Abstract
Tumor necrosis factor (TNF)-related apop-tosis-inducing ligand (TRAIL), a type II transmembrane protein, is a part of the TNF superfamily of cytokines. Cantharidin, a type of terpenoid, is extracted from the blister beetles (Mylabris genus) used in Traditional Chinese Medicine. Cantharidin elicits antibiotic, antiviral and antitumor effects, and can affect the immune response. The present study demonstrated that a cantharidin and TRAIL combination treatment regimen elicited a synergistic outcome in TRAIL-resistant DU145 cells. Notably, it was also identified that cantharidin treatment initiated the downregulation of cellular FLICE-like inhibitory protein (c-FLIP) and upregulation of death receptor 5 (DR-5), and sensitized cells to TRAIL-mediated apoptosis by initiating autophagy flux. In addition, cantharidin treatment increased lipid-modified microtubule-associated proteins 1A/1B light chain 3B expression and significantly attenuated sequestosome 1 expression. Attenuation of autophagy flux by a specific inhibitor such as chloroquine and genetic modification using ATG5 small interfering RNA abrogated the cantharidin-mediated TRAIL-induced apoptosis. Overall, the results of the present study revealed that cantharidin effectively sensitized cells to TRAIL-mediated apoptosis and its effects are likely to be mediated by autophagy, the downregulation of c-FLIP and the upregulation of DR-5. They also suggested that the combination of cantharidin and TRAIL may be a successful therapeutic strategy for TRAIL-resistant prostate cancer.
Keywords: cantharidin, autophagy, cellular FLICE-like inhibitory protein, tumor necrosis factor-related apoptosis-inducing ligand, death receptor 5, apoptosis
Introduction
Prostate cancer (PCa) is one of the major types of malignancy in men, with a high incidence rate (1). Currently, the primary treatment strategies for these tumors are surgical castration combined with androgen deprivation therapy or antiandrogens, radiotherapy, chemotherapy, immunotherapy and other combined treatments (2,3). In patients with PCa, the aim of these treatments is initiation of apoptosis in tumor cells, thereby inhibiting cancer growth and spread.
Cantharidin, a type of terpenoid, is extracted from the blister beetles (Mylabris genus) and is used in Traditional Chinese Medicine (4,5). Cantharidin possesses antibiotic, antiviral and antitumor qualities, and can affect immune responses (4,6). Cantharidin has been used as a medicinal agent for >2,000 years and has a number of applications, including treatment of edema and warts (4). Cantharidin treatment initiates cell cycle arrest and attenuates apoptosis in various types of carcinomas including those of the bladder (7), breast (8), liver (9,10), and colon (11), buccal mucosa and leukemia (12,13).
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is the only identified protein that can specifically initiate apoptosis of tumor cells without affecting noncancerous cells (3). TRAIL, a type II transmembrane protein, is a part of the TNF superfamily of cytokines identified in 1995 by Wiley et al (14). TRAIL is able to bind the death receptors (DR)-4 and DR-5, promoting the death-inducing signaling complexes activity of caspase-3 and caspase-8, thereby initiating tumor cell death (15). However, downregulation of the death receptors and pro-apoptotic proteins, and upregulation of the anti-apoptotic proteins such as cellular FLICE-like inhibitory protein (c-FLIP), leads to TRAIL-mediated apoptosis resistance (16-19). Therefore, identification of TRAIL sensitizers is may help to overcome the barrier of TRAIL resistance.
Autophagy is a multi-step cellular process in which cytoplasmic constituents are targeted to lysosomes for degradation, and is associated with several diseases including cancer (20). Recent studies have revealed that autophagy has a dual mechanism, having either pro-survival or pro-death characteristics depending on the cell type and stimulus strength (21-23). Autophagy acts in a pro-survival manner through cytoprotective events that favor cell survival and decrease the incidence of cell death. Conversely, autophagy can augment a pro-death signaling system and ultimately result in cell death in cancer (24). The complete autophagic flux engages microtubule-associated proteins 1A/1B light chain 3B (LC3), which serves an important role in cargo elimination, phagophore elongation and autophagosome composition, and the sequestosome 1 (p62)-cargo system is selectively attached to autophagosomes through the interaction of p62 and LC3 (20).
In the present study, the effectiveness of cantharidin in sensitization of PCa cells to TRAIL was examined. We aimed to elucidate the molecular pathways underlying the effect of cantharidin and synergistic effect of cantharidin and TRAIL in DU145 cells. It was identified that cantharidin treatment sensitizes DU145 tumor cells to TRAIL-mediated apoptosis via autophagy flux. The combined treatment of cantharidin and TRAIL may represent a viable therapeutic strategy for certain cases of TRAIL-resistant cancer.
Materials and methods
Cell culture
Human prostate DU145 and LNCaP cell lines were maintained in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. During the experiments, the culture medium was changed to RPMI-1640 containing 1% FBS. In the present study, TRAIL treatment intervals of 1 and 2 h were used for the western blot analysis and the cell viability assay, respectively. It was identified that a 2 h treatment period for the combination of TRAIL and cantharidin induced a high level of cell death that affected the detection of certain proteins. The cells were maintained at 37°C and 5% CO2 in a humidified incubator.
Reagents
Cantharidin was acquired from Sigma-Aldrich; Merck KGaA, while TRAIL was attained from AbFrontier Co., Ltd.
Cell viability assay
The DU145 and LNCaP cells were plated 2×105 cells/well in 12-well plates and treated with cantharidin (0, 0.25, 0.50 and 1 μM) for 18 h and were further exposed to TRAIL (200 ng/ml) for an additional 2 h. For autophagy inhibition, cells were treated 10 μM chloroquine (CQ) 1 h prior to cantharidin or TRAIL treatment at 37°C. Cell morphology was assessed under a light microscope (Nikon Corporation) at magnification, ×400. Cell viability was determined by crystal violet staining method as previously described (25).
