Abstract
Alginate hydrogels have been widely validated for controlled release of growth factors and cytokines, but studies exploring sustained release of small hydrophobic lipids are lacking. Sphingosine-1-phosphate (S1P), a bioactive lipid, is an appealing small molecule for inducing blood vessel formation in the context of ischemic conditions. However, there are numerous biological and engineering challenges associated with designing biomaterial systems for controlled release of this lipid. Thus, the objective of this study was to design an injectable, alginate hydrogel formulation that provides controlled release of S1P to establish locally sustained concentration gradients that promote neovascularization. Herein, we varied the molecular weight distribution of alginate polymers within the hydrogel to alter the resultant mechanical properties in a manner that provides control over S1P release. With increasing high molecular weight (HMW) content, the hydrogels exhibited stiffer material properties and released S1P at slower rates. Accordingly, S1P released from hydrogels with 100% HMW content led to enhanced directed migration of outgrowth endothelial cells (OECs) and blood vessel development assessed using a chick chorioallantoic membrane (CAM) assay as compared to hydrogels with less HMW content. Overall, this study describes how alginate hydrogels of varied molecular weight may be used to control S1P release kinetics for therapeutic applications.
Keywords: endothelial cell migration, chick chorioallantoic membrane (CAM) assay, neovascularization, polymeric biomaterials, lipid delivery
INTRODUCTION
Polymeric biomaterials are widely used as delivery vehicles for controlled presentation of proangiogenic factors in promotion of neovascularization via therapeutic angiogenesis.1,2 Previous work in this field has centered attention on the delivery of growth factors and cytokines, such as vascular endothelial growth factor (VEGF) and stromal cell-derived factor-1 (SDF-1), which play critical roles in regulating tissue homeostasis and regeneration.1–3 However, recombinant proteins can be expensive to produce and results thus far have been underwhelming.3–5 In alternative, enthusiasm regarding the pursuit of pharmacological strategies involving small molecule lipid mediators, such as sphingosine-1-phosphate (S1P), has thus exhibited a prominent increase due to their wide range of effects over multiple cell types and ease of scale-up in manufacturing.4 Therefore, the development of strategies involving the design of biomaterial systems for lipid delivery presents a growing field of research.
The ability to develop delivery vehicles that control sustained presentation of S1P is particularly important, as the lipid’s function is highly dependent upon extensively regulated, endogenous concentration gradients.6 However, research efforts to present S1P in a controlled fashion have thus far been limited in part due to difficult challenges associated with the lipid’s small size, strong hydrophobicity, and rapid metabolic turnover.4,6,7 Thus, there is a growing need for a better understanding of how these material systems may be formulated and tuned to control bioactive lipid release.
Alginate hydrogels are broadly used carriers in controlled delivery systems for therapeutic applications given their low toxicity, strong biocompatibility, ease of tuning, and minimally invasive delivery.8–10 Alginate is an anionic copolymer derived from brown seaweed containing blocks of (1,4)-linked β-D-mannuronate (M) and α-L-guluronate (G) residues.11 The M and G block contents and proportions differ depending on the source the alginate is extracted from and more than 200 different alginates are currently being manufactured.12 The G blocks of alginate polymers may be ionically crosslinked via mild gelation with the addition of divalent cations such as Ca2+.11 Alginate is a particularly attractive biomaterial for the delivery of therapeutic factors as it provides a highly tunable gelation scheme that may be altered to control the physical properties of the hydrogels and the rates of payload release.11 The mechanical properties of alginate gels could be enhanced by increasing G block content and the overall molecular weights of the polymer chains.11,12 Importantly, alginate hydrogels can be assigned specific rates of factor release by controlling the molecular weight distribution of alginate polymers within the hydrogel.2,13 Indeed, injectable alginate hydrogels have been designed for controlling sustained release of several proangiogenic factors, including but not limited to VEGF,2,14–16 PDGF-BB,14 SDF-1,15 and lentivectors.13 However, it is unclear whether this interdependency between altered mechanical properties and release holds true for smaller molecules and lipids.
This work tests the hypothesis that varying the molecular weight distribution within alginate hydrogels may provide a means to control and tune lipid release kinetics in a manner that is favorable for promoting neovascularization. Alginate hydrogels of varying molecular weight distribution were formulated, lipid release was measured over time, and the subsequent potential to induce therapeutic angiogenesis was tested using a variety of in vitro and in vivo assays. In particular, outgrowth endothelial cell (OEC) sprouting and directed migration/invasion under hypoxic conditions in vitro and blood vessel development in a chick chorioallantoic membrane (CAM) assay in vivo were assayed to identify the material formulation with the greatest potential for therapeutic efficacy.
MATERIALS AND METHODS
Hydrogel Formulation
LF20/40 polymer containing a higher G-block content (> 60% as specified by the manufacturer; MVG; Novamatrix) was used as the high molecular weight (HMW) component to prepare gels. LF10/60 polymer was used as the low molecular weight (LMW) component unless otherwise specified. Ultra-pure (UP) MVG alginate polymer was used for in vivo and in vitro assays involving cells, as well as for S1P release studies for consistency. UP MVG LMW alginate was prepared by gamma (γ)-irradiating the HMW UP MVG alginate as previously described.2,16 All alginates were oxidized with sodium periodate (Sigma) to an extension of 1% of the sugar residues in the polymer as previously described.17 The oxidized alginate solutions were then dialyzed, sterile filtered, lyophilized, and stored at −20°C until use.
