Abstract
Background
Studies have shown racial differences in cancer outcomes. We investigate whether survival differences existed in Hispanic Glioblastoma (GBM) patients compared to other ethnicities from our modern radiotherapy series, as no study to date has focused on outcomes in this group after radiation therapy (RT).
Methods
We retrospectively evaluated 428 patients diagnosed with GBM from 1996–2014 at our institution, divided into four groups based on self-report: white, black, Hispanic, and Asian/Indian. The primary outcome was overall survival (OS). We analyzed differences in prognostic factors among the whole cohort compared to the Hispanic cohort alone.
Results
Baseline characteristics of the four racial groups were comparable. With a median follow-up of 387 days, no survival differences were seen by Kaplan-Meier analysis. Median OS for Hispanic patients was 355 days versus 450 days for the entire cohort. Factors significant for patient outcomes in the entire cohort differed slightly to those specific to Hispanic patients. Low Karnofsky Performance Status (KPS) was significant on multivariate analysis in the whole population, but not in Hispanic patients. Extent of resection, Recursive partitioning analysis (RPA) class, and RT total dose were significant on multivariate analysis in both the whole population and Hispanic patients.
Conclusions
We found Hispanic GBM patients had no difference in survival compared to other ethnicities in our cohort. Differences exist in factors associated with outcomes on single and multivariate analysis for Hispanic GBM patients compared to the entire cohort. Additional studies focusing on Hispanic patients will aid in more personalized treatment approaches in this group.
Keywords: Glioblastoma, Radiation Therapy, Hispanic, Ethnicity, Race
Introduction
Each year, glioblastoma (GBM) accounts for approximately 70% of new cases of malignant primary gliomas diagnosed in the United States.1 The annual incidence of GBM is 3.19 cases per 100,000 in the United States alone, with 10,110 predicted cases in 2015.2 Since the publication of the landmark study by Stupp and colleagues, the standard of treatment for GBM includes surgery followed by adjuvant chemoradiation with temozolomide.3 Despite the multimodality treatment strategy, GBMs are associated with high morbidity and mortality, with a median survival ranging from 1.25 to 1.5 years.1
Ethnic and racial disparities in cancer outcome have been observed across many types of cancers, including GBM.4–7 There are many possible contributors to these differences, including cultural and socioeconomic differences between ethnicities that can affect access to care and the type of treatment received, and thus contribute to differences in outcome.8–10 Evidence also suggests that molecular variability occurs between ethnic groups.11–13 Epidemiological and molecular studies have shown multiple factors correlated to differences in incidence rates of GBM as well as prognostic factors for outcome, including ethnicity, age at diagnosis, intracranial location, performance status, degree of resection and molecular/genetic variations.14,15
Hispanics have a much lower incidence of GBM in the United States, but represent the fastest-growing population in this country. The relative lack of studies focusing on outcomes in this population reveals a need for studies examining whether these patients have differences in prognostic factors and outcomes. Columbia Medical University Center (CUMC) is located in Washington Heights, New York, the population demographics of which include a 71.0% Hispanic population based on the US Census Bureau 2000 and 2010.16 CUMC plays a large role in serving minority populations, and the Herbert Irving Comprehensive Cancer Center at CUMC is one of twelve recipients of the Minority/Underserved Community Site grants.17 Our particular population distribution thus allows the opportunity to examine the outcomes of Hispanic GBM patients due to a higher volume of these patients being seen at our medical center.
Material and Methods
We reviewed all patients who underwent radiation therapy for GBM at Columbia University Medical Center from 1996 to 2014 from an Institutional Review Board-approved database. We collected baseline demographics as well as treatment related variables including: age at diagnosis, gender, marital status, Karnofsky Performance Status (KPS), extent of surgical resection, Recursive Partitioning Analysis (RPA) class, RT total dose, laterality, mulicentricity, isocitrate dehydrogenase 1 (IDH-1) status, O6-methylguanine–DNA methyltransferase (MGMT) status, and temozolomide usage. The definition of race and ethnicity was based on patients’ self-identification of their background. Extent of surgery was categorized as biopsy only, subtotal resection (STR), or gross total resection (GTR) by two study-blinded radiologists who reviewed the immediate pre- and post-operative MRI scans with comparison to pre-contrast T1 images. Residual tumor was defined as a single area of enhancement measuring 0.175 cm3 or greater.18 The primary outcome was overall survival (OS), determined as the time from surgery until death or last follow-up. Patients were stratified by ethnicity determined by patient-reported intake sheets: white, black, Hispanic, or Asian/Indian. Patients identifying as Hispanic were not included in the “white” and “black” populations, even if they self-identified in more than one group. We also analyzed differences in prognostic factors among the whole cohort and compared these to prognostic factors in the Hispanic cohort alone.
Statistical Methods
To compare baseline characteristics between the different ethnicities, contingency tables were generated using Pearson’s chi-squared test for categorical variables and Fisher’s Exact test when necessary. Actuarial survival curves were generated using the Kaplan-Meier survival model, and OS was compared by ethnicity using the Kaplan-Meier estimator and log-rank test. Univariate Cox regression analysis was performed among our cohort on variables expected to be significant predictors of mortality based on historical outcomes and existing literature. Variables found to be statistically significantly associated with survival on univariate analysis, as well as variables with expected clinical significance based on historical outcomes (even when p-values were bordering on significant) were considered for inclusion in the multivariate Cox regression model. Covariates in the final multivariate analysis were included in the model by step-wise selection. A p-value < .05 was considered significant for both univariate and multivariate analyses. The p-value was not adjusted for multiple comparisons. All analyses were performed using SPSS, version 22.
