Abstract
Study Objectives
Sleep deprivation and low sleep quality are widespread among adolescents, and associate with obesity risk. Plausible mediators include diet and physical activity. Another potential interrelated pathway, as yet unexplored in adolescents, could involve epigenetic modification of metabolism genes.
Methods
In a cohort of 351 Mexico City adolescents (47% male; mean [SD] age = 14 [2] years), 7-day actigraphy was used to assess average sleep duration, sleep fragmentation, and movement index. DNA isolated from blood leukocytes was bisulfite-converted, amplified, and pyrosequenced at four candidate regions. Linear mixed models evaluated sex-stratified associations between sleep characteristics (split into quartiles [Q]) and DNA methylation of each region, adjusted for potential confounders.
Results
Mean sleep duration was 8.5 [0.8] hours for boys and 8.7 [1] hours for girls. There were sex-specific associations between sleep duration and LINE-1 (long interspersed nuclear element) methylation. Boys with longer sleep duration (Q4) had lower LINE-1 methylation than boys in the 3rd quartile reference category, while girls with both longer and shorter sleep duration had higher LINE-1 methylation compared to Q3. Longer sleep duration was associated with higher H19 methylation among girls (comparing highest to third quartile, −0.9% [−2.2, 0.5]; p, trend = 0.047). Sleep fragmentation was inversely associated with peroxisome proliferator-activated receptor alpha (PPARA) methylation among girls (comparing highest to lowest fragmentation quartile, 0.9% [0.1 to 1.8]). Girls also showed an inverse association between sleep fragmentation and hydroxysteroid (11-beta) dehydrogenase 2 (HSD11B2; Q4 to Q1, 0.6% [−1.2%, 0%]).
Conclusions
Sleep duration and fragmentation in adolescents show sex-specific associations with leukocyte DNA methylation patterns of metabolism genes.
Keywords: epigenetics, metabolism, actigraphy, genes, cardiometabolic, sleep quality
Statement of Significance.
Insufficient and low-quality sleep among adolescents associate with obesity risk. Several mediating pathways may be involved, including epigenetic modification of genes related to metabolism. In a cohort of adolescents, we examined sleep duration, fragmentation, and movement with actigraphy. DNA was isolated from blood leukocytes to measure DNA methylation in four regions. We found sex-specific associations between sleep duration and LINE-1 methylation, a marker of global methylation. For individual candidate genes, sleep duration and fragmentation were associated with metabolic genes among girls only. Adolescent sleep duration and fragmentation is associated with DNA methylation of metabolism genes in a sex-specific manner.
Introduction
Suboptimal sleep, including short sleep duration and low sleep quality, is a widespread public health issue among adolescents. In the United States, estimates suggest that 58% and 73% of middle and high school-aged adolescents, respectively, do not meet American Academy of Sleep Medicine sleep duration recommendations [1]. In addition to problems associated with learning, memory, mental health, and motor vehicle crashes [2], the sleep debt epidemic carries implications for obesity and chronic disease risk. One recent meta-analysis estimated that children/adolescents with short sleep duration have a twofold greater odds of developing obesity over time [3]. Similarly, although data were mostly cross-sectional, another meta-analysis concluded that lower sleep quality was associated with a 1.5-fold higher odds of obesity [4].
Multiple mechanisms are hypothesized to link short or inadequate sleep to obesity and cardiometabolic disease risk. Among well-established predictors of obesity, diet and physical activity are at the forefront. Short sleep and poor quality sleep have been associated with poorer dietary quality and higher total energy intake [5], and with lower physical activity [6]. Evidence also supports mechanisms independent of diet and physical activity, as even one night of sleep restriction has been associated with increased markers of insulin resistance in controlled experiments [7].
Another possible interrelated pathway by which sleep could affect cardiometabolic health in adolescents is through altered expression of genes involved with metabolism and growth. Gene expression may be altered without modification to the genetic code itself via epigenetic changes. Examples include DNA methylation, histone modification, and chromatin remodeling. The epigenome is not static throughout life; it changes with age and as a result of nutritional and environmental exposures [8]. No studies to date have examined whether sleep is associated with epigenetic changes of relevant candidate genes in a healthy population of adolescents. However, shift work and experimentally induced sleep restriction (in mice and/or human adults) are associated with particular epigenetic markers, including DNA methylation (measured in blood and in specific target tissues) [9–11]. In addition, DNA methylation profiles among children with obstructive sleep apnea appear to differ from those of controls [12].