To prepare bimodal (i.e. varied molecular weight) alginate hydrogels, modified alginates were reconstituted to 2% w/v in phosphate buffered saline containing calcium and magnesium ions (PBS++; Life Technologies). Combinations of LMW and HMW alginate solutions were then mixed together to create final hydrogels with varied bimodal distributions. The solutions were then cross-linked with calcium sulfate slurry (0.21 g/mL CaSO4 in water; Sigma) such that the ratio between calcium and the carboxyl units of the alginate polymer chains was maintained at 0.6. Herein, a syringe containing 2% LMW alginate solution was connected to a syringe containing 2% HMW alginate solution via a syringe connector and the contents were mixed together. The syringes were then disconnected, calcium sulfate slurry was added for crosslinking, and the syringes were then reconnected and mixed. All contents were then collected in one syringe and the mixture was dispensed onto a glass plate set with 1-mm spacers, sandwiched with another glass plate, and incubated for at least 25 minutes at room temperature to ensure full gelation. Hydrogel disks were then cut using a 10-mm biopsy punch (Acuderm Inc.). For S1P-loaded hydrogels, 10 μL S1P (stock reconstituted at 1 mM in methanol; Tocris) was added per mL of alginate prior to mixing the two solutions together. For blank hydrogels, methanol was incorporated as a carrier control. Hydrogels containing 75% LMW and 25% HMW (75/25) were used as the blank control for all experiments. A new set of hydrogels was prepared for each experiment.
Mechanical characterization of bimodal alginate hydrogels
The equilibrium swelling ratios were determined as previously described.7 Herein, hydrogel disks were cast, swelled in PBS++ for 24 hours, and the mass of the swollen gel (Ws) was determined after removing excess liquid with a damp kimwipe. The gels were then frozen and lyophilized, and the dry weight (Wd) was measured. The swelling ratio (Q) was defined as Q following Equation 1,
| (Eqn. 1) |
The storage moduli (G’) of the hydrogels were determined as previously described.7 Briefly, the gel disks were swollen in PBS++ overnight, pressed with a damp kimwipe to remove excess fluid, trimmed to a diameter of 8 mm using a biopsy punch (Miltex), and placed between parallel plates (gap distance set at 0.9 mm) in a rheometer (HR2, TA Instruments). The gels were then strained over a range within 0.4–5% at a frequency of 10 rad/sec. An average G’ value was reported for each hydrogel condition using at least 9 points within the linear viscoelastic region for each disk.
The initial mesh sizes (ξ) of the hydrogel networks were calculated as previously described using the measured values of storage moduli and swelling ratios at 24h.7,18–20 In particular, the molecular weight between crosslinks (Mc) was determined using Equation 2,
| (Eqn. 2) |
where cp is the total concentration of the polymer solution, R is the gas constant (8.314 m3·Pa·mol−1·K−1), and T is the temperature at which the measurement was performed (296.15 K). The polymer volume fraction (v2) was determined using Equation 3,
| (Eqn. 3) |
where ρp is the density of the polymer (1.601 g·cm−3)18 and ρ is the density of water (1.0 g·cm−3). The mesh size was then calculated as shown in Equation 4,
| (Eqn. 4) |
where Mr is the molecular weight of the monomer units (194 g/mol), L is the carbon-carbon bond length of the monomer unit (5.15 Å), and Cn is the characteristic ratio (Cn = 0.021Mn + 17.95 calculated as for alginate).18,21
To measure dissolution of the hydrogels over time, gel disks were first cast, immersed in sterile PBS++, and incubated at 37°C until collected at the indicated time points. For initial 0h measurements, disks were immediately collected and not immersed in PBS++. To obtain a dry weight for each disk, the collected disks were frozen overnight, lyophilized, and weighed. The percent loss of dry weight over time was then calculated as compared to the initial dry weight of the gels.
Lipid release from bimodal alginate hydrogels
Hydrogels were loaded with octadecyl rhodamine B chloride (R18; 1 mM stock in dimethyl sulfoxide; ThermoFisher). Hydrogel disks were cast as previously described (1mm in height, 10mm in diameter), immersed in 1mL of PBS++, incubated at 37°C, and samples were collected at various time points (3 and 16h, 1, 3, 7, 19, 28, and 61 days) for quantification via fluorescence detection using a plate reader (Spectramax i3, Molecular Devices). At each time point, collected eluent was replaced with fresh PBS++ and incubated at 37°C until the next time point.
For measurement of S1P release, hydrogels were incorporated with radiolabeled S1P33 (1:3 ratio of S1P33:S1P) and release was quantified via liquid scintillation counting as previously described.7 Herein, 0.5mL of hydrogel was dispensed onto the bottom of each liquid scintillation vial and allowed to gel for 30 minutes. The gels were then topped with 2 mL of eluent buffer consisting of PBS++ with 5% fatty acid free bovine serum albumin (FAF-BSA; Gemini Bio Products) and incubated at 37°C. At each time point (3, 12, 24, and 72 hours, and 1 and 2 weeks), the entire eluent buffer was collected and then replaced with fresh buffer. Prior to reading on the liquid scintillation counter (Packard), 1 mL of collected eluent buffer was reacted with 3 mL of scintillation fluid (EcoLite+). Samples taken from blank hydrogels were used to subtract background readings and a mock solution was used to calibrate the amount of R18 or S1P present at each time point.