Results
A total of 428 patients were included in our analysis. Baseline demographics are in Table 1. Most patients were over the age of 50 (N=326, 76.2%) and 247 patients (57.7%) were men. There were 313 white (73.1%), 21 black (4.9%), 77 Hispanic (18.0%), and 17 Asian/Indian (4.0%) patients. Baseline characteristics were similar across all ethnicities with the notable exception of marital status: more white and Asian/Indian patients were married compared to black and Hispanic cohorts.
Table 1:
Baseline Characteristics
| Total | % | White | % | Black | % | Hispanic | % | Asian/Indian | % | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at Diagnosis (N) | 428 | 313 | 73.1 | 21 | 4.9 | 77 | 18.0 | 17 | 4.0 | 0.379 | |
| <50 | 102 | 23.8 | 74 | 23.6 | 7 | 33.3 | 15 | 19.5 | 6 | 35.3 | |
| ≥50 | 326 | 76.2 | 239 | 76.4 | 14 | 66.7 | 62 | 80.5 | 11 | 64.7 | |
| Gender (N) | 428 | 313 | 73.1 | 21 | 4.9 | 77 | 18.0 | 17 | 4.0 | 0.911 | |
| Male | 247 | 57.7 | 181 | 57.8 | 11 | 52.4 | 46 | 59.7 | 9 | 52.9 | |
| Female | 181 | 42.3 | 132 | 42.2 | 10 | 47.6 | 31 | 40.3 | 8 | 47.1 | |
| Marital Status (N) | 425 | 311 | 73.2 | 21 | 4.9 | 76 | 17.9 | 17 | 4.0 | 0.001 | |
| Married | 299 | 70.4 | 235 | 75.6 | 9 | 42.9 | 40 | 52.6 | 15 | 88.2 | |
| Unmarried | 126 | 29.6 | 76 | 24.4 | 12 | 57.1 | 36 | 47.4 | 2 | 11.8 | |
| KPS (N) | 428 | 313 | 73.1 | 21 | 4.9 | 77 | 18.0 | 17 | 4.0 | 0.459 | |
| ≥70 | 346 | 80.8 | 258 | 82.4 | 16 | 76.2 | 58 | 75.3 | 14 | 82.4 | |
| <70 | 82 | 19.2 | 55 | 17.6 | 5 | 23.8 | 19 | 24.7 | 3 | 17.6 | |
| Extent of Surgical Resection (N) | 302 | 217 | 71.9 | 16 | 5.3 | 57 | 18.9 | 12 | 4.0 | 0.411 | |
| Gross Total Resection | 193 | 63.9 | 139 | 64.1 | 13 | 81.3 | 32 | 56.1 | 9 | 75.0 | |
| Subtotal Resection | 45 | 14.9 | 29 | 13.4 | 2 | 12.5 | 13 | 22.8 | 1 | 8.9 | |
| Biopsy | 64 | 21.2 | 49 | 22.6 | 1 | 6.3 | 12 | 21.1 | 2 | 16.7 | |
| RPA Class (N) | 366 | 264 | 72.1 | 18 | 4.9 | 68 | 18.6 | 16 | 4.4 | 0.203 | |
| III | 38 | 10.4 | 30 | 11.4 | 1 | 5.6 | 5 | 7.4 | 2 | 12.5 | |
| IV | 224 | 61.2 | 163 | 44.5 | 15 | 4.1 | 36 | 52.9 | 10 | 62.5 | |
| V/VI | 104 | 28.4 | 71 | 26.9 | 2 | 11.1 | 27 | 39.7 | 4 | 25.0 | |
| RT Technique (N) | 421 | 308 | 73.2 | 20 | 4.8 | 76 | 18.1 | 17 | 4.0 | 0.001 | |
| IMRT | 231 | 54.9 | 169 | 54.9 | 12 | 60.0 | 38 | 50.0 | 12 | 70.6 | |
| 3D CRT | 170 | 40.4 | 133 | 43.2 | 7 | 35.0 | 26 | 34.2 | 4 | 23.5 | |
| 2D | 20 | 4.8 | 6 | 1.9 | 1 | 5.0 | 12 | 15.8 | 1 | 5.9 | |
| RT Total Dose (N) | 428 | 313 | 73.1 | 21 | 4.9 | 77 | 18.0 | 17 | 4.0 | 0.617 | |
| <36 Gy | 42 | 9.8 | 32 | 10.2 | 2 | 9.5 | 8 | 10.4 | 0 | 0 | |
| 36–54 Gy | 41 | 9.6 | 31 | 9.9 | 0 | 0 | 9 | 11.7 | 1 | 5.9 | |
| >54 Gy | 345 | 80.6 | 250 | 29.9 | 19 | 90.5 | 60 | 77.9 | 16 | 94.1 | |
| Laterality (N) | 399 | 290 | 72.7 | 20 | 5.0 | 73 | 18.3 | 16 | 4.0 | 0.973 | |
| Unilateral | 331 | 83.0 | 241 | 83.1 | 17 | 85.0 | 60 | 82.2 | 13 | 81.3 | |
| Bilateral | 68 | 17.0 | 49 | 16.9 | 3 | 15.0 | 13 | 17.8 | 3 | 18.8 | |
| Multicentricity (N) | 399 | 290 | 72.7 | 20 | 5.0 | 73 | 18.3 | 16 | 4.0 | 0.278 | |
| No | 314 | 78.7 | 222 | 76.6 | 17 | 85.0 | 63 | 86.3 | 12 | 75.0 | |
| Yes | 85 | 21.3 | 68 | 23.4 | 3 | 15.0 | 10 | 13.7 | 4 | 25.0 | |
| IDH-1 Status (N) | 93 | 68 | 73.1 | 3 | 3.2 | 16 | 17.2 | 6 | 6.5 | 0.406 | |
| Negative | 87 | 93.5 | 63 | 92.6 | 3 | 100 | 16 | 100 | 5 | 83.3 | |
| Positive | 6 | 6.5 | 5 | 7.4 | 0 | 0 | 0 | 0 | 1 | 16.7 | |
| MGMT Status (N) | 65 | 46 | 70.8 | 2 | 3.1 | 12 | 18.5 | 5 | 7.7 | 0.259 | |
| Unmethylated | 38 | 58.5 | 28 | 60.9 | 2 | 100 | 7 | 58.3 | 1 | 20.0 | |
| Methylated | 27 | 41.5 | 18 | 29.1 | 0 | 0 | 5 | 41.7 | 4 | 80.0 | |
| Temozolomide (N) | 286 | 208 | 72.7 | 15 | 5.2 | 51 | 17.8 | 12 | 4.2 | 0.216 | |
| Yes | 265 | 92.7 | 191 | 91.8 | 13 | 86.7 | 50 | 98.0 | 11 | 91.7 | |
| No | 21 | 7.3 | 17 | 18.2 | 2 | 13.3 | 1 | 2.0 | 1 | 8.3 |
OS and survival stratified by ethnicity was assessed with Kaplan-Meier curves (Figure 1 and Table 2). Median follow up for the entire cohort was 387 days. There was no difference in OS between ethnic groups. The median OS for the entire cohort was 450 days with 95% confidence interval (CI) of 410–490 days. Median OS for white patients were 457 days (CI 414–500 days), black patients 449 days (CI 268–630 days), Hispanic patients 355 days (CI 275–435 days), and Asian/Indian patients 602 days (CI 495–709 days).
