Abstract
Subchondral sclerosis is considered the main characteristic of advanced osteoarthritis, in which bone remodeling mediated by transforming growth factor β (TGFβ) signaling plays an indispensable role in the metabolism. Osteocytes have been identified as pivotal regulators of bone metabolism, due to their mechanosensing and endocrine function. Therefore, the aim of the present study was to investigate the association between osteocyte TGFβ signal and subchondral sclerosis. Knee tibia plateau samples were collected from osteoarthritic patients and divided into three groups: The full cartilage, partial cartilage and full defect groups. Next, changes in osteocyte TGFβ signaling and subchondral bone structure underlying various types of cartilage erosion were detected. Bone mineral density (BMD) assay, histology [hematoxylin and eosin, Safranin-O/Fast green, and tartrate resistant acid phosphatase (TRAP) staining], and reverse transcription-quantitative PCR mainly detected structural alterations, osteogenic and osteo-clastic activity in the cartilage and subchondral bone. The activation of the TGFβ signaling pathway in the subchondral bone was detected by immunohistochemistry and western blotting. The association between osteocyte TGFβ and the regulation of bone metabolism was analyzed by correlation analysis, and further proven in vitro. It was confirmed that the BMD of the subchondral bone increased and underwent sclerosis in the partial cartilage and full defect groups. Additional observation included the thinning of the area of calcified cartilage, in which a bone island formed locally, with subchondral bone plate thickening and increased trabecular bone volume. TRAP staining suggested an increase in bone resorption in subchondral underlying areas of the partial cartilage and full defect groups. Immunohistochemistry results confirmed the activation of osteocyte TGFβ in subchondral underlying areas with severe cartilage erosion. Moreover, osteocyte phosphorylated-Smad2/3 was positively correlated with subchondral BMD, alkaline phosphatase and osteopontin mRNA expression, but it was negatively correlated with TRAP+ cells. Furthermore, it was confirmed in vitro that osteocyte TGFβ signaling could regulate the osteogenic and osteoclastic activity of the mesenchymal stem cells. This study illustrated that osteocyte TGFβ signaling is positively associated with the remodeling of subchondral bone in advanced osteoarthritis and provides a preliminary theoretical basis for further investigations of the role and mechanism of osteocyte TGFβ in subchondral of osteoarthritis.
Keywords: transforming growth factor β, osteocytes, osteoarthritis, subchondral bone, sclerosis
Introduction
Knee osteoarthritis affects millions of people worldwide (1). It is mainly characterized by cartilage lesions, subchondral bone sclerosis and aseptic inflammation of the synovium (2). Previous studies have identified that the pathogenesis of osteoarthritis (OA) is initially caused by structural modification in the subchondral bone in response to changes in the mechanical environment (3-5). It has also been reported that subchondral bone of OA shows dysregulated bone modified by osteoblast and osteoclast activity (6), finally resulting in the formation of sclerosis (7).
Transforming growth factor β (TGFβ), canonically binds to its cell surface receptor and then directly phosphorylates Smad2/3 to translocate the signaling molecule into the nucleus and interact with other transcriptional factors, playing an important role in the development of OA (8-13). However, the effect of TGFβ on OA varies depending on the sites it acts on. Regarding cartilage, the inhibition of TGFβ1 in chondrocytes could decrease the expression of collagen and proteoglycans in developing knee joints (14-16), suggesting that TGFβ1 might be a therapeutic agent for OA. Conversely, in adult knee joints, chondrocyte TGFβ1 could induce extracellular matrix-degrading enzymes (17). Yet, in the subchondral bone of OA, a large amount of TGFβ was activated in the extracellular matrix (8). Moreover, it has been suggested that osteoblastic TGFβ signaling in subchondral bone might play an important role in the pathogenesis of OA by altering mesenchymal stem cell (MSC) osteogenic activity (9).
Several studies have emphasized that osteocytes play an important regulatory role in maintaining bone metabolism (18,19). It has been found that osteocytes, the most abundant mechanosensory cells, are involved in loading-increased anabolism of cortical bone via inhibition of the TGFβ-Smad2/3 pathway (20). Moreover, osteocyte morphology in OA has been found to be altered, exhibiting rough and rounded cell bodies with fewer and disorganized dendrites (21). A dysregulated expression of the osteocyte markers (22), apoptosis (23), and degradative enzymes (24) was also observed in OA.
Although osteocytes have been identified to play vital roles in OA development, it is unclear how osteocytes respond to abnormally activated TGFβ in the extracellular matrix of osteochondral bone in the late OA. Therefore, the aim of the present study was to explore the association between osteocyte TGFβ signaling and the remodeling of the subchondral bone in relation to different cartilage damage, which will lay the foundation for further research on the role of osteocyte TGFβ signaling in OA.
Materials and methods
Patients
Chinese patients who had undergone total knee arthroplasty due to OA rather than rheumatoid arthritis, included 7 males and 13 females (21 knees), with an average age of 68 years (age range, 60-78 years). The mean body mass index of males and females was 25.18±1.81 and 26.34±2.05, respectively. A total of 21 tibial plateau specimens were collected from The First Affiliated Hospital of Chongqing Medical University (Chongqing, China) between May 2018 and May 2019 (Table I). Standard surgical procedures were performed during knee arthroplasty and specimen collection did not involve any clinical intervention. The collection of human specimens and animal experiments in the present study were approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (Permit number: 2015-316), under which the effect of Smad7 on chondrogenesis and endochondral ossification (25), osteocyte TGFβ-Smad4 on osteoblastic and osteoclastic differentiation (26) had been previously have identified. Written informed consent was obtained from participants.
Table I.
Basic characteristics of participants.
| Gender | Population | Right knee | Left knee | BMI | Joint instability historya |
|---|---|---|---|---|---|
| Male | 7 | 5 | 2 | 25.18±1.81 | 4 |
| Female | 13 | 8 | 6 | 26.34±2.05 | 7 |
Including knee meniscus injury or resection, anterior and posterior cruciate ligament and medial or lateral collateral ligament injury or fracture history. BMI, body mass index.
Specimen collection and processing
The tibial plateaus of patients were removed during surgery and rapidly transferred to the laboratory at on-ice. After collecting gross images, the tibial plateaus were aseptically cut into pieces of ~0.5x0.5x0.2 cm using bone scissors. The areas of cartilage wear were divided into full cartilage, partial cartilage and full defect groups. Specimens were radiographed using a bone density instrument (kubtecNC), 50% of which then underwent RNA and protein isolation and the other half of which was soaked in 4% paraformaldehyde at room temperature for 24 h, decalcified in 10% EDTA for 3 weeks, dehydrated in graded alcohol, embedded and cut into 5-mm thick sections.
Bone mineral density (BMD) analysis
Small pieces of specimens were radiographed using a kubtecNC instrument with automatic exposure at 45 KV, 500 mA. The BMD of the subchondral bone area was measured and analyzed using kubtec Digicom BMD Processor.
Hematoxylin and eosin (H&E) and safranin O staining H&E staining
Paraffin sections were baked at 59°C for 30 min, dewaxed in xylene and hydrated in a series of gradient alcohol, followed by immersion in hematoxylin dye solution at room temperature for 5 min to stain the nuclei, in hydrochloric acid for 5 sec for differentiation, and in lithium carbonate to blue the nuclei. They were then immersed in eosin at room temperature for 5 min to stain the cytoplasm. Finally, once they were dehydrated and transparent, they were sealed with resin.
