considering that COVID-19 itself can have cardiac manifestations [16], periodic QT interval should be monitored in COVID-19 patients on HCQ. At the same time, it is necessary to define when a treated patient can be considered as no longer contagious after treatment with HCQ and the viral load can come handy [57]. There is enough rationale to justify the continued investigation of the efficacy and safety of HCQ in COVID-19 patients [58]. Based on the preliminary trial results, some countries, in fact, have already incorporated HCQ into their treatment protocols for certain patients with COVID-19 [47].
There are certain limitations to our review. To date, there is a dearth of adequate data from well-designed studies on this topic of interest. There was some heterogeneity in the HCQ treatment regimens across the clinical studies. Some non-peer-reviewed studies from pre-print servers were included, the final version of which are likely to change after publication. Pre-clinical and clinical studies are ongoing, and most likely new information will be rapidly be added to the existing literature shortly. As of 5 May 2020, 171 and 107 clinical studies on HCQ in COVID-19 have been registered in ClinicalTrials.gov [59] and the International Clinical Trials Registry Platform (ICTRP) of the World Health Organization (WHO) [60]. Further, our review mostly focused on the adult population, and hence, the generalizability to the special populations (pregnant, lactating, pediatric, or geriatric) and those with systemic co-morbidities affecting the pharmacokinetics and pharmacodynamics of HCQ is questionable. However, a recent study with CQ, having a similar pharmacokinetic profile as that of HCQ, has shown that the influence of renal function, critical illness, or obesity on its action is probably limited [61]. Notwithstanding these limitations, this systematic review included a large sample size (3543) of COVID-19 patients and the results of this study will add to the knowledge of the treating clinicians who are using HCQ in COVID-19 patients in their care.
Conclusion
In this systematic review, we have found that the results of efficacy and safety of HCQ in COVID-19, as obtained from 12 clinical studies, is not satisfactory, although many of these studies had major methodological limitations. Stronger evidence from well-designed robust randomized clinical trials is required before conclusively determining the role of HCQ in the treatment of COVID-19. Clinical prudence is required to advocate HCQ as an unmitigated therapeutic armamentarium in COVID-19. Also, the potential of HCQ as a chemo-prophylactic agent against COVID-19 needs to be explored.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
SD and SB conceptualized the review; SD and SB were involved in literature search and study selection; SD and SB were involved in disagreement resolution and finalization of the included studies; SD and ST extracted data from the studies for qualitative synthesis of evidence; SD, SB, ST, and SS interpreted the analyses; SD, SB, and ST drafted the review; ST revised the manuscript; SS provided expert input and updated the final review.
Funding
No funding or support was received for the work.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- 1.Countries where Coronavirus has spread—Worldometer. https://www.worldometers.info/coronavirus/countries-where-coronavirus-has-spread/. Accessed 10 May 2020
- 2.Sahu KK, Mishra AK, Lal A. COVID-2019: update on epidemiology, disease spread and management. Monaldi Arch Chest Dis Arch Monaldi Mal Torace. 2020 doi: 10.4081/monaldi.2020.1292. [DOI] [PubMed] [Google Scholar]
- 3.Devaux CA, Rolain J-M, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golden EB, Cho H-Y, Hofman FM, Louie SG, Schönthal AH, Chen TC. Quinoline-based antimalarial drugs: a novel class of autophagy inhibitors. Neurosurg Focus. 2015;38(3):E12. doi: 10.3171/2014.12.FOCUS14748. [DOI] [PubMed] [Google Scholar]
- 5.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3(11):722–727. doi: 10.1016/s1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comparison of random and site directed mutation effects on the efficacy between lead SARS-CoV2 anti-protease drugs. Indinavir and Hydroxychloroquine. 2020; 10.21203/rs.3.rs-22082/v1
