Abstract
Sevoflurane (Sevo) is one of the most frequently used volatile anesthetic agents in surgical oncology and has various effects on tumors, including inhibiting tumor growth, recurrence, and metastases; however, the molecular mechanisms are unknown. This study tried to investigate the influence of Sevo on hepatocellular carcinoma (HCC) cells and its possible mechanisms of action. The present study found that Sevo suppressed both the proliferative and invasive capabilities of both HCCLM3 and Huh7 cells in a dose-dependent manner. Moreover, 53 differentially expressed microRNAs (miRNAs/miRs) in HCC cells that resulted from Sevo were screened out using miRNA microarray assay. In particular, miR-25-3p displayed a significant decrease in response to Sevo treatment. Further studies showed that Sevo's inhibitory actions on HCC cells were attenuated by overexpression of miR-25-3p but enhanced by its inhibitor. Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN (PTEN), a tumor suppressor gene, was directly targeted by miR-25-3p and its expression was upregulated by Sevo. In addition, Sevo suppressed the expression of phosphorylated-protein kinase B (p-Akt) (S473), glycogen synthase kinase (GSK) 3β (p-GSK3β) (S9), β-catenin, c-Myc and matrix metalloproteinase 9; whereas these inhibitory effects were reversed by miR-25-3p overexpression. More importantly, Sevo's tumor-suppressive effects were enhanced by LY294002 (a PI3-kinase inhibitor) but weakened by insulin growth factor-1 (an agonist of the Akt signaling pathway). These data suggest that Sevo's antitumor effects on HCC could be explained, in part, by Sevo inhibiting the miR-25-3p/PTEN/Akt/GSK-3β/β-catenin signaling pathway.
Keywords: sevoflurane; microRNA-25-3p; phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN/protein kinase B/glycogen synthase kinase-3β/β-catenin signaling pathway
Introduction
Hepatocellular carcinoma (HCC) has been recognized as one of the major causes of cancer-associated death worldwide, affecting ~600,000 people annually (1,2). The high incidence of postoperative recurrence and metastasis contribute to a low survival rate for HCC patients (3,4); therefore, exploring new and effective therapeutic approaches against HCC metastasis is urgently needed.
Sevoflurane (Sevo) is a volatile anesthetic agent clinically, which exerts a suppressive role in varying cancers. For example, Liang et al (5) have shown that Sevo inhibited the proliferation, induced apoptosis and blocked cell cycle progression of lung carcinoma cells. Liu et al (6) have found that Sevo exerts a suppressive role on breast cancer cell proliferation and cell cycle. Yang et al (7) simulated the effects of clinical use of Sevo on colon cancer cells in vitro and found that Sevo inhibited tumor cell growth and induced apoptosis. In contrast, several preclinical studies reported that Sevo could promote breast cancer growth and enhances renal cancer survival (8,9). However, few studies have addressed the influence of Sevo on HCC; therefore, this study explored the role of Sevo on the various biological aspects of HCC cells.
MicroRNAs (miRNAs/miRs) are a family of short, small, noncoding RNAs (an average size of 22 nucleotides), which negatively regulate target gene expression (10,11). Increasing evidence has demonstrated the important role of miRNAs in Sevo-mediated mediated processes in numerous cancers. For example, Sevo inhibited the migration and invasion of colorectal cancer cells by upregulating miR-203 (12). Another study from Sun et al (13) showed that Sevo inhibited migration and invasion of colorectal cancer cells by regulating miR-34a. Notably, Song et al (14) found that Sevo restored the expression of miR-29 and in turn miR-29a inhibition abolished the antitumor property of Sevo in HCC cells. Nonetheless, whether Sevo exerts its antitumor effect by regulating miRNA in HCC is not fully clear.
The present study analyzed the miRNA expression profile following exposure to Sevo using microarray assay and investigated the roles of Sevo in HCC cells. Subsequently, the regulatory role and relevant mechanism of miR-25-3p in the antitumor effect of Sevo were explored. The present findings may provide a potential theoretical basis for the development of new therapies for HCC.
Materials and methods
Cell culture and drugs
The human HCC cell lines HCCLM3 and Huh7, and 293T cells were obtained from the American Type Culture Collection. All cells were grown in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA), 100 IU/ml penicillin and 100 mg/ml streptomycin at 37°C and 5% CO2 incubator. Sevo was obtained from Sigma-Aldrich; Merck KGaA.
Sevo treatment
HCCLM3 and Huh7 cells were divided into 4 groups: Control group, 1.7% Sevo group, 3.4% Sevo group and 5.1% Sevo group. According to the experimental protocol as previously described (15,16), cultured HCCLM3 and Huh7 cells were placed in an air-tight glass chamber with inflow and outflow connectors. The chamber atmosphere was kept continuously saturated with water at 37°C. The entrance port of the chamber was connected to anesthetic machine (Cicero-EM 8060, Drägerwerk AG & Co. KGaA). Sevoflurane was delivered into the chamber by a Sevo vaporizer (SEVORANE®; Abott Pharmaceutical Co. Ltd.) attached to the anesthesia machine. The concentrations of Sevo in the chamber were detected at the chamber exit port by a gas monitor (PM 8060, Drägerwerk AG & Co. KGaA) that inlayed with the anesthetic machine. The control group was exposed to 95% air/5% CO2 at 6 l/min for 6 h. The sevoflurane group was exposed to 1.7, 3.4, or 5.1% of sevoflurane mixed with 95% air/5% CO2 at 6 l/min for 6 h. A stable sevoflurane concentration was achieved within 5 min.
Cell proliferation
The antiproliferative effect of Sevo against HCC cells was measured using MTT assay. At the end of transfection, 20 µl MTT solution (Sigma-Aldrich; Merck KGaA) was added to each well (1×105/well) and cultured for 4 h. Subsequently, MTT solution was aspirated and dimethylsulfoxide (200 µl/well) was added. The optical density absorbance of the samples at 570 nm was detected by a micro-plate reader (Bio-Rad Laboratories, Inc.).
Lactate dehydrogenase (LDH) release assay
A colorimetric assay kit (Nanjing Jiancheng Bioengineering Institute; http://www.njjcbio.com/) was used to quantify the LDH released from the cultured HCCLM3 and Huh7 cells. After treated with Sevo, HCCLM3 and Huh7 cells were collected, then centrifuged at 400 × g for 5 min at 4°C. The supernatant was removed and 150 µl LDH release reagent was added, mixed completely, then incubated at 37°C 5% CO2 for 1 h. Finally, 120 µl supernatant was added to 96-well plates and cell cytotoxicity was measured by the absorbance at 490 nm by a micro-plate reader (Bio-Rad Laboratories, Inc.).