Safranin O staining
Slices were baked, dewaxed and hydrated as previously described. Next, they were immersed in freshly prepared Weigert stain for 5 min, differentiated in acid for 15 sec and immersed in the green staining solution for 5 min and in the Safranin O dye solution for 5 min at room temperature, according to the manufacturer's protocol (G1371; Beijing Solarbio Science & Technology Co., Ltd.). They were then washed with a weak acid solution for 30 sec to remove the remaining solid green. Finally, once they were dehydrated and transparent, they were sealed with resin.
Immunohistochemistry
Sections were baked, dewaxed and hydrated as previously described. The antigen was then repaired by proteinase K (0.3 mg/ml; Merck KGaA) treatment at 37°C for 30 min. Endogenous enzyme activity was extinguished using 3% hydrogen peroxide for 10 min. Slices were blocked with goat serum (cat. no. 5425; Cell Signaling Technology, Inc.) at 37°C for 1 h and incubated with primary antibody overnight at 4°C [rabbit anti-TGFβ1, cat. no. 3709, 1:100; rabbit anti-phosphorylated (p)-Smad2/3, cat. no. #8828S, 1:200; Cell Signaling Technology, Inc.; rabbit anti-a disintegrin and metalloproteinase with thrombospondin motifs 4 (Adamts4), cat. no. #ab185722, 1:200; Abcam], incubated with secondary antibody for 1 h at room temperature [horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody, cat. no. #7074, 1:1,000; Cell signaling Technology, Inc.; goat anti-rabbit lgG/Cy3, cat. no. bs-0295G-Cy3; 1:1,000; Bio-synthesis Inc.]. 3′-Diaminobenzidine (DAB, Bio-synthesis Inc.) was added for 5 min and then 4′, 6-diamidino-2-phenylindole or hematoxylin was used to counterstain the nuclei at room temperature for 5 min. The slides were observed under a light or fluorescence microscope (magnification, ×40, ×100 or ×200).
RT-qPCR
Subchondral bones from human knees were ground within liquid nitrogen. Next, total RNA extraction was performed using TRIzol (Thermo Fisher Scientific, Inc.) and reverse transcribed into cDNA with PrimeScript RT Reagent kit (Takara Bio, Inc.) with the following conditions: 37°C for 15 min and 85°C for 5 sec. qPCR analysis of alkaline phosphatase (ALP), osteopontin (OPN) and osteocalcin (OCN) mRNA was performed using the CFX96 Real time PCR Detection system (Bio-Rad Laboratories, Inc.) with SYBR Premix Ex Taq II (Takara Bio, Inc.). The thermocycling conditions of qPCR were as follows: 95°C for 30 sec; and 40 cycles of 95°C for 5 sec and 60°C for 5 sec. GAPDH was used as a reference gene. All sample values were normalized to GAPDH expression using the 2−ΔΔCq method as described previously (27). The qPCR primer sequences were as follows: ALP forward, 5′-ACA CCA ATG TAG CCA AGA ATG TCA-3′ and reverse, 5′-GAT TCG GGC AGC AGC GGT TAC T-3; OPN forward, 5′-ACA CTT TCA CTC CAA TCG TCC-3′ and reverse, 5′-TGC CCT TTC CGT TGT TGT CC-3′; OCN forward, 5′-CAG CGG CCC TGA GTC TGA-3′ and reverse, 5′-GCC GGA GTC TGT TCA CTA CCT TA-3′; and Gapdh forward, 5′-CTA CAC TGA GGA CCA GGT TGT CT-3′ and reverse, 5′-TTG TCA TAC CAG GAA ATG AGC TT-3′.
Isolation and co-culture mouse osteocytes and bone marrow stromal cells (BMSCs)
Mice were sacrificed by cervical dislocation after inhaling 2.5% concentration of isoflurane (RWD Life Science Co., Ltd.) and all efforts were made to minimize the suffering of the animals included in present study. Death was confirmed when the mice were ceased breathing and had no heartbeat and dilated pupils. The tibial and femoral diaphyses were dissected from 5, 10-week-old mice, cut into 1-2-mm pieces, and incubated in collagenase type I and EDTA 9 times, as previously described (28). Next, primary mouse osteocytes were cultured in α-minimal essential medium (Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (TBD0130HYT; Tianjing TBD Science Co., Ltd.), 1% penicillin and streptomycin (GE Healthcare Life Sciences), and 50 µM β-Mercaptoethanol (Merck KGaA). Next, osteocytes were digested with trypsin and seeded at a density of ~40,000 cells per well (24 well plate; Wuxi Nest Biology & Technology Co., Ltd.), and TGFβ1 protein (PeproTech, Inc.) or LY2109761 (MedChemExpress, Inc.) was added for 1 h, prior to co-culture with wild-type mouse BMSCs flushed from the femur and tibia. The method of isolating mouse bone marrow cells was previously described (29).
Alkaline phosphatase (ALP) and tartrate resistant acid phosphatase (TRAP) staining
The co-cultured mouse cells were fixed with 4% paraformaldehyde at room temperature for 10 min and the dye solution was added following the manufacturer's protocol of ALP (catalog no. C3206; Beyotime Institute of Biotechnology) and TRAP (catalog no. 387; Sigma-Aldrich; Merck KGaA) staining kits. ALP-stained cells were incubated at room temperature for 30 min and TRAP-stained cells at 37°C for 1 h.
Western blotting
Protein extraction from mouse osteocytes and human knee subchondral bone was performed using 2% SDS lysis buffer containing 100 mM Tris-HCL and 100 mM β-Mercaptoethanol. Total protein was determined using a BCA protein Assay kit (Beyotime Institute of Biotechnology). Total protein (20 µg) was electrophoresed on 5-10% Bis-Tris gels (Thermo Fisher Scientific, Inc.) and transferred to polyvinylidene fluoride membranes (EMD Millipore). Membranes were blocked with 5% skimmed milk for 1 h at room temperature and incubated with primary antibodies against p-Smad2/3, Smad2 and β-actin (rabbit anti-p-Smad2/3, cat. no. #8828S; 1:1,000; rabbit anti-Smad2; cat. no. #5339; 1:1,000; rabbit anti-β-actin; cat. no. #4970, 1:1,000; Cell signaling Technology, Inc.) overnight at 4°C, followed by incubation with corresponding HRP-conjugated secondary antibody (goat anti-rabbit secondary antibody, cat. no. #7074, 1:1,000; Cell Signaling Technology, Inc.) at room temperature for 1 h. The blots were visualized using Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore) and analyzed by Image Lab 6.0.1. software (Bio-Rad Laboratories, Inc.).
Image and statistical analysis
All images were obtained using a light or fluorescence microscope (Olympus Corporation) and analyzed by Image J Pro (National Institutes of Health; version 1.50 g). Data are expressed as the mean ± standard deviation and analyzed with SPSS software (version 21; IBM, Corp.). One-way analysis of variance followed by Student-Newman Keuls post hoc test was performed to analyze inter- and intra-group differences. A t-test was used to compare two groups. The correlations between osteocyte p-Smad2/3 expression and other bone turnover parameters were calculated with Pearson's correlation coefficients. P<0.05 was considered to indicate a statistically significant difference.