- 8.Yao X, Ye F, Zhang M, et al. In Vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6(1):1–4. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreani J, Le Bideau M, Duflot I, et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog. 2020 doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3(4):e208857–e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastick KA, Okafor EC, Wang F, et al. Review: hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19) Open Forum Infect Dis. 2020 doi: 10.1093/ofid/ofaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferner RE, Aronson JK. Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020;369:m1432. doi: 10.1136/bmj.m1432. [DOI] [PubMed] [Google Scholar]
- 14.Coronavirus: we are now receiving patients suffering from chloroquine poisoning, says Lagos govt, NCDC cautions Nigerians. https://tribuneonlineng.com/coronavirus-we-are-now-receiving-patients-suffering-from-chloroquine-poisoning-says-lagos-govt-ncdc-cautions-nigerians/. Accessed 15 Apr 2020
- 15.Man fatally poisons himself while self-medicating for coronavirus, doctor says—the New York times. https://www.nytimes.com/2020/03/24/us/chloroquine-poisoning-coronavirus.html. Accessed 15 Apr 2020
- 16.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Altman DG, Gøtzsche PC, et al. The cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ottawa Hospital Research Institute. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 8 May 2020
- 19.Gautret P, Lagier J-C, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial. MedRxiv. 2020 doi: 10.1101/2020.03.16.20037135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Chen Jun LD, Chen Jun LD. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ Med Sci. 2020 doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molina JM, Delaugerre C, Le Goff J, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Médecine Mal Infect. 2020 doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Million M, Lagier J-C, Gautret P, et al. Full-length title: early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille. France Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.03.22.20040758. [DOI] [Google Scholar]
- 27.Mahevas M, Tran V-T, Roumier M, et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. medRxiv. 2020 doi: 10.1101/2020.04.10.20060699. [DOI] [Google Scholar]
- 28.Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv. 2020 doi: 10.1101/2020.04.16.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial. medRxiv. 2020 doi: 10.1101/2020.04.10.20060558. [DOI] [Google Scholar]
- 30.Ramireddy A, Chugh HS, Reinier K, et al. Experience with hydroxychloroquine and azithromycin in the COVID-19 pandemic: implications for QT interval monitoring. medRxiv. 2020 doi: 10.1101/2020.04.22.20075671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Biot C, Daher W, Chavain N, et al. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem. 2006;49(9):2845–2849. doi: 10.1021/jm0601856. [DOI] [PubMed] [Google Scholar]
- 33.Shiryaev SA, Mesci P, Pinto A, et al. Repurposing of the anti-malaria drug chloroquine for Zika Virus treatment and prophylaxis. Sci Rep. 2017;7(1):15771. doi: 10.1038/s41598-017-15467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowall SD, Bosworth A, Watson R, et al. Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig model. J Gen Virol. 2015;96(12):3484–3492. doi: 10.1099/jgv.0.000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falzarano D, Safronetz D, Prescott J, Marzi A, Feldmann F, Feldmann H. Lack of protection against ebola virus from chloroquine in mice and hamsters. Emerg Infect Dis. 2015;21(6):1065–1067. doi: 10.3201/eid2106.150176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roques P, Thiberville S-D, Dupuis-Maguiraga L, et al. Paradoxical effect of chloroquine treatment in enhancing Chikungunya virus infection. Viruses. 2018 doi: 10.3390/v10050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L-F, Lin Y-S, Huang N-C, et al. Hydroxychloroquine-inhibited dengue virus is associated with host defense machinery. J Interferon Cytokine Res Off J Int Soc Interferon Cytokine Res. 2015;35(3):143–156. doi: 10.1089/jir.2014.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borges MC, Castro LA, de Fonseca BAL. Chloroquine use improves dengue-related symptoms. Mem Inst Oswaldo Cruz. 2013;108(5):596–599. doi: 10.1590/s0074-02762013000500010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paton NI, Lee L, Xu Y, et al. Chloroquine for influenza prevention: a randomised, double-blind, placebo controlled trial. Lancet Infect Dis. 2011;11(9):677–683. doi: 10.1016/S1473-3099(11)70065-2. [DOI] [PubMed] [Google Scholar]