Transwell invasion assay
Transwell chambers (8-µm pore; BD Biosciences; Becton, Dickinson and Company) coated with Matrigel (BD Biosciences; Becton, Dickinson and Company) were used for invasion assay. Briefly, 1×105 HCCLM3 and Huh7 cells were added in the top chamber with DMEM, while the lower chamber added DMEM containing 20% FBS. After 24 h incubation, the invasion cells was counted and images were captured of five independent visual fields under the fluorescence microscope (Olympus Corp.) at ×200 magnification.
Wound-healing assay
When HCCLM3 and Huh7 cells reached ~80% confluence, cells were serum starved overnight and the monolayer was scratched with a 10 µl pipette tip, and then the wound area was measured at 0 and 24 h under the fluorescence microscope (Olympus Corp.) and Image J analysis software 1.46 (National Institute of Health) was used to calculated the migration distances.
Transfection assay
When HCCLM3 and Huh7 cells in six-well plate grown to ~80% confluence, miR-25-3p mimics (20 nmol/l), miR-25-3p inhibitor (20 nmol/l) and their corresponding negative controls (NC-mimic/NC-inhibitor; 20 nmol/l) were transfected into cells at 37°C for 24 h, using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). miR-25-3p mimics, mimics NC, miR-25-3p inhibitor and inhibitor NC were obtained from Guangzhou RiboBio Co., Ltd. The miR-25 mimics sequence is 5′-CAU UGC ACU UGU -CUC GGU CUG A-3′. The control RNA mimics sequence is 5′-UCA CAA CCU CCU AGA AAG AGU AGA-3′. The miR-25 inhibitor sequence is 5′-UCA GAC CGA GAC AAG UGC A AU G-3′. The control miRNA inhibitor sequence is 5′-UUU GUA CUA CAC AAA AGU ACU G-3′.
miRNA microarray analysis
Total RNA from HCCLM3 cells treated with or without 3.4% Sevo were extracted using the miRNeasy Mini kit (Qiagen, Inc.). The samples were assessed using the miRCURY LNA™ Array v. 18.0 (Agilent Technologies, Inc.). The procedure and imaging processes were as described previously (17).
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA from cells were isolated by using TRIzol reagent (Takara Biotechnology, Co., Ltd.). Reverse transcription of miR-25-3p was performed at 42°C using the miScript II RT kit (cat. no. 4366597; Thermo Fisher Scientific, Inc.). miR-25-3p expression was measured using the iCycler iQ Multicolor RT-qPCR System (Bio-Rad Laboratories, Inc.). The primers for RT-qPCR analysis were as follows: MiR-25-3p forward, 5′-TCT GGT CTC CCT CAC AGG AC-3′ and reverse 5′-CAT GGG TCG CCT ACT CAC-3′; U6 forward, 5′-TGC GGG TGC TCG CTT CGC AGC-3′ and reverse, 5′-CCA GTG CAG GGT CCG AGGT-3′. The PCR thermocycling conditions were as follows: 5 min at 95°C, and 36 cycles of 10 sec at 95°C, 10 sec at 58°C and 20 sec at 72°C. The miRNA relative expression was analyzed using the 2−ΔΔCq method (18) and determined by normalization to U6.
Target gene analyses of miR-25-3p
Bioinformatics tools, including TargetScan 7.0 (targetscan.org/) and miRanda (microrna.org/), were used to predict the potential target genes of miR-25-3p.
Luciferase reporter assay
miRNA target prediction tools, including PicTar version 2007 (https://pictar.mdc-berlin.de/), Miranda (http://miranda.org.uk) and TargetScan Release 7.0 (http://targetscan.org/) were used to search for the putative targets of miR-25-3p. The dual-luciferase reporter assay was performed as described previously (19). 293T cells were co-transfected with miR-25-3p mimics, miR-25-3p inhibitor and the luciferase reporter plasmids using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). At 48 h post-transfection, luciferase activities were measured with the dual luciferase reporter kit (Beyotime Institute of Biotechnology). Normalization of firefly luciferase activity to Renilla luciferase activity was subsequently performed.
Western blotting
Western blot was performed as previously described (19,20). Briefly, 40 µg extracted protein samples were transferred onto a polyvinylidene difluoride (EMD Millipore) membrane and then blocked with 5% skim milk for 2 h at 4°C. Then each membrane was probed with primary antibodies against PTEN, phosphorylated-protein kinase B (p-Akt) (S473) (cat. no. OMA1-03061; 1:1,000), Akt (cat. no. 44-609G; 1:1,000), p-glycogen synthase kinase (GSK)-3β (S9) (cat. no. MA5-14873; 1:1,000), GSK-3β (cat. no. MA3-038; 1:1,000), β-catenin (cat. no. 71-2700; 1:1,000), c-Myc (cat. no. MA5-27025; 1:1,000), matrix metallopro-teinase-9 (MMP-9; cat. no. MA5-15886; 1:1,000) and β-actin (cat. no. MA5-15739-D800; 1:1,000) (all primary antibodies were obtained from Thermo Fisher Scientific, Inc.) at 4°C for 20 h, followed by horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:10,000; cat. no. 205718; Abcam). β-actin served as the loading control and for normalization of protein expression. The protein bands were developed using ECL kit (GE Healthcare) and blot bands were quantified with ImageJ (version 1.46; Rawak Software, Inc.).