Figure 1:
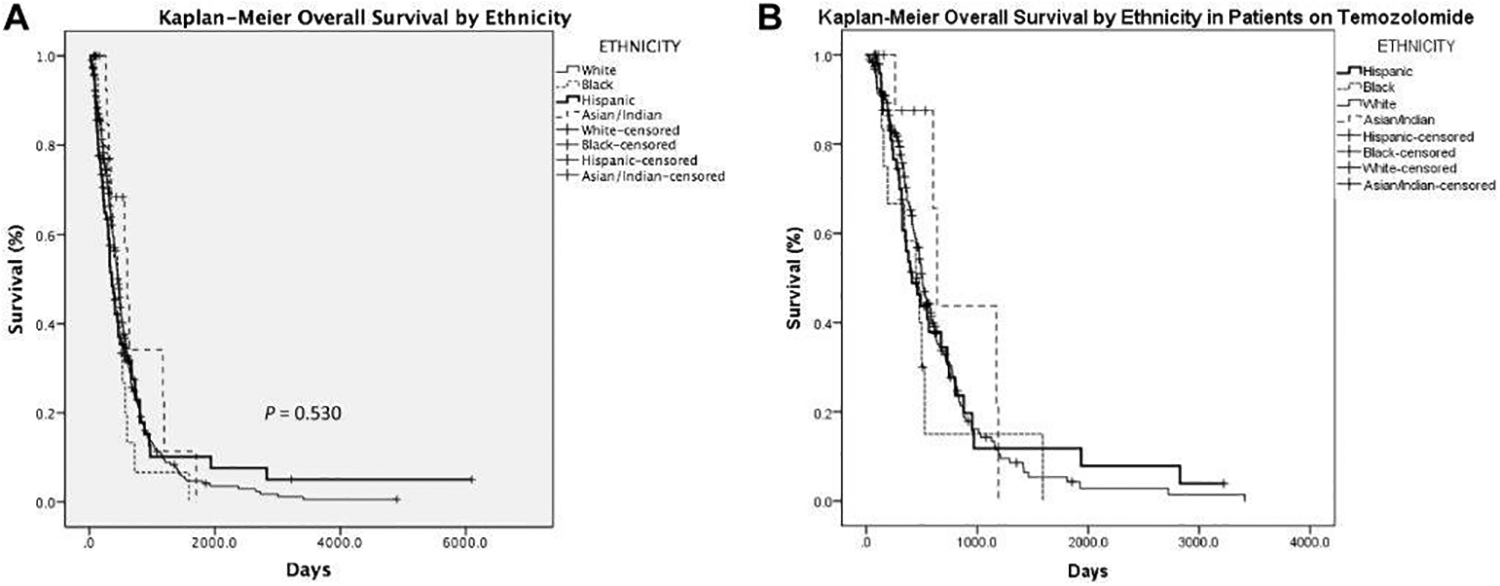
Kaplan-Meier curves to assess Overall Survival by Ethnicity.
Table 2.
Kaplan Meier Median OS by Ethnicity
| Days | 95% CI | |
|---|---|---|
| White | 457 | 414–500 |
| Black | 449 | 268–630 |
| Hispanic | 355 | 275–435 |
| Asian | 602 | 495–709 |
| Overall | 450 | 410–490 |
Univariate Cox regression analysis for the entire cohort showed no difference between ethnic groups. Increased hazard ratio for death (HR) was seen with age greater than or equal to 50 years of age (HR 1.826; CI 1.409–2.368); KPS less than 70 (HR 3.233; CI 2.452–4.262); extent of surgical resection: subtotal resection or biopsy versus gross total resection (HR 1.698; CI 1.182–2.440 and HR 2.992; CI 2.180–4.107, respectively); RPA class: IV and V/VI versus III (HR 2.357; CI 1.511–3.675 and HR 7.815; CI 4.816–12.681, respectively); RT total dose: less than 36 Gy and 36 to 54 Gy versus greater than 54 Gy (HR 4.526; CI 3.202–6.396 and HR 1.787; CI 1.158–2.757); and no temozolomide (TMZ) use versus TMZ use (HR 2.285; CI 1.451–3.598). Decreased HR for death was seen with Asian/Indian ethnicity versus Hispanic ethnicity (HR 0.439; CI 0.223–0.866). On multivariate analysis, KPS less than 70 (HR 3.040; CI 1.402–6.594); extent of surgical resection: subtotal resection or biopsy versus gross total resection (HR 1.560; CI 1.016–2.394 and HR 2.059; CI 1.087–3.903, respectively); RPA class IV and V/VI versus III (HR 3.609; CI 1.404–9.273 and HR 4.384; CI 1.264–15.209, respectively); RT total dose less than 36 Gy versus greater than 54 Gy (HR 3.270; CI 1.685–6.348); and no TMZ use versus TMZ use (HR 2.976; CI 1.207–7.336) were associated with worse outcomes (Table 4).
