Abstract
The aim of the present study was to explore the mechanism by which Notch1-small activating (sa)RNA restored androgen sensitivity in human metastatic castration-resistant prostate cancer (CRPC). After transfection of Notch1-saRNA-1480 in PC3 cells, the expression of Notch1 and androgen receptor (AR) was investigated by reverse transcription quantitative PCR (RT-qPCR) and western blotting. Furthermore, the protein expression level of vascular endothelial growth factor (VEGF) was measured. Then, flow cytometry was used to analyze the cell cycle and apoptosis after transfection. Moreover, the migration and invasion ability of PC3 cells were assessed by transwell assays. Then, angio-genesis experiments were conducted to analyze the abilities of PC3 cells to form blood vessels. Furthermore, in vivo experiments detected the antitumor activity of Notch1-saRNA-1480. The mRNA and protein expression levels of Notch1 were significantly increased after transfection, while the expression levels of AR and VEGF were decreased. After transfection, the cell cycle was arrested at the G0/G1 checkpoint. Notch1-saRNA-1480 significantly increased the proportion of apoptotic cells after transfection. In addition, transwell assay results showed that PC3 cell migration and invasion were inhibited. The total vessel length was significantly decreased based on angiogenesis experiments, which indicated that PC3 cell angiogenesis was inhibited. In vivo experiments showed that Notch1-saRNA-1480 could inhibit tumor growth and volume. The protein expression of Notch1, AR, VEGF receptor 2 (VEGFR2) and VEGF in tumor tissues was consistent with in vitro levels. Notch1-saRNA-1480 could significantly inhibit the proliferation of PC3 cells in vitro and the growth of tumors in vivo, which is associated with the inhibition of the AR and VEGF pathways.
Keywords: Notch1-saRNA-1480, castration-resistant prostate cancer, PC3, androgen receptor, vascular endothelial growth factor
Introduction
Prostate cancer has become the third leading cause of death in male cancer patients (1). Clinically, most patients with advanced prostate cancer receive androgen deprivation therapy (ADT) targeting the androgen receptor (AR) (2). However, most tumors develop metastatic castration-resistant prostate cancer (CRPC) after ADT (3). Currently, treatments for CRPC include second-generation androgen inhibitors, chemotherapeutic agents and immunotherapy. However, these therapies only increase the median overall survival of the patient by 4 months (4). Therefore, there is an urgent need for more effective treatment for CRPC.
Notch is a family of evolutionarily highly conserved transmembrane receptor proteins that are widely expressed in numerous species, from invertebrates to mammals (5). Notch signaling consists of four receptors (Notch 1-4) and five ligands. Growing evidence has found that the Notch signaling pathway is involved in prostate cancer cell proliferation, apoptosis, migration and invasion, and affects angiogenesis and other processes (6). Increased AR plays a key role in promoting prostate cancer development (7). Additionally, mutations in the AR gene allow other endogenous hormones or antiandrogens as AR agonists to promote prostate cancer cell proliferation (8,9). Therefore, blocking and inhibition of the AR signaling pathway has been a critical node for the treatment of CRPC. Increasing evidence suggests that it exists as a mutual negative feedback regulation mechanism between the AR and Notch pathways (10,11). The vascular system of tumors has become a new and promising target for anti-tumor therapy. Vascular endothelial growth factor (VEGF) has been considered as a key factor in angiogenesis during malignant tumor metastasis (12). Hematogenous metastasis of prostate cancer is the most common, but overexpression of activated Notch1 can inhibit the proliferation of prostate cancer cells (13). Prostate cancer secretes elevated VEGF and this promotes endothelial cell proliferation and migration through autocrine or paracrine pathways, which are closely related to prostate cancer metastasis (14). In addition, Notch signaling is important in determining how endothelial cells respond to VEGF (15).
Activation of the Notch pathway by small activating RNA (saRNA) can simultaneously regulate AR and VEGF, which could provide new ideas for CRPC development and gene therapy, and the present study has important theoretical value and clinical guiding significance.
Materials and methods
saRNA technology synthesis
Three saRNAs (including saRNA-571, saRNA-947 and saRNA-1480) were designed to target non-CpG islands and Alu regions of the promoters of human Notch1 gene (GenBank: NM_017617), and were chemically synthesized by the Bioneer Corporation. Then the saRNA were screened for the highest activation rate of Notch1. The sequence of the three saRNAs is listed in Table I.
Table I.
Sequences of three saRNAs and scramble RNA.
| saRNAs | Sequence |
|---|---|
| saRNA-571 | 5′-GCUGUUUCCAGAGUGCUCATT-3′ |
| 5′-UGAGCACUCUGGAAACAGCTT-3′ | |
| saRNA-947 | 5′-GGCAUCGUGGUGGAGAAAUTT-3′ |
| 5′-AUUUCUCCACCACGAUGCCTT-3′ | |
| saRNA-1480 | 5′-CCGCUUAUUCACAUGCAAATT-3′ |
| 5′-UUUGCAUGUGAAUAAGCGGTT-3′ | |
| Scramble RNA | 5′-UUCUCCGAACGUGUCACGU-3′ |
| 5′-ACGUGACACGUUCGGAGAATT-3′ |
sa, small activating.
Cell culture and transfection
PC3 cells (prostate cancer cells from the bone metastasis site cell line) were obtained from the Shanghai Cell Bank of Chinese Academy of Sciences. PC3 cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml penicillin/streptomycin in a humidified CO2 incubator at 37°C. Cells were seeded into 12-well plates the day before cell transfection, with a density of 2×105 cell/ml the next day. Transfections of saRNA and scramble RNA were carried out at a concentration of 25 nM/well using Lipofectamine 3000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and lasted for 24, 48 and 72 h, respectively.
Quantitative (q)PCR
PC3 cells were collected at 24, 48 and 72 h after treating with saRNA and untreated cells were used as untreated reference group. After washing twice with PBS, total RNA was isolated using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and then transcribed into cDNA by PrimeScript RT Master Mix (Takara Biotechnology Co., Ltd.) according to the following conditions: 25°C for 10 min, 37°C for 60 min, 70°C for 10 min. PCR amplification included an initial denaturation step (95°C for 10 min), 38 cycles of denaturation (95°C for 10 sec), and annealing (60°C for 1 min). The experimental results were automatically calculated by reverse transcription (RT)-qPCR analysis software CFX Manager 3.1 (Bio-Rad Laboratories, Inc.). The primer sequences of Notch1, AR and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are shown in Table II. All experiments were repeated 3 times independently. According to above methods, the mRNA expression level of KISS1, MKK4 and KAI1 was calculated by RT-qPCR. Table II lists the primer sequences. The relative expression of each gene was converted into 2-∆∆Cq (16).
Table II.