The examination of sleep and epigenetic regulation of genes related to metabolism assumes particular relevance during the sensitive developmental period of adolescence. The pubertal transition is a critical period for sleep because it coincides with shifts in sleep timing, duration, and quality [2]; and it may also represent a sensitive window for epigenetic alterations. Therefore, utilizing a well-characterized cohort of children from Mexico City, we sought to evaluate associations between actigraphy-assessed sleep duration and quality and leukocyte DNA methylation. In particular, we focused on a marker of global DNA methylation (LINE-1), as well as individual candidate genes that were related to metabolism (peroxisome proliferator-activated receptor alpha [PPARA], hydroxysteroid (11-beta) dehydrogenase 2 [HSD11B2], and H19).
Methods
Participants
The analytic sample includes adolescent participants from three sequentially-enrolled cohorts of the Early Life Exposure in Mexico to ENvironmental Toxicants (ELEMENT) study [13, 14]. Between 1997 and 2004, 1012 mother/child dyads were recruited from prenatal clinics of the Mexican Social Security Institute in Mexico City, which serves low- to middle-income populations formally employed in the private sector. Beginning in 2015, a subset of 550 participants from the original birth cohorts 2 and 3 who were in the midst of pubertal transition (ages 9 to 17 years) were selected to participate in a follow-up study. The present study includes the 351 of these children who had sleep actigraphy measures and at least one marker of venous blood DNA methylation at the same time point. The institutional review boards at the Mexico National Institute of Public Health and the University of Michigan approved research protocols. Informed consent was obtained from parents of all participants, and assent was received from each adolescent.
Sleep measures
At the clinic visit, each adolescent was given an actigraph (ActiGraph GT3X+; ActiGraph LLC, Pensacola, Florida) to wear on the non-dominant wrist continuously for 7 days.
Nightly sleep duration was estimated from the actigraphic data with a fused lasso (least absolute shrinkage and selection operator)-based algorithm. The obtained estimates were highly correlated with manual sleep duration detection in a validation subset of 50 participants (r = 0.95; data available upon request). We examined sleep duration (averaged over the wear period), as well as two measures of sleep quality: sleep fragmentation index and movement index. In accordance with the Actilife software (ActiGraph LLC, Pensacola, FL) and used in previous research [15], sleep fragmentation index was calculated as the percentage of 1-minute (or shorter) periods of sleep out of the total number of sleep bouts of any length, with higher values representing more fragmented sleep. The sleep versus wake episodes (during the previously determined night-time sleep duration window) were identified with the Sadeh algorithm [16]. Movement index was calculated as the percentage of minutes with movement count > 0 out of the total number of minutes in the nightly sleep period. All sleep measures were categorized into quartiles, with higher quartiles representing longer sleep duration, higher sleep fragmentation, or higher movement index, respectively. For sleep duration, quartile 3 (mean = 8.9 hours) was selected as the reference group to evaluate the possibility of U-shaped associations. For sleep fragmentation and movement index, the group with the lowest sleep fragmentation or movement (quartile 1) was used as the reference group.
DNA methylation analysis
We focused on four regions that have been studied by our group before and are associated with environmental exposures [17–19] and/or with pubertal markers [20]. The first was LINE-1 (long interspersed nuclear elements) repetitive elements, which serve as a marker of global levels of DNA methylation. We also decided to evaluate three specific candidate genes, PPARA, H19, and HSD11B2. These genes were selected as they are metabolically related and may be susceptible to environmental exposures [17, 21, 22]. In particular, PPARA is a gene involved in fatty acid oxidation and could play a protective role in adiposity gain [23]. HSD11B2 plays a role in converting cortisol to its inactive form of cortisone, and has been related to hypertension [24]. H19 is gene for a long noncoding RNA, which is genomically imprinted (parent-of-origin monoallelic expression) and involved in weight regulation, especially during in-utero life [25].