Table 4:
Multivariate Cox Regression Analysis for Overall Survival Over Entire Cohort
| Variable | Hazard Ratio | 95% CI (Lower) | 95% CI (Upper) | p-value |
|---|---|---|---|---|
| Age at Diagnosis | ||||
| <50 | 1 | Reference | ||
| ≥50 | 1.045 | 0.627 | 1.743 | 0.865 |
| Ethnicity | ||||
| White | 1 | Reference | ||
| Black | 0.790 | 0.372 | 1.677 | 0.540 |
| Hispanic | 1.035 | 0.657 | 1.632 | 0.881 |
| Asian/Indian | 0.501 | 0.179 | 1.405 | 0.501 |
| KPS | ||||
| ≥70 | 1 | Reference | ||
| <70 | 3.702 | 1.608 | 8.520 | 0.002 |
| Extent of Surgical Resection | ||||
| Gross Total Resection | 1 | Reference | ||
| Subtotal Resection | 1.532 | 0.992 | 2.366 | 0.054 |
| Biopsy | 2.209 | 1.129 | 4.320 | 0.021 |
| RPA Class | ||||
| III | 1 | Reference | ||
| IV | 3.178 | 1.223 | 8.255 | 0.018 |
| V/VI | 3.499 | 0.979 | 12.508 | 0.054 |
| RT Technique | ||||
| IMRT | 1 | Reference | ||
| 3D CRT | 0.618 | 0.363 | 1.055 | 0.078 |
| 2D | 2.261 | 0.278 | 18.402 | 0.446 |
| RT Total Dose | ||||
| <36 Gy | 3.978 | 1.836 | 8.621 | 0.001 |
| 36–54 Gy | 1.214 | 0.651 | 2.264 | 0.542 |
| >54 Gy | 1 | Reference | ||
| Laterality | ||||
| Unilateral | 1 | Reference | ||
| Bilateral | 1.468 | 0.908 | 2.372 | 0.117 |
| Multicentricity | ||||
| No | 1 | Reference | ||
| Yes | 1.130 | 0.736 | 1.735 | 0.576 |
| Temozolomide | ||||
| Yes | 1 | Reference | ||
| No | 3.908 | 1.526 | 10.008 | 0.004 |
Subset analysis of only the Hispanic population showed increased HR for death in patients with KPS less than 70 (HR 2.236; CI 1.208–4.140); extent of surgical resection: subtotal resection or biopsy versus gross total resection (HR 3.856; CI 1.623–9.164 and HR 5.284; CI 2.341–11.926, respectively); RPA class IV and V/VI versus III (HR 6.029; CI 0.806–45.079 and HR 16.981; CI 2.192–131.559, respectively); and RT total dose less than 36 Gy versus greater than 54 Gy (HR 9.128; CI 3.612–23.067) (Table 4). On multivariate analysis, extent of surgical resection: subtotal resection or biopsy versus gross total resection (HR 3.011; CI 1.139–7.963 and HR 12.086; CI 2.965–49.269, respectively); RPA class IV versus III (HR 9.327; CI 1.100–79.109); and RT total dose less than 36 Gy versus greater than 54 Gy (HR 5.391; CI 1.162–25.004) were associated with worse outcomes (Table 5). Multivariate analysis limited to the Hispanic population included KPS, extent of surgical resection, RPA class, and RT total dose in the analysis (Table 6). After including these variables together in the Cox regression model, the following were associated with statistically significantly worse OS: both biopsy and subtotal surgical resection compared to gross total resection; RPA Class IV compared to RPA Class III; and RT total dose < 36 Gy compared to RT total dose > 54 Gy.