Primer sequences for quantitative PCR.
| Target gene | Primer sequence | Product size in bp |
|---|---|---|
| Homo Notch1 | 5′-GAGGCGTGGCAGACTATGC-3′ | 140 |
| 5′-CTTGTACTCCGTCAGCGTGA-3′ | ||
| Homo AR | 5′-CCAGGGACCATGTTTTGCC-3′ | 226 |
| 5′-CGAAGACGACAAGATGGACAA-3′ | ||
| Homo KISS1 | 5′-CTCACTGGTTTCTTGGCAGC-3′ | 100 |
| 5′-GCCTGTGGGTCTAGAATTCCC-3′ | ||
| Homo MKK4 | 5′-TGCAGGGTAAACGCAAAGCA-3′ | 93 |
| 5′-CTCCTGTAGGATTGGGATTCAGA-3′ | ||
| Homo MTA1 | 5′-ACGCAACCCTGTCAGTCTG-3′ | 104 |
| 5′-GGGCAGGTCCACCATTTCC-3′ | ||
| Homo KAI1 | 5′-TGTCCTGCAAACCTCCTCCA-3′ | 80 |
| 5′-CCATGAGCATAGTGACTGCCC-3′ | ||
| GAPDH | 5′-CTCGCTTCGGCAGCACA-3′ | 90 |
| 5′-AACGCTTCACGAATTTGCGT-3′ |
AR, androgen receptor.
Western blotting analysis
Cells were collected at 24, 48 and 72 h after treatment. Cells were rinsed twice with ice-cold PBS and were lysed using 200 μl of RIPA lysis buffer (Beyotime Institute of Biotechnology) per 1×106 cells. Lysates were allowed to stand on ice for 5 min, transferred to a 1.5-ml centrifuge tube and lysed for 30 min on ice. Cell lysates were clarified by centrifugation at 12,000 × g for 15 min at 4°C and protein concentrations were determined using the bicinchoninic acid protein assay reagent (Pierce, Thermo Fisher Scientific, Inc.). Cell lysates were denatured in a 100°C water for 5 min and then collected by centrifugation at 13,000 × g at 4°C for 30 min. Cell lysates (35 μg) were subjected to 12% SDS-PAGE gels and electrophoretically transferred to Invitrolon™ polyvinylidene difluoride membranes (Thermo Fisher Scientific, Inc.). Membranes were blocked with 5% BSA (Beyotime Institute of Biotechnology) and then incubated overnight with the appropriate primary antibodies including anti-VEGF (1:1,000; cat. no. SAB1402390; Sigma-Aldrich; Merck KGaA), anti-Notch1 (1:1,000; cat. no. 3608; Cell Signaling Technology, Inc.), anti-VEGFR2 (1:1,000; cat. no. ab134191; Abcam), anti-AR (1:1,000; cat. no. 5153S; Cell Signaling Technology, Inc.) and anti-GAPDH (1:1,000; cat. no. 2118L; Cell Signaling Technology, Inc.) followed by matching horseradish peroxidase-conjugated secondary antibodies (1:2,000; cat. nos. ab205719 or ab7090; Abcam). After washing each sample three times for 10 min with TBS-0.05% Tween 20, Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore) and a ChemiDoc™ Touch Imaging System (Bio-Rad Laboratories, Inc.) were used to visualize bands; the intensity of bands was quantified with Image Lab™ Software (version 4.0; Bio-Rad Laboratories, Inc.).
Nuclear run-on RT-qPCR
Notch1 transcriptional activity was measured by nuclear run-on experiments, as previously described (17). PC3 cells were used to determine transcriptional activity after transfection with Notch1-saRNA-1480.
Cell cycle analysis
An AnnexinV detection kit (BD Biosciences) was used to analyze cell cycle. PC3 cells were seeded in 6-well plates and the density was 50% the next day. The serum-free medium was replaced and synchronized for 24 h, and then the normal medium was restored for subsequent experiments. The cells were transfected with 25 nM saRNA using Lipofectamine 3000. Scramble RNAs were used as reference. A total of 48 h after treatment, cells were harvested, rinsed with PBS twice and fixed in 4% paraformaldehyde phosphate buffer for 10 min at room temperature. Cold ethanol (750 μl) was added to resuspend the cells and fix at 4°C overnight. After centrifugation at 300 × g for 5 min at 4°C, the cells were resuspended in 1 ml PBS and allowed to stand at room temperature for 30 min to recover. A total of 500 μl propidium iodide (PI) staining solution was added into each tube, incubated for 30 min at room temperature in the darkness. The distribution of cell cycle was shown as the percentage of cells in G0/G1, G2/M and S populations using a CytoFLEX flow cytometer (Beckman Coulter, Inc.) and FlowJo software (version 11.0; FlowJo LLC).
Apoptosis analysis
The culture supernatant and cells were collected, washed twice with pre-cooled PBS and the supernatant was discarded. After resuspending in 500 μl apoptosis positive control solution, the cells were incubated on ice for 30 min. An appropriate amount of pre-cooled 1X Binding Buffer was added, followed by the same amount of untreated live cells. After adding 1.5 ml pre-cooled 1X Binding Buffer, the cells were divided into 3 tubes, one of which was a blank control tube and the two tubes were single-dyed tubes. Then, 5 μl Annexin V-FITC or 10 μl PI was added into the single-stained tube and the cells were incubated at room temperature for 5 min in the dark. Finally, the results were measured via flow cytometry.
Cell proliferation
The cells were seeded in 6-well plates, which was transfected with saRNA the next day. Then the cells were seeded at a density of 1×103/well in 96-well plates on the third day in triplicate. Cell Counting Kit-8 values were determined at 0, 24, 48, 72 and 96 h according to the manufacturer's protocol. The growth curves were plotted according to the results.
Colony formation assay
The cells were seeded in 6-well plates and transfected with the corresponding saRNAs the next day. On the third day, the cells were seeded at a density of 1×103 cell into 6-well plates and the medium was changed every 3 days. After 2 weeks of culture, the cells were fixed with paraformaldehyde 4°C for 20 min. After 20 min, crystal violet stained for 15 min at room temperature and the count was observed under a light microscope.
Cell migration and invasion assay
Transwell chambers (BD Biosciences) were inserted into 6-well plates. In the invasion experiment, Matrigel was diluted 40 times with PBS on ice and added to the upper chamber. Then, cells transfected with saRNA for 48 h were seeded in 6-well plates supplemented with serum-free medium. The migration assay was performed in the same manner except without the Matrigel. After incubating at 37°C for 1 h, the supernatant was aspirated. The cells were centrifuged at 300 × g for for 5 min at 4°C, resuspended and counted in serum-free medium. Then 2×102 inoculated into the upper chamber of the Transwell chamber, and 600 μl of complete medium in the lower chamber. After incubation for 24 h, the paraformaldehyde was fixed for 20 min at 4°C and crystal violet staining was performed for 15 min at room temperature. After 15 min, an image was captured under a light microscope.
Tube formation
Normally cultured cells were centrifuged at 300 × g for 5 min at 4°C and inoculated into 6-well plates (1×103 cells/well). The next day, different saRNAs were transfected as required. After 48 h, the cells were digested, centrifuged at 300 × g for 5 min at 4°C, adjusted to a cell density of 5×104 cells/ml and plated into a 96-well plate in which Matrigel matrix was pre-coated, and 0.1 ml of cell suspension was added to each well, to give a final density of 5×103 cells/well. After 5-6 h of serum-free culture, the cells of each group were observed under a light microscope; the total length of blood vessels was calculated by Image J software (version 1.8.0, National Institute of Health), and the angiogenic ability of each group was analyzed.