Results
Increased sclerosis of subchondral bone underneath the eroding cartilage
The degrees of cartilage degeneration were distinct in the medial and lateral plateaus, especially when it came to 67% (14:21) of patients with valgus and varus deformities. The tibial plateaus of human with 'normal' cartilage had a milky color and smooth surface, with mild cartilage degeneration showing as yellowish and coarse surface, and severe degeneration as a full thickness cartilage defect, sclerotic subchondral bone and extensive fractures (Fig. 1A). Next, the alternation of sclerosis in subchondral bone was confirmed by BMD assay and sclerosis was shown to increase with the increasing cartilage erosion severity (0.029±0.006 vs. 0.050±0.012 vs. 0.075±0.011; respectively; P<0.05; Fig. 1B and C).
Figure 1.
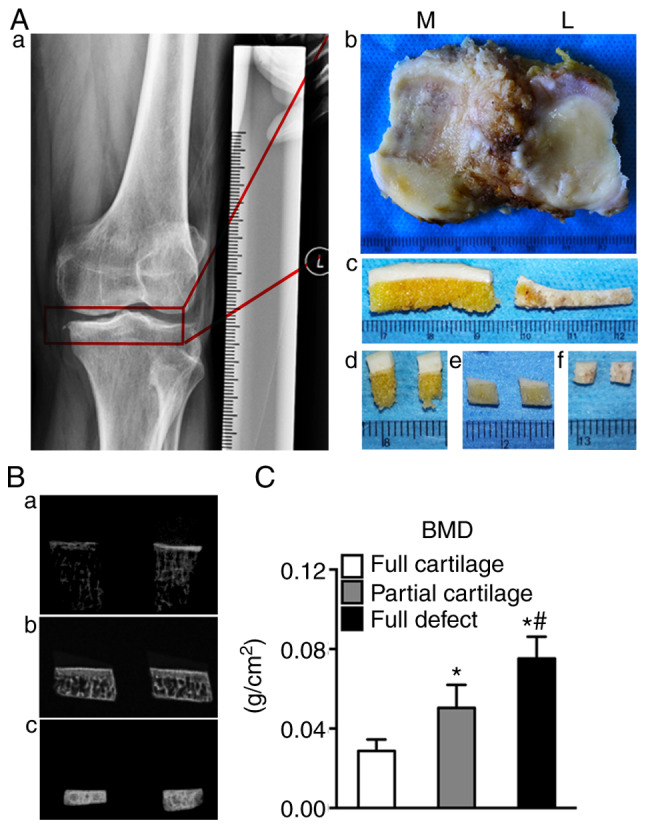
Schematic diagram of specimen processing. (Aa and b) The human tibial plateau was removed during surgery; (Aa) the X-ray image of human osteoarthritic knee and (Ab) the plateau specimen removed from osteoarthritic knee. (Ac) According to the severity of cartilage erosion, the specimens were divided into 3 groups: The (Ad) full cartilage, (Ae) partial cartilage and (Af) full defect groups. (Ba-c) Subchondral bone X-ray inspection was performed in three groups of specimens; (Ba) the X-ray image of the full cartilage group, (Bb) the X-ray image of the partial cartilage group and (Bc) the X-ray image of the full defect group. (C) BMD analysis was performed in three groups of specimens. *P<0.05 vs. the full cartilage and #P<0.05 vs. the partial cartilage groups, n=21. M, medial; L, lateral; BMD, bone mineral density.
Altered structure of subchondral bone underneath the worn cartilage
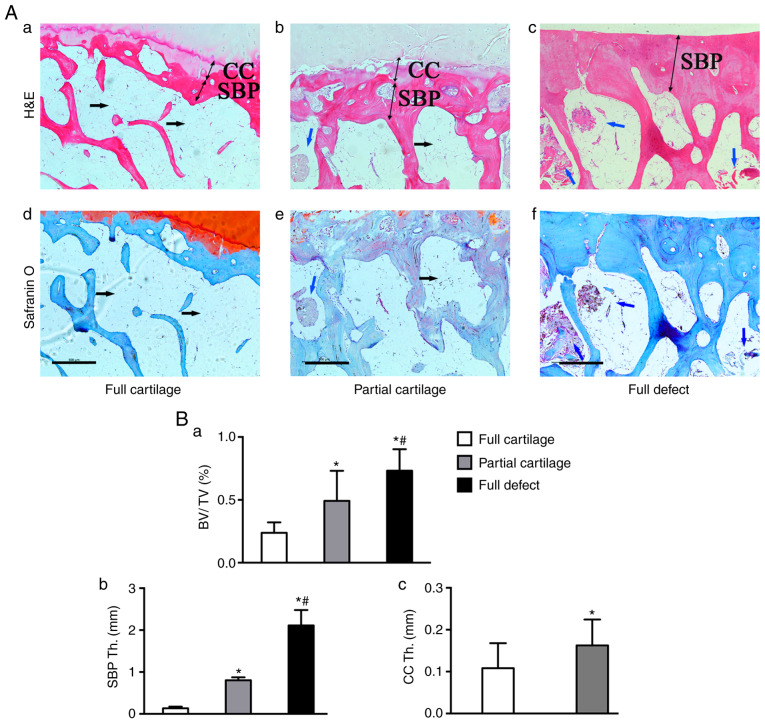
The structural changes in the subchondral bone from human knees were further examined (Fig. 2Ba-c). It was found that, along with elevated cartilage defects, the bone volume/total tissue volume ratio (BV/TV) was also increased (0.24±0.08 vs. 0.49±0.24 vs. 0.73±0.17; P<0.05). The thickness of the subchondral bone plate (SBP Th.) was significantly increased in the partial cartilage group compared with that in the full cartilage (0.80±0.07 vs. 0.13±0.04; P<0.05) and full defect groups. There was a significant decrease in the partial cartilage group compared with the full defect group (2.11±0.37 vs. 0.13±0.04; P<0.05). Similarly, the thickness of the calcified cartilage (CC Th.) in the partial cartilage group was increased compared with that in the full cartilage group (0.16±0.06 vs. 0.11±0.06; P<0.05). In addition, the bone marrow cavity was mainly filled with adipocytes in 'normal' subchondral bone (black arrow), which were gradually replaced with bone marrow cells, in which some new trabecular bone (blue arrow) was formed locally (Fig. 2Aa-f). These results indicated that the more obvious the cartilage destruction, the more severe the subchondral bone structure changes, eventually leading to the formation of subchondral bone sclerosis.
Figure 2.
Alternation of the microstructure in subchondral bone from human knee. (A) H&E staining of the (a) full, (b) partial cartilage and (c) full defect. (Ad-f) Safranin O/fast green staining in (d) full, (e) partial cartilage and (f) full defect groups. In the full cartilage group, the bone marrow cavity was mainly filled with adipose tissue (black arrow), but in the partial cartilage and especially in full defect groups, it was composed of bone marrow cells (blue arrow), in which new trabecular bone formed locally (red color in H&E and blue in safranin O staining). Quantitative results showing that subchondral bone possesses a (Ba) higher BV/TV fraction, (Bb) SBP Th. and CC Th. in cartilage wear groups, as compared with (Bc) the full cartilage group. *P<0.05 vs. the full cartilage and #P<0.05 vs. the partial cartilage groups, n=21. Scale bar, 500 µm. H&E, hematoxylin and eosin; BV/TV, Bone volume/total tissue volume; CC Th., Thickness of calcified cartilage; SBP Th., Thickness of subchondral bone plate.