Table 5:
Univariate Cox Regression Analysis for Overall Survival Over Hispanic Population
| Variable | Hazard Ratio | 95% CI (Lower) | 95% CI (Upper) | p-value |
|---|---|---|---|---|
| Age at Diagnosis | ||||
| <50 | 1 | Reference | ||
| ≥50 | 1.922 | 0.968 | 3.817 | 0.062 |
| Gender | ||||
| Male | 1 | Reference | ||
| Female | 1.351 | 0.801 | 2.278 | 0.260 |
| Marital Status | ||||
| Married | 1 | Reference | ||
| Unmarried | 1.407 | 0.836 | 2.370 | 0.199 |
| KPS | ||||
| ≥70 | 1 | Reference | ||
| <70 | 2.236 | 1.208 | 4.140 | 0.010 |
| Extent of Surgical Resection | ||||
| Gross Total Resection | 1 | Reference | ||
| Subtotal Resection | 3.856 | 1.623 | 9.164 | 0.002 |
| Biopsy | 5.284 | 2.341 | 11.926 | <0.001 |
| RPA Class | ||||
| III | 1 | Reference | ||
| IV | 6.029 | 0.806 | 45.079 | 0.080 |
| V/VI | 16.981 | 2.192 | 131.559 | 0.007 |
| RT Technique | ||||
| IMRT | 1 | Reference | ||
| 3D CRT | 1.563 | 0.877 | 2.784 | 0130 |
| 2D | 3.026 | 1.411 | 6.487 | 0.004 |
| RT Total Dose | ||||
| <36 Gy | 9.128 | 3.612 | 23.067 | <0.001 |
| 36–54 Gy | 0.704 | 0.251 | 1.975 | 0.505 |
| >54 Gy | 1 | Reference | ||
| Laterality | ||||
| Unilateral | 1 | Reference | ||
| Bilateral | 0760 | 0.370 | 1.562 | 0.455 |
| Multicentricity | ||||
| No | 1 | Reference | ||
| Yes | 1.581 | 0.705 | 3.543 | 0.266 |
Table 6:
Multivariate Cox Regression Analysis for Overall Survival Over Hispanic Population
| Variable | Hazard Ratio | 95% CI (Lower) | 95% CI (Upper) | p-value |
|---|---|---|---|---|
| KPS | ||||
| ≥70 | 1 | Reference | ||
| <70 | 1.153 | 0.795 | 12.628 | 0.102 |
| Extent of Surgical Resection | ||||
| Gross Total Resection | 1 | Reference | ||
| Subtotal Resection | 2.948 | 1.111 | 7.825 | 0.030 |
| Biopsy | 15.890 | 3.292 | 76.696 | 0.001 |
| RPA Class | ||||
| III | 1 | Reference | ||
| IV | 9.094 | 1.046 | 79.095 | 0.045 |
| V/VI | 4.041 | 0.457 | 35.762 | 0.209 |
| RT Technique | ||||
| IMRT | 1 | Reference | ||
| 3D CRT | 0.656 | 0.257 | 1.673 | 0.377 |
| 2D | 0.482 | 0.114 | 2.035 | 0.321 |
| RT Total Dose | ||||
| <36 Gy | 9.611 | 1.420 | 65.039 | 0.020 |
| 36–54 Gy | 0.978 | 0.297 | 3.220 | 0.970 |
| >54 Gy | 1 | Reference |
Subset analysis limited to the 265 patients on TMZ revealed moderate deviations from the results in the entire cohort. Median OS in days was slightly longer in this group (median OS for the TMZ group was 497 days (95% CI: 455–539)). Univariate Cox regression analysis revealed a less significant effect of age (bordering on significant), and no longer showed a significant effect of Asian/Indian ethnicity, tumor bilaterality, or tumor multicentricity on OS in the TMZ group. Multivariate analysis in the TMZ group included age at diagnosis, KPS, extent of surgical resection, RPA Class, and RT total dose. RPA Class V/VI was no longer associated with significantly worse OS compared to RPA Class III or RT total dose of <36 Gy compared to RT total dose of >54 Gy. However, in the TMZ group, RT total dose between 36–54 Gy was significantly associated with worse OS compared to RT total dose >54 Gy, unlike the pattern seen in the overall cohort.
Discussion:
Recent advances in oncological research have led to interest in personalized medicine. Personalized oncology is evidence-based and involves the use of genomic analysis, targeted drugs, biological and molecular markers, and gender, as well as ethnic variation to determine optimized treatment for patients.19 Randomized clinical trials (RCT) play an important role in determining the treatment strategy for various diseases. Randomized trial designs typically require and assume a homogenous population. When RCTs are designed to study cancer, selection criteria are usually based on the site and stage of the disease.20–22 Assuming a homogeneous population in this cohort would result in a limited understanding of the role of individualized criteria such as race and ethnicity. Ultimately, such an approach may miss opportunities to better understand how these patient characteristics affect cancer prognosis and how to optimize treatment in different patient populations with the same disease site and stage. Race and ethnicity have an important impact on molecular pathways in cancer. Differences in race and ethnicity have been shown to contribute to differences in molecular pathways in various human malignancies.12,23 Furthermore, racial disparities in cancer risk factor, screening, incidence, therapies, and mortality have also been observed in the United States.24–28
In the United States, Hispanics represent the largest and fastest growing population, and are a highly heterogeneous group.29,30 The incidence rate for GBM in the Hispanic population is 2.45 in 100,000.15 Studies have examined GBM-specific cancer characteristics in the Hispanic population.13,31–33 However, little is known to date about the survival outcomes of Hispanic patients with GBM. Barnholtz-Sloan and colleagues examined the role of racial and ethnic differences in survival among elderly patients with primary GBM using the population-based Surveillance, Epidemiology and End Results (SEER) Program-Medicare linked database. Although a subset population analysis included the white-Hispanic population, no further analysis for survival was done on this population.7 Despite these studies, it remains unclear which population-specific outcomes, if any, are to be expected in Hispanic patients that receive radiation therapy.
Some studies have begun to explore this question. In an interesting population study, Aizer and colleagues examined the utilization of radiation therapy in 22,777 patients diagnosed with GBM using the SEER database. Results from their analysis showed that in the entire cohort, the use of radiation was associated with improved OS, as expected. However, a multivariable logistic regression model revealed that the Hispanic population had a significantly higher risk of omitting radiation therapy (odds ratio 1.34, CI 1.19–1.50) compared to the non-Hispanic white population.10 Possible explanations offered for this association included markers of underserved status, such as lower income and education, being associated with both non-white ethnicity and omission of RT. This group also proposed the possibility of barriers to effective communication in the patient-physician relationship associated with factors such as age, income, and race. The question remains whether Hispanic GBM patients that receive radiation treatment have similar outcomes compared to white patients, and whether variables such as access to care or toleration of treatment toxicity may play a larger role in this group.