In vivo antitumor activity of Notch1-saRNA-1480
The PC3 cell line in the logarithmic growth phase was prepared as a single cell suspension by adding serum-free medium, and counted in a flow cytometer (the number of viable cells was >95%). The cell density was adjusted to 7×107/ml and 0.1 ml was inoculated into the left armpit of 10 male Balb/c mice (weight, 20±2 g; Shanghai Slack Laboratory Animals Co., Ltd.) at 5-6 weeks of age. All mice were reared under the following conditions: Temperature, 21±2°C; relative humidity, 30-70%; light and dark cycle, 12/12 h. All rats received food and water ad libitum. The tumor formation of the mice was closely observed every day after inoculation. From the fourth day of inoculation, the long diameter (a) and short diameter (b) of the tumor were measured with a vernier caliper every other day. The tumor volume was calculated according to the formula V=axb2×0.5 and the tumor growth curve was drawn. The saRNA powder (5OD) was dissolved with 20 μl of ddH2O. A total of 14.4 μl of RNAiMax was added to 14.4 μl of Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.) and mixed well. After adding 16 μl of the diluted saRNA solution, the cells were allowed to stand at room temperature for 5-20 min. A total of four days after tumor cell inoculation, intratumoral injection was performed for 9 μl/3 days. After 5 weeks, all mice were euthanized with sodium pentobarbital (150 mg/kg). Then, tumors were removed and weighed. The current study complied with the ARRIVE guidelines and the AVMA euthanasia guidelines 2013. To observe the internal structure of the tumor, hematoxylin staining for 20 min at room temperature and eosin staining for 1 min at room temperature was performed. At the same time, the protein expression levels of Notch1, AR, VEGFR2 and VEGF were also detected in tumor tissues as described in the Western blotting analysis section. The present study was approved by the Ethics Committee of Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine (permit no. 2019421).
Statistical analysis
All statistical analyses were performed using GraphPad Prism software 8.0 (GraphPad Software, Inc.). All values were expressed as means ± SD. Comparisons between two groups were analyzed using unpaired Student's t-test. Furthermore, multiple comparisons were performed using one-way analysis of variance with Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
SaRNA-1480 has the highest functional effect of activating Notch1
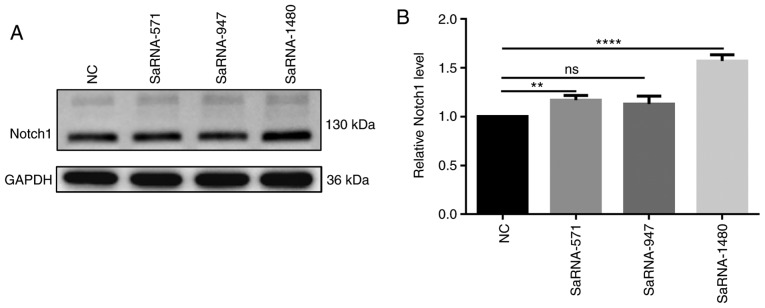
After transfection of saRNA-571, saRNA-947 and saRNA-1480 into PC3 cells, the results of western blotting assay showed the highest expression level of Notch1 in PC3 cells transfected with saRNA-1480 (Fig. 1A and B). Therefore, saRNA-1480 had the highest functional effect of activating Notch1.
Figure 1.
Expression level of Notch 1 for PC3 cells transfected by saRNA-571, saRNA-947 and saRNA-1480 through western blotting. (A) Representative images of the protein blot. (B) Western blot analysis showing the expression levels of Notch1. **P<0.01 and ****P<0.0001. NC, negative control; ns, not significant; sa, small activating.
Expression levels of Notch1, AR, VEGF and metastasis suppressor genes in the PC3 cells after transfection of saRNA
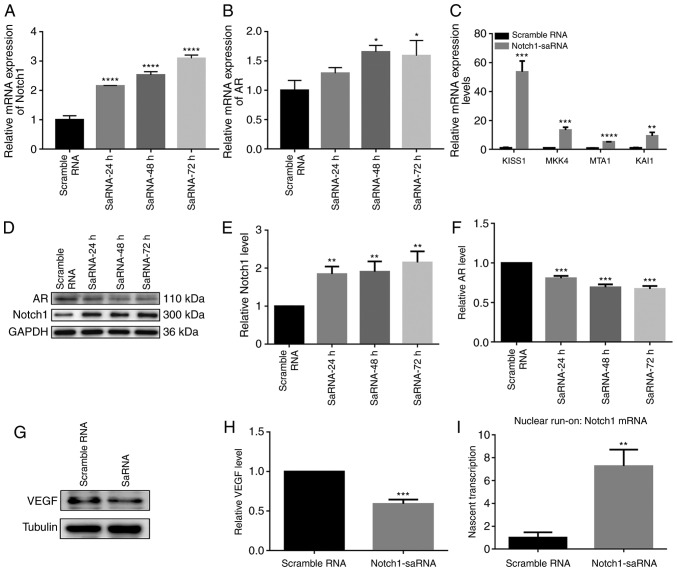
Gene and protein expression after transfection of saRNA in the PC3 cell line is shown in Fig. 2. RT-qPCR results demonstrated that after saRNA transfection, the mRNA expression of Notch1 was upregulated. Notch1 expression increased with increasing duration (Fig. 2A). Previous studies found that AR was expressed in PC3 cells (18,19). Furthermore, the mRNA expression of AR was significantly upregulated by Notch1-saRNA (Fig. 2B). Moreover, compared with the control group, RT-qPCR results showed that metastasis suppressor genes including KISS1, MKK4, MTA1 and KAI1 were upregulated in Fig. 2C. At the protein level, Notch1-saRNA significantly increased the expression levels of AR and Notch1 in Fig. 2D-F. Western blotting analysis depicted a decrease in VEGF expression levels in Notch1-saRNA compared with the scramble RNA (Fig. 2G and H).
Figure 2.
Expression levels of Notch1, AR, VEGF and metastasis suppressor genes with prostate cancer in the PC3 cells after transfection of saRNA. Reverse transcription-quantitative PCR analysis showing the mRNA expression levels of (A) Notch1, (B) AR and (C) metastasis suppressor genes associated with prostate cancer. (D) Western blotting results and analysis showing the expression levels of (E) Notch 1 and (F) AR after transfection. (G) Western blot results and (H) analysis showing the expression level of VEGF after transfection. (I) Quantitative PCR for Notch1 mRNA on nascent transcripts isolated from nuclear run-on after PC3 cells transfected by Notch1-saRNA-1480. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. VEGF, vascular endothelial growth factor; AR, androgen receptor; sa, small activating.
Notch1-saRNA-1480 could activate Notch1 transcription
To determine whether Notch1-saRNA-1480 could activate Notch1 transcription, nascent Notch1 mRNA transcription was measured by a nuclear run-on experiment. After transfection of Notch1-saRNA-1480 in PC3 cells, nascent Notch1 mRNA level was significantly higher compared with the scramble RNA (Fig. 2I), indicating activation of transcription.