Whole blood was collected during the visit in tubes with ethylenediaminetetraacetic acid (EDTA) preservative (BD Vacutainer), and the Flexigene kit (Qiagen) was used to isolate DNA from blood leukocytes (approximately 500 ng). The DNA was then sodium bisulfite-treated which converts unmethylated cytosines to uracil while leaving the methylated cytosines intact with EZ DNA Methylation kits (Zymo Research, Irvine, CA) [58]. Methylation (percent of methylated cells) was quantified at global repetitive elements (long interspersed element 1, LINE-1) and the three genes previously mentioned: H19, HSD11B2, and PPARA via pyrosequencing. Percent methylation was quantified at multiple CpG sites (region of DNA where a cytosine nucleotide is followed by a guanine nucleotide) for each gene/repetitive element (LINE-1 and H19 at 4 CpG sites, HSD11B2 at 5 CpG sites, and PPARA at 2 CpG sites) using assays previously developed by us and others [17, 26, 27]. See Supplementary Table S1 for location information of the genomic regions analyzed and the primer sequences. For all regions, sequences were amplified with HotStarTaq Master Mix (Qiagen) from approximately 50 ng bisulfite-converted DNA. Positive controls of known methylation status (0%, 25%, 50%, 75%, and 100%) and negative controls were included in all PCR plates (batches). Samples were randomized across five batches (96-well plates). The percentage of methylated cells was quantified by a PyroMark ID Pyrosequencer (Qiagen) [28]. Pyro Q-CpG Software computes percent methylation and performs internal quality control checks to ensure each sample was completely bisulfite converted before analysis, the sample did not deviate from the expected sequence, and the sample generated an adequate amount of signal over background noise. A random subset of samples (>10% of samples and all controls) were run in duplicate, and in this case duplicate reads were averaged.
Potential confounders
Potential confounders examined included age, pubertal status, maternal education, and smoking history, physical activity, screen time, sedentary time spent commuting (bus, car, etc.), alcohol consumption, and smoking behavior. Sexual maturation status, assessed by Tanner staging and testicular volume assessment (for boys), were completed by trained physicians during the visit using standard methods [29]. Girls were also asked whether they had started menstruating. To assess pubertal status, participants were classified into those who had reached the latter stages of puberty (testicular volume ≥15 mL for boys and onset of menarche for girls) and those who had not. Physical activity and sedentary behavior were assessed with a questionnaire adapted for and validated in Mexican adolescents [30]. To calculate average hours of moderate and vigorous physical activity per week, the self-reported hours spent in all physical activities were summed (e.g. soccer, volleyball, running). The average number of hours spent in moderate/vigorous physical activity per week, average number of hours of screen time (including television, movies/DVDs, and video games) per week, and average hours spent seated while commuting per week were each categorized into quartiles. Alcohol and smoking behaviors were classified as dichotomous variables: for alcohol, whether they had self-reported consuming alcohol in the past year, and for smoking, whether they had ever tried smoking. Maternal education and smoking history information was abstracted from questionnaires that mothers completed during the original cohort enrollment visit. Maternal education was categorized as <9 years, 9 to <12 years, 12 years, or >12 years. Maternal smoking was a three-category variable for never smoker, past smoker, or current smoker.
Statistical analysis
Of 528 children with actigraphy data for >4 nights, a sample of 385 children were selected for DNA methylation analysis (on the basis of biospecimen availability from earlier time points). Of these, 351 children had PPARA methylation that met quality control requirements. The final sample size for LINE-1 was 320, for HSD11B2 was 279, and for H19 was 335, due to further quality control exclusions. To describe the study population and to assess potential confounders, average DNA methylation of LINE-1 and candidate genes (averaged across sites) were examined according to categories of sociodemographic and lifestyle characteristics. Differences in methylation across covariate categories were evaluated using linear regression models with average DNA methylation as continuous outcomes and sociodemographic and lifestyle characteristics as predictors. To evaluate whether any of these crude associations were sex-specific, interaction terms for sex and each potential confounder were included in each model.