Trypan blue exclusion assay
Cell viability was evaluated by trypan blue exclusion assay using a hemocytometer. The DU145 and LNCaP cells were seeded 1×105 cells/well in 24-well plate, after treatment the cells were dissociated with trypsin-EDTA are were then suspended in 1 ml PBS per well and 1 ml trypan blue solution (Sigma-Aldrich; Merck KGaA). After 5 min at 20°C, the cells were counted using a hemocytometer (Marienfeld Corp.) under a light microscope (Nikon Corp.) at ×400 magnification. Untreated cells were used as control, and the cell viability was compared to the control. Each treatment was performed in triplicate.
Immunofluorescence staining
DU145 cells were cultured on poly-L-lysine-coated coverslips. Following cantharidin treatment with or without CQ or ATG5 siRNA, the cells were were fixed with 4% paraformaldehyde at 4°C for 10 min and permeabilized with 0.1% Triton X-100. The cells were then incubated for 1 h at room temperature in a blocking solution (5% FBS in TBS) followed by overnight incubation at 4°C with anti p62 antibody (1:250; cat. no. PAS-20839; Invitrogen; Thermo Fisher Scientific, Inc.). Following washing with PBS, the cells were incubated with the Alexa Fluor® 488-conjugated donkey polyclonal anti-rabbit secondary antibody (1:500; cat. no. A-21206; Thermo Fisher Scientific, Inc.) for 2 h in the dark. Finally, immunostaining was visualized under a fluorescence microscope at magnification, ×200.
Western blot analysis
Western blot analysis was performed as described previously (26). Immunoprecipitation assay buffer (Qiagen, Inc.) was used for total protein extraction. The supernatant was collected by centrifugation at 11,200 × g and 4°C for 10 min. The protein concentration was determined using a BCA protein assay kit (Thermo Fisher Scientific; Inc.). Protein (30 μg) were separated on 10% SDS-PAGE gels and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% non-fat dried milk at 25°C for 1 h. Then membranes were incubated with primary antibodies for 1 h at 25°C, the following antibodies were used: DR-5 (1:1,000; cat. no. ab181846; Abcam); β-actin (1:10,000; cat. no. A5441; Sigma-Aldrich; Merck KGaA); c-FLIP (1:1,000; cat. no. ADI-AAP-440; Enzo life sciences, USA); LC3A/B (1:1,000; cat. no. 4108; Cell Signaling Technology, Inc.); p62 (1:250; cat. no. PAS-20839; Invitrogen; Thermo Fisher Scientific, Inc.); ATG5 (1:1,000; cat. no. c.s12994s; Cell Signaling Technology, Inc.); and cleaved caspase-8 (1:1,000; cat. no. 551242; BD Pharmingen; BD Biosciences). Then membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5,000; cat. nos. ADI-SAB-100 and ADI-SAB-300; Enzo Life Science, Inc.) at 25°C for 1 h. The immune reactive protein bands were visualized using enhanced chemiluminescence detection system (GE Healthcare Life Sciences) and detected with chemiluminiscence imaging system (Fusion FX7; Viber Lourmat). The intensities of the protein bands were determined using Image J Java 1.8.0 software (National Institutes of Health).
Transmission electron microscopy (TEM) analysis
Following the fixation of cells in 2% glutaraldehyde (Electron Microscopy Sciences) and 2% paraformaldehyde (Electron Microscopy Sciences) in 0.05 M sodium cacodylate (pH 7.2; Electron Microscopy Sciences) for 2 h at 4°C, the specimens were fixed in 1% osmium tetroxide (Electron Microscopy Sciences) for 1 h at 4°C, dehydrated in increasing ethanol concentration (25, 50, 70, 90 and 100%) for 5 min each, and embedded in Embed 812 epoxy resin (Electron Microscopy Sciences) for 48 h at 60°C according to the manufacturers' protocol. Ultrathin sections (60 nm) were prepared using a LKBIII ultratome (Leica Microsystems GmbH) and stained with 0.5% uranyl acetate (Electron Microscopy Sciences) for 20 min and 0.1% lead citrate (Electron Microscopy Sciences) for 7 min at room temperature. Images were captured using a Hitachi H7650 electron microscope (Hitachi, Ltd.) at magnification, ×10,000, installed at the Center for University-Wide Research Facilities (CURF), Jeonbuk National University.
Small interfering RNA transfection
DU145 cells were seeded 1×105 cells/well in 24-well plates for 24 h and then transfected with Silencer Select small interfering RNA (ATG5 siRNA; oligo ID HSS114103; Sequence GGU UUG GAC GAA UUC CAA CUU GUU U; Invitrogen; Thermo Fisher Scientific, Inc.) using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Concomitantly, non-targeting siRNA was transfected as a negative control. The cells were incubated with ATG5 siRNA or negative control siRNA for 6 h and the medium was then changed to RPMI-1640 with 10% FBS for 24 h. The cells were then treated with neferine, or neferine in combination with TRAIL.
Statistical analysis
Statistical analyses were performed using GraphPad v.5.01 software (GraphPad Software Inc.). All experiments were performed in triplicate, and the data are expressed as the mean ± standard error of the mean. Significant differences between control and treated samples were analyzed by one-way analysis of variance followed by Tukey's post hoc test.