Cell apoptosis and cell cycle distribution in the PC3 cells after Notch1-saRNA-1480
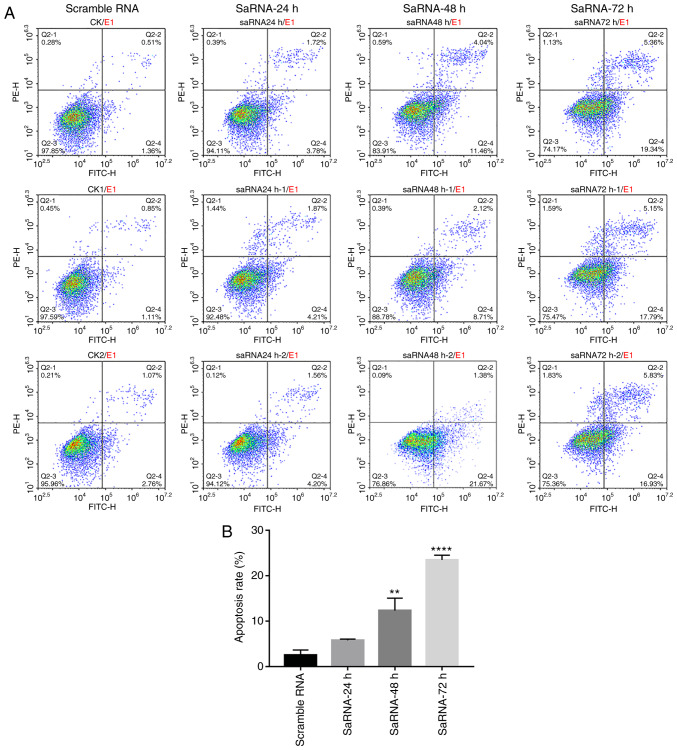
Apoptosis of PC3 cells after transfection of saRNA was analyzed by flow cytometry (Fig. 3A and B). PC3 cells were labeled with PI and Annexin V, and the results showed that Notch1-saRNA-1480 significantly increased the proportion of apoptotic cells after transfection. As the transfection time increased, the proportion of apoptotic cells increased. A total of 24 h after transfection with Notch1-saRNA-1480, the apoptotic rate was 5.78% compared with the control group. The apoptosis rate was 12.35% compared with the control group 48 h after transfection, which was 2.14 times that of the 24-h apoptosis rate. A total of 72 h after transfection, the apoptotic rate was 23.48% compared with the control group, which was 4.06 times the 24-h apoptotic rate.
Figure 3.
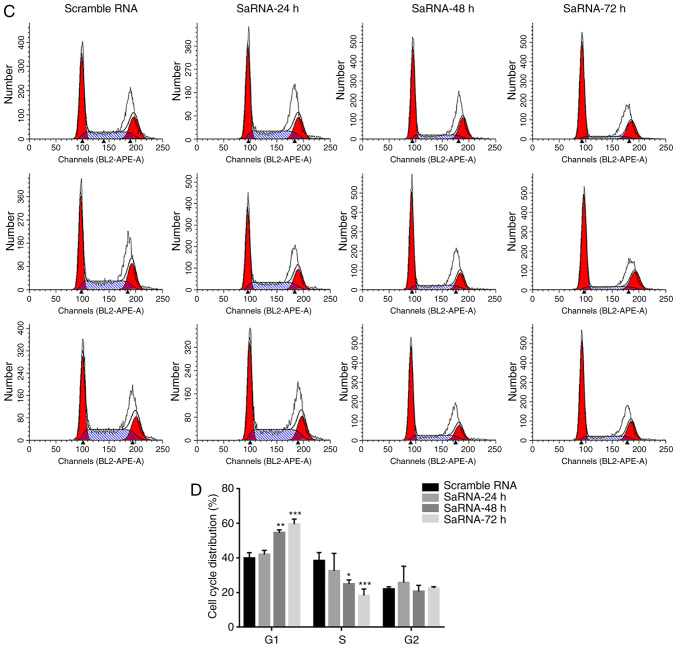
Notch1-saRNA-1480 could promote PC3 cell apoptosis and cell cycle arrest. (A) Flow cytometry data and (B) analysis showed that Notch1-saRNA-1480 significantly promoted PC3 cell apoptosis. (C) Flow cytometry data and (D) analysis showed that Notch1-saRNA-1480 significantly induced PC3 cell cycle arrest. The flow cytometry plots from repeat experiments were shown for each experimental group. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. sa, small activating.
Notch1-saRNA-1480 could affect the cell cycle distribution of PC3 cells. As shown in Fig. 3C and D, the percentage of G0/G1 phase cells in the Notch1-saRNA-1480 treated group increased, and the proportion of S phase cells decreased with the increase of transfection time. The proportion of cells in the G0/G1 phase increased from 39.77% in 24 h to 41.99%, increased to 54.51% in 48 h and increased to 59.45% in 72 h. Correspondingly, the percentage of cells in S phase decreased from 39.36 to 32.4% in 24 h, decreased to 24.93% in 48 h, decreased to 18.14% in 72 h and only 0.47 times in 0 h S phase. This indicated that the cell cycle was arrested at the G0/G1 checkpoint. These results were consistent with previous studies (13,20,21).
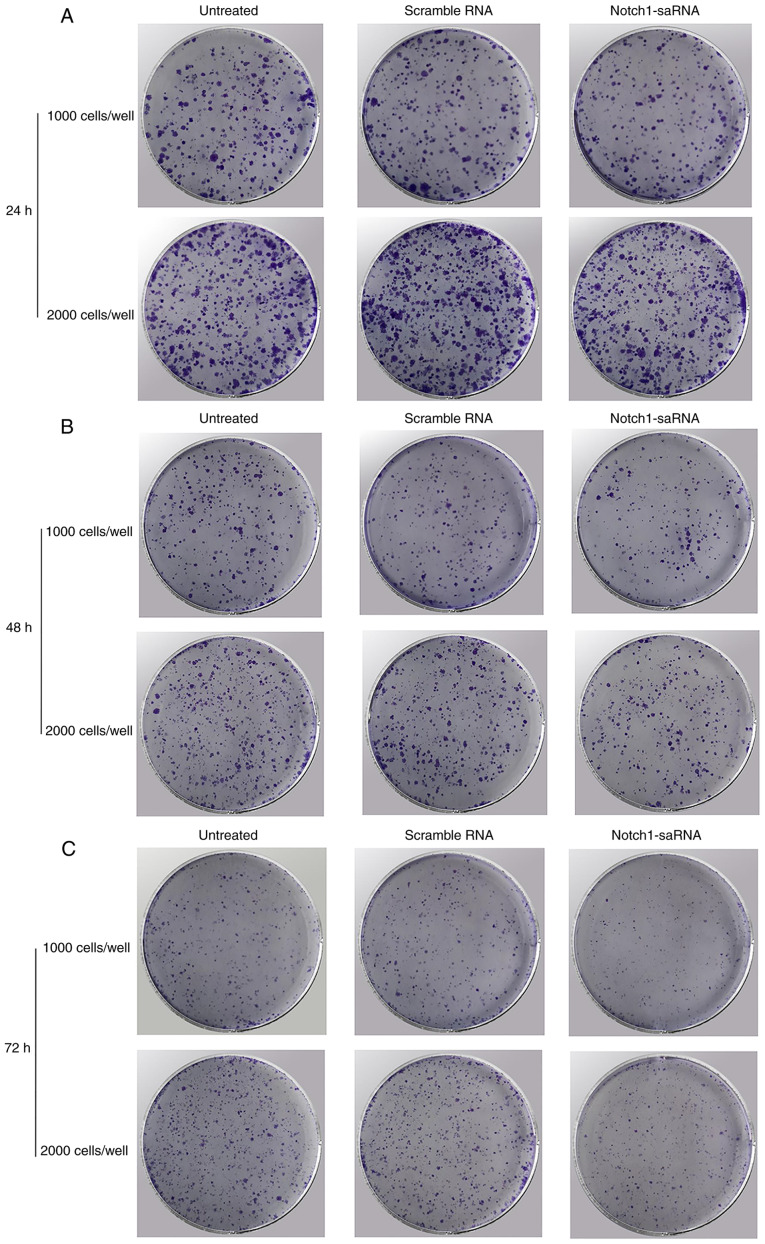
Notch1-saRNA-1480 could inhibit PC3 cell proliferation and colony formation
Then whether Notch1-saRNA-1480 could affect cell proliferation and colony formation was investigated. The results depicted that Notch1-saRNA-1480 could inhibit cell proliferation and colony formation of PC3 cells (Fig. 4A-C). As shown in the Fig. 4D, the growth rate of cells transfected with Notch1-saRNA-1480 was significantly inhibited compared with the control group. The transfected Notch1-saRNA-1480 group showed lower colony formation compared with the control group.