To evaluate the primary study question, a general linear modeling approach was used that takes into account the correlation between CpG sites within the same region with an unstructured variance-covariance matrix. In these models, methylation at all CpG sites of a given region was the continuous outcome with individual CpG sites treated as repeated measures, and sleep quartiles (separate models for duration, fragmentation, and movement index) were included as indicator variables. In adjusted models, the following variables were included as potential confounders: age, maternal education, child’s smoking, and screen time. These variables were selected based on prior knowledge and associations from Table 1. p-values for trend were obtained by replacing the indicator variables with ordinal variable representing sleep quartiles. All analyses were sex-stratified, a decision made a priori, due to sex-specific DNA methylation associations observed previously in this cohort as well as biological plausibility. To investigate the possibility that the sex-specific findings were driven by outliers, each region was checked for possible outliers and sensitivity analyses were conducted by excluding potential outliers. An additional set of sensitivity analyses were conducted by adjusting for proportion of neutrophils and leukocytes, to account for cell type variation. Analyses were conducted in SAS version 9.4 (SAS Institute Inc., Cary, NC), and p values < 0.05 were considered statistically significant.
Table 1.
Associations between DNA methylation and sociodemographic and lifestyle characteristics among Mexican adolescents
| Sociodemographic predictors | % | Mean ±SD LINE-1 methylation, averaged across four sites N = 320 | Mean ±SD PPARA methylation, averaged across two sites N = 351 | Mean ± SD HSD11B2 methylation, averaged across five sites N = 279 | Mean ± SD H19 methylation, averaged across four sites N = 335 |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 46.4 | 75.8 ± 4.1 | 10.6 ± 2.1 | 2.3 ± 3.5 | 49.7 ± 3.5 |
| Female | 53.6 | 74.6 ± 4.6 | 10.7 ± 2.1 | 2.1 ± 3.5 | 48.9 ± 2.9 |
| p value | 0.04 | 0.60 | 0.73 | 0.06 | |
| Age group, years (y) | |||||
| 9.5 to <12 y | 20.5 | 74.6 ± 4.1 | 10.8 ± 1.9 | 2.2 ± 3.6 | 48.4 ± 3.0 |
| 12 to <14 y | 33.9 | 75.4 ± 4.8 | 10.8 ± 2.3 | 2.1 ± 3.3 | 49.3 ± 2.9 |
| 14 to <16 y | 20.5 | 76.6 ± 4.1 | 10.2 ± 2.6 | 2.2 ± 2.4 | 50.3 ± 3.8 |
| 16 to 18 y | 25.1 | 74.4 ± 3.9 | 10.7 ± 1.5 | 2.4 ± 4.2 | 49.1 ± 3.1 |
| p value | 0.91 | 0.29 | 0.61a | 0.11 | |
| Testicular volume (boys only) | |||||
| <15 mL | 19.0 | 77.3 ± 4.3 | 10.3 ± 2.4 | 2.6 ± 2.3 | 49.8 ± 3.2 |
| ≥15 mL (latter stages of puberty) | 81.0 | 75.4 ± 3.9 | 10.6 ± 2.0 | 2.3 ± 3.8 | 49.7 ± 3.6 |
| p value | 0.07 | 0.54 | 0.91 | 0.90 | |
| Menarche status (girls only) | |||||
| Had not experienced | 18.3 | 74.1 ± 4.5 | 10.9 ± 1.8 | 1.3 ± 1.2 | 49.0 ± 3.0 |