- 40.Gustafsson LL, Walker O, Alván G, et al. Disposition of chloroquine in man after single intravenous and oral doses. Br J Clin Pharmacol. 1983;15(4):471–479. doi: 10.1111/j.1365-2125.1983.tb01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guastalegname M, Vallone A. Could chloroquine/hydroxychloroquine be harmful in coronavirus disease 2019 (COVID-19) treatment? Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Kofahi M, Jacobson P, Boulware DR, et al. Finding the dose for hydroxychloroquine prophylaxis for COVID-19; the desperate search for effectiveness. Clin Pharmacol Ther. 2020 doi: 10.1002/cpt.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sperber K, Hom C, Chao CP, Shapiro D, Ash J. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J. 2009;7:9. doi: 10.1186/1546-0096-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sapp JL, Alqarawi W, MacIntyre CJ, et al. Guidance on minimizing risk of drug-induced ventricular arrhythmia during treatment of COVID-19: a statement from the Canadian Heart Rhythm society. Can J Cardiol. 2020 doi: 10.1016/j.cjca.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asli R, Abdullah MS, Chong PL, et al. Case report: right bundle brunch block and QTc prolongation in a patient with novel coronavirus disease (COVID-19) treated with hydroxychloroquine. Am J Trop Med Hyg. 2020 doi: 10.4269/ajtmh.20-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang D, Saleh M, Gabriels J, et al. Inpatient use Of Ambulatory Telemetry Monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh AK, Singh A, Shaikh A, Singh R, Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr. 2020;14(3):241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gbinigie K, Frie K. Should chloroquine and hydroxychloroquine be used to treat COVID-19? A rapid review. BJGP Open. 2020 doi: 10.3399/bjgpopen20X101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCreary EK, Pogue JM. Coronavirus disease 2019 treatment: a review of early and emerging options. Open Forum Infect Dis. 2020;7(4):105. doi: 10.1093/ofid/ofaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubin EJ, Harrington DP, Hogan JW, Gatsonis C, Baden LR, Hamel MB. The urgency of care during the Covid-19 pandemic—learning as we go. N Engl J Med. 2020 doi: 10.1056/NEJMe2015903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taccone FS, Gorham J, Vincent J-L. Hydroxychloroquine in the management of critically ill patients with COVID-19: the need for an evidence base. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 53.AdvisoryontheuseofHydroxychloroquinasprophylaxisforSARSCoV2infection.pdf
- 54.Shah S, Das S, Jain A, Misra DP, Negi VS. A systematic review of the prophylactic role of chloroquine and hydroxychloroquine in Coronavirus Disease-19 (COVID-19) Int J Rheum Dis. 2020 doi: 10.1111/1756-185X.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rathi S, Ish P, Kalantri A, Kalantri S. Hydroxychloroquine prophylaxis for COVID-19 contacts in India. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D’Cruz M. The ICMR bulletin on targeted hydroxychloroquine prophylaxis for Covid-19: need to interpret with caution. Indian J Med Ethics. 2020;5(2):100–102. doi: 10.20529/IJME.2020.040. [DOI] [PubMed] [Google Scholar]
- 57.La Scola B, Le Bideau M, Andreani J, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2020 doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim AHJ, Sparks JA, Liew JW, et al. A rush to judgment? Rapid reporting and dissemination of results and its consequences regarding the use of hydroxychloroquine for COVID-19. Ann Intern Med. 2020 doi: 10.7326/M20-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Search of: Hydroxychloroquine | COVID 19 - List Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=COVID+19&term=Hydroxychloroquine&cntry=&state=&city=&dist=. Accessed 5 May 2020
- 60.WHO | Welcome to the WHO ICTRP. In: WHO. https://www.who.int/ictrp/en/. Accessed 5 May 2020
- 61.Smit C, Peeters MYM, van den Anker JN, Knibbe CAJ. Chloroquine for SARS-CoV-2: implications of its unique pharmacokinetic and safety properties. Clin Pharmacokinet. 2020 doi: 10.1007/s40262-020-00891-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.