To our knowledge, ours is the first study which focuses on the outcomes of Hispanic patients with newly-diagnosed GBM treated with radiation therapy. The cohort of patients examined in this database includes 428 patients, in which 18% (77 patients) constituted Hispanic patients. Comparing baseline characteristics among the ethnicities (white, black, Hispanic, and Asian/Indian), the cohorts are relatively similar with differences noted in marital status. Initial examination of the patients for OS with Kaplan-Meier curves showed no survival difference between the four groups by ethnicity. The median survival of the Hispanic population was 355 days (approximately 12 months). This value is similar to the reported median survival for all GBM patients.1 Although the median survivals were not statistically significant across ethnicities, the median OS of the Hispanic cohort was relatively lower. Perhaps a larger, more highly powered study may be able to elucidate differences in survival. Furthermore, as suggested by the Aizer paper, there may be differences in access to medical resources related to the Hispanic population that may affect timing of treatment initiation (including standard of care measures such as surgery, RT, and chemotherapy), compliance to care, and toxicity reporting, and as a result, outcomes. Several studies have explored factors associated with effectiveness of treatment and treatment compliance in the Hispanic population for a variety of health concerns, examining ethnic differences in pain management,34 adherence in setting of treatment side effects,35 willingness to discuss issues of treatment tolerability and dissatisfaction,36 and non-adherence when treated for diseases associated with social stigma.37 All of these studies acknowledged barriers specific to differences in culture, language, and medical literacy in the experiences of Hispanic patients in receiving optimal treatment, and encourage further emphasis on understanding these topics. Given our unique cohort, we are currently looking into these possibilities, and additional follow-up and research is necessary.
Disparities in healthcare pertaining to Hispanic populations exist; to what extent this affects OS remains unclear. Differences were observed in factors significant for patient outcome in the entire cohort and those in the Hispanic cohort alone. For example, KPS was a significant factor found on multivariate analysis in the whole population, but was not in the Hispanic patients. Despite a larger relative proportion of Hispanic patients in our population, the absolute number in this cohort may be insufficient for examining KPS and OS, further follow up is needed. Extent of resection, RPA class, and RT total dose were significant on multivariate analysis in both the whole population and the Hispanic patients alone. Limitations of this study include its retrospective nature, which could lead to confounding factors related to data collection as well as patient treatment selection bias. Despite this limitation, the baseline characteristics of the patient population were relatively similar. An additional limitation related to the retrospective nature of this study is that the term “Hispanic” encompasses a diverse and widely variable population (among whom are included individuals who could also identify as white, black, or both), thus presenting a limitation in how patients were classified. Our groupings were based on patient self-reported race/ethnicity, and patients were assigned to the Hispanic group based on their self-identification. Examining further sub-divided groups within the broad category of Hispanic patients will allow for more homogenous cohorts, and could address the limitation of classifying a heterogeneous population into one ethnic group. However, further delineation of Hispanic ethnicity into these sub-categories is difficult given the long history of immigrants in the United States and the ensuing merging of ethnic backgrounds, resulting in rich and complex family trees that continue to be shaped by socioeconomic, sociopolitical, and geographic factors.30
In addition, there is recent interest in the molecular characteristics of patients with GBM including IDH-1 status and MGMT methylation status, limited data has been collected, as patients have been routinely screened for the past few years. Although we analyzed molecular characteristics in patients who had this information recorded, the absence of this information in patients seen earlier made this analysis less fruitful than it may be in the future, when patients are more routinely screened for IDH-1 and MGMT status. Further follow-up examining molecular characteristics related to our Hispanic cohort could reveal associations with OS that cannot be adequately assessed at this time.
Conclusion:
GBM is a devastating disease in which outcomes are poor. Radiation therapy is part of the current treatment strategy. Despite an increasing interest in personalized medicine, very little is currently known about the outcomes of Hispanic patients with newly diagnosed GBM treated with radiation. In our study population, no differences in survival were seen between the different ethnicities. Hispanic patients had different prognostic factors compared to the entire cohort. Future studies focusing on biological and social characteristics associated with race and ethnicity may help determine how they affect cancer prognosis, with the goal of optimizing treatment in different patient populations with the same disease site and stage.
Table 3:
Univariate Cox Regression Analysis for Overall Survival Over Entire Cohort
| Variable | Hazard Ratio | 95% CI (Lower) | 95% CI (Upper) | p-value |