Results
Effect of cantharidin on TRAIL-mediated apoptosis in PCa cells
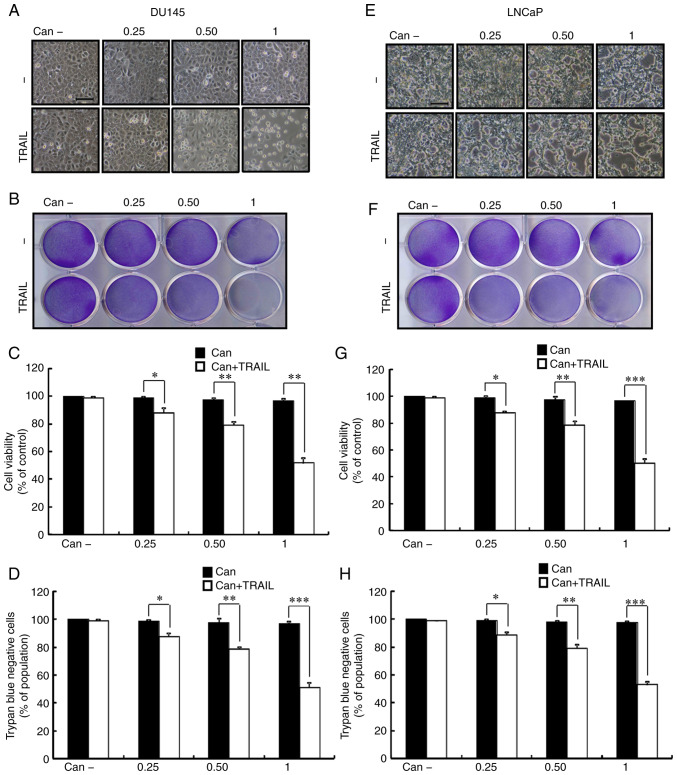
To observe the potential suppressive effect of cantharidin on PCa cell growth, DU145 and LNCaP cells were treated consecutively with cantharidin at different concentrations for 18 h and then TRAIL for an additional 2 h. As shown in Fig. 1, TRAIL or cantharidin alone only marginally induced cell death, and no morphological changes were observed. In addition, the combined regimen of cantharidin and TRAIL significantly attenuated cell viability, whereas treatment with cantharidin or TRAIL alone had no effect on apoptosis (Fig. 1A-H). The results suggested that cantharidin effectively sensitized human PCa cells to TRAIL-mediated apoptosis.
Figure 1.
Effect of Can on TRAIL-mediated apoptosis in prostate cancer cells. DU145 cells were treated with Can (0, 0.25, 0.50 and 1 μM) for 18 h and then with TRAIL (200 ng/ml) for an additional 2 h. (A) The morphology of DU145 cells was evaluated by interference light microscopy (scale bar=100 μm; magnification, ×100). (B) Cellular viability was assessed with crystal violet staining in DU145 cells. (C) Quantification of the average density of crystal violet staining in DU145 cells. (D) Cellular viability was evaluated with trypan blue dye exclusion assays in DU145. (E) Morphology, (F and G) crystal violet cellular viability and (H) trypan blue staining in LNCaP cells. *P<0.05, **P<0.01 and ***P<0.0001. Can, cantharidin; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Cantharidin induced autophagy and enhanced cells to TRAIL-mediated apoptosis
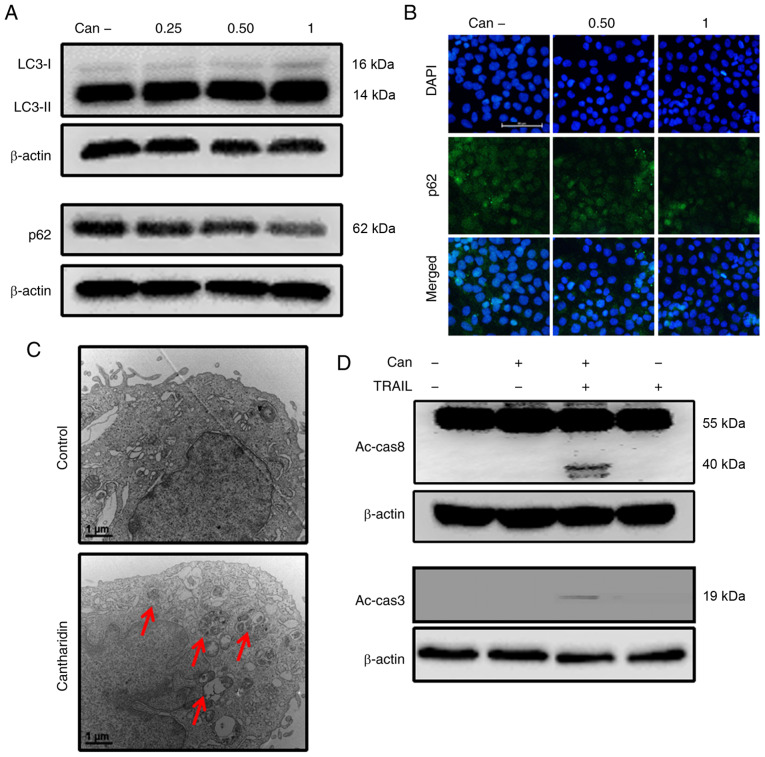
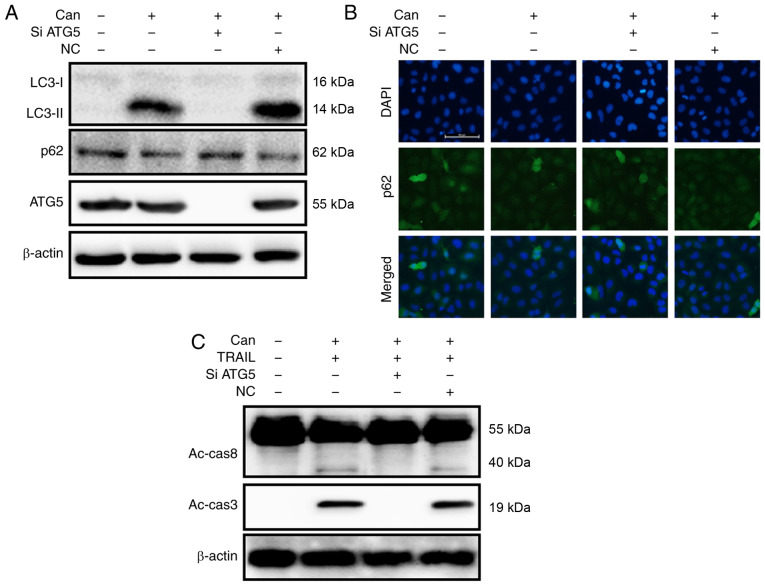
In response to cantharidin treatment, DU145 cells exhibited significantly upregulated LC3-II expression levels and attenuated p62 expression levels (Figs. 2A, and S1A and B). The protein levels assayed by immunofluorescence staining were consistent with the results of western blot analysis (Figs. 2B and S1C). TEM demonstrated that both autophagic and vacant vacuoles were secreted in cantharidin-treated cells (Fig. 2C). Cells treated with cantharidin and TRAIL in combination exhibited increased expression of activated caspase (Ac-cas)-3 and Ac-cas8, respectively (Fig. 2D). These data suggest that cantharidin can initiate autophagy in DU145 cells.