Turnover of osteogenic and osteoclastic activities of the subchondral bone significantly increases with the increasing severity of OA
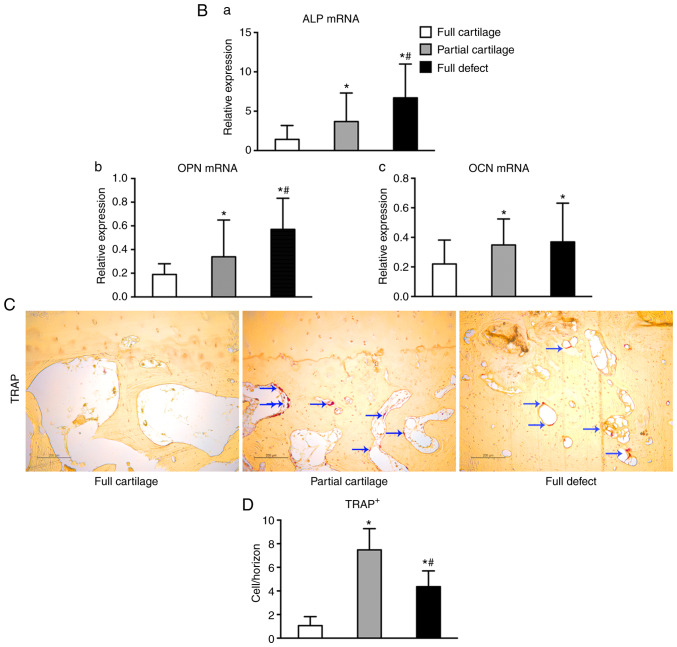
To explore the causes leading to structural changes in subchondral bone, the metabolic parameters of the bone were detected. It was found that, even in the full cartilage group, some bone islands had begun to form locally in the calcified cartilage zone and proteoglycans in the surrounding cartilage matrix were gradually lost (Fig. 3Aa-c). Moreover, chondrocytes degenerated more severely in the partial cartilage group, multiple cell clusters developed and were sparsely distributed, and more proteoglycans were lost (Fig. 3Ad-f).
Figure 3.
Turnover of osteogenic and osteoclastic activities in subchondral bone from human knee. Safranin O/fast green staining for architecture alternation in the (Ab) full and (Ae) partial cartilage groups. Alternation in the architecture of (Aa and d) cartilage and bone island (red arrow) formation (Ac and f) locally at the calcified cartilage zone. (B) The expression of osteogenic markers (a) ALP, (b) OPN and (c) OCN in subchondral bone was analyzed by reverse transcription-quantitative PCR. Bone resorption in subchondral bone was assayed by (C) TRAP stain and positive cells were stained red (blue arrow). (D) Quantitative analysis was performed by counting TRAP+ cells per horizon. *P<0.05 vs. the full cartilage and #P<0.05 vs. the partial cartilage groups, respectively, n=21. Scale bar in Ab and e, 1,000 µm and in others, 200 µm. TRAP, tartrate resistant acid phosphatase; ALP, alkaline phosphatase; OPN, osteopontin; OCN, osteocalcin.
The mechanism that leads to subchondral bone sclerosis was further explored. The mRNA expression of the osteogenic marker ALP and OPN (Fig. 3Ba and b) in the subchondral bone increased significantly with the severity of cartilage defects (1.41±1.77 vs. 3.68±3.63 vs. 6.69±4.30; P<0.05; 0.19±0.09 vs. 0.34±0.31 vs. 0.57±0.26; P<0.05). However, the expression of OCN mRNA (Fig. 3Bc) was only increased in the partial cartilage and full defect groups, as compared with the full cartilage group (0.35±0.18, 0.37±0.26 vs. 0.22±0.16; P<0.05), but there was no significant difference between the full defect group and the partial cartilage group (0.37±0.26 vs. 0.35±0.18; P=0.74). Similarly, it was found that the number of TRAP+ cells per horizon in both the partial cartilage and full defect groups was significantly increased compared with that in the full cartilage group (1.06±0.76, 7.48±1.80 vs. 4.37±1.34; P<0.05), but in the full defect group it was decreased compared with that in the partial cartilage group (4.37±1.34 vs. 7.48±1.80, P<0.05) (Fig. 3C and D). These results indicated that abnormal knee joint stress can significantly enhance the turnover of the osteogenic and osteoclastic activity in the subchondral bone, and that subchondral bone sclerosis occurs to cope with the abnormal mechanical loading.
Activation of osteocyte TGFβ signaling in subchondral bone underneath the partial and full defect cartilage
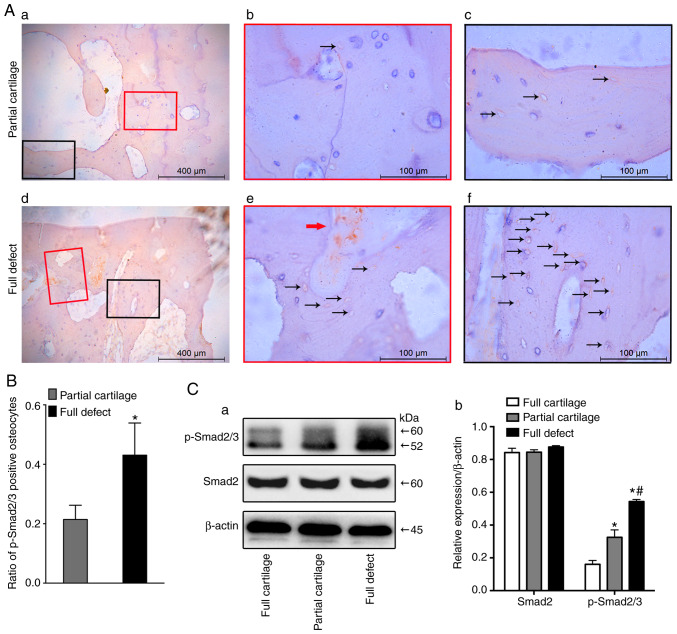
In order to investigate whether osteocyte TGFβ signaling is activated in subchondral bone throughout the development of human OA, the expression of activated TGFβ1 in three different groups was first examined. The results showed that the higher the degree of cartilage defect, the higher the expression of activated TGFβ1 in the subchondral bone marrow matrix (Fig. S1). Furthermore, the activation of the TGFβ signaling pathway was detected with immunohistochemistry (Fig. 4A). Results showed that a much higher number of p-Smad2/3+ osteocytes existed in the full defect group, compared with the partial cartilage group (0.43±0.11 vs. 0.21±0.05; P<0.001; Fig. 4B). It was also found that the osteocyte TGFβ signaling pathway was activated in an osteoarthritic mouse model by excising the anterior cruciate ligament (data not shown). Consistent with a previous result (9), the TGFβ signaling pathway of bone marrow stromal cells (red arrow) was also activated in the subchondral bone of human osteoarthritic specimens (Fig. 4Ad and e). Furthermore, the western blotting results for p-Smad2/3 showed that, as the degree of cartilage erosion increased, the activation of the TGFβ signal pathway in subchondral bone became more obvious (Fig. 4Ca and b).