Isolation and culture of OECs
Human umbilical cord blood was obtained from the UC Davis Umbilical Cord Blood Collection Program (UCBCP) for isolation of OECs. Cells were isolated from female cord blood within 12 hours of collection following protocols as previously described.7,17,22 Colonies of OECs appeared after 7 to 21 days of culture. OECs were between P3-P4 for all experiments.
Endothelial Cell Growth Media −2 Microvascular (EGM-2MV) was prepared as instructed by the vendor (Lonza). Cells were cultured in EGM-2MV under ambient oxygen tension until stimulation with S1P was provided at which point N media, defined as EGM-2MV without the addition of growth factors, was used instead. For culture under low oxygen, cells were put in a hermetically sealed, modular incubator chamber (Billups-Rothenberg) widely used for hypoxic studies.5,7,23 The chamber was flushed with a medical grade 1%-O2, 5%-CO2, 94%-N2 gas mixture for three minutes at 30–40 L/min to establish hypoxia according to the manufacturer’s instructions. A plastic petri dish containing 10 mL of sterile water was placed on the chamber bottom to maintain humidity. The chamber was then flushed again every 24h to assure maintenance of hypoxia.
OEC angiogenesis in response to released S1P in vitro
A modified sprouting assay was used to analyze the 3D sprout formation by OECs in response to S1P released from the hydrogels of interest as previously described in detail.7 Briefly, microcarrier beads coated with OECs were first embedded within fibrin gels as previously described.2,7,17 Herein, cell-laden beads suspended in N media were combined with fibrinogen (Sigma) solution supplemented with aprotinin (Sigma) and distributed in 24-well plates. A second solution containing thrombin (Sigma) was then added at a 4:5 ratio and the plates were incubated at 37°C for 30 minutes to allow for fibrin gelation. The rightmost third of the fibrin gel was then gently aspirated and 50 μL of alginate hydrogel loaded either with or without S1P was dispensed through a needle (18G; BD) into the newly made space. All alginate hydrogels were first crosslinked within the syringe (~30 minutes) and blank 75/25 hydrogels served as the negative control. The dispensed alginate hydrogel was then covered with blank pre-mixed fibrin solution and incubated at 37°C for 30 minutes to allow for full gelation to seal all air gaps. The sealed gels were then topped with 0.5 mL of N media and cultured under hypoxia for 4 days. The average number of sprouts per bead was calculated as previously described and normalized to the average value for the negative control.5
OEC directed migration and 3D matrix invasion in response to released S1P
A modified Transwell chemotaxis assay was used as previously described.5,7 Briefly, hydrogel disks loaded either with or without S1P were prepared and placed at the bottom of each well in a 24-well plate. The disks were then covered with fibrin gel solution and incubated at 37°C for 40 minutes to complete gelation. The gels were then topped with 0.5 mL of N media, a Transwell insert (5 μm pores; polycarbonate; Corning Inc.) was placed in slight contact with the fibrin gel, and 30,000 cells (labeled with Hoechst 33342) in 0.1 mL of N media were seeded within the insert. The plates were then incubated under hypoxia for 4 days without media changes. In order to report an average degree of directed migration through the Transwell insert membrane, the inserts were removed, a cotton swab was used to remove any non-migrated cells in the top chamber, and the number of migrated cells was quantified in at least ten representative 20X fields of view (FOV; Nikon Eclipse TE2000-S microscope). In order to assess the degree of 3D fibrin matrix invasion, each gel was fixed in 4% formaldehyde at 4°C overnight. The fixed gel was then removed from the well and sandwiched between two glass slides (VWR) prior to fluorescent imaging. The total number of cells was manually quantified using ImageJ (NIH).
CAM assay to assess physiological blood vessel development
The physiological response to released S1P was assessed in vivo via a modified open-shell CAM assay.7,24 Fertilized hy-line white leghorn chicken eggs (E0) were purchased from the UC Davis Avian Facility. The eggs were placed in a vertical position and incubated at 37.8°C for 3 days with 55–65% humidity and six rotations per day. After three days, the eggs (E3) were positioned horizontally, cracked open into square weigh boats (Fisher Scientific) under a biosafety cabinet, and the embryos were subsequently incubated at 37.8°C with 90% humidity. Hydrogel disks were prepared as previously described. On day 10 (E10), either blank (negative control; 75/25) or S1P-loaded hydrogel disks (75/25, 50/50, 0/100) were placed on the CAM in areas void of any major blood vessels. Regional pictures of each hydrogel were taken with a 12-megapixel camera (Galaxy S7 Edge; Samsung Inc.) at 0h and after 24h of incubation. The number of blood vessels surrounding the gel within a 2 mm radius was then quantified as previously described in order to assess the angiogenic response.7 The percent change in the number of blood vessels after 24h was then calculated for each CAM and the percent difference from the blank control was reported as an average for each hydrogel condition.
Statistical Analysis
Data are presented as mean ± standard deviation (SD). Comparisons were assessed by one-way ANOVA followed by Tukey’s post hoc test to correct for multiple comparisons. Differences between conditions were considered statistically significant if P < 0.05. All analyses were performed using GraphPad Prism software (GraphPad Software Inc.).