Statistical analyses
Statistical analysis was performed by SPSS 18.0 (SPSS, Inc.). Each experiment was repeated three times. All data were presented as mean ± standard deviation. The comparisons among data were calculated by one-way analysis of variance followed by Tukey's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Sevo inhibits the proliferation, invasion and migration of HCC cells
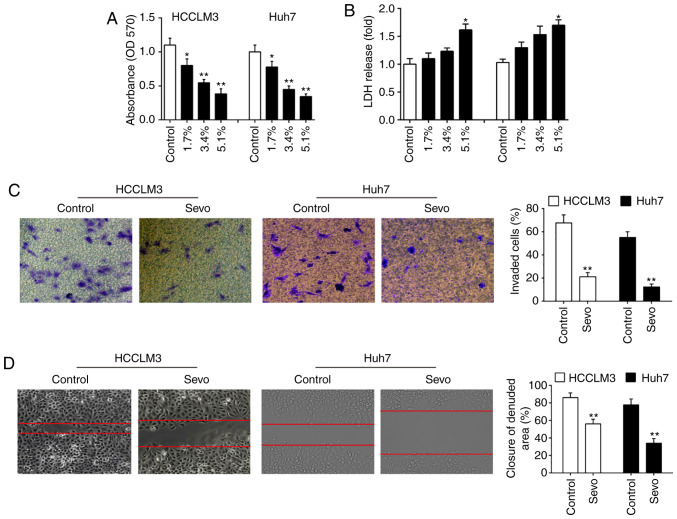
To explore the effects of Sevo on HCC cells, HCCLM3 and Huh7 cells were exposed to different concentrations of Sevo (1.7, 3.4, and 5.1%) for 6 h, and then the cell viability was assessed. The results showed that Sevo treatment suppressed the HCCLM3 and Huh7 cells viability in a dose-dependent manner (Fig. 1A). Cytotoxicity of Sevo on HCCLM3 and Huh7 cells was measured by LDH assays. It was shown that LDH release in 5.1% Sevo group was slightly increased, compared with that in control group (Fig. 1B) indicating that the decreased cell viability induced by Sevo is associated with the some degree of toxicity. The invasiveness of both HCCLM3 and Huh7 cells was further tested using the Transwell assay. It was found that Sevo treatment significantly suppressed the invasive ability of both HCCLM3 and Huh7 cells (Fig. 1C). Moreover, the wound healing assay showed that Sevo treatment significantly inhibited wound closures compared with the control group (Fig. 1D). Collectively, these data indicated that Sevo displays the anti-tumor activity on HCC cells.
Figure 1.
Sevo could suppress the proliferation and invasion capabilities of hepatocellular carcinoma cells. HCCLM3 and Huh7 cells were treated with different concentrations (0, 1.7, 3.4, and 5.1%) of Sevo for 6 h. (A) Cell proliferation was determined by MTT assay. (B) Cytotoxicity was measured by LDH assays. (C) Quantification of the invading and migratory cells. (D) Transwell and wound healing assays were used to examine cell invasion and migration in HCCLM3 and Huh7 cells (magnification, ×200). Data are the mean ± standard deviation. (n=3) of three representative experiment, *P<0.01 and **P<0.01 vs. NC group. Sevo, sevoflurane; NC, negative control; LDH, lactate dehydrogenase; OD, optical density.
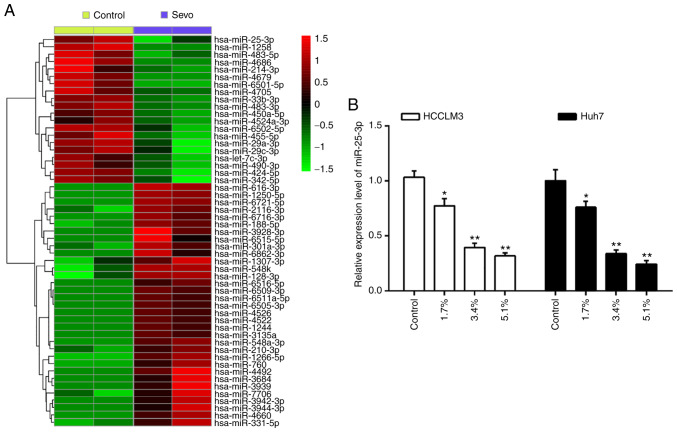
Sevo downregulates the expression of miR-25-3p in hepatocellular carcinoma cells
Several studies have addressed the pharmacological activities of Sevo, especially its anticancer effects, through the regulation of cell miRNAs (6,21). To explore the potential role of miRNAs in the anti-tumor activity of Sevo, the differentially expressed miRNAs in HCCLM3 cells treated with or without Sevo were identify using a miRNA microarray. As shown in Fig. 2A, of the 53 miRNAs screened, 20 were downregulated and 33 were upregulated by Sevo in HCCLM3 cells. Of interest, miR-25-3p displays a significant decrease after Sevo treatment. Additionally, miR-25-3p is well known oncogenic miRNA in several types of human cancers, including HCC (22-24); therefore, it appears plausible that Sevo's observed anticancer effects might be exerted through miR-25-3p in HCC cells.
Figure 2.
Sevo downregulates the expression of miR-25-3p in hepatocellular carcinoma cells. (A) miRNA microarray analysis was performed to compare the miRNA expression profiles in HCCLM3 cells treated with or without Sevo. (B) HCCLM3 and Huh7 cells were treated with different concentrations (0, 1.7, 3.4, and 5.1%) of Sevo for 6 h and then the expression of miR-25-3p was measured by reverse transcription-quantitative polymerase chain reaction. Data are the mean ± standard deviation. (n=3) of three representative experiment, *P<0.01 and **P<0.01 vs. control group. Sevo, sevoflurane; miR/miRNA, microRNA.
Using RT-qPCR, the expression of miR-25-3p was detected in Sevo treated HCC cells. The results showed that Sevo significantly downregulated miR-25-3p expression in both HCCLM3 and Huh7 cells, and this effect was dose-dependent (Fig. 2B). These results further suggested that miR-25-3p may play important roles in the anticancer effects of Sevo on HCC.
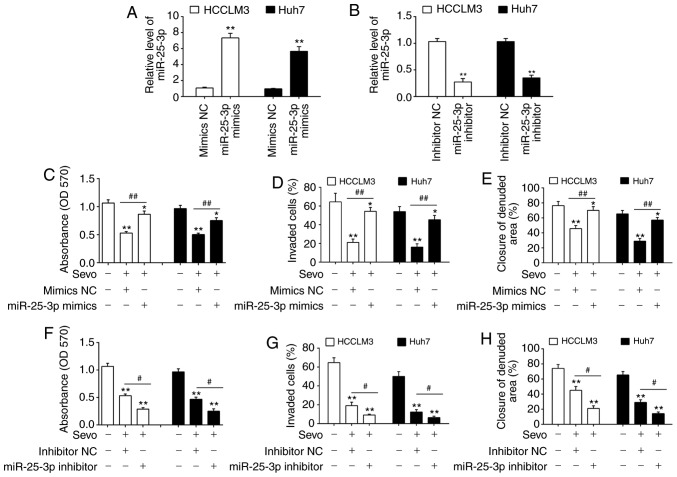
miR-25-3p is involved in the anticancer effects of Sevo on HCC cells
To determine whether miR-25-3p was required for Sevo-mediated processes in HCC cells, miR-25-3p mimics and miR-25-3p inhibitor were added into the HCCLM3 and Huh7 cells at 6 h after administering Sevo, and incubated for 48 h. miR-25-3p levels were significantly increased or decreased in HCCLM3 and Huh7 cells after transfection with the mimic or inhibitor, respectively (Fig. 3A and B). Subsequently, cell viability, invasion and migration were assessed. It was observed that 3.4% Sevo treatment significantly inhibited the proliferation, invasion and migration of HCCLM3 and Huh7 cells, but these inhibitory effects were attenuated by miR-25-3p mimics (Fig. 3C-E). In contrast, Sevo's inhibitory effects on cell proliferation, invasion and migration were enhanced by the miR-25-3p inhibitor (Fig. 3F-H). Overall, these data support miR-25-3p as an essential target of Sevo for mediating the anticancer effects of HCC cells in vitro.