Figure 4.
Activation of osteocyte TGFβ signaling in human subchondral bone. The activation of osteocyte TGFβ signaling was detected by immunohistochem-istry staining with phosphorylated smad2/3 antibody in (Aa-c) partial cartilage defects and (Ad-f) full defect groups. (Ab) Magnification of the super-layer structure in subchondral bone of Aa. (Ac) Magnification of the deep-layer structure in subchondral bone of Aa. (Ae) Magnification of the super-layer structure in subchondral bone of Ad. (Af) Magnification of the deep-layer structure in subchondral bone of Ad. (B) Quantitative assay was performed by ratio of p-Smad2/3+ osteocytes. Positive cell stained brown in osteocytes (black arrow) and bone marrow (red arrow). (Ca) Western blotting was performed to test the activation of the TGFβ-Smad2/3 signaling pathway in subchondral bone from three different groups and (Cb) quantitative analysis for the relative expression of Smad2 and p-Smad2/3 was performed. *P<0.05 vs. the full cartilage and #P<0.05 vs. the partial cartilage groups n=21. Scale bar in Aa and d, 400 µm; in others, 100 µm. TGFβ, transcriptional growth factor β; p, phosphorylated.
Occasionally, it was observed that the activated p-Smad2/3 of chondrocytes in the full cartilage group was mainly located at the hypertrophic zone (Fig. S2Aa-c). However, the activated p-Smad2/3 was activated throughout the layers in the partial cartilage group (Fig. S2Ad-f). Mechanistically, the expression of chondrocyte matrix-degrading enzymes is the main manifestation of articular cartilage degeneration (30,31). Therefore, the expression of Adamts4, an important cartilage matrix-degrading enzyme, was then examined in the cartilage, in the partial and full cartilage groups. Of note, the expression of chondrocyte Adamts4 in the partial cartilage group showed the same trend as that of p-Smad2/3 (Fig. S2B). These results indicated that abnormal TGFβ may be correlated with the occurrence of OA.
Association between osteocyte p-Smad2/3 and BMD, osteogenic markers, and TRAP+ cells in subchondral bone
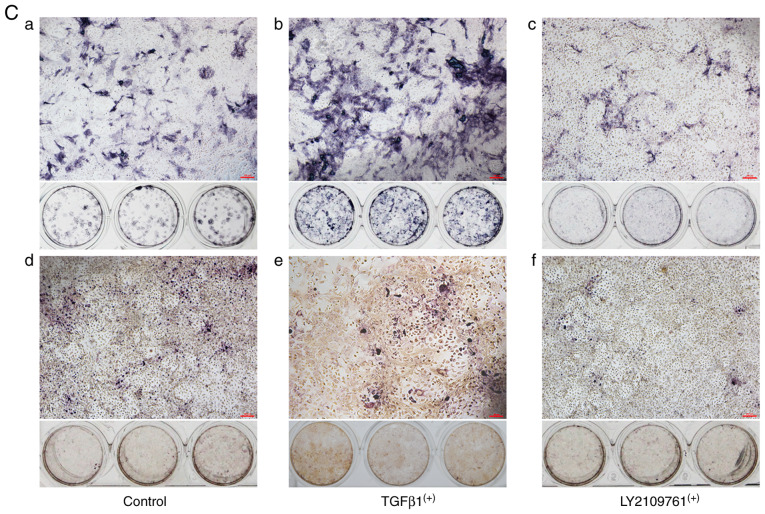
Pearson's correlation analysis revealed that the ratios of p-Smad2/3+ osteocytes were positively correlated with subchondral BMD, ALP and OPN mRNA expression, suggesting that osteocyte p-Smad2/3 activation in subchondral bone was positively associated with the severity of OA (Fig. 5Aa-c, Table II). However, it was negatively correlated with TRAP+ cells (Fig. 5Ad, Table II). To further verify the role of osteocyte TGFβ signaling, mouse osteocytes were isolated and purified in vitro and co-cultured with mouse bone marrow MSCs. TGFβ1 protein or the selective inhibitor of TGFβ receptor type 1/2 (LY2109761) was added prior to co-culture with MSCs to activate or inhibit the TGFβ signaling pathway in osteocytes, respectively (Fig. 5Ba and b). It was found that the exogenously over-activated TGFβ-Smad2/3 signaling in osteocytes could enhance the expression of ALP in bone marrow stromal cells (Fig. 5Cb) and promote the formation of TRAP+ cells (Fig. 5Ce). In addition, the inhibition of its TGFβ signaling pathway can suppress the basal osteogenic and osteoclastic promoting effects (Fig. 5Cc and f).
Figure 5.
Association between p-Smad2/3+ osteocytes and bone turnover states. Scatter plot diagrams of the association between the ratio of p-Smad2/3+ osteocytes and (Aa) ALP mRNA, (Ab) OPN mRNA, (Ac) BMD of human subchondral bone, and (Ad) TRAP+ cells in the partial cartilage and full defect groups. Exogenous TGFβ1 protein and the selective inhibitor of TGFβ receptor type 1/2 (LY2109761) respectively activated and inhibited the expression of p-Smad2/3 in mouse osteocytes in vitro, as compared with the control group using (Ba) western blotting and (Bb) its quantitative analysis. ALP staining and TRAP staining assays were performed in the (Ca and d) control, (Cb and e) osteocyte TGFβ1 overexpression and (Cc and f) osteocyte TGFβ inhibited co-cultured groups. The results showed that (Cb and e) p-Smad2/3+ osteocytes promoted mouse MSCs osteogenic and osteoclastic differentiation, (Cc and f) while p-Smad2/3- mouse osteocytes suppressed them when co-cultured for 11 days. *P<0.05 vs. the control and #P<0.05 vs. the LY2109761 groups, n=3. Scale bar, 200 µm. ALP, alkaline phosphatase; OPN, Osteopontin; BMD, bone marrow density; TRAP, tartrate resistant acid phosphatase; TGFβ, transcriptional growth factor β.
Table II.
Correlation coefficients.
| Bone turnover parameter | Ratio of p-smad2/3-positive osteocytes
|
|
|---|---|---|
| R | P-value | |
| ALP mRNA | 0.3056 | 0.0491 |
| OCN mRNA | 0.1176 | 0.4582 |
| OPN mRNA | 0.3257 | 0.0353 |
| BMD | 0.7041 | <0.0001 |
| TRAP+ cells | −0.8517 | <0.0001 |
ALP, alkaline phosphatase; OCN, osteocalcin; OPN, osteopontin; BMD, bone mineral density; TRAP+, tartrate resistant acid phosphatase positive; p, phosphorylated.