RESULTS
Mechanical characterization of bimodal alginate hydrogels
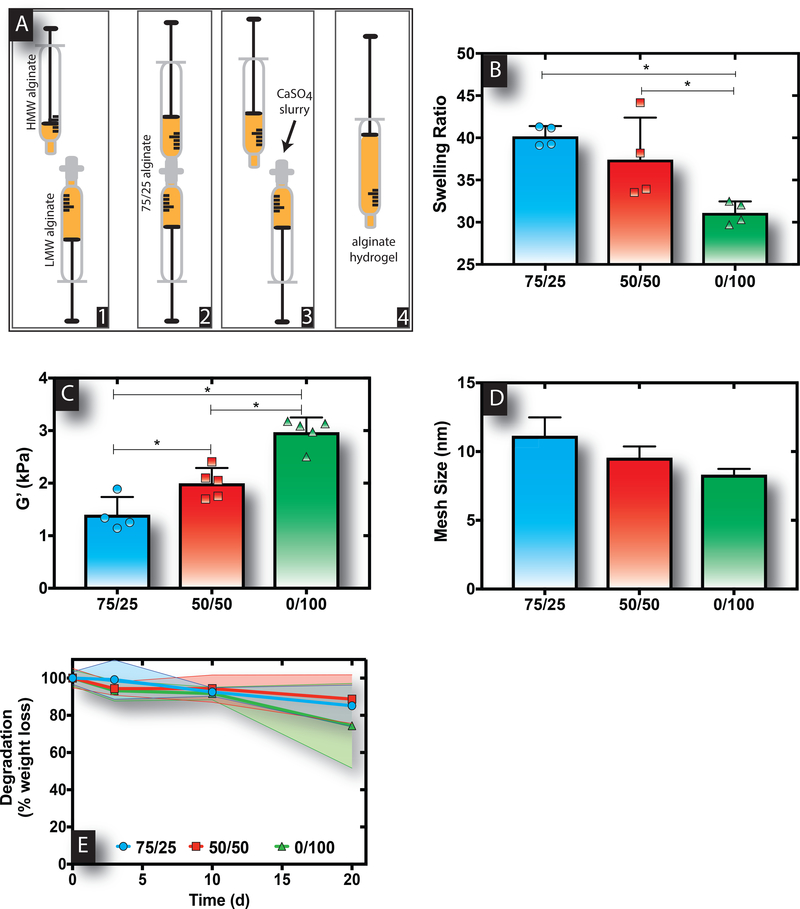
Alginate hydrogels of varying molecular weight composition were formed to create hydrogels with varied mechanical properties following procedures as exampled in Figure 1A. The swelling ratio of bimodal alginate hydrogels was decreased as greater HMW content was introduced within the hydrogel formulation (Figure 1B). Correspondingly, the storage modulus (G’) was significantly increased, wherein 0/100 gels were roughly two times stiffer than 75/25 gels (Figure 1C). Similarly, the calculated mesh size (ξ) exhibited a decreasing trend with increasing HMW content (Table 1 and Figure 1D). Finally, alginate hydrogels of varying molecular weight distributions exhibited comparable rates of degradation assessed via dry weight loss, with roughly 10–20% loss within 3 weeks (Figure 1E).
Figure 1. Alginate hydrogels with greater proportions of HMW polymer content exhibit stiffer material properties.
A schematic of the process followed to form alginate hydrogels of varied molecular weight is provided as an example for 75/25 hydrogels (A). Swelling ratios (B) and storage moduli (C) decreased and increased, respectively, for alginate hydrogels with increasing HMW content. Calculated mesh sizes for the various bimodal alginate hydrogels decreased with increasing HMW content (D). Dissolution of the various bimodal alginate hydrogels over 3 weeks (E). Bar represents mean, scatter dot plots display individual measurements and error bars represent standard deviation (B-D, n=4–5). For B and C, asterisks indicate statistically significant differences (P < 0.05) between conditions indicated by capped bar. Data represent mean ± SD (E; n=4).
Table 1.
Formulations for 1-mL Bimodal Alginate Hydrogels and Corresponding Mesh Sizes
| LMW/HMW | Hydrogel Formulation | Mesh Size (nm) | ||
|---|---|---|---|---|
| 2% LMW Solution (mL) | 2% HMW Solution (mL) | 0.21 g/mL CaSO4 Slurry (μL) | ||
| 75/25 | 0.75 | 0.25 | 40 | 11.2±1.3 |
| 50/50 | 0.5 | 0.5 | 40 | 9.6±0.8 |
| 0/100 | – | 1 | 40 | 8.3±0.4 |
Controlled lipid release from bimodal alginate hydrogels
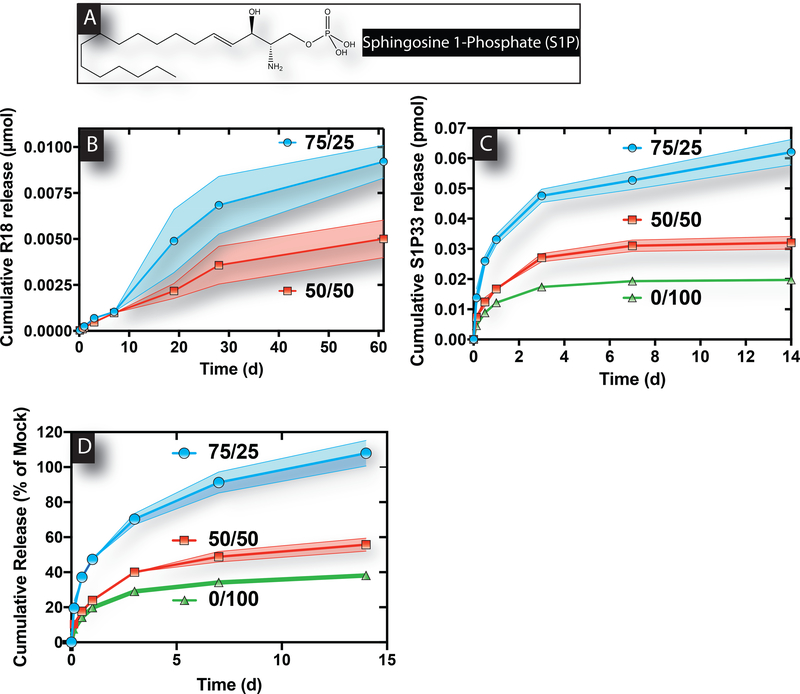
The ability of the bimodal alginate hydrogels to serve as an injectable system for hydrophobic lipid delivery in a localized manner was next examined. The hydrophobic structure of S1P is shown in Figure 2A. Importantly, modifying the molecular weight distributions of alginate hydrogels allowed for sustained and tunable release of both lipids, R18 and S1P33, in vitro. Release of R18 from 75/25 and 50/50 hydrogels was measured as a similar lipid substitute for S1P with comparable molecular weight and hydrophobicity. Interestingly, 50/50 alginate hydrogels displayed retarded R18 release over the course of 2 months compared to 75/25 alginate hydrogels (Figure 2B). Additionally, sustained release of S1P33 was further delayed by increased HMW content within the bimodal alginate hydrogel (Figure 2C–D). Specifically, 0/100 gels resulted in approximately 3.1- and 1.6-fold less cumulative release of S1P33 than 75/25 gels after 2 weeks. This delayed release is in accordance with the stiffer material properties (lower swelling ratios and greater G’) of bimodal alginate hydrogels containing greater HMW content.