| Had experienced | 81.7 | 74.8 ± 4.6 | 10.7 ± 2.2 | 2.3 ± 3.8 | 48.9 ± 2.9 |
| p value | 0.45 | 0.47 | 0.25 | 0.86 | |
| BMI-for-age z scores | |||||
| <0 | 10.9 | 76.0 ± 4.9 | 10.8 ± 2.0 | 2.0 ± 2.1 | 49.9 ± 2.9 |
| 0 to <1 | 22.6 | 74.8 ± 4.4 | 10.8 ± 2.1 | 2.1 ± 3.2 | 49.3 ± 2.7 |
| 1 to <2 | 28.7 | 75.6 ± 3.6 | 10.5 ± 1.8 | 2.8 ± 4.5 | 49.5 ± 3.4 |
| ≥2 | 37.8 | 75.0 ± 4.7 | 10.7 ± 2.4 | 1.9 ± 2.9 | 48.9 ± 3.4 |
| p value | 0.53 | 0.52 | 0.77 | 0.15 | |
| Maternal education, years (y) | |||||
| 8 y or less (secondary or primary) | 11.5 | 74.1 ± 4.6 | 10.9 ± 1.4 | 2.2 ± 3.6 | 48.8 ± 3.1 |
| 9 to 11 y (some high school) | 39.8 | 75.2 ± 4.1 | 10.8 ± 2.0 | 2.4 ± 4.3 | 49.5 ± 3.0 |
| 12 y (completed high school) | 35.2 | 75.8 ± 4.1 | 10.5 ± 2.1 | 1.9 ± 1.6 | 49.5 ± 3.5 |
| >12 y | 13.5 | 74.6 ± 5.3 | 10.4 ± 2.9 | 2.4 ± 4.2 | 48.4 ± 3.2 |
| p value | 0.41 | 0.13 | 0.68 | 0.60 | |
| Maternal smoking historyb | |||||
| Never smoked | 51.8 | 75.1 ± 4.6 | 10.7 ± 2.1 | 1.9 ± 2.6 | 49.3 ± 3.3 |
| Ever smoked | 48.2 | 75.3 ± 4.1 | 10.6 ± 2.3 | 2.6 ± 4.3 | 49.3 ± 3.2 |
| p value | 0.96 | 0.98 | 0.10 | 0.92 | |
| Physical activity, quartiles | |||||
| Q1, 0 to 6 hours/week (h/wk) | 27.4 | 75.9 ± 4.2 | 10.4 ± 2.5 | 2.0 ± 1.3 | 49.8 ± 3.5 |
| Q2, 6.3 to 10 h/wk | 23.9 | 74.8 ± 4.1 | 10.9 ± 2.0 | 1.9 ± 2.5 | 48.6 ± 3.2 |
| Q3, 10.3 to 14.3 h/wk | 23.9 | 75.2 ± 4.5 | 10.7 ± 2.2 | 2.9 ± 5.3 | 49.4 ± 2.8 |
| Q4, 14.5 to 29 h/wk | 24.8 | 75.1 ± 4.4 | 10.7 ± 1.8 | 2.4 ± 4.0 | 49.5 ± 3.3 |
| p value | 0.70 | 0.54 | 0.20 | 0.89 | |
| Screen time, quartiles | |||||
| Q1, 2 to 19.5 hours/week (h/wk) | 25.6 | 75.2 ± 4.2 | 11.0 ± 1.7 | 2.8 ± 4.5 | 49.2 ± 3.0 |
| Q2, 20 to 30.5 h/wk | 26.2 | 75.1 ± 4.1 | 10.5 ± 2.1 | 2.4 ± 3.2 | 49.3 ± 3.2 |
| Q3, 31 to 40.5 h/wk | 23.7 | 75.5 ± 4.1 | 10.7 ± 1.8 | 2.1 ± 2.8 | 49.9 ± 3.3 |
| Q4, 41 to 116 h/wk | 24.5 | 75.2 ± 4.9 | 10.5 ± 2.8 | 1.9 ± 3.5 | 48.9 ± 3.5 |
| p value | 0.52 | 0.26 | 0.11 | 0.90 | |
| Time spent commuting, quartiles | |||||
| Q1, ≤3.5 hours/week (h/wk) | 42.7 | 75.1 ± 4.4 | 10.5 ± 2.3 | 2.4 ± 3.9 | 49.1 ± 3.3 |
| Q2, >3.5 to 5.5 h/wk | 11.7 | 76.3 ± 4.7 | 10.8 ± 1.7 | 2.2 ± 1.4 | 50.2 ± 3.4 |
| Q3, 7.5 to 9.5 h/wk | 21.9 | 74.9 ± 3.9 | 10.7 ± 2.0 | 1.6 ± 1.1 | 49.6 ± 3.6 |
| Q4, 10.5 to 27.5 h/wk | 2.7 | 75.4 ± 4.2 | 10.9 ± 2.2 | 2.7 ± 4.5 | 49.1 ± 2.6 |
| p value | 0.77 | 0.22 | 0.93 | 0.96 | |
| Consumed alcohol in the past year | |||||
| No | 80.6 | 75.1 ± 4.5 | 10.6 ± 2.1 | 2.4 ± 3.8 | 49.1 ± 3.2 |
| Yes | 19.4 | 75.8 ± 4.0 | 10.7 ± 2.2 | 2.6 ± 4.7 | 50.0 ± 3.6 |
| p value | 0.95 | 0.69 | 0.84 | 0.37 | |
| Ever smoked cigarettes | |||||
| No | 85.0 | 75.1 ± 4.3 | 10.6 ± 2.1 | 2.0 ± 2.8 | 49.2 ± 3.2 |
| Yes | 15.0 | 75.6 ± 4.7 | 10.7 ± 2.1 | 3.0 ± 5.2 | 49.5 ± 3.2 |
| p value | 0.77a | 0.88 | 0.06 | 0.91 |
aInteraction with sex (line= higher methylation in boys, slightly lower in girls; exactly opposite for HSD11B2, 0.049; 0.03, respectively).