Discussion
Subchondral bone sclerosis is an important pathological feature in advanced OA (32). Mechanically, altered bone remodeling finally leads to subchondral sclerosis, which causes uneven stress on the overlying cartilage and ultimately accelerates its degeneration (10). Therefore, preventing altered remodeling can help delay the progression of cartilage degeneration, thereby elevating the average age of OA patients that need surgical treatment. Moreover, it has been found that osteoblast TGFβ signaling in subchondral bone plays an important role in the pathogenesis of OA by altering MSC osteogenic activity (9). It has also found that osteocyte morphology changes in the specimens from OA patients, with the secreted cytokine of sclerositin (SOST) decreasing and dentin matrix acidic phosphoprotein 1 increasing (21). Although a large number of studies have emphasized the key regulatory role of osteocytes in the maintenance of metabolism (33-36), the expression of osteocyte TGFβ signaling in the subchondral of advanced OA remains unclear. In the present study, it was found osteocyte TGFβ was abnormally activated under the cartilage wear region and was closely associated with the subchondral bone structural alternation and cartilage wearing. To the best of our knowledge, this is the first study to explore the association of osteocyte TGFβ with OA. This provides a preliminary basis and theoretical guidance for further exploration of the role and mechanism of osteocyte TGFβ signaling in OA.
Although cartilage degeneration is a characteristic pathological change in OA, subchondral bone structural changes caused by bone remodeling disorders are considered to be an important pathological change that precedes cartilage degeneration. This was further confirmed by MRI examination of bone marrow damage in subchondral bone, where it was found that bone marrow lesions in the subchondral bone had occurred before cartilage changes (37). Moreover, in early OA, the subchondral bone is mainly characterized by bone resorption (38), which is characterized by a decrease in the mass fraction of trabecular bone, with its increase appearing during late OA along with sclerosis (39). The present study also found an increase in the bone mass parameters and sclerosis in subchondral bone from human knee of middle-advanced OA. Studies have shown that cartilage degeneration in OA is strongly associated with subchondral bone sclerosis, regardless of whether the increase (40) or decrease (38) in sclerosis will result in cartilage degeneration. Therefore, simply reducing subchondral bone remodeling by inhibiting bone resorption does not necessarily attenuate the progression of OA. The double-balanced regulation of bone resorption and bone formation may be an effective method to cope with the increase in the turnover of subchondral bone during OA. Namely, inhibiting bone remodeling if high sclerosis is the underlying cause of OA may be beneficial, since that may prevent an increase in bone stiffness. However, if decreased mineralization is the underlying cause, inhibiting remodeling may have a negative effect, since it may prevent 'normalization' of the apparent bone sclerosis.
It should also be noted that other factors may play a role as well, as TGFβ has been detected to be abundantly expressed in OA bone (11) and synovial tissue (41). In the present experiments, it was found that activated TGFβ1 was also expressed in subchondral bone in the middle and late stage of OA, and it showed an increased expression trend with the increase in cartilage defect. Furthermore, neutralizing the TGFβ protein in subchondral bone can effectively delay the OA process (12). Micro-fractures occur in the subchondral bone of early OA due to loadbearing, which increases the activity of osteoclasts (42) and activates latent TGFβ in the extracellular matrix (43). It has been suggested that activated TGFβ in the bone marrow can regulate the migration of MSCs via osteoblasts and induce osteogenic differentiation (44), which cause early non-coupled osteogenesis and osteoclastogenesis, accelerating the formation of local bone islands. Moreover, the conditional inhibition of osteoblast TGFβ signaling can alleviate the progression of OA (9).
How do the osteocytes, the most abundant mechanosensory cells, respond to abnormally activated TGFβ in the extracellular matrix when there is an abnormal stress distribution on the subchondral bone in advanced OA? It has been found that loading can cause an increase in the anabolism of cortical bone and that its mechanism is associated with the inhibition of the TGFβ-smad2/3 pathway in osteocytes (20). However, in the present study, it was first discovered that the osteocyte TGFβ pathway in the subchondral bone of OA patients was activated. Moreover, as previously reported (3,5,37), the structure of the subchondral bone significantly changed in the middle and late stages of OA, bone formation increased, and bone sclerosis was enhanced in the region of the subchondral bone under cartilage wear, further indicating that subchondral bone sclerosis is associated with cartilage.
Bone resorption in the subchondral bone of OA is also enhanced, especially in the early stage of OA, which was manifested by a decrease in bone density (38). Zhen et al (9) found that the osteoblast TGFβ signaling only increased the activity of osteoclasts at an early stage in the animal model of OA. It was also found that the osteoclast activity was stronger in the subchondral bone in the area underneath severely worn cartilage, especially full defect cartilage, which indicated bone resorption by osteoclasts is still elevated in the middle and late stages of OA. At the same time, it was verified in vitro that the activation of osteoblast TGFβ signaling can induce osteogen-esis and osteoclast differentiation of MSCs co-cultured with it. It is suggested that osteocyte TGFβ signaling may regulate the coupled bone resorption and bone formation metabolism. These results indicated that abnormal bone remodeling occurs in the subchondral bone underneath the loadbearing cartilage and eventually leads to the formation of sclerosis locally in the subchondral bone, since bone formation is stronger than bone resorption. During this process, osteocytes play an central role in regulating this dynamic bone metabolism.
It has been found that the reduction of BMD in the subchondral bone in early OA and sclerosis in late OA was associated with an increased expression of SOST (32,45). Moreover, knocking out SOST increased the development of sclerosis in subchondral bone (46). Furthermore, as the degree of OA progresses, the expression of SOST in the subchondral bone decreases (46). In the late stage of osteo-blasts, TGFβ regulates the expression of its SOST via its ECR5 enhancer (47). However, it was found that PTH can inhibit the expression of SOST (48). This suggests that in late-stage OA, TGFβ may inhibit the expression of SOST through the PTH pathway, Ansari et al (49) demonstrated that osteocyte PTHrP/PTH regulates their gene expression, which is involved in matrix mineralization. Although direct evidence that links the aberrant activation of osteocyte TGFβ to subchondral bone sclerosis is still lacking, the present observations suggested a possible relationship.
In addition to the effect that TGFβ signaling has on bone, it may influence cartilage. Although TGFβ1-Smad2/3 signaling is required to control chondrocyte hypertrophic differentiation in immature articular cartilage (14), the activation of TGFβ signaling in mature articular cartilage will result in an extremely high expression of metalloproteinase 13, resulting in the loss of proteoglycans and increased expression of type X collagen (13). Occasionally, it was found that, in the process of degenerative changes in mature cartilage, the TGFβ-activated chondrocytes exhibited significant temporal and spatial characteristics, by which chondrocyte TGFβ signaling activation varies in different degrees of OA cartilage (temporal) and in different layers of cartilage (spatial). Initially this was only in the hypertrophic zone in the early stage, followed by a gradual spread to the superficial layer, and finally the activation of the TGFβ-Smad2/3 signaling pathway in throughout the chondrocyte layer. Mechanistically, the expression of cartilage-matrix degrading enzymes is the main manifestation of articular cartilage degeneration (30,31). Therefore, the expression of Adamts4, an important cartilage matrix-degrading enzyme, in cartilage was then examined in the partial and full cartilage groups. Of note, the expression of chondrocytic Adamts4 in the partial cartilage group showed the same trend as that of p-Smad2/3. However, the specific role and mechanism of the spatial distribution of TGFβ in articular cartilage, was not studied in depth in the present study, and the current research group will further explore this phenomenon in the future.