Figure 3.
miR-25-3p is involved in the anticancer effects of Sevo on hepatocellular carcinoma cells. (A) miR-25-3p mimics or (B) miR-25-3p inhibitor were transfected into HCCLM3 and Huh7 cells for 24 h, and then cells were treated with Sevo for 6 h. The expression of miR-25-3p was determined by reverse transcription quantitative-PCR. Data are presented as the mean ± SD. (n=3) of one representative experiment, **P<0.01 vs. mimics NC or inhibitor NC group. The effect of the miR-25-3p mimic on (C) cell proliferation was determined by MTT assay, (D) cell invasion was measured by Transwell assay and (E) cell migration was detected by wound healing assay. The effect of the inhibitor was tested on (F) cell proliferation, (G) cell invasion and (H) Cell migration. Data are the mean ± SD. (n=3) of three representative experiment, *P<0.05 and **P<0.01 vs. mimics NC or inhibitor NC group. #P<0.05 and ##P<0.01 vs. Sevo + mimics NC or Sevo + inhibitor NC. Sevo, sevoflurane; NC, negative control; SD, standard deviation; miR/miRNA, microRNA; OD, optical density.
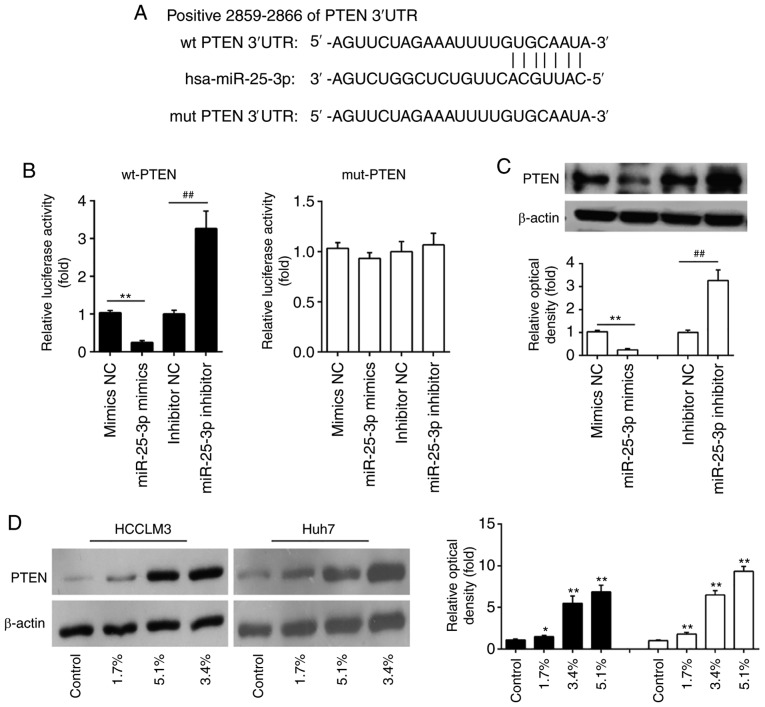
PTEN is a direct target of miR-25-3p
To examine the molecular mechanism by which miR-25-3p mediated the anticancer effects of Sevo in HCC, candidate target genes of miR-25-3p were computationally screened using TargetScan 7.0 and miRanda. The bioinformatics analysis showed that a putative target site of miR-25-3p in the 3'-UTR of PTEN (Fig. 4A). To validate whether miR-25-3p targeted PTEN, a luciferase reporter assay was performed. The luciferase reporter assay showed that the miR-25-3p mimics significantly inhibited the luciferase activity in the PTEN-3'UTR wild type reporter and that the miR-25-3p inhibitor caused an increased luciferase activity; however, no changes were observed in the cells co-transfected with PTEN 3'-UTR-mutant with miR-25-5p (Fig. 4B). Western blot analyses demonstrated that PTEN was decreased in miR-25-3p mimics transfected HCC cells, while increased in miR-25-3p inhibitor transfected HCC cells (Fig. 4C). These data suggest that PTEN is a direct target of miR-25-3p.
Figure 4.
PTEN is a direct target of miR-25-3p in hepatocellular carcinoma cells. (A) The putative binding site of miR-25-3p and PTEN is shown. (B) 293T cells were cotransfected with the PTEN wt or mut 3'-UTRs and miR-25-3p mimics and miR-25-3p inhibitor, and then the luciferase activities were quantified (n=3). (C) The expression of PTEN was measured using western blotting. Data are the mean ± SD. (n=3) of one representative experiment, **P<0.01 vs. mimics NC, ##P<0.01 vs. inhibitor NC. (D) HCCLM3 and Huh7 cells were treated with different concentrations (0, 1.7, 3.4, and 5.1%) of Sevo for 6 h and the expression of PTEN was measured using western blotting. β-actin served as the loading control. Data are presented as the mean ± SD. (n=3) of three representative experiment, *P<0.01 and **P<0.01 vs. NC. Sevo, sevoflurane; NC, negative control; SD, standard deviation; miR/miRNA, microRNA; OD, optical density; PTEN, phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN; UTR, untranslated region; wt, wild type; mut, mutant.
It is well-known that PTEN acts as a tumor suppressor in various human cancers (25,26). Given the relationship between Sevo and miR-25-3p, the present study further examined whether the expression of PTEN is regulated by Sevo. The expression of PTEN in HCCLM3 and Huh7 cells treated with Sevo was measured by western blotting. As shown in Fig. 4D, Sevo treatment dose-dependently upregulated the expression levels of PTEN in HCCLM3 and Huh7 cells. These findings support the possible roles of the miR-25-3p/PTEN axis in the anticancer effects of Sevo on HCC cells.