bAssessed during baseline prenatal visit.
Results
The average (SD) age of participants in the analytic sample was 14.1 (2.0) years, and 54% were female. Average (SD) BMI-for-age Z scores 0.52 (1.27), and approximately 83% of the sample was in the latter stages of puberty (i.e. girls had experienced menarche or boys had testicular volume > 15 mL).
The average (SD) sleep duration among boys was 8.5 (0.8) hours while the average sleep fragmentation and movement indices were 12.2% (4.1) and 14.4% (3.3), respectively. Among girls, the average (SD) sleep duration was 8.7 (1) hours, the average sleep fragmentation index was 11.3% (3.4) and average movement index was 13.0% (3.3). Fragmentation index and movement index were positively correlated in both boys and girls (sex-combined Spearman ρ = 0.62). Sleep duration was also positively correlated with sleep fragmentation and movement indices (Spearman ρ = 0.20 and 0.23, respectively). To illustrate, adolescents with chronically insufficient sleep (meaning they had shorter than American Academy of Sleep Medicine guidelines for sufficient sleep and low night-to-night variability [31]; includes 20% of the sample) had 1.2% lower sleep fragmentation (95% confidence interval −2.3, −0.1) and 1.3% lower movement than adolescents with consistently sufficient sleep (−2.3, −0.3).
The mean (SD) LINE-1 methylation (marker of global methylation), averaged across four sites, was 75.2% (4.4%). The mean (SD) candidate gene methylation was 10.7% (2.1%) for PPARA, 2.2% (3.5%) for HSD11B2, and 49.3% (3.2%) for H19. Males had higher methylation of LINE-1 and H19 than girls (Table 1). In addition, adolescents who reported ever smoking had higher HSD11B2 methylation than those who had never smoked. A few associations were modified by sex. Among boys, those who reported ever smoking had higher LINE-1 methylation than those who never smoked, while girls who smoked had lower LINE-1 methylation (interaction term p = 0.049). In addition, among girls, older age was associated with HSD11B2 methylation, while boys showed an inverse association (interaction term p = 0.03).