Figure 2. Greater HMW alginate composition within bimodal alginate hydrogels results in slower lipid release.
The chemical structure of the hydrophobic lipid, S1P (A). Release of R18 from 75/25 hydrogels was faster than that from 50/50 hydrogels over time (B). Measured S1P33 release from 75/25, 50/50, and 0/100 alginate hydrogels over the course of 2 weeks showed that greater HMW content resulted in slower release (C-D). Data represent mean ± SD (n=4).
Angiogenic response to locally generated gradients of S1P in vitro
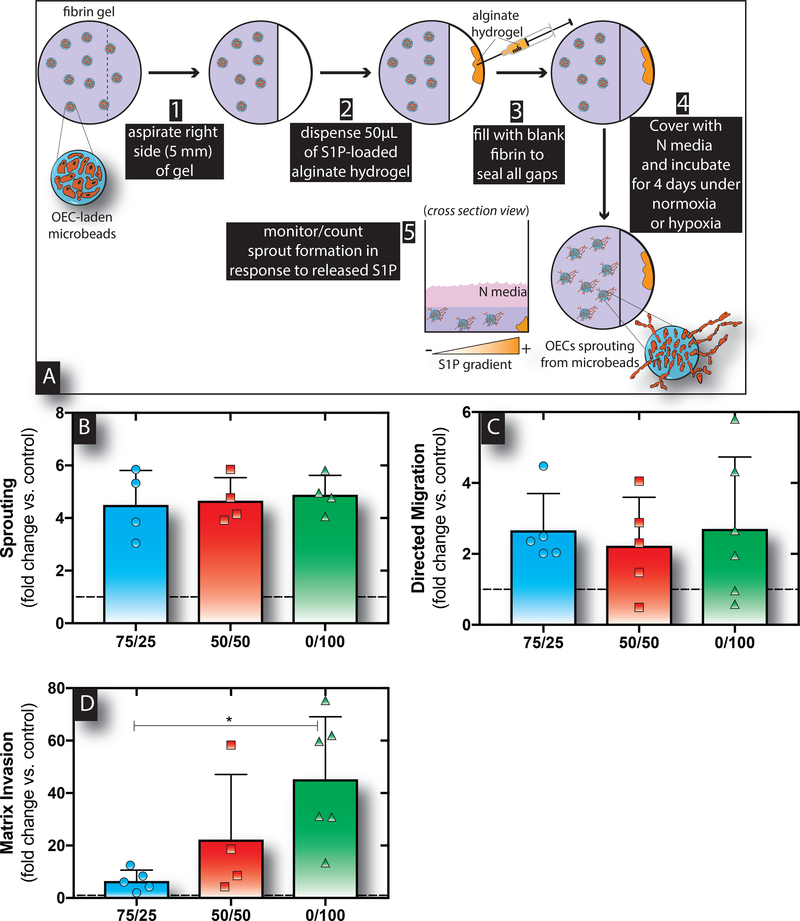
The ability for delivered S1P to stimulate local OEC angiogenic activity was assessed using a modified sprouting assay (Figure 3A) to determine which material system induced the greatest response. In all cases, OECs significantly sprouted in response to S1P released from hydrogels as compared to the blank control (Figure 3B). However, there were no significant differences in sprouting between the various S1P-loaded alginate hydrogels. Subsequently, direction migration and 3D matrix invasion were tested next as another tool to assess angiogenic activity. Herein, a modified Transwell assay was used to test directed migration and invasion into 3D fibrin matrices towards gradients of S1P established by release from the alginate delivery systems over the course of 4 days as previously described.7 S1P released from all three gels resulted in similar degrees of OEC migration through the Transwell insert (Figure 3C) compared to the blank control. In contrast, S1P released from 0/100 hydrogels led to significantly enhanced invasion of OECs into the tissue-like fibrin matrix below compared to both the blank control and 75/25 hydrogels (Figure 3D). Furthermore, hydrogels containing 100% HMW content exhibited statistically significantly increased 3D directed matrix invasion compared to 25% HMW alginate hydrogels.