Sevo blocks the PTEN/Akt/GSK-3β/β-catenin signaling pathway by modulating the expression of miR-25-3p in HCC cells
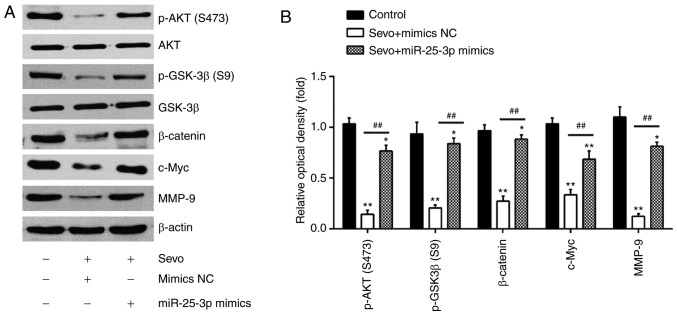
As the Akt/GSK-3β/β-catenin pathway is associated with cell migration and invasion in numerous cancers (20,27), the effect of Sevo on the Akt/GSK-3β/β-catenin pathway was investigated in HCC cells. The results of western blot-ting showed that Sevo treatment reduced the expression of key proteins, including p-Akt (S473), p-GSK3β (S9), c-Myc, β-catenin and MMP9, but the inhibitory effects of Sevo on protein expression were partly restored by miR-25-3p overexpression (Fig. 5A and B). These data suggest that Sevo blocks the PTEN/Akt/GSK-3β/β-catenin pathway by downregulating the expression of miR-25-3p in HCC cells.
Figure 5.
Sevo blocks the PTEN/Akt/GSK-3β/β-catenin signaling pathway by modulating the expression of miR-25-3p in HCC cells. HCCLM3 and Huh7 cells were transfected with miR-25-3p mimics or miR-25-3p inhibitor for 24 h followed by Sevo treatment for 6 h. (A) Protein levels of p-Akt, Akt, p-GSK-3β, GSK-3β, β-catenin, c-Myc and MMP9 were measured using western blotting. β-actin served as the loading control. (B) The bands were semi quantitatively analyzed using ImageJ and normalized to β-actin density. Data are presented as the mean ± standard deviation. (n=3) of three representative experiments. *P<0.01 and **P<0.01 vs. NC group. ##P<0.01 vs. Sevo + mimics NC. PTEN, phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN; Sevo, sevoflurane; NC, negative control; SD, standard deviation; miR/miRNA, microRNA; MMP, matrix metalloproteinase; PTEN, phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN; GSK, glycogen synthase kinase; p-Akt, phosphorylated-protein kinase B.
Sevo exerts its antitumor effects by blocking the PTEN/Akt/GSK-3β/β-catenin signaling pathway in HCC cells
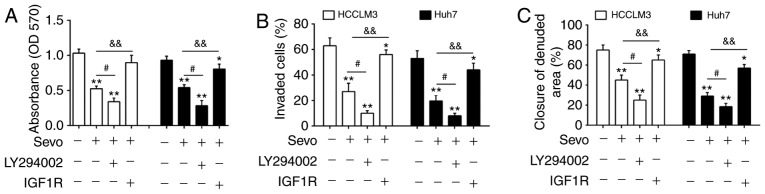
To validate the role of the PTEN/Akt/GSK-3β/β-catenin pathway on Sevo's observed anticancer effects on HCC cells, HCCLM3 and Huh7 cells were treated with 1 µM PI3K inhibitor LY294002 and 100 ng/ml PI3K signaling activator IGF-1. The results indicated that LY294002 enhanced Sevo's inhibitory effects on cell proliferation, invasion and migration in HCCLM3 and Huh7 cells. In contrast, IGF-1 reversed these inhibitory effects (Fig. 6A-C). Taken together, the present findings suggest that Sevo's anticancer effects on HCC cells might be modulated by blocking the PTEN/Akt/GSK-3β/β-catenin signaling pathway (Fig. 7).
Figure 6.
Sevo exerts its antitumor effects by blocking the PTEN/Akt/GSK-3β/β-catenin signaling pathway through the downregulation of miR-25-3p in hepatocellular carcinoma cells. HCCLM3 and Huh7 cells were treated with 1 µM PI3K inhibitor LY294002 or 100 ng/ml PI3K signaling activator insulin-like growth factor-1 for 24 h followed by Sevo treatment for 6 h. (A) Cell proliferation was determined by MTT assay. (B) Cell invasion was measured by Transwell assay. (C) Cell migration was detected by wound healing assay. Data are the mean ± standard deviation. (n=3) of three representative experiments, *P<0.01 and **P<0.01 vs. negative control group. #P<0.05 vs. Sevo, &&P<0.01 vs. Sevo. Sevo, sevoflurane; NC, negative control; miR/miRNA, microRNA; OD, optical density; PTEN, phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN;GSK, glycogen synthase kinase; p-Akt, phosphorylated-protein kinase B; PI3K, phosphatidylinositol 3 kinase.
Figure 7.
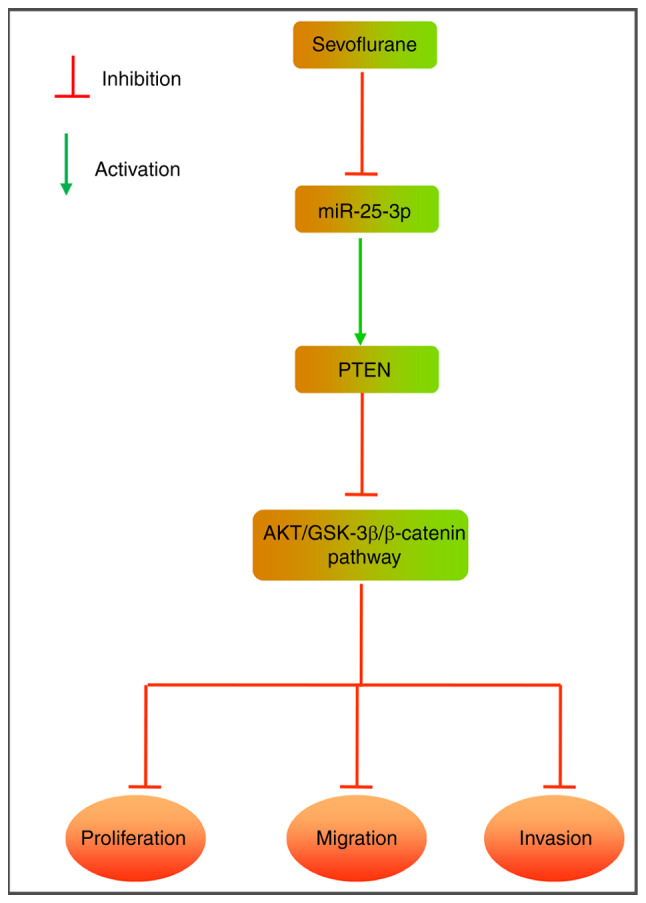
Schematic representation shows the potential mechanism of Sevo-mediated anti-proliferation and anti-invasion roles in hepatocellular carcinoma cells. Sevo inhibits the PTEN/Akt/GSK-3β/β-catenin signaling pathway, leading to decreased migration and invasion via regulating miR-25-3p in HCCLM3 and Huh7 cells. Sevo, sevoflurane; miR/miRNA, microRNA; PTEN, phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN; GSK, glycogen synthase kinase; Akt, protein kinase B.