Associations between sleep and DNA methylation in boys
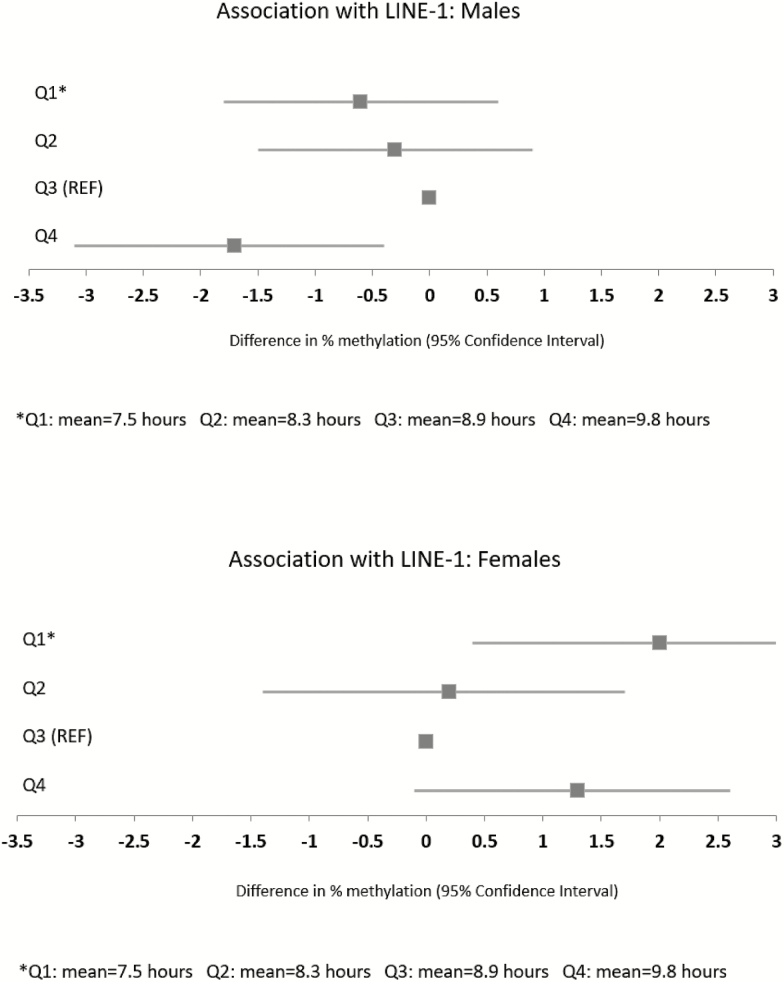
Longer sleep duration was associated with less LINE-1 methylation, such that boys in the 4th quartile (Q4) of sleep duration (average = 9.8 hours) had 1.7% lower adjusted LINE-1 methylation than did boys in the reference group (Q3, average = 8.9 hours [0.4%, 3.1%], p = 0.0097; Figure 1). There were no statistically significant associations of methylation with fragmentation or movement index (Table 2).
Figure 1.
Sex-stratified associations between sleep duration and LINE-1 DNA methylation (see Supplementary Table S2 for crude and confounder-adjusted estimates and 95% confidence intervals).
Table 2.
Associations between sleep measures and leukocyte DNA methylation among Mexico City boys
| Sleep measures | PPARA, % methylated unadjusted | PPARA, % methylated adjusteda | HSD11B2, % methylated unadjusted | HSD11B2, % methylated adjusteda | H19, % methylated unadjusted | H19, % methylated adjusteda |
|---|---|---|---|---|---|---|
| Sleep duration | ||||||
| Q1 (mean = 7.5 hours) | 0.5 (−0.3, 1.2) | 0.5 (−0.3, 1.4) | 0.1 (−0.5, 0.6) | 0.1 (−0.5, 0.6) | 0.4 (−1.1, 2.0) | 0.6 (−1.0, 2.3) |
| Q2 (8.3 hours) | 0.2 (−0.5, 1.0) | 0.2 (−0.6, 1.0) | 0.3 (−0.3, 0.8) | 0.3 (−0.3, 0.8) | 1.0 (−0.6, 2.5) | 0.8 (−0.6, 2.6) |
| Q3 (8.9 hours) | Reference | Reference | Reference | Reference | Reference | Reference |
| Q4 (9.8 hours) | 0.3 (−0.5, 1.2) | 0.3 (−0.6, 1.2) | 0.5 (−0.1, 1.1) | 0.5 (−0.2, 1.1) | 0.5 (−1.2, 2.2) | 0.4 (−1.4, 2.2) |
| p, trend | 0.56 | 0.47 | 0.36 | 0.41 | 0.78 | 0.50 |
| Fragmentation indexb | ||||||
| Q1 (mean = 7.2%) | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 (10.4%) | −0.1 (−0.9, 0.8) | −0.1 (−1.1, 0.8) | 0.1 (−0.5, 0.7) | 0.0 (−0.7, 0.6) | 0.1 (