Finally, the present study must admit several limitations. First, only 21 participants were enrolled. Yet, the aim of this preliminary study was to provide evidence of the association between osteocyte TGFβ and bone turnover state in subchondral bone of OA, and the authors believe that the sample qualities were sufficient. Secondly, TGFβ signaling through the smad2/3 pathway is its canonical pathway. Therefore, in present study, the expression of TGFβ1 and pSmad2/3 was detected by immunohistochemistry with reference to previous research findings (8,9,12). Although statistical correlation and in vitro analysis were performed to determine the effect of activating osteocyte TGFβ signaling pathway on osteogenesis and osteoclast differentiation, a direct relationship between osteocyte TGFβ signal and the development of OA remains to be elucidated. In future studies, the authors will further investigate the effect of osteocyte TGFβ signaling on the progression of OA by constructing transgenic mouse models that conditionally regulate TGFβ signaling in osteocytes, which would ultimately unveil the function of osteocyte TGFβ in OA progression. Also, in order to clarify that TGFβ influences which process of Smad2/3 pathway, the authors will next perform the transfection experiments using miRNA and siRNA. Lastly, although in the present study it was found that activated TGFβ signaling is mainly found in osteocytes, bone marrow stromal cells and osteoblasts, the role and localization of osteoclasts is unclear because it is difficult to distinguish this from the cell shape without special staining. Whether TGFβ signaling of osteoclasts is activated may require osteoclast-specific markers and p-smad2/3 double-labeled immunohistochemical staining to confirm. The authors consider these experiments outside the scope of the present study, but they are valuable and the authors wish to finish these assays in their future study.
Supplementary Data
Acknowledgments
The authors would like to thank Dr Zhang Chen (The General Hospital of Chongqing Iron and Steel, Chongqing, China) for critical reading the manuscript.
Funding
This study was supported by National Natural Science Foundation Program of China (grant no. 81572142).
Availability of data and materials
The data used and analyzed in this study are available from the corresponding author upon reasonable request.
Authors' contributions
GD and WH conceived and designed the study. GD, HX and WH analyzed and interpreted the data. GD drafted the manuscript. JL critically revised the manuscript for important intellectual content. GD, HX, WeiX and XL obtained the study materials and collected patient samples. GD and HX performed the statistical analysis. NZ, CZ and WeiX provided administrative, technical or logistic support. GD and HX collected data and assembled the figures GD, HX, JL, NZ, WenX, XL and CZ performed the experiments. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The ethics committee of the First Affiliated Hospital of Chongqing Medical University approved the collection of human specimens and the animal experiments in present study (Permit number: 2015-316). Written informed consent was obtained from the patients before surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 2.Hugle T, Geurts J. What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatology (Oxford) 2017;56:1461–1471. doi: 10.1093/rheumatology/kew389. [DOI] [PubMed] [Google Scholar]
- 3.Adebayo OO, Ko FC, Wan PT, Goldring SR, Goldring MB, Wright TM, van der Meulen MCH. Role of subchondral bone properties and changes in development of load-induced osteoarthritis in mice. Osteoarthritis Cartilage. 2017;25:2108–2118. doi: 10.1016/j.joca.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwan Tat S, Lajeunesse D, Pelletier JP, Martel-Pelletier J. Targeting subchondral bone for treating osteoarthritis: What is the evidence? Best Pract Res Clin Rheumatol. 2010;24:51–70. doi: 10.1016/j.berh.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayami T, Pickarski M, Wesolowski GA, McLane J, Bone A, Destefano J, Rodan GA, Duong LT. The role of subchondral bone remodeling in osteoarthritis: Reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50:1193–1206. doi: 10.1002/art.20124. [DOI] [PubMed] [Google Scholar]
- 6.Bertuglia A, Lacourt M, Girard C, Beauchamp G, Richard H, Laverty S. Osteoclasts are recruited to the subchondral bone in naturally occurring post-traumatic equine carpal osteoarthritis and may contribute to cartilage degradation. Osteoarthritis Cartilage. 2016;24:555–566. doi: 10.1016/j.joca.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Bianco D, Todorov A, Čengić T, Pagenstert G, Schären S, Netzer C, Hügle T, Geurts J. Alterations of subchondral bone progenitor cells in human knee and hip osteoarthritis lead to a bone sclerosis phenotype. Int J Mol Sci. 2018;19:E475. doi: 10.3390/ijms19020475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao W, Wang T, Luo Q, Chen Y, Leung VY, Wen C, Shah MF, Pan H, Chiu K, Cao X, Lu WW. Cartilage degeneration and excessive subchondral bone formation in spontaneous osteoarthritis involves altered TGF-β signaling. J Orthop Res. 2016;34:763–770. doi: 10.1002/jor.23079. [DOI] [PubMed] [Google Scholar]
- 9.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhen G, Cao X. Targeting TGFβ signaling in subchondral bone and articular cartilage homeostasis. Trends Pharmacol Sci. 2014;35:227–236. doi: 10.1016/j.tips.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui Z, Crane J, Xie H, Jin X, Zhen G, Li C, Xie L, Wang L, Bian Q, Qiu T, et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-beta activity and H-type vessel formation in subchondral bone. Ann Rheum Dis. 2016;75:1714–1721. doi: 10.1136/annrheumdis-2015-207923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie L, Tintani F, Wang X, Li F, Zhen G, Qiu T, Wan M, Crane J, Chen Q, Cao X. Systemic neutralization of TGF-β attenuates osteoarthritis. Ann N Y Acad Sci. 2016;1376:53–64. doi: 10.1111/nyas.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aref-Eshghi E, Liu M, Harper PE, Doré J, Martin G, Furey A, Green R, Rahman P, Zhai G. Overexpression of MMP13 in human osteoarthritic cartilage is associated with the SMAD-independent TGF-β signalling pathway. Arthritis Res Ther. 2015;17:264. doi: 10.1186/s13075-015-0788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, Hoedemaekers YM, Willemsen R, Severijnen LA, Venselaar H, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43:121–126. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 15.Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen J, Li J, Wang B, Jin H, Wang M, Zhang Y, Yang Y, Im HJ, O'Keefe R, Chen D. Deletion of the transforming growth factor beta receptor type II gene in articular chondrocytes leads to a progressive osteoarthritis-like phenotype in mice. Arthritis Rheum. 2013;65:3107–3119. doi: 10.1002/art.38122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakker AC, van de Loo FA, van Beuningen HM, Sime P, van Lent PL, van der Kraan PM, Richards CD, van den Berg WB. Overexpression of active TGF-beta-1 in the murine knee joint: Evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthritis Cartilage. 2001;9:128–136. doi: 10.1053/joca.2000.0368. [DOI] [PubMed] [Google Scholar]