Figure 3. S1P released from alginate hydrogels stimulates OEC angiogenic activity under hypoxia in vitro.
Schematic of the modified sprouting assay process used to evaluate OEC sprout formation in response to S1P concentration gradients established from varied release regimens (A). Sustained release of S1P from all three bimodal alginate hydrogels resulted in enhanced angiogenic sprouting by OECs under hypoxia (B). The number of cells that migrated through the Transwell insert was similar for all three hydrogels (C). The number of cells that directly migrated and invaded into the fibrin matrix was significantly enhanced in response to S1P release from 0/100 hydrogels (D). Bars represent mean, scatter dot plots display individual measurements and error bars represent standard deviation (B-D, n=5–6). Asterisks indicate statistically significant differences (P < 0.05).
Alginate hydrogels exhibiting slower release of S1P maximally enhanced blood vessel formation in a CAM assay
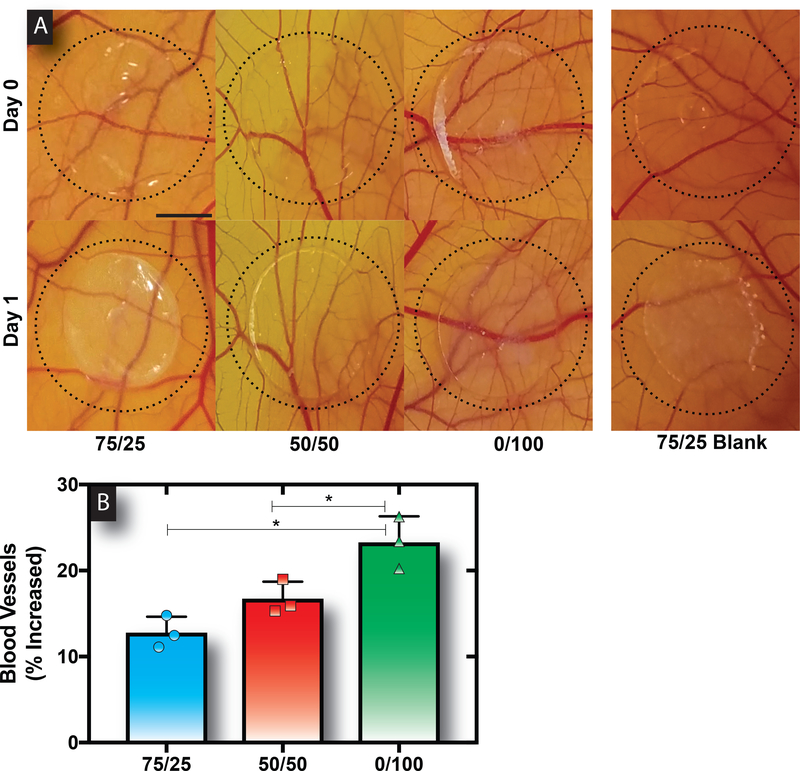
The optimal release rate and best suited hydrogel composition for stimulating blood vessel development was probed via a CAM assay. The biological relevance in the context of angiogenesis and the simplicity, rapidity, and low cost of the chick embryos motivates the use of this assay for this work.25 Importantly, the CAM assay has been described as a useful tool to screen and evaluate the effects of S1P in vivo.7,26,27 Overall, S1P release from all hydrogels resulted in an increase in angiogenesis compared to the blank control over a 24-hour period (Figure 4A). Consistent with a slower release rate and increased OEC directed matrix invasion, the delivery of S1P from 0/100 gels showed maximal angiogenic potential with statistically significant increases in blood vessel formation as compared to all the other tested conditions (Figure 4B).
Figure 4. Sustained release of S1P from 100% HMW alginate hydrogels induced maximal blood vessel development.
Representative images of the various hydrogels placed on the CAMs at 0h and after 24h of incubation (A). Scale bar represents 3mm. The quantified number of blood vessels developed showed that 0/100 hydrogels resulted in a statistically significant increase in response as compared to the other hydrogels (B). Bars represent mean, scatter dot plots display individual measurements and error bars represent standard deviation (B, n=3). Asterisks indicate statistically significant differences (P < 0.05) between conditions as indicated by capped bar.
DISCUSSION
This work investigates the utility of altering the molecular weight composition of alginate polymers to create hydrogels that enable tunable and controlled delivery of a bioactive lipid, S1P. The results of this work confirm the hypothesis that increasing the HMW alginate content, which correspondingly increases the mechanical stiffness within the hydrogels, provides a means to manipulate the release of S1P. Furthermore, this work provides evidence that slower release of S1P optimally enhances directed migratory invasion of OECs under hypoxia in vitro and leads to greater blood vessel development in vivo. Overall, this report demonstrates that injectable alginate hydrogels may be designed and used for controlled hydrophobic lipid release in therapeutic applications.
We demonstrated, for the first time to our knowledge, that altering the molecular weight distribution of alginate hydrogels provides a means to control sustained release of two hydrophobic lipids, R18 and S1P. The use of 2% 75/25 alginate hydrogels has been extensively characterized for the delivery of growth factors given its binding capacity to sequester heparin-binding proteins.2,14–17 While lipid release studies are still lacking, we recently showed that composite alginate-chitosan hydrogels provide controlled release of S1P by varying the chitosan content within the alginate (75/25) hydrogel.7 However, the molecular interactions between the polymers within the hydrogel network and S1P were not fully elucidated. Furthermore, achieving homogeneity without precipitation is challenging to achieve when crosslinking two polymers of opposing charge28 and may impact the ability to predictably control release. Thus, it was hypothesized here that the material properties of the homogenous alginate hydrogel, controlled via molecular weight distribution, might provide a means to control and sustain lipid release without the need for a second polymer addition.