Figure 4.
Notch1-saRNA-1480 could inhibit PC3 cell proliferation and colony formation. Colony formation assays at (A) 24, (B) 48 and (C) 72 h showing the effect of Notch1-saRNA-1480 on PC3 cell proliferation. (D) The growth rate of PC3 cells transfected with Notch1-saRNA-1480. ****P<0.0001. sa, small activating; OD, optical density.
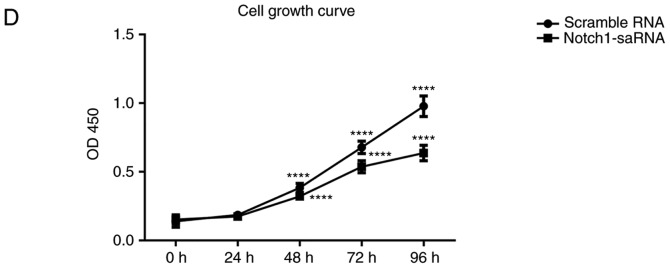
Notch1-saRNA-1480 could inhibit PC3 cell migration, invasion and epithelial-mesenchymal transition (EMT)
To assess the cell migration and invasion ability of PC3 cells after Notch1-saRNA-1480, transwell analysis was performed. After the crystal violet staining, the transwell chamber was imaged and the number of migrated or invaded cells was calculated for significant analysis. The results showed that the ability of PC3 cell migration after Notch1-saRNA-1480 was significantly decreased compared with the control group (Fig. 5A and B). With increasing time, the number of PC3 cell invasion was significantly higher than of that PC3 cells after Notch1-saRNA-1480 (Fig. 5C-F).
Figure 5.
Notch1-saRNA-1480 inhibits PC3 cell migration and invasion. (A) Images and (B) analysis showing that Notch1-saRNA-1480 significantly inhibited the migration ability of PC3 cells after 24 h. (C and D) Notch1-saRNA-1480 significantly inhibited the invasion ability of PC3 cells after 24 h. (E and F) Notch1-saRNA-1480 significantly inhibited the invasion ability of PC3 cells after 48 h. **P<0.01, ***P<0.001 and ****P<0.0001. sa, small activating.
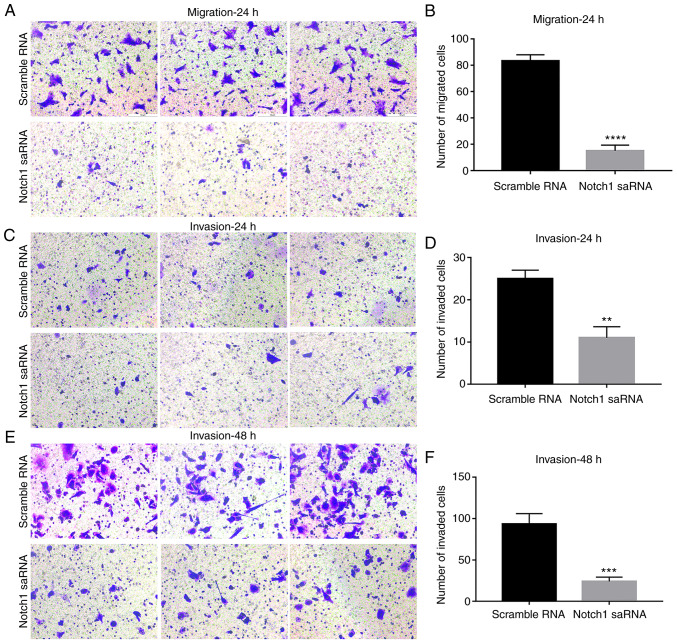
Next, whether Notch1-saRNA-1480 could affect the expression levels of EMT related proteins was observed (Fig. 6A). The results showed that Notch1-saRNA-1480 significantly increased E-cadherin expression (Fig. 6B) and inhibited the expression levels of N-cadherin (Fig. 6C), β-catenin (Fig. 6D), Twist (Fig. 6E) and vimentin (Fig. 6F). Above results indicated that Notch1-saRNA-1480 could inhibit the EMT process of PC3 cells.
Figure 6.
Notch1-saRNA-1480 could mediate the expression levels of EMT related proteins. (A) Western blot analysis was used to examine the expression levels of (B) E-cadherin expression, (C) N-cadherin, (D) β-catenin, (E) Twist and (F) vimentin after transfection of Notch1-saRNA-1480 in PC3 cells. ***P<0.001 and ****P<0.0001. sa, small activating.
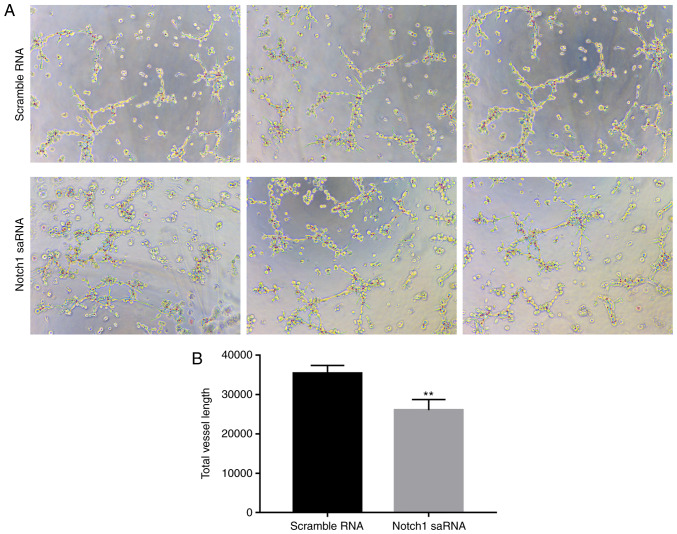
Notch1-saRNA-1480 could inhibit PC3 cell angiogenesis
Then a PC3 cell angiogenesis test was performed. Compared with the control group, total vessel length in PC3 cell after Notch1-saRNA-1480 treatment was decreased. The results indicated that Notch1-saRNA-1480 could inhibit PC3 cell angiogenesis in Fig. 7.