Figure 2.
Can induces autophagy and enhances TRAIL-mediated apoptosis of cells. DU145 cells were treated with Can (0, 0.25, 0.50 and 1 μM) for 18 h. (A) The levels of LC3-II and p62 were assessed by western blot analysis. (B) DU145 cells were treated with Can (0, 0.50 and 1 μM) for 18 h. Cells were then immunostained with p62 (green) and evaluated for fluorescence (scale bar=50 μm; magnification, ×200). (C) The formation of autophagosomes in treated cells was examined by transmission electron microscopy. Red arrows indicate autophagosomes. Scale bar=1 μm. (D) DU145 cells were treated with Can (1 μM) for 18 h and then TRAIL for an additional 1 h. The levels of Ac-cas3 and Ac-cas8 were assessed by western blot analysis. β-actin was used as the control. TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; can, cantharidin; Ac-cas, activated caspase; p62, sequestosome 1; LC3-I, cytoplasmic microtubule-associated proteins 1A/1B light chain 3B; LC3-II, lipid-modified microtubule-associated proteins 1A/1B light chain 3B.
Autophagy inhibition rescues TRAIL-mediated apoptosis sensitization through cantharidin
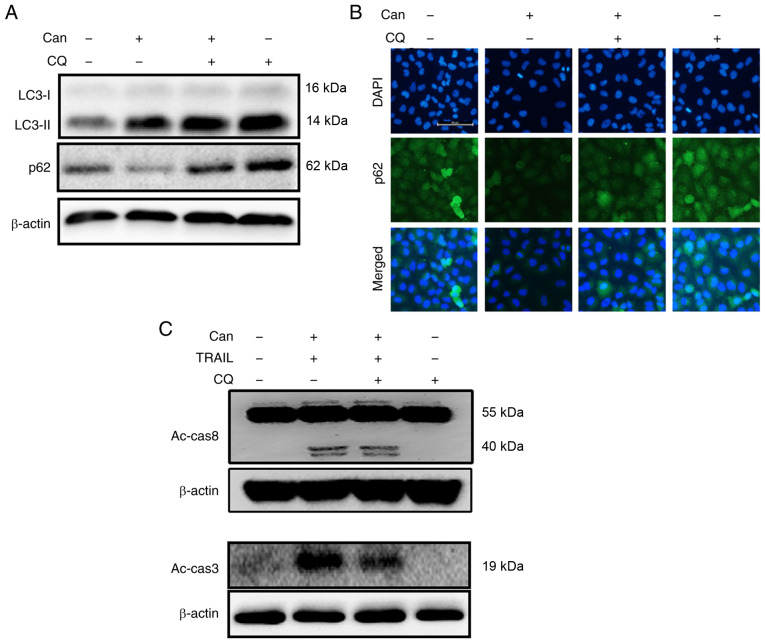
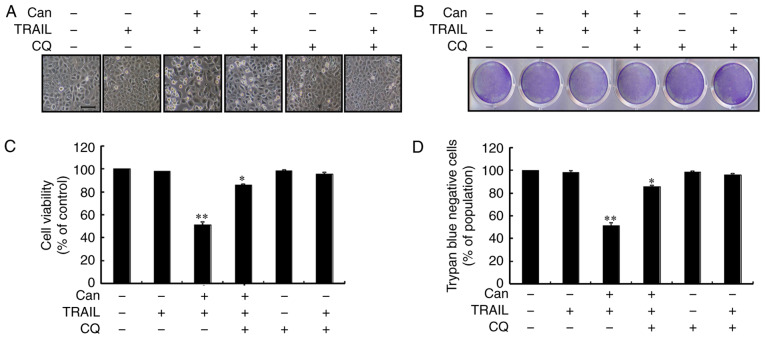
Autophagy control was further confirmed by examining the autophagy flux following treatment with CQ. CQ promoted the accumulation of membrane-bound LC3-II and led to an increase in p62 levels (Figs. 3A, and S2A and B). Immunofluorescence staining confirmed the increase in p62 expression (Figs. 3B and S2C). However, co-treatment with cantharidin, TRAIL and CQ attenuated the upregulation of Ac-cas3 and Ac-cas8 (Fig. 3C). The effects of cantharidin on the viability of the DU145 cells in response to CQ were also investigated. Cell morphology analysis confirmed that CQ treatment more effectively attenuated the cell death compared with the combined regimen of cantharidin and TRAIL (Fig. 4A). Co-treatment with cantharidin, TRAIL and CQ markedly increased the viability of DU145 cells (Fig. 4B-D). This suggests that cantharidin sensitized TRAIL-mediated apoptosis may be attenuated through CQ by blocking autophagy flux.