Discussion
The present study found that Sevo inhibited the proliferation, invasion and migration of HCC cells. Moreover, Sevo was found to downregulate the level of miR-25-3p in HCC cell lines and proved that the miR-25-3p/PTEN/Akt/GSK-3β/β-catenin axis was responsible for the antitumor actions of Sevo in HCC cells. It is of clinical significance for anesthesiologists to select volatile anesthetics for surgical resection of HCC.
Previous studies have indicated that Sevo displays its anticancer activity in numerous human cancers. For example, Liang et al (28) have shown that Sevo actually inhibited lung cancer cell invasion by suppressing the activation of the p38 mitogen associated protein kinase pathway. Liang et al (5) have also found that Sevo inhibited proliferation by inducing cell apoptosis in A549 cells. Müller-Edenborn et al (29) found that Sevo reduced the invasiveness of colon cancer cells in vitro by downregulating MMP9. Sevo could suppress hypoxia-induced growth and metastasis of lung cancer cells via inhibiting hypoxia-inducible factor-1α (30). Sevo has an inhibitory effect on the migration and MMP-2 activity in glioma cells (31). In addition, Sevo exerts its antiproliferative effect on colon cancer cells (32,33); therefore, taking into account the inhibitory properties of Sevo against the tumor cells, Sevo is a better choice of anesthetics during cancer surgery. However, few reports have addressed the roles of Sevo in HCC. The present results indicate that Sevo markedly suppressed cell proliferation, invasion and migration of HCCLM3 and Huh7 cells, which supports the efficacy of Sevo on tumor growth and invasion.
Several studies have also reported that miRNAs are implicated in processes affected by Sevo in numerous conditions (34,35). For example, Shao and Xia (36) showed that Sevo repressed neurogenesis by regulating miR-183 expression in newborn rats. Zhao et al (34) found that Sevo upregulated the expression of miR-19-3p and inhibited the expression of CCNA2, thus resulting in the impairment of learning and memory in neonatal rats. Otsuki et al (37) found that Sevo exhibited a protective effect against endotoxin-induced acute injury in rats by regulating inflammation-associated miRNAs. Moreover, increasing evidence has indicated that Sevo exerts antitumor effects on several cancers by modulating the expression of several miRNAs. For example, Sevo inhibited cell migration and invasion by the induction of miR-637 in glioma (21). Liu et al (6) found that Sevo suppresses breast cancer cell growth by modulating miR-203 expression. Therefore the present study hypothesized that Sevo inhibited cell migration and invasion of HCC cells by regulating miRNAs. This study found that a large number of miRNAs were differently expressed following exposure to Sevo in HCC cells and the most downregulated miR-25-3p were selected for further study.
A number of miRNAs have been revealed to be involved in the pathogenesis of HCC (38). For example, upregulation of miR-21 could enhance cell proliferation, reduce cell apoptosis and favor invasion (39). These findings suggest that miRNAs could become novel molecular targets for HCC treatment. The oncogenic roles of miR-25-3p in numerous types of cancers have previously been investigated (40,41). In HCC, one previous study demonstrated that miR-25-3p was upregulated in HCC tissues when compared with adjacent normal tissues and that the upregulation of miR-25-3p is of predictive value on poor prognosis (22). Another study from Wang et al (23) revealed that miR-25-3p dramatically stimulated HCC cell growth and activated the epithelial-mesenchymal transition. Thus, the present study hypothesized that miR-25-3p has an important role in Sevo's observed anticancer effects on HCC cells. As expected, the current results showed that the antitumor effects by Sevo was suppressed by miR-25-3p overexpression, while enhanced by miR-25-3p inhibition. These findings suggest that Sevo's anti-HCC effects are mediated by downregulating miR-25-3p.
PTEN is a tumor suppressor gene, which could negatively regulate the Akt/GSK-3β/β-catenin pathway (42). In addition, PTEN could regulate proliferation, migration and invasion of HCC (43,44). Moreover, Wan et al (45) showed that miR-25-3p promoted malignant phenotypes of retinoblastoma by targeting PTEN and activating Akt signaling pathway. In the present study, PTEN was directly targeted by miR-25-3p in HCC cells and its expression was upregulated after exposure to Sevo. Moreover, it was also found that Sevo decreased p-Akt, p-GSK3β, β-catenin, c-Myc and MMP-9 expression; whereas, overexpression of miR-25-3p obviously reversed this inhibitory effect, which suggested that Sevo suppressed this signaling pathway by downregulating the miR-25-3p/PTEN axis. In addition, the PI3K inhibitor LY294002 enhanced Sevo's inhibitory effects on HCC cell proliferation, invasion and migration, while these inhibitory effects were reversed with the PI3K signaling activator IGF-1. The above results suggest that Sevo inhibits the proliferation, invasion and migration of HCC cells in part by downregulating miR-25-3p through suppressing the PTEN/Akt/GSK-3β/β-catenin signaling pathway.
There are still some limitations in the present study. For example, this exploratory study summarizes the novel role of Sevo in regulating the miR-25-3p/PTEN/Akt/GSK-3β/β-catenin pathway and subsequently exerts its anti-tumor activity in vitro. However, more experiments in vivo should be performed to confirm that miR-25-3p mediated the anti-tumor activity of Sevo in HCC. Moreover, further study is needed to explore the regulatory functions of miR-25-3p in the other signaling pathways, such as Rho GDP dissociation inhibitor α/Wnt/β-catenin pathway (23). Thus, future research is needed to gain deeper insight into these questions.