Alginate hydrogels indeed provide sustained release of hydrophobic lipids, wherein increasing the HMW content from 25% to 50% or 100% retarded the lipid release profiles. Importantly, altering the molecular weight distribution resulted in varied mechanical properties that in fact correlated with this altered lipid release. Bimodal alginate hydrogels with greater HMW proportions exhibited less swelling, higher storage moduli, and correspondingly smaller mesh sizes that are indicative of more densely arranged polymer networks.18,19 With only a 10–20% weight loss over the course of 3 weeks in vitro, the stability of these bimodal gels also supported previous findings.29 Albeit similar dissolution profiles, hydrogels with greater HMW content displayed overall stiffer material properties that coupled with retarded lipid release over time. In particular, S1P release from 75/25 hydrogels was 1.7 and 1.9 times faster than that from 50/50 hydrogels within the first one and two weeks, respectively. A similar trend was also observed for R18 release over greater periods of time. Of importance, previous works have only shown sustained biomaterial-based release of S1P over a short period of 1–7 days.6,30,31 In contrast, these combined results indicated that long-term lipid release over at least 2 weeks might be finely tuned depending on the lipid of interest and molecular weight distribution of the alginate hydrogel.
Importantly, the results of this work suggest that release of S1P from bimodal alginate hydrogels can be tuned to potentially enhance therapeutic benefit via enhanced blood vessel formation. We first tested the capacity for varied gradients of S1P, established via hydrogel release, to modulate the angiogenic activity of a prominent cell type involved in new blood vessel formation, OECs. It is well established that S1P gradients are crucial to its function, particularly with regards to regulating cellular trafficking,32 and numerous works have demonstrated that S1P delivery stimulates therapeutic angiogenesis.30,33,34 However, the ability to enhance the angiogenic response by altering the S1P release regimen had yet to be demonstrated until our recent findings using alginate-chitosan hydrogels.7 Herein, it was shown that a slower release profile generated by composite 75/25 alginate and 0.5% chitosan hydrogels resulted in about 1.6-fold greater cumulative migration and matrix invasion than 75/25 alginate hydrogels.7 Interestingly, the release regimens established using the varied bimodal alginate hydrogels tested in this work resulted in drastically greater 3D matrix invasion of OECs with release from 0/100 hydrogels exhibiting about 7- and 45-fold more invasion than that from the 75/25 hydrogels and the negative control, respectively. Furthermore, S1P release from 0/100 hydrogels induced roughly 10% and 6% more CAM blood vessel formation in vivo as compared to control than 75/25 and 50/50 hydrogels did, respectively, after 24h. Thus, these collective results highlight how this bimodal alginate hydrogel system can be used and manipulated to control release of S1P in a manner that provides gradients suitable for inducing greater therapeutic angiogenesis.
Overall, this work provides first time evidence that alginate-based delivery systems may be used to provide and manipulate sustained release of lipids by altering the molecular weight distribution of the alginate polymers. Herein, the data presented here suggests that bimodal alginate formulations are beneficial for both maintaining injectable hydrogels and controlling tunable lipid release for enhanced therapeutic angiogenesis both in vitro and in vivo.
ACKNOWLEDGEMENTS
We thank the American Heart Association (15BGIA25730057 and 15PRE22930044) and the Hellman Family for the funding support for this work.
Footnotes
Conflict of Interest Statement: 3) No benefit of any kind will be received either directly or indirectly by the author(s).
REFERENCES
- 1.Anderson EM, Kwee BJ, Lewin SA, Raimondo T, Mehta M, Mooney DJ. Local delivery of VEGF and SDF enhances endothelial progenitor cell recruitment and resultant recovery from ischemia. Tissue Eng Part A 2015;21:1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost 2007;5:590–598. [DOI] [PubMed] [Google Scholar]
- 3.Ouma GO, Jonas RA, Usman MH, Mohler ER, 3rd. Targets and delivery methods for therapeutic angiogenesis in peripheral artery disease. Vasc Med 2012;17:174–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binder BYK, Williams PA, Silva EA, Leach JK. Lysophosphatidic acid and sphingosine-1-phosphate: A concise review of biological function and applications for tissue engineering. Tissue Eng Part B Rev 2015;21:531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams PA, Stilhano RS, To VP, Tran L, Wong K, Silva EA. Hypoxia augments outgrowth endothelial cell (OEC) sprouting and directed migration in response to sphingosine-1-phosphate (S1P). PLoS One 2015;10:e0123437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogle ME, Sefcik LS, Awojoodu AO, Chiappa NF, Lynch K, Peirce-Cottler S, Botchwey EA. Engineering in vivo gradients of sphingosine-1-phosphate receptor ligands for localized microvascular remodeling and inflammatory cell positioning. Acta Biomater 2014;10:4704–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams PA, Campbell KT, Gharaviram H, Madrigal JL, Silva EA. Alginate-chitosan hydrogels provide a sustained gradient of sphingosine-1-phosphate for therapeutic angiogenesis. Ann Biomed Eng 2017;45:1003–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to Bionanotechnology. Adv Mater 2006;18:1345–1360. [Google Scholar]