Figure 3.
Autophagy inhibition rescues TRAIL-mediated apoptosis sensitization by Can by promoting autophagy flux. DU145 cells were pretreated with CQ (10 μM) for 1 h prior to their exposure to Can (1 μM) for 18 h. (A) The levels of LC3-II and p62 were assessed by western blot analysis. (B) Cells were immunostained with p62 (green) and evaluated for fluorescence (scale bar=50 μm; magnification, ×200). (C) DU145 cells were pretreated with CQ (10 μM) for 1 h prior to their exposure to Can (1 μM) for 18 h and then to TRAIL for an additional 1 h. The levels of Ac-cas3 and Ac-cas8 were then assessed by western blot analysis. TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; CQ, chloroquine; can, cantharidin; LC3, microtubule-associated proteins 1A/1B light chain 3B; p62, sequestosome 1; Ac-cas, activated caspase.
Figure 4.
Can-mediated sensitization of TRAIL-mediated apoptosis is rescued by autophagy attenuation. DU145 cells were pretreated with CQ (10 μM) for 1 h prior to their exposure to Can (1 μM) for 18 h and then to TRAIL for an additional 2 h. (A) The morphology of the cells was evaluated by interference light microscopy (scale bar=100 μm; magnification, ×100). (B) Cellular viability was assessed by crystal violet staining. (C) Bar chart demonstrating the average density of crystal violet staining. (D) Cellular viability was evaluated with trypan blue dye exclusion assays. *P<0.05 and **P<0.0001 vs. control and each treatment group, respectively. TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; CQ, chloroquine; Can, cantharidin.
Genetic attenuation of autophagy rescues cantharidin-sensitized TRAIL-mediated apoptosis
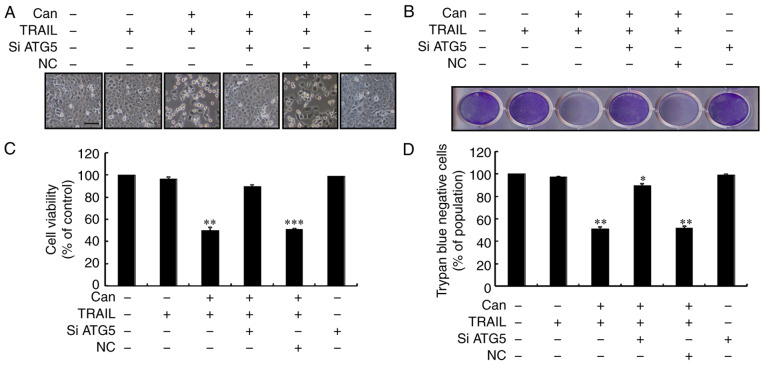
To investigate the inhibition of autophagic flux, the autophagy-related 5 (ATG5) gene was knocked down. ATG5 knockdown decreased cantharidin-initiated expression of LC3-II and markedly increased p62 protein levels (Figs. 5A and S3A). The protein levels of p62, determined by immunofluorescence staining, were consistent with those determined by western blot analysis (Figs. 5B and S3B). However, co-treatment with cantharidin, ATG5 siRNA and TRAIL attenuated the increase in Ac-cas3 and Ac-cas8 expression (Fig. 5C). Cell morphology analysis confirmed that the ATG5 siRNA regimen regulated cell death more effectively compared with combined treatment of cantharidin and TRAIL (Fig. 6A). Co-treatment with cantharidin, TRAIL and ATG5 siRNA markedly increased the viability of DU145 cells and significantly attenuated cell death (Fig. 6B-D). These data indicate that cantharidin-sensitized prolongation of TRAIL-mediated apoptosis can be facilitated by genetic attenuation of autophagy and induction of autophagy flux.
Figure 5.
Genetic attenuation of autophagy rescues Can-sensitized TRAIL-mediated apoptosis through the activation of autophagy flux. DU145 cells were pretreated with ATG5 siRNA or negative control siRNA for 24 h prior to their exposure to Can (1 μM) for 18 h. (A) The levels of LC3-II, p62 and ATG5 were assessed by western blot analysis. (B) Cells were immunostained with p62 antibody (green) and evaluated for fluorescence (scale bar=50 μm; magnification, ×200). DU145 cells were pretreated with ATG5 siRNA or negative control siRNA for 24 h prior to their exposure to Can (1 μM) for 18 h and then to TRAIL for an additional 1 h. (C) The levels of Ac-cas3 and Ac-cas8 were assessed by western blot analysis. TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; Can, cantharidin; ATG5, autophagy-related 5; siRNA, small interfering RNA; LC3-II, lipid-modified microtubule-associated proteins 1A/1B light chain 3B; p62, sequestosome 1; NC, negative control.
Figure 6.
Can-enhanced TRAIL-mediated apoptosis is rescued by genetic attenuation of autophagy. DU145 cells were pretreated with ATG5 siRNA or negative control siRNA for 24 h prior to their exposure to Can (1 μM) for 18 h and then to TRAIL for an additional 2 h. (A) The morphology of the cells was evaluated by interference light microscopy (magnification, ×100). (B) Cellular viability was assessed by crystal violet staining. (C) Bar diagram demonstrating the average density of crystal violet staining. (D) Cellular viability was evaluated by trypan blue dye exclusion assays. *P<0.05, **P<0.01 and ***P<0.0001 vs. control group and each treatment group, respectively. TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; Can, cantharidin; ATG5, autophagy related 5; siRNA, small interfering RNA; NC, negative control.