Figure 7.
Notch1-saRNA-1480 could inhibit PC3 cell angiogenesis. (A) Angiogenesis experiments showing the decreased angiogenesis of PC3 cells transfected by Notch1-saRNA-1480. (B) The total vessel length after transfection of Notch1-saRNA-1480 in PC3 cells was significantly decreased. **P<0.01. sa, small activating.
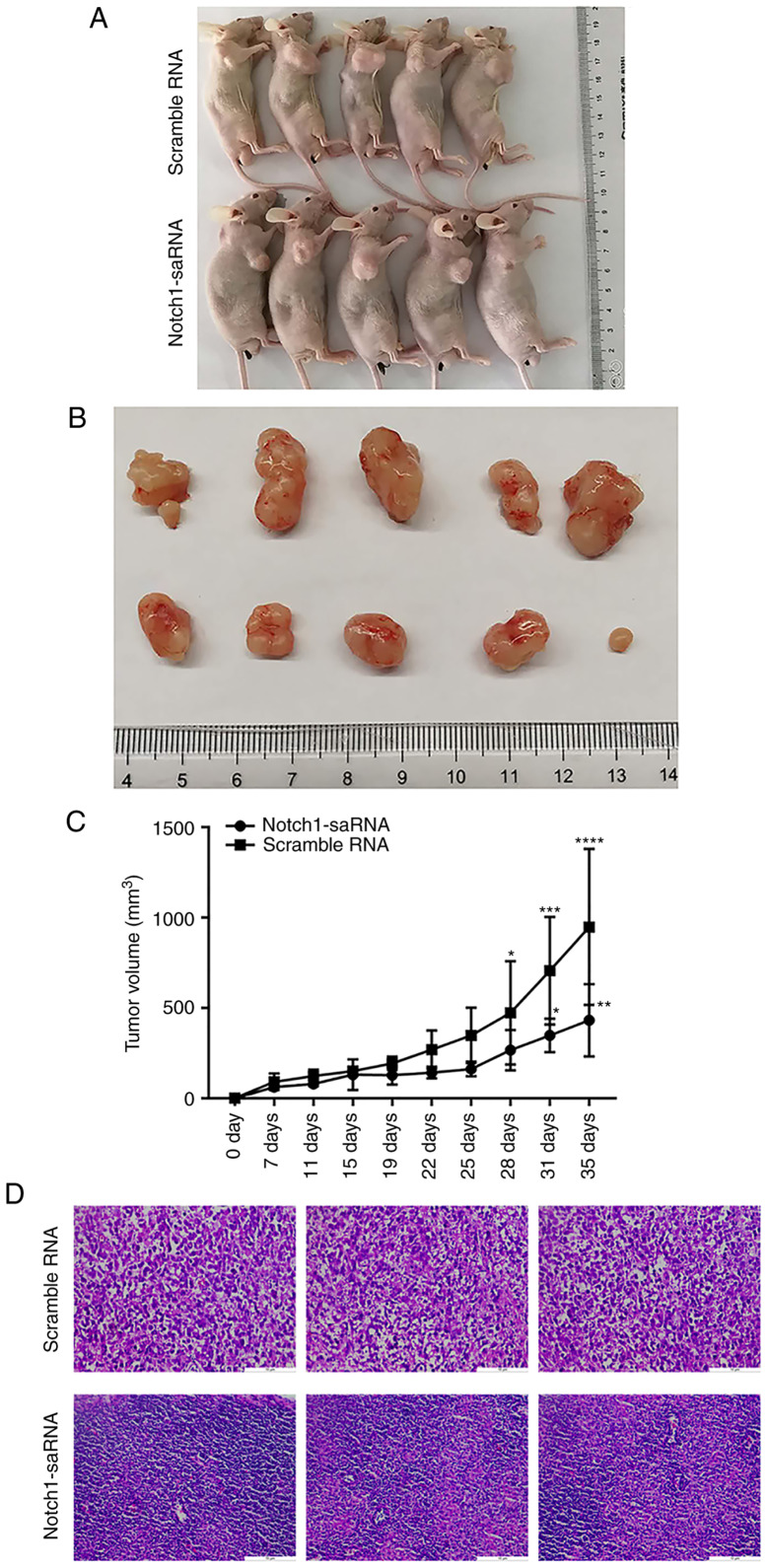
In vivo anti-tumor activity of Notch1-saRNA-1480
A total of 10 nude mice were randomly divided into two groups, including the negative control group and the Notch1-saRNA-1480 group. The administration of the tumor cells was started 4 days after the inoculation and performed by intratumoral injection. It was administered once every 3 days for a total of 5 weeks. The tumor diameter was measured every other day. The nude mice were sacrificed on the 35th day and the tumor tissue was taken out for subsequent experiments. Photographs of mice sacrificed and corresponding tumor mass images are shown in Fig. 8A, and the volume of the tumor is recorded in Fig. 8B. Tumor volume statistics of the Notch1-saRNA-1480 treated group and the control group showed that the tumor diameter of the Notch1-saRNA-1480 treated group was smaller than that of the control group, and there was a significant difference. Additionally, Fig. 8C exhibits the tumor growth curve. Tumor growth in the Notch1-saRNA-1480 treated group was inhibited compared with the control group.
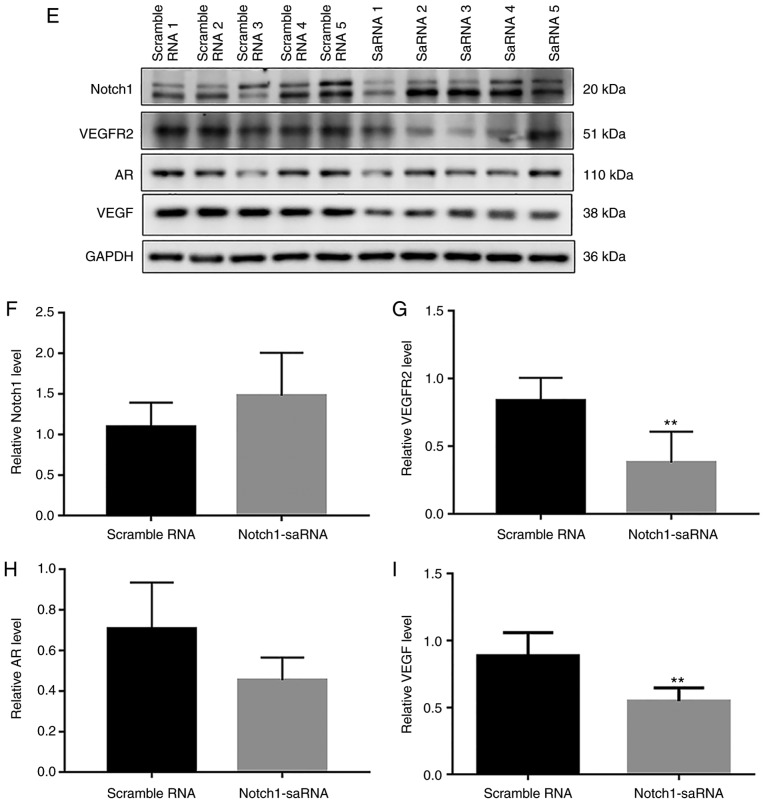
Figure 8.