|---|---|---|---|---|
| Age at Diagnosis | ||||
| <50 | 1 | Reference | ||
| ≥50 | 1.826 | 1.409 | 2.368 | 0.001 |
| Gender | ||||
| Male | 1 | Reference | ||
| Female | 0.989 | 0.799 | 1.223 | 0.918 |
| Ethnicity | ||||
| White | 1 | Reference | ||
| Black | 1.192 | 0.738 | 1.926 | 0.472 |
| Hispanic | 1.039 | 0.781 | 1.384 | 0.791 |
| Asian/Indian | 0.675 | 0.358 | 1.270 | 0.222 |
| Marital Status | ||||
| Married | 1 | Reference | ||
| Unmarried | 1.117 | 0.882 | 1.415 | 0.358 |
| KPS | ||||
| ≥70 | 1 | Reference | ||
| <70 | 3.23 | 2.452 | 4.262 | 0.001 |
| Extent of Surgical Resection | ||||
| Gross Total Resection | 1 | Reference | ||
| Subtotal Resection | 1.698 | 1.182 | 2.440 | 0.004 |
| Biopsy | 2.992 | 2.180 | 4.107 | 0.001 |
| RPA Class | ||||
| III | 1 | Reference | ||
| IV | 2.357 | 1.511 | 3.675 | 0.001 |
| V/VI | 7.815 | 4.816 | 12.681 | 0.001 |
| RT Technique | ||||
| IMRT | 1 | Reference | ||
| 3D CRT | 1.417 | 1.136 | 1.766 | 0.002 |
| 2D | 4.033 | 2.452 | 6.633 | 0.001 |
| RT Total Dose | ||||
| <36 Gy | 4.526 | 3.202 | 6.396 | 0.001 |
| 36–54 Gy | 1.787 | 1.158 | 2.757 | 0.009 |
| >54 Gy | 1 | Reference | ||
| Laterality | ||||
| Unilateral | 1 | Reference | ||
| Bilateral | 1.317 | 0.983 | 1.764 | 0.065 |
| Multicentricity | ||||
| No | 1 | Reference | ||
| Yes | 1.311 | 0.995 | 1.727 | 0.054 |
| IDH-1 Status | ||||
| Negative | 26.362 | 0.296 | 2351.589 | 0.153 |
| Positive | 1 | Reference | ||
| MGMT Status | ||||
| Unmethylated | 1.136 | 0.338 | 3.816 | 0.836 |
| Methylated | 1 | Reference | ||
| Temozolomide | ||||
| Yes | 1 | Reference | ||
| No | 2.285 | 1.451 | 3.598 | 0.001 |
Table 7:
Kaplan-Meier median Overall Survival by Ethnicity in Patients on Temozolomide
| Days | 95% CI | |
|---|---|---|
| White | 507 | 460–554 |
| Black | 449 | 244–654 |
| Hispanic | 411 | 254–568 |
| Asian | 638 | 565–711 |
| Overall | 497 | 455–539 |
Table 8:
Univariate Cox Regression Analysis for Overall Survival in Patients on Temozolomide
| Variable | Hazard Ratio | 95% CI (Lower) | 95% CI (Upper) | p-value |
|---|---|---|---|---|
| Age at Diagnosis | ||||
| <50 | 1 | Reference | ||
| ≥50 | 1.399 | 0.973 | 2.011 | .070 |
| Gender | ||||
| Male | 1 | Reference | ||
| Female | 0.985 | 0.740 | 1.310 | .917 |
| Ethnicity | ||||
| Hispanic | 1 | Reference | ||
| Black | 1.343 | 0.663 | 2.720 | .413 |
| White | 0.999 | 0.689 | 1.446 | .994 |
| Asian/Indian | 0.599 | 0.234 | 1.533 | .285 |
| Marital Status | ||||
| Married | 1 | Reference | ||
| Unmarried | 1.204 | 0.873 | 1.659 | .258 |
| KPS | ||||
| ≥70 | 1 | Reference | ||
| <70 | 3.680 | 2.551 | 5.308 | <.0001 |
| Extent of Surgical Resection | ||||
| Gross Total Resection | 1 | Reference | ||
| Subtotal Resection | 1.515 | 1.014 | 2.264 | .043 |
| Biopsy | 3.051 | 1.958 | 4.754 | <.0001 |
| RPA Class | ||||
| III | 1 | Reference | ||
| IV | 2.398 | 1.167 | 4.927 | .017 |
| V/VI | 7.314 | 3.391 | 15.774 | <.0001 |
| RT Total Dose | ||||
| <36 Gy | 4.457 | 2.673 | 7.432 | <.0001 |
| 36–54 Gy | 1.750 | 1.015 | 3.016 | .044 |
| >54 Gy | 1 | Reference | ||
| Laterality | ||||
| Unilateral | 1 | Reference | ||
| Bilateral | 1.327 | 0.892 | 1.973 | .162 |
| Multicentricity | ||||
| No | 1 | Reference | ||
| Yes | 1.247 | 0.861 | 1.807 | .243 |
| IDH-1 Status | ||||
| Negative | 26.362 | 0.296 | 2351.589 | .153 |
| Positive | 1 | Reference | ||
| MGMT Status | ||||
| Unmethylated | 1.136 | 0.338 | 3.816 | .836 |
| Methylated | 1 | Reference |
Table 9:
Multivariate Cox Regression Analysis for Overall Survival in Patients on Temozolomide
| Variable | Hazard Ratio | 95% CI (Lower) | 95% CI (Upper) | p-value |
|---|---|---|---|---|
| Age at Diagnosis | ||||
| <50 | 1 | Reference | ||
| ≥50 | 1.036 | 0.630 | 1.705 | .888 |
| KPS | ||||
| ≥70 | 1 | Reference | ||
| <70 | 4.178 | 1.905 | 9.160 | <.0001 |
| Extent of Surgical Resection | ||||
| Gross Total Resection | 1 | Reference | ||
| Subtotal Resection | 1.610 | 1.063 | 2.438 | .024 |
| Biopsy | 2.798 | 1.478 | 5.296 | .002 |
| RPA Class | ||||
| III | 1 | Reference | ||
| IV | 2.396 | 0.956 | 6.003 | .062 |
| V/VI | 2.014 | 0.560 | 7.248 | .284 |
| RT Total Dose | ||||
| <36 Gy | 1.345 | 0.748 | 2.417 | .322 |
| 36–54 Gy | 2.795 | 1.443 | 5.415 | .002 |
| >54 Gy | 1 | Reference |
References:
- 1.Wen PY, Kesari S. Malignant gliomas in adults. The New England journal of medicine. July 31 2008;359(5):492–507. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-oncology. October 2014;16 Suppl 4:iv1–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet. Oncology May 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 4.Sze M, Butow P, Bell M, et al. Migrant health in cancer: outcome disparities and the determinant role of migrant-specific variables. The oncologist. May 2015;20(5):523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grenade C, Phelps MA, Villalona-Calero MA. Race and ethnicity in cancer therapy: what have we learned? Clinical pharmacology and therapeutics. April 2014;95(4):403–412. [DOI] [PubMed] [Google Scholar]
- 6.Allard JE, Maxwell GL. Race disparities between black and white women in the incidence, treatment, and prognosis of endometrial cancer. Cancer control : journal of the Moffitt Cancer Center. January 2009;16(1):53–56. [DOI] [PubMed] [Google Scholar]
- 7.Barnholtz-Sloan JS, Maldonado JL, Williams VL, et al. Racial/ethnic differences in survival among elderly patients with a primary glioblastoma. Journal of neuro-oncology. November 2007;85(2):171–180. [DOI] [PubMed] [Google Scholar]
- 8.Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE. Patterns of care in elderly glioblastoma patients. Annals of neurology. December 2008;64(6):628–634. [DOI] [PubMed] [Google Scholar]