Downregulation of c-FLIP and upregulation of DR-5 by cantharidin rescues TRAIL resistance
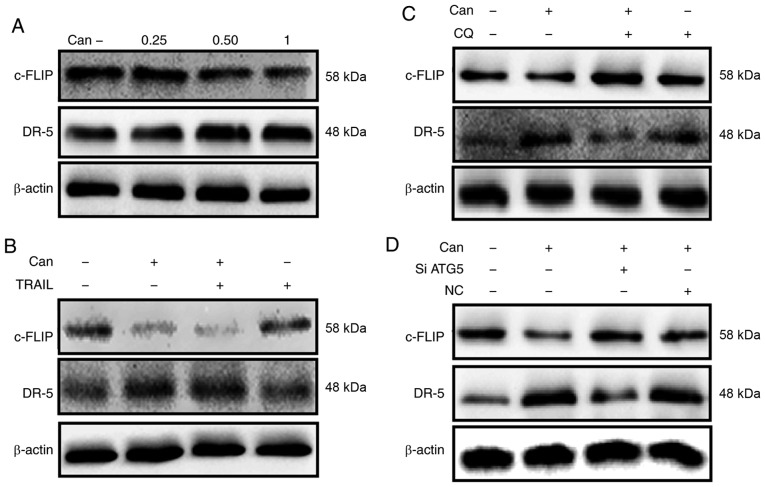
The results from the western blot analysis revealed that cantharidin attenuated the expression of c-FLIP and upregulated the expression of DR-5 (Figs. 7A and S4A and B). The combined regimen of canthar-idin and TRAIL decreased c-FLIP and increased DR-5 levels (Figs. 7B and S4C and D). c-FLIP expression was increased and DR-5 upregulation was partially inhibited by combined treatment with CQ and cantharidin (Fig. 7C). Furthermore, a combined regimen of ATG5 siRNA and cantharidin increased c-FLIP levels while DR-5 upregulation was partially inhibited (Fig. 7D). These data suggest that cantharidin down-regulated c-FLIP expression and upregulated DR-5 expression and thereby sensitized TRAIL-mediated apoptosis through the initiation of autophagy flux.
Figure 7.
Downregulation of c-FLIP and upregulation of DR-5 by Can attenuates TRAIL resistance. DU145 cells were treated with Can (0, 0.25, 0.50 and 1 μM) for 18 h. (A) The levels of c-FLIP and DR-5 were assessed by western blot analysis. DU145 cells were treated with Can (1 μM) for 18 h and then TRAIL (200 ng/ml) for an additional 1 h. (B) The levels of c-FLIP and DR-5 were assessed by western blot analysis. DU145 cells were pretreated with CQ (10 μM) for 1 h prior to exposure to Can (1 μM) for 18 h. (C) The levels of c-FLIP and DR-5 were assessed by western blot analysis. DU145 cells were pretreated with ATG5 siRNA or negative control siRNA for 24 h prior to their exposure to Can (1 μM) for 18 h. (D) The levels of c-FLIP and DR-5 assessed by western blot analysis. c-FLIP, cellular FLICE-like inhibitory protein; DR-5, death receptor 5; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; ATG5, autophagy-related 5; siRNA, small interfering RNA; Can, cantharidin; CQ, chloroquine; NC, negative control.
Discussion
The present study investigated the mechanism of cantharidin-mediated TRAIL sensitization. Cantharidin treatment induced autophagy flux, decreased c-FLIP expression and increased DR-5 expression. It also enhanced the cleavage of caspase-8 and caspase-3 leading to the induction of apoptosis. Furthermore, cantharidin treatment sensitized TRAIL-mediated apoptosis in multiple cancer cells. Therefore, the results suggested that cantharidin treatment may be used for suppression of TRAIL-resistance.
TRAIL can specifically initiate apoptosis in human malignant and transformed cells (27), whereas normal cells can resist this cell death process. The precise understanding of the function of TRAIL may present a novel therapy regimen for cancer treatment (28). Although various types of tumor cells are sensitive to TRAIL-mediated apoptosis, other cells including PCa are resistant (29). Cantharidin is a known inhibitor of protein phosphatases 1 and 2A (PP1 and PP2A) (30). It is a terpenoid that has been reported to exhibit promising biological and clinical effects (30). Cantharidin sensitized TRAIL-mediated apoptosis in prostate cancer cells. In the present study, the combined treatment of cantharidin and TRAIL enhanced cancer cell death both in DU145 and LNCaP cell lines, suggesting that cantharidin has the ability to decrease TRAIL-resistance in prostate cancer cells.
Certain studies have reported that cantharidin attenuates tumor cell propagation and induction of autophagy (31-34). Autophagy has been widely investigated in response to various stimuli, including particular anticancer agents (35). In the present study, cantharidin treatment induced an increase in LC-3II expression and a decrease in p62 expression, which indicated that cantharidin treatment enhanced autophagy flux. However, cantharidin treatment alone cannot induce cancer cell apoptosis; the combined treatment of cantharidin and TRAIL led to cleavage of caspase-8 and caspase-3. The autophagy mechanism is controlled by evolutionarily conserved autophagy-associated ATG proteins, which are engaged in the enucleation and elongation of autophagosome composition (36). The present study also confirmed that a diacritic pharmacological and genetic autophagy inhibitor can rescue cantharidin-sensitized apoptosis initiation through TRAIL. These data indicated that cantharidin treatment sensitizes TRAIL-mediated activation of caspase through the activation of autophagy.