In vivo anti-tumor activity of Notch1-saRNA-1480. (A) Representative images of nude mice. (B) Representative images of tumors. (C) Tumor growth curves. (D) The internal structure of the tumor by hematoxylin and eosin staining. (E) Western blot results and analysis showing the expression levels of (F) Notch1, (G) VEGFR2, (H) AR and (I) VEGF in tumor tissues. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. sa, small activating; VEGFR, vascular endothelial growth factor receptor; AR, androgen receptor
The results of hematoxylin and eosin staining showed that the tumor cells in the control group were evenly distributed as a whole, arranged in a different order and the nuclei were deeply stained. In the Notch1-saRNA group, there were few tumors and a large number of macrophages appeared (Fig. 8D).
The expression of Notch1, AR and VEGFR2 protein in tumor tissues was detected by western blotting (Fig. 8E). The results showed that the expression of Notch1 was increased in the saRNA group (Fig. 8F). Furthermore, the expression of VEGFR2 and AR proteins in the Notch1-saRNA-1480 treated group was significantly downregulated (Fig. 8G and H). The study also found that Notch1-saRNA significantly inhibited the expression of VEGF (Fig. 8I).
Discussion
Currently, the treatment of prostate cancer includes radiation therapy, surgery and chemotherapy. If treated with conventional chemotherapy, it leads to the resistance and development of androgen-independent prostate cancer, which further complicates the situation. It has been reported that ADT treatment significantly increases the expression of AR in prostate cancer cells (9), and AR reactivation is also observed in recurrent diseases. VEGF and Notch1 have been shown to play important roles in epithelial-mesenchymal transition (EMT) (22). Activation of Notch1 is known to be involved in the development and progression of human malignancies. Emerging evidence suggests that the acquisition of the EMT phenotype is associated with induction of cancer stem cells or cancer stem cell-like phenotypes and contributes to tumor recurrence and drug resistance (23). E-cadherin is one of the important indicators of EMT. Moreover, E-cadherin-saRNA can induce migration and invasion of PC3 cells, which is related to the relocalization of β-catenin from the nucleus to the plasma membrane and β-catenin-mediated transactivation (24). In this experiment, it was found that Notch1-saRNA also had a similar effect, which can inhibit the migration, invasion and EMT process of PC3 cells. The saRNA could activate the related genes in cancer cells during the cell cycle and apoptosis. For example, p21 saRNA could cause the changes in the proliferation of T24 cells in a time- and dose-dependent manner. Furthermore, the same p21 saRNA could induce cell cycle arrest and cell apoptosis in the G1 phase (25). In this experiment, Notch1-saRNA-1480 also induced cell cycle changes and promoted apoptosis.
Notch signaling plays a key role in angiogenesis (26). The activin receptor-like kinase 1 signaling pathway acts in concert with the Notch signaling pathway to inhibit angiogenesis. The Notch signaling pathway together induces the target genes HEY1 and HEY2, thereby inhibiting the VEGF signaling pathway, cell formation and endothelial cell formation (27). One study found that emodin inhibits VEGF expression in PC3 cells via the Notch signaling pathway, thereby reducing angiogenesis (20,28). In the present study, Notch1-saRNA-1480 could induce Notch1 protein expression, which in turn affected cell apoptosis. To investigate the relationship between Notch1 and VEGF, the expression of the VEGF protein was examined. Under the stimulation of Notch1-saRNA-1480, the expression of VEGF decreased and then the angiogenesis experimental results showed that Notch1-saRNA-1480 could inhibit the ability of PC3 cells to form tubes, thereby destroying the formation of blood vessels. The treatment of prostate cancer is linked to vascular targeted therapy and the simultaneous regulation of AR and VEGF is achieved by activation of Notch1 (29,30).
The protein expression of Notch1 was increased 15 days after transfection of Notch1-saRNA-1480 in PC3 cells, which contrasted with the short silencing effect of siRNA. Therefore, the current study concluded that saRNA may act on the promoter region of the target gene. Demethylation of histones is induced, altering chromatin structure and leading to long-term increases in gene expression (31-33). Then in vivo experiments were conducted. Nude mice were injected with Notch1-saRNA-1480 after subcutaneous tumor formation. The experimental results showed that the tumor diameter was reduced. Western blot analysis showed that the expression levels of VEGFR2 and VEGF were both decreased in the tumor tissues. Finally, the anticancer effect of Notch1-saRNA-1480 was successfully validated on xenograft animal models. Based on this, the next step is to focus on clinically relevant studies to investigate the effects of Notch-saRNA-1480 on prostate cancer. The present study believes this is an important step in the future possible application in humans.
Notch1-saRNA-1480 could significantly inhibit the proliferation of PC3 cells in vitro and the growth of tumor in vivo, which is related to the inhibition of AR and VEGF pathways. However, the current study lacks analysis on the expression of Notch1-saRNA-1480 and clinicopathological features in patients with prostate cancer. Thus, the next step is to perform clinically relevant studies to investigate the effects of Notch-saRNA-1480 on prostate cancer.
Acknowledgments
Not applicable.
Abbreviations
- CRPC
castration-resistant prostate cancer
- AR
androgen receptor
- RT-qPCR
reverse transcription-quantitative PCR
- VEGF
vascular endothelial growth factor
- saRNA
small activating RNA
Funding
The current study was funded by the Zhejiang Province Natural Science Foundation (grant nos. LY17H050002 and Y2111329); Zhejiang Province Science and Technology Plan Projects (grant no. 2014C37016); Zhejiang Province Chinese Medicine Science and Technology Plan Projects (grant nos. 2016ZB099, 2013ZA107, 2011ZB099 and 2020ZA087); Zhejiang Province Medicine and Health Science and Technology Plan Projects (grant nos. 2011KYB066 and 2015KYB295); Hangzhou Science and Technology Plan Projects (grant nos. 2017A05, 2018A09, 20110833B05 and 20110733Q12).
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
GD, LM conceived and designed the study. KJ, PJ conducted most of the experiments and data analysis, and wrote the manuscript. HH, KC and JS participated in data acquisition and helped to draft the manuscript. All authors reviewed and approved the manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine (permit no. 2019421).