The current study demonstrates that Sevo exerts its anti-tumor activity through inactivation of PTEN/Akt/GSK-3β/β-catenin pathway by the downregulation of miR-25-3p. The present study provides new clinical implications for the potential role of Sevo in HCC surgery.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YC, WL and WD performed the experiments, contributed to data analysis and wrote the paper. YC, WL and WD analyzed the data. JL conceptualized the study design, contributed to data analysis and experimental materials. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the No. 6 Medical Center, General Hospital of PLA Ethics Committees.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. doi: 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: Present and future. Clin Liver Dis. 2011;15:223–243. vii–x. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang ZY. Hepatocellular carcinoma-cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang Z, Zhou X, Lin Z, Yang B, Ma Z, Ye S, Wu Z, Fan J, Liu Y, Liu K, et al. Surgical treatment of hepatocellular carcinoma and related basic research with special reference to recurrence and metastasis. Chin Med J (Engl) 1999;112:887–891. [PubMed] [Google Scholar]
- 5.Liang H, Gu MN, Yang CX, Wang HB, Wen XJ, Zhou QL. Sevoflurane inhibits proliferation, induces apoptosis, and blocks cell cycle progression of lung carcinoma cells. Asian Pac J Cancer Prev. 2011;12:3415–3420. [PubMed] [Google Scholar]
- 6.Liu J, Yang L, Guo X, Jin G, Wang Q, Lv D, Liu J, Chen Q, Song Q, Li B. Sevoflurane suppresses proliferation by upregulating microRNA-203 in breast cancer cells. Mol Med Rep. 2018;18:455–460. doi: 10.3892/mmr.2018.8949. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, Zheng YT, Rong W. Sevoflurane induces apoptosis and inhibits the growth and motility of colon cancer in vitro and in vivo via inactivating Ras/Raf/MEK/ERK signaling. Life Sci. 2019;239:116916. doi: 10.1016/j.lfs.2019.116916. [DOI] [PubMed] [Google Scholar]
- 8.Ciechanowicz S, Zhao H, Chen Q, Cui J, Mi E, Mi E, Lian Q, Ma D. Differential effects of sevoflurane on the metastatic potential and chemosensitivity of non-small-cell lung adenocarcinoma and renal cell carcinoma in vitro. Br J Anaesth. 2018;120:368–375. doi: 10.1016/j.bja.2017.11.066. [DOI] [PubMed] [Google Scholar]
- 9.Ecimovic P, McHugh B, Murray D, Doran P, Buggy DJ. Effects of sevoflurane on breast cancer cell function in vitro. Anticancer Res. 2013;33:4255–4260. [PubMed] [Google Scholar]
- 10.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 11.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 12.Fan L, Wu Y, Wang J, He J, Han X. Sevoflurane inhibits the migration and invasion of colorectal cancer cells through regulating ERK/MMP-9 pathway by up-regulating miR-203. Eur J Pharmacol. 2019;850:43–52. doi: 10.1016/j.ejphar.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Sun SQ, Ren LJ, Liu J, Wang P, Shan SM. Sevoflurane inhibits migration and invasion of colorectal cancer cells by regulating microRNA-34a/ADAM10 axis. Neoplasma. 2019;66:887–895. doi: 10.4149/neo_2018_181213N962. [DOI] [PubMed] [Google Scholar]
- 14.Song G, Tian L, Cheng Y, Liu J, Wang K, Li S, Li T. Antitumor activity of sevoflurane in HCC cell line is mediated by miR-29a-induced suppression of Dnmt3a. J Cell Biochem. 2019;120:18152–18161. doi: 10.1002/jcb.29121. [DOI] [PubMed] [Google Scholar]
- 15.Roesslein M, Frick M, Auwaerter V, Humar M, Goebel U, Schwer C, Geiger KK, Pahl HL, Pannen BH, Loop T. Sevoflurane-mediated activation of p38-mitogen-activated stresskinase is independent of apoptosis in Jurkat T-cells. Anesth Analg. 2008;106:1150–1160. doi: 10.1213/ane.0b013e3181683d37. table of contents. [DOI] [PubMed] [Google Scholar]
- 16.Loop T, Scheiermann P, Doviakue D, Musshoff F, Humar M, Roesslein M, Hoetzel A, Schmidt R, Madea B, Geiger KK, et al. Sevoflurane inhibits phorbolmyristate-acetate-induced activator protein-1 activation in human T lymphocytes in vitro: Potential role of the p38-stress kinase pathway. Anesthesiology. 2004;101:710–721. doi: 10.1097/00000542-200409000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Mei LL, Wang WJ, Qiu YT, Xie XF, Bai J, Shi ZZ. MiR-125b-5p functions as a tumor suppressor gene partially by regulating HMGA2 in esophageal squamous cell carcinoma. PLoS One. 2017;12:e0185636. doi: 10.1371/journal.pone.0185636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Yao Y, Sun F, Lei M. MiR-25 inhibits sepsis-induced cardiomyocyte apoptosis by targetting PTEN. Biosci Rep. 2018;38:BSR20171511. doi: 10.1042/BSR20171511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Q, Xu HX, Li JP, Wang S, Fu Z, Jia J, Wang L, Zhu ZF, Lu R, Yao Z. Growth differentiation factor 15 induces growth and metastasis of human liver cancer stem-like cells via AKT/GSK-3β/β-catenin signaling. Oncotarget. 2017;8:16972–16987. doi: 10.18632/oncotarget.15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi W, Li D, Guo Y, Zhang Y, Huang B, Li X. Sevoflurane inhibits the migration and invasion of glioma cells by upregulating microRNA-637. Int J Mol Med. 2016;38:1857–1863. doi: 10.3892/ijmm.2016.2797. [DOI] [PubMed] [Google Scholar]