TRAIL has 2 death receptors, DR-4 and DR-5, which initiate cancer cell death signals (15). Previous studies have demonstrated that the drug-initiated downregulation of c-FLIP and upregulation of DR-5 promotes apoptosis in tumor cells (37-39). The results of the present study also indicated that cantharidin mediated the downregulation of c-FLIP and upregulation of DR-5, and that this effect could be rescued by pharmacological and genetic suppression of autophagy. These results indicated that cantharidin-induced autophagy sensitized TRAIL-mediated cancer cell apoptosis through the downregulation of c-FLIP and upregulation of DR-5.
In summary, the downregulation of c-FLIP and upregulation of DR-5 by cantharidin treatment sensitized TRAIL-mediated apoptosis in DU145 tumor cells via autophagy flux. The combined regimen of cantharidin and TRAIL may become an adequate therapeutic strategy for the treatment of certain cases of TRAIL-resistant cancer.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by a grant from the National Research Foundation of Korea funded by the Ministry of Education (grant no. 2019R1A2B5B02069765).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article or are available from the corresponding author upon reasonable request.
Authors' contributions
UMN, HY and SYP designed, executed the study and analyzed the data. UMN and HY wrote the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Gamat M, McNeel DG. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocr Relat Cancer. 2017;24:T297–T310. doi: 10.1530/ERC-17-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hao L, Zhao Y, Li ZG, He HG, Liang Q, Zhang ZG, Shi ZD, Zhang PY, Han CH. Tumor necrosis factor-related apoptosis-inducing ligand inhibits proliferation and induces apoptosis of prostate and bladder cancer cells. Oncol Lett. 2017;13:3638–3640. doi: 10.3892/ol.2017.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorn DC, Kou CA, Png KJ, Moore MA. The effect of cantharidins on leukemic stem cells. Int J Cancer. 2009;124:2186–2199. doi: 10.1002/ijc.24157. [DOI] [PubMed] [Google Scholar]
- 5.Nickolls LC, Teare D. Poisoning by cantharidin. Br Med J. 1954;2:1384–1386. doi: 10.1136/bmj.2.4901.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huh JE, Kang KS, Chae C, Kim HM, Ahn KS, Kim SH. Roles of p38 and JNK mitogen-activated protein kinase pathways during cantharidin-induced apoptosis in U937 cells. Biochem Pharmacol. 2004;67:1811–1818. doi: 10.1016/j.bcp.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Huan SK, Lee HH, Liu DZ, Wu CC, Wang CC. Cantharidin-induced cytotoxicity and cyclooxygenase 2 expression in human bladder carcinoma cell line. Toxicology. 2006;223:136–143. doi: 10.1016/j.tox.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Williams LA, Moller W, Merisor E, Kraus W, Rosner H. In vitro anti-proliferation/cytotoxic activity of cantharidin (Spanish Fly) and related derivatives. West Indian Med J. 2003;52:10–13. [PubMed] [Google Scholar]
- 9.Xiong X, Wu M, Zhang H, Li J, Lu B, Guo Y, Zhou T, Guo H, Peng R, Li X, et al. Atg5 siRNA inhibits autophagy and enhances norcantharidin-induced apoptosis in hepatocellular carcinoma. Int J Oncol. 2015;47:1321–1328. doi: 10.3892/ijo.2015.3103. [DOI] [PubMed] [Google Scholar]
- 10.Chen YN, Chen JC, Yin SC, Wang GS, Tsauer W, Hsu SF, Hsu SL. Effector mechanisms of norcantharidin-induced mitotic arrest and apoptosis in human hepatoma cells. Int J Cancer. 2002;100:158–165. doi: 10.1002/ijc.10479. [DOI] [PubMed] [Google Scholar]
- 11.Kok SH, Cheng SJ, Hong CY, Lee JJ, Lin SK, Kuo YS, Chiang CP, Kuo MY. Norcantharidin-induced apoptosis in oral cancer cells is associated with an increase of proapoptotic to antiapoptotic protein ratio. Cancer Lett. 2005;217:43–52. doi: 10.1016/j.canlet.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 12.Su CC, Lee KI, Chen MK, Kuo CY, Tang CH, Liu SH. Cantharidin induced oral squamous cell carcinoma cell apoptosis via the JNK-regulated mitochondria and endoplasmic reticulum stress-related signaling pathways. PLoS One. 2016;11:e0168095. doi: 10.1371/journal.pone.0168095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi SN, Wass J, Vincent P, Iland H. Inhibitory effect of norcantharidin on K562 human myeloid leukemia cells in vitro. Leuk Res. 1991;15:883–886. doi: 10.1016/0145-2126(91)90163-N. [DOI] [PubMed] [Google Scholar]
- 14.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava RK. TRAIL/Apo-2L: Mechanisms and clinical applications in cancer. Neoplasia. 2001;3:535–546. doi: 10.1038/sj.neo.7900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walczak H, Bouchon A, Stahl H, Krammer PH. Tumor necrosis factor-related apoptosis-inducing ligand retains its apoptosis-inducing capacity on Bcl-2- or Bcl-xL-overexpressing chemotherapy-resistant tumor cells. Cancer Res. 2000;60:3051–3057. [PubMed] [Google Scholar]
- 17.Kelly MM, Hoel BD, Voelkel-Johnson C. Doxorubicin pretreatment sensitizes prostate cancer cell lines to TRAIL induced apoptosis which correlates with the loss of c-FLIP expression. Cancer Biol Ther. 2002;1:520–527. doi: 10.4161/cbt.1.5.169. [DOI] [PubMed] [Google Scholar]