- 9.Williams PA, Silva EA. The role of synthetic extracellular matrices in endothelial progenitor cell homing for treatment of vascular disease. Ann Biomed Eng 2015;43:2301–2313. [DOI] [PubMed] [Google Scholar]
- 10.Bidarra SJ, Barrias CC, Fonseca KB, Barbosa MA, Soares RA, Granja PL. Injectable in situ crosslinkable RGD-modified alginate matrix for endothelial cells delivery. Biomaterials 2011;32:7897–7904. [DOI] [PubMed] [Google Scholar]
- 11.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci 2012;37:106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonnesen HH, Karlsen J. Alginate in drug delivery systems. Drug Dev Ind Pharm 2002;28:621–630. [DOI] [PubMed] [Google Scholar]
- 13.Stilhano RS, Madrigal JL, Wong K, Williams PA, Martin PK, Yamaguchi FS, Samoto VY, Han SW, Silva EA. Injectable alginate hydrogel for enhanced spatiotemporal control of lentivector delivery in murine skeletal muscle. J Control Release 2016;237:42–49. [DOI] [PubMed] [Google Scholar]
- 14.Hao X, Silva EA, Mansson-Broberg A, Grinnemo KH, Siddiqui AJ, Dellgren G, Wardell E, Brodin LA, Mooney DJ, Sylven C. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res 2007;75:178–185. [DOI] [PubMed] [Google Scholar]
- 15.Anderson EM, Mooney DJ. The Combination of Vascular Endothelial Growth Factor and Stromal Cell-Derived Factor Induces Superior Angiogenic Sprouting by Outgrowth Endothelial Cells. J Vasc Res 2015;52:62–69. [DOI] [PubMed] [Google Scholar]
- 16.Silva EA, Mooney DJ. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials 2010;31:1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva EA, Kim ES, Kong HJ, Mooney DJ. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci USA 2008;105:14347–14352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BH, Li B, Guelcher SA. Gel microstructure regulates proliferation and differentiation of MC3T3-E1 cells encapsulated in alginate beads. Acta Biomater 2012;8:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maia FR, Fonseca KB, Rodrigues G, Granja PL, Barrias CC. Matrix-driven formation of mesenchymal stem cell-extracellular matrix microtissues on soft alginate hydrogels. Acta Biomater 2014;10:3197–3208. [DOI] [PubMed] [Google Scholar]
- 20.Neves SC, Gomes DB, Sousa A, Bidarra SJ, Petrini P, Moroni L, Barrias CC, Granja PL. Biofunctionalized pectin hydrogels as 3D cellular microenvironments. J Mater Chem B 2015;3:2096–2108. [DOI] [PubMed] [Google Scholar]
- 21.Chan AW, Neufeld RJ. Modeling the controllable pH-responsive swelling and pore size of networked alginate based biomaterials. Biomaterials 2009;30:6119–6129. [DOI] [PubMed] [Google Scholar]
- 22.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004;104:2752–2760. [DOI] [PubMed] [Google Scholar]
- 23.Abaci HE, Truitt R, Luong E, Drazer G, Gerecht S. Adaptation to oxygen deprivation in cultures of human pluripotent stem cells, endothelial progenitor cells, and umbilical vein endothelial cells. Am J Physiol Cell Physiol 2010;298:C1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh S, Wu BM, Dunn JC. Delivery of VEGF using collagen-coated polycaprolactone scaffolds stimulates angiogenesis. J Biomed Mater Res A 2012;100:720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowak-Sliwinska P, Segura T, Iruela-Arispe ML. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014;17:779–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vargas A, Zeisser-Labouebe M, Lange N, Gurny R, Delie F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv Drug Deliv Rev 2007;59:1162–1176. [DOI] [PubMed] [Google Scholar]
- 27.Rivera-Lopez CM, Tucker AL, Lynch KR. Lysophosphatidic acid (LPA) and angiogenesis. Angiogenesis 2008;11:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khong TT, Aarstad OA, Skjak-Braek G, Draget KI, Varum KM. Gelling concept combining chitosan and alginate-proof of principle. Biomacromolecules 2013;14:2765–2771. [DOI] [PubMed] [Google Scholar]
- 29.Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005;26:2455–2465. [DOI] [PubMed] [Google Scholar]
- 30.Wacker BK, Scott EA, Kaneda MM, Alford SK, Elbert DL. Delivery of sphingosine 1-phosphate from poly(ethylene glycol) hydrogels. Biomacromolecules 2006;7:1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Song J. Amphiphilic degradable polymers for immobilization and sustained delivery of sphingosine 1-phosphate. Acta Biomater 2014;10:3079–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Hsu A, Lee JF, Cramer DE, Lee MJ. To stay or to leave: Stem cells and progenitor cells navigating the S1P gradient. World J Biol Chem 2011;2:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oyama O, Sugimoto N, Qi X, Takuwa N, Mizugishi K, Koizumi J, Takuwa Y. The lysophospholipid mediator sphingosine-1-phosphate promotes angiogenesis in vivo in ischaemic hindlimbs of mice. Cardiovasc Res 2008;78:301–307. [DOI] [PubMed] [Google Scholar]
- 34.Sefcik LS, Petrie Aronin CE, Wieghaus KA, Botchwey EA. Sustained release of sphingosine 1-phosphate for therapeutic arteriogenesis and bone tissue engineering. Biomaterials 2008;29:2869–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]