- 9.Formenti SC, Meyerowitz BE, Ell K, et al. Inadequate adherence to radiotherapy in Latina immigrants with carcinoma of the cervix. Potential impact on disease free survival. Cancer. March 1 1995;75(5):1135–1140. [DOI] [PubMed] [Google Scholar]
- 10.Aizer AA, Ancukiewicz M, Nguyen PL, Shih HA, Loeffler JS, Oh KS. Underutilization of radiation therapy in patients with glioblastoma: predictive factors and outcomes. Cancer January 15 2014;120(2):238–243. [DOI] [PubMed] [Google Scholar]
- 11.Wrensch M, Jenkins RB, Chang JS, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nature genetics. August 2009;41(8):905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen P, Aldape K, Wiencke JK, et al. Ethnicity delineates different genetic pathways in malignant glioma. Cancer research. May 15 2001;61(10):3949–3954. [PubMed] [Google Scholar]
- 13.Yang H, Wei D, Yang K, Tang W, Luo Y, Zhang J. The prognosis of MGMT promoter methylation in glioblastoma patients of different race: a meta-analysis. Neurochemical research. December 2014;39(12):2277–2287. [DOI] [PubMed] [Google Scholar]
- 14.Thakkar JP, Dolecek TA, Popa AM, Villano JL. Racial disparities in survival of glioblastoma patients not treated with radiation. 2013 ASCO Annual Meeting; 2013. [Google Scholar]
- 15.Thakkar JP, Dolecek TA, Horbinski C, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. October 2014;23(10):1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart RG, Davenport J. Diagnosis of acoustic neuroma. Neurosurgery. October 1981;9(4):450–463. [DOI] [PubMed] [Google Scholar]
- 17.Stangerup SE, Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. Otolaryngologic clinics of North America. April 2012;45(2):257–268, vii. [DOI] [PubMed] [Google Scholar]
- 18.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. The Lancet. Oncology May 2006;7(5):392–401. [DOI] [PubMed] [Google Scholar]
- 19.Kalia M Personalized oncology: recent advances and future challenges. Metabolism: clinical and experimental. January 2013;62 Suppl 1:S11–14. [DOI] [PubMed] [Google Scholar]
- 20.Umscheid CA, Margolis DJ, Grossman CE. Key concepts of clinical trials: a narrative review. Postgraduate medicine. September 2011;123(5):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tejeda HA, Green SB, Trimble EL, et al. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. Journal of the National Cancer Institute. June 19 1996;88(12):812–816. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain RM, Winter KA, Vijayakumar S, et al. Sociodemographic analysis of patients in radiation therapy oncology group clinical trials. International journal of radiation oncology, biology, physics. January 1 1998;40(1):9–15. [DOI] [PubMed] [Google Scholar]
- 23.Wiencke JK. Impact of race/ethnicity on molecular pathways in human cancer. Nature reviews. Cancer January 2004;4(1):79–84. [DOI] [PubMed] [Google Scholar]
- 24.O’Keefe EB, Meltzer JP, Bethea TN. Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000–2010. Frontiers in public health. 2015;3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haile RW, John EM, Levine AJ, et al. A review of cancer in U.S. Hispanic populations. Cancer prevention research. February 2012;5(2):150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. October 15 2006;107(8):1711–1742. [DOI] [PubMed] [Google Scholar]
- 27.Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA: a cancer journal for clinicians. Sep-Oct 2012;62(5):283–298. [DOI] [PubMed] [Google Scholar]
- 28.Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. August 2009;18(8):2162–2169. [DOI] [PubMed] [Google Scholar]
- 29.Caballero AE. Understanding the Hispanic/Latino patient. The American journal of medicine. October 2011;124(10 Suppl):S10–15. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez Burchard E, Borrell LN, Choudhry S, et al. Latino populations: a unique opportunity for the study of race, genetics, and social environment in epidemiological research. American journal of public health. December 2005;95(12):2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehrer S, Green S, Ramanathan L, Rosenzweig K, Labombardi V. No consistent relationship of glioblastoma incidence and cytomegalovirus seropositivity in whites, blacks, and Hispanics. Anticancer research. March 2012;32(3):1113–1115. [PubMed] [Google Scholar]
- 32.Krishnamachari B, Il’yasova D, Scheurer ME, et al. A pooled multisite analysis of the effects of atopic medical conditions in glioma risk in different ethnic groups. Annals of epidemiology. April 2015;25(4):270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang J, Shao W, Dorak MT, et al. Positive and negative associations of human leukocyte antigen variants with the onset and prognosis of adult glioblastoma multiforme. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. August 2005;14(8):2040–2044. [DOI] [PubMed] [Google Scholar]
- 34.Campbell CM, Edwards RR. Ethnic differences in pain and pain management. Pain management. May 2012;2(3):219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delgado PL, Alegría M, Cañive JM, et al. Depression and Access to Treatment Among U.S. Hispanics: Review of the Literature and Recommendations for Policy and Research. FOCUS. 2006;4(1):38–47. [Google Scholar]
- 36.Sleath B, Rubin RH, Wurst K. The influence of Hispanic ethnicity on patients’ expression of complaints about and problems with adherence to antidepressant therapy. Clinical therapeutics. June 2003;25(6):1739–1749. [DOI] [PubMed] [Google Scholar]
- 37.Sleath B, Rubin RH, Huston SA. Hispanic ethnicity, physician-patient communication, and antidepressant adherence. Comprehensive psychiatry. May-Jun 2003;44(3):198–204. [DOI] [PubMed] [Google Scholar]