- 18.Ng CP, Zisman A, Bonavida B. Synergy is achieved by complementation with Apo2L/TRAIL and actinomycin D in Apo2L/TRAIL-mediated apoptosis of prostate cancer cells: Role of XIAP in resistance. Prostate. 2002;53:286–299. doi: 10.1002/pros.10155. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang B. TRAIL resistance of breast cancer cells is associated with constitutive endocytosis of death receptors 4-5. Mol Cancer Res. 2008;6:1861–1871. doi: 10.1158/1541-7786.MCR-08-0313. [DOI] [PubMed] [Google Scholar]
- 20.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 22.Kondo Y, Kondo S. Autophagy and cancer therapy. Autophagy. 2006;2:85–90. doi: 10.4161/auto.2.2.2463. [DOI] [PubMed] [Google Scholar]
- 23.Moretti L, Yang ES, Kim KW, Lu B. Autophagy signaling in cancer and its potential as novel target to improve anticancer therapy. Drug Resist Updat. 2007;10:135–143. doi: 10.1016/j.drup.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: Apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–975. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 25.Nazim UM, Moon JH, Lee JH, Lee YJ, Seol JW, Eo SK, Lee JH, Park SY. Activation of autophagy flux by metformin downregulates cellular FLICE-like inhibitory protein and enhances TRAIL- induced apoptosis. Oncotarget. 2016;7:23468–23481. doi: 10.18632/oncotarget.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nazim UM, Jeong JK, Park SY. Ophiopogonin B sensitizes TRAIL-induced apoptosis through activation of autophagy flux and downregulates cellular FLICE-like inhibitory protein. Oncotarget. 2017;9:4161–4172. doi: 10.18632/oncotarget.23647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elmallah MI, Micheau O. Marine drugs regulating apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) Mar Drugs. 2015;13:6884–6909. doi: 10.3390/md13116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei RJ, Zhang XS, He DL. Andrographolide sensitizes prostate cancer cells to TRAIL-induced apoptosis. Asian J Androl. 2018;20:200–204. doi: 10.4103/aja.aja_30_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuckey DW, Shah K. TRAIL on trial: Preclinical advances in cancer therapy. Trends Mol Med. 2013;19:685–694. doi: 10.1016/j.molmed.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honkanen RE. Cantharidin, another natural toxin that inhibits the activity of serine/threonine protein phosphatases types 1 and 2A. FEBS Lett. 1993;330:283–286. doi: 10.1016/0014-5793(93)80889-3. [DOI] [PubMed] [Google Scholar]
- 31.Li HC, Xia ZH, Chen YF, Yang F, Feng W, Cai H, Mei Y, Jiang YM, Xu K, Feng DX. Cantharidin inhibits the growth of triple-negative breast cancer cells by suppressing autophagy and inducing apoptosis in vitro and in vivo. Cell Physiol Biochem. 2017;43:1829–1840. doi: 10.1159/000484069. [DOI] [PubMed] [Google Scholar]
- 32.Ren Y, Zhang SW, Xie ZH, Xu XM, Chen LL, Lou ZG, Weng GB, Yao XP. Cantharidin induces G2/M arrest and triggers apoptosis in renal cell carcinoma. Mol Med Rep. 2016;14:5614–5618. doi: 10.3892/mmr.2016.5963. [DOI] [PubMed] [Google Scholar]
- 33.Hsiao YP, Tsai CH, Wu PP, Hsu SC, Liu HC, Huang YP, Yang JH, Chung JG. Cantharidin induces G2/M phase arrest by inhibition of Cdc25c and Cyclin A and triggers apoptosis through reactive oxygen species and the mitochondriadependent pathways of A375.S2 human melanoma cells. Int J Oncol. 2014;45:2393–2402. doi: 10.3892/ijo.2014.2689. [DOI] [PubMed] [Google Scholar]
- 34.Han Z, Li B, Wang J, Zhang X, Li Z, Dai L, Cao M, Jiang J. Norcantharidin inhibits SK-N-SH neuroblastoma cell growth by induction of autophagy and apoptosis. Technol Cancer Res Treat. 2017;16:33–44. doi: 10.1177/1533034615624583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poornima P, Weng CF, Padma VV. Neferine from Nelumbo nucifera induces autophagy through the inhibition of PI3K/Akt/mTOR pathway and ROS hyper generation in A549 cells. Food Chem. 2013;141:3598–3605. doi: 10.1016/j.foodchem.2013.05.138. [DOI] [PubMed] [Google Scholar]
- 36.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park MH, Kim JH, Chung YH, Lee SH. Bakuchiol sensitizes cancer cells to TRAIL through ROS- and JNK-mediated upregulation of death receptors and downregulation of survival proteins. Biochem Biophys Res Commun. 2016;473:586–592. doi: 10.1016/j.bbrc.2016.03.127. [DOI] [PubMed] [Google Scholar]
- 38.Liu YJ, Lin YC, Lee JC, Kuo SC, Ho CT, Huang LJ, Kuo DH, Way TD. CCT327 enhances TRAIL-induced apoptosis through the induction of death receptors and downregulation of cell survival proteins in TRAIL-resistant human leukemia cells. Oncol Rep. 2014;32:1257–1264. doi: 10.3892/or.2014.3317. [DOI] [PubMed] [Google Scholar]
- 39.Sophonnithiprasert T, Nilwarangkoon S, Nakamura Y, Watanapokasin R. Goniothalamin enhances TRAIL-induced apoptosis in colorectal cancer cells through DR5 upregulation and cFLIP downregulation. Int J Oncol. 2015;47:2188–2196. doi: 10.3892/ijo.2015.3204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during the present study are included in this published article or are available from the corresponding author upon reasonable request.