- 18.Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S. Mechanosensation and transduction in osteocytes. Bone. 2013;54:182–190. doi: 10.1016/j.bone.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Senda T, Kubo KY. The osteocyte plays multiple roles in bone remodeling and mineral homeostasis. Med Mol Morphol. 2015;48:61–68. doi: 10.1007/s00795-015-0099-y. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen J, Tang SY, Nguyen D, Alliston T. Load regulates bone formation and Sclerostin expression through a TGFβ-dependent mechanism. PLoS One. 2013;8:e53813. doi: 10.1371/journal.pone.0053813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaiprakash A, Prasadam I, Feng JQ, Liu Y, Crawford R, Xiao Y. Phenotypic characterization of osteoarthritic osteocytes from the sclerotic zones: A possible pathological role in subchondral bone sclerosis. Int J Biol Sci. 2012;8:406–417. doi: 10.7150/ijbs.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasadam I, Farnaghi S, Feng JQ, Gu W, Perry S, Crawford R, Xiao Y. Impact of extracellular matrix derived from osteoarthritis subchondral bone osteoblasts on osteocytes role of integrinβ1 and focal adhesion kinase signaling cues. Arthritis Res Ther. 2013;15:R150. doi: 10.1186/ar4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolan EB, Haugh MG, Voisin MC, Tallon D, McNamara LM. Thermally induced osteocyte damage initiates a remodelling signaling cascade. PLoS One. 2015;10:e0119652. doi: 10.1371/journal.pone.0119652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meo Burt P, Xiao L, Dealy C, Fisher MC, Hurley MM. FGF2 high molecular weight isoforms contribute to osteoarthropathy in male mice. Endocrinology. 2016;157:4602–4614. doi: 10.1210/en.2016-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao C, Jiang W, Zhou N, Liao J, Yang M, Hu N, Liang X, Xu W, Chen H, Liu W, et al. Sox9 augments BMP2-induced chondro-genic differentiation by downregulating Smad7 in mesenchymal stem cells (MSCs) Genes Dis. 2017;4:229–239. doi: 10.1016/j.gendis.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai Guangming RL, Chen H, Liu W, Chen Y, He X, Liu W, Tu X, Huang W. Down-regulation of osteoeytic TGFβ/Smad4 inhibits the osteoblastic and osteoelastic differentiation in mouse BMSCs. Basic Clin Med. 2017;37:786–791. [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Stern AR, Stern M, Van Dyke ME, Jähn K, Prideaux M, Bonewald LF. Isolation and culture of primary osteocytes from the long bones of skeletally mature and aged mice. BioTechniques. 2012;52:361–373. doi: 10.2144/0000113876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boregowda SV, Krishnappa V, Phinney DG. Isolation of mouse bone marrow mesenchymal stem cells. Methods Mol Biol. 2016;1416:205–223. doi: 10.1007/978-1-4939-3584-0_11. [DOI] [PubMed] [Google Scholar]
- 30.Yatabe T, Mochizuki S, Takizawa M, Chijiiwa M, Okada A, Kimura T, Fujita Y, Matsumoto H, Toyama Y, Okada Y. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann Rheum Dis. 2009;68:1051–1058. doi: 10.1136/ard.2007.086884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 32.Wu L, Guo H, Sun K, Zhao X, Ma T, Jin Q. Sclerostin expression in the subchondral bone of patients with knee osteoarthritis. Int J Mol Med. 2016;38:1395–1402. doi: 10.3892/ijmm.2016.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood CL, Pajevic PD, Gooi JH. Osteocyte secreted factors inhibit skeletal muscle differentiation. Bone Rep. 2017;6:74–80. doi: 10.1016/j.bonr.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, Kurahara C, Gao Y, Cao J, Gong J, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 36.Rosser J, Bonewald LF. Studying osteocyte function using the cell lines MLO-Y4 and MLO-A5. Methods Mol Biol. 2012;816:67–81. doi: 10.1007/978-1-61779-415-5_6. [DOI] [PubMed] [Google Scholar]
- 37.Kazakia GJ, Kuo D, Schooler J, Siddiqui S, Shanbhag S, Bernstein G, Horvai A, Majumdar S, Ries M, Li X. Bone and cartilage demonstrate changes localized to bone marrow edema-like lesions within osteoarthritic knees. Osteoarthritis Cartilage. 2013;21:94–101. doi: 10.1016/j.joca.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siebelt M, Waarsing JH, Groen HC, Müller C, Koelewijn SJ, de Blois E, Verhaar JA, de Jong M, Weinans H. Inhibited osteoclastic bone resorption through alendronate treatment in rats reduces severe osteoarthritis progression. Bone. 2014;66:163–170. doi: 10.1016/j.bone.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 39.Nakasa T, Ishikawa M, Takada T, Miyaki S, Ochi M. Attenuation of cartilage degeneration by calcitonin gene-related paptide receptor antagonist via inhibition of subchondral bone sclerosis in osteoarthritis mice. J Orthop Res. 2016;34:1177–1184. doi: 10.1002/jor.23132. [DOI] [PubMed] [Google Scholar]
- 40.Xie L, Ding F, Jiao J, Kan W, Wang J. Total Hip and Knee arthroplasty in a patient with osteopetrosis: A case report and review of the literature. BMC Musculoskelet Disord. 2015;16:259. doi: 10.1186/s12891-015-0716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remst DF, Blom AB, Vitters EL, Bank RA, van den Berg WB, Blaney Davidson EN, van der Kraan PM. Gene expression analysis of murine and human osteoarthritis synovium reveals elevation of transforming growth factor beta-responsive genes in osteoarthritis-related fibrosis. Arthritis Rheumatol. 2014;66:647–656. doi: 10.1002/art.38266. [DOI] [PubMed] [Google Scholar]
- 42.Li ZC, Dai LY, Jiang LS, Qiu S. Difference in subchondral cancellous bone between postmenopausal women with hip osteoarthritis and osteoporotic fracture: Implication for fatigue microdamage, bone microarchitecture, and biomechanical properties. Arthritis Rheum. 2012;64:3955–3962. doi: 10.1002/art.34670. [DOI] [PubMed] [Google Scholar]
- 43.Hinz B. The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol. 2015;47:54–65. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zarei A, Hulley PA, Sabokbar A, Javaid MK. Co-expression of DKK-1 and sclerostin in subchondral bone of the proximal femoral heads from osteoarthritic hips. Calcif Tissue Int. 2017;100:609–618. doi: 10.1007/s00223-017-0246-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouaziz W, Funck-Brentano T, Lin H, Marty C, Ea HK, Hay E, Cohen-Solal M. Loss of sclerostin promotes osteoarthritis in mice via β-catenin-dependent and -independent Wnt pathways. Arthritis Res Ther. 2015;17:24. doi: 10.1186/s13075-015-0540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loots GG, Keller H, Leupin O, Murugesh D, Collette NM, Genetos DC. TGF-β regulates sclerostin expression via the ECR5 enhancer. Bone. 2012;50:663–669. doi: 10.1016/j.bone.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li C, Wang W, Xie L, Luo X, Cao X, Wan M. Lipoprotein receptor-related protein 6 is required for parathyroid hormone-induced Sost suppression. Ann N Y Acad Sci. 2016;1364:62–73. doi: 10.1111/nyas.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ansari N, Ho PW, Crimeen-Irwin B, Poulton IJ, Brunt AR, Forwood MR, Divieti Pajevic P, Gooi JH, Martin TJ, Sims NA. Autocrine and paracrine regulation of the murine skeleton by osteocyte-derived parathyroid hormone-related protein. J Bone Miner Res. 2018;33:137–153. doi: 10.1002/jbmr.3291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used and analyzed in this study are available from the corresponding author upon reasonable request.