- 22.Su ZX, Zhao J, Rong ZH, Geng WM, Wu YG, Qin CK. Upregulation of microRNA-25 associates with prognosis in hepatocellular carcinoma. Diagn Pathol. 2014;9:47. doi: 10.1186/1746-1596-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Wang X, Su Z, Fei H, Liu X, Pan Q. MiR-25 promotes hepatocellular carcinoma cell growth, migration and invasion by inhibiting RhoGDI1. Oncotarget. 2015;6:36231–36244. doi: 10.18632/oncotarget.4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Gong X, Tian K, Chen D, Sun J, Wang G, Guo M. MiR-25 promotes glioma cell proliferation by targeting CDKN1C. Biomed Pharmacother. 2015;71:7–14. doi: 10.1016/j.biopha.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S, Navab R. MicroRNA-21 (miR-21) regulates cellular proliferation, invasion, migration, and apoptosis by targeting PTEN, RECK and Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS One. 2014;9:e103698. doi: 10.1371/journal.pone.0103698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song MS, Salmena L, Pandolfi PP. The functions and regu-lation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 27.Park NR, Cha JH, Jang JW, Bae SH, Jang B, Kim JH, Hur W, Choi JY, Yoon SK. Synergistic effects of CD44 and TGF-β1 through AKT/GSK-3β/β-catenin signaling during epithelial-mesenchymal transition in liver cancer cells. Biochem Biophys Res Commun. 2016;477:568–574. doi: 10.1016/j.bbrc.2016.06.077. [DOI] [PubMed] [Google Scholar]
- 28.Liang H, Gu M, Yang C, Wang H, Wen X, Zhou Q. Sevoflurane inhibits invasion and migration of lung cancer cells by inactivating the p38 MAPK signaling pathway. J Anesth. 2012;26:381–392. doi: 10.1007/s00540-011-1317-y. [DOI] [PubMed] [Google Scholar]
- 29.Müller-Edenborn B, Roth-Z'graggen B, Bartnicka K, Borgeat A, Hoos A, Borsig L, Beck-Schimmer B. Volatile anesthetics reduce invasion of colorectal cancer cells through down-regulation of matrix metalloproteinase-9. Anesthesiology. 2012;117:293–301. doi: 10.1097/ALN.0b013e3182605df1. [DOI] [PubMed] [Google Scholar]
- 30.Liang H, Yang CX, Zhang B, Wang HB, Liu HZ, Lai XH, Liao MJ, Zhang T. Sevoflurane suppresses hypoxia-induced growth and metastasis of lung cancer cells via inhibiting hypoxia-inducible factor-1α. J Anesth. 2015;29:821–830. doi: 10.1007/s00540-015-2035-7. [DOI] [PubMed] [Google Scholar]
- 31.Hurmath FK, Mittal M, Ramaswamy P, Umamaheswara Rao GS, Dalavaikodihalli Nanjaiah N. Sevoflurane and thiopental preconditioning attenuates the migration and activity of MMP-2 in U87MG glioma cells. Neurochem Int. 2016;94:32–38. doi: 10.1016/j.neuint.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Kvolik S, Glavas-Obrovac L, Bares V, Karner I. Effects of inhalation anesthetics halothane, sevoflurane, and isoflurane on human cell lines. Life Sci. 2005;77:2369–2383. doi: 10.1016/j.lfs.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 33.Kvolik S, Dobrosevic B, Marczi S, Prlic L, Glavas-Obrovac L. Different apoptosis ratios and gene expressions in two human cell lines after sevoflurane anaesthesia. Acta Anaesthesiol Scand. 2009;53:1192–1199. doi: 10.1111/j.1399-6576.2009.02036.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhao X, Jin Y, Li H, Jia Y, Wang Y. Sevoflurane impairs learning and memory of the developing brain through post-transcriptional inhibition of CCNA2 via microRNA-19-3p. Aging (Albany NY) 2018;10:3794–3805. doi: 10.18632/aging.101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu C, Niu JJ, Zhou JF, Wei YS. MicroRNA-96 is responsible for sevoflurane-induced cognitive dysfunction in neonatal rats via inhibiting IGF1R. Brain Res Bull. 2019;144:140–148. doi: 10.1016/j.brainresbull.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Shao CZ, Xia KP. Sevoflurane anesthesia represses neurogenesis of hippocampus neural stem cells via regulating microRNA-183-mediated NR4A2 in newborn rats. J Cell Physiol. 2018;234:3864–3873. doi: 10.1002/jcp.27158. [DOI] [PubMed] [Google Scholar]
- 37.Otsuki T, Ishikawa M, Hori Y, Goto G, Sakamoto A. Volatile anesthetic sevoflurane ameliorates endotoxin-induced acute lung injury via microRNA modulation in rats. Biomed Rep. 2015;3:408–412. doi: 10.3892/br.2015.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gramantieri L, Fornari F, Callegari E, Sabbioni S, Lanza G, Croce CM, Bolondi L, Negrini M. MicroRNA involvement in hepatocellular carcinoma. J Cell Mol Med. 2008;12:2189–2204. doi: 10.1111/j.1582-4934.2008.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C, Yu J, Yu S, Lavker RM, Cai L, Liu W, Yang K, He X, Chen S. MicroRNA-21 acts as an oncomir through multiple targets in human hepatocellular carcinoma. J Hepatol. 2010;53:98–107. doi: 10.1016/j.jhep.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Pan H, Qian Y, Zhou W, Liu X. MiR-25-3p promotes the proliferation of triple negative breast cancer by targeting BTG2. Mol Cancer. 2018;17:4. doi: 10.1186/s12943-017-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao H, Wang Y, Yang L, Jiang R, Li W. MiR-25 promotes gastric cancer cells growth and motility by targeting RECK. Mol Cell Biochem. 2014;385:207–213. doi: 10.1007/s11010-013-1829-x. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Qi X, Li Z, Jin S, Xie Y, Zhong H. lncRNA CADM1-AS1 inhibits cell-cycle progression and invasion via PTEN/AKT/GSK-3β axis in hepatocellular carcinoma. Cancer Manag Res. 2019;11:3813–3828. doi: 10.2147/CMAR.S197673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He J, Mu M, Luo Y, Wang H, Ma H, Guo S, Fang Q, Qian Z, Lu H, Song C. MicroRNA-20b promotes proliferation of H22 hepatocellular carcinoma cells by targeting PTEN. Oncol Lett. 2019;17:2931–2936. doi: 10.3892/ol.2019.9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han Y, Chen M, Wang A, Fan X. STAT3-induced upregulation of lncRNA CASC11 promotes the cell migration, invasion and epithelial-mesenchymal transition in hepatocellular carcinoma by epigenetically silencing PTEN and activating PI3K/AKT signaling pathway. Biochem Biophys Res Commun. 2019;508:472–479. doi: 10.1016/j.bbrc.2018.11.092. [DOI] [PubMed] [Google Scholar]
- 45.Wan W, Wan W, Long Y, Li Q, Jin X, Wan G, Zhang F, Lv Y, Zheng G, Li Z, Zhu Y. MiR-25-3p promotes malignant phenotypes of retinoblastoma by regulating PTEN/Akt pathway. Biomed Pharmacother. 2019;118:109111. doi: 10.1016/j.biopha.2019.109111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.