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Filson CP, Marks LS, Litwin MS. Expectant management for men with early stage prostate cancer. CA Cancer J Clin. 2015;65:265–282. doi: 10.3322/caac.21278. [DOI] [PubMed] [Google Scholar]
- 3.Chen WY, Tsai YC, Yeh HL, Suau F, Jiang KC, Shao AN, Huang J, Liu YN. Loss of SPDEF and gain of TGFBI activity after androgen deprivation therapy promote EMT and bone metastasis of prostate cancer. Sci Signal. 2017;10:eaam6826. doi: 10.1126/scisignal.aam6826. [DOI] [PubMed] [Google Scholar]
- 4.Stoyanova T, Riedinger M, Lin S, Faltermeier CM, Smith BA, Zhang KX, Going CC, Goldstein AS, Lee JK, Drake JM, et al. Activation of Notch1 synergizes with multiple pathways in promoting castration-resistant prostate cancer. Proc Natl Acad Sci USA. 2016;113:E6457–E6466. doi: 10.1073/pnas.1614529113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho FL, Simons BW, Eberhart CG, Berman DM. Notch signaling in prostate cancer: A moving target. Prostate. 2014;74:933–945. doi: 10.1002/pros.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng G, Ma L, Meng Q, Ju X, Jiang K, Jiang P, Yu Z. Notch signaling in the prostate: Critical roles during development and in the hallmarks of prostate cancer biology. J Cancer Res Clin Oncol. 2016;142:531–547. doi: 10.1007/s00432-015-1946-x. [DOI] [PubMed] [Google Scholar]
- 7.Nabbi A, McClurg UL, Thalappilly S, Almami A, Mobahat M, Bismar TA, Binda O, Riabowol KT. ING3 promotes prostate cancer growth by activating the androgen receptor. BMC Med. 2017;15:103. doi: 10.1186/s12916-017-0854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamoto H, Meming EM, Chang C. Androgen deprivation therapy for prostate cancer: Current status and future prospects. Prostate. 2004;61:332–353. doi: 10.1002/pros.20115. [DOI] [PubMed] [Google Scholar]
- 9.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Wu X, Ou L, Yang X, Wang X, Tang M, Chen E, Luo C. PLCε knockdown inhibits prostate cancer cell proliferation via suppression of Notch signalling and nuclear translocation of the androgen receptor. Cancer Lett. 2015;362:61–69. doi: 10.1016/j.canlet.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Belandia B, Powell SM, García-Pedrero JM, Walker MM, Bevan CL, Parker MG. Hey1, a mediator of notch signaling, is an androgen receptor corepressor. Mol Cell Biol. 2005;25:1425–1436. doi: 10.1128/MCB.25.4.1425-1436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadick H, Naim R, Gössler U, Hörmann K, Riedel F. Angiogenesis in hereditary hemorrhagic telangiectasia: VEGF165 plasma concentration in correlation to the VEGF expression and microvessel density. Int J Mol Med. 2005;15:15–19. [PubMed] [Google Scholar]
- 13.Shou J, Ross S, Koeppen H, de Sauvage FJ, Gao WQ. Dynamics of notch expression during murine prostate development and tumorigenesis. Cancer Res. 2001;61:7291–7297. [PubMed] [Google Scholar]
- 14.D'Amore PA, Ng YS. Won't you be my neighbor? Local induction of arteriogenesis. Cell. 2002;110:289–292. doi: 10.1016/S0092-8674(02)00869-3. [DOI] [PubMed] [Google Scholar]
- 15.Siekmann AF, Covassin L, Lawson ND. Modulation of VEGF signalling output by the Notch pathway. Bioessays. 2008;30:303–313. doi: 10.1002/bies.20736. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Roberts TC, Hart JR, Kaikkonen MU, Weinberg MS, Vogt PK, Morris KV. Quantification of nascent transcription by bromouridine immunocapture nuclear run-on RT-qPCR. Nat Protoc. 2015;10:1198–1211. doi: 10.1038/nprot.2015.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alimirah F1, Chen J, Basrawala Z, Xin H, Choubey D. DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: Implications for the androgen receptor functions and regulation. FEBS Lett. 2006;580:2294–2300. doi: 10.1016/j.febslet.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 19.Davis R, Jia D, Cinar B, Sikka SC, Moparty K, Zhau HE, Chung LW, Agrawal KC, Abdel-Mageed AB. Functional androgen receptor confers sensitization of androgen-independent prostate cancer cells to anticancer therapy via caspase activation. Biochem Biophys Res Commun. 2003;309:937–945. doi: 10.1016/j.bbrc.2003.08.096. [DOI] [PubMed] [Google Scholar]
- 20.Deng G, Ju X, Meng Q, Yu ZJ, Ma LB. Emodin inhibits the proliferation of PC3 prostate cancer cells in vitro via the Notch signaling pathway. Mol Med Rep. 2015;12:4427–4433. doi: 10.3892/mmr.2015.3923. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Sha J, Yang G, Huang X, Bo J, Huang Y. Activation of Notch pathway is linked with epithelial-mesenchymal transition in prostate cancer cells. Cell Cycle. 2017;16:999–1007. doi: 10.1080/15384101.2017.1312237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang JH, Wylie-Sears J, Bischoff J. Opposing actions of Notch1 and VEGF in post-natal cardiac valve endothelial cells. Biochem Biophys Res Commun. 2008;374:512–516. doi: 10.1016/j.bbrc.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad A, Banerjee S, Azmi AS, Miele L, Sarkar FH. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307:26–36. doi: 10.1016/j.canlet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Mao Q, Zheng X, Yang K, Qin J, Bai Y, Jia X, Li Y, Xie L. Suppression of migration and invasion of PC3 prostate cancer cell line via activating E-cadherin expression by small activating RNA. Cancer Invest. 2010;28:1013–1018. doi: 10.3109/07357900802620844. [DOI] [PubMed] [Google Scholar]
- 25.Yang K, Zheng XY, Qin J, Wang YB, Bai Y, Mao QQ, Wan Q, Wu ZM, Xie LP. Up-regulation of p21WAF1/Cip1 by saRNA induces G1-phase arrest and apoptosis in T24 human bladder cancer cells. Cancer Lett. 2008;265:206–214. doi: 10.1016/j.canlet.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: Implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larrivée B, Prahst C, Gordon E, del Toro R, Mathivet T, Duarte A, Simons M, Eichmann A. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell. 2012;22:489–500. doi: 10.1016/j.devcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Wang C, Zhang Z, Chen X, Jia Y, Wang B, Kong T. Curcumin inhibits the survival and metastasis of prostate cancer cells via the Notch-1 signaling pathway. APMIS. 2017;125:134–140. doi: 10.1111/apm.12650. [DOI] [PubMed] [Google Scholar]
- 29.Sahara M, Hansson EM, Wernet O, Lui KO, Später D, Chien KR. Manipulation of a VEGF-Notch signaling circuit drives formation of functional vascular endothelial progenitors from human pluripotent stem cells. Cell Res. 2015;25:148. doi: 10.1038/cr.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zang M, Hu L, Zhang B, Zhu Z, Li J, Zhu Z, Yan M, Liu B. Luteolin suppresses angiogenesis and vasculogenic mimicry formation through inhibiting Notch1-VEGF signaling in gastric cancer. Biochem Biophys Res Commun. 2017;490:913–919. doi: 10.1016/j.bbrc.2017.06.140. [DOI] [PubMed] [Google Scholar]
- 31.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 32.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Place RF, Jia ZJ, Pookot D, Dahiya R, Li LC. Antitumor effect of dsRNA-induced p21(WAF1/CIP1) gene activation in human bladder cancer cells. Mol Cancer Ther. 2008;7:698–703. doi: 10.1158/1535-7163.MCT-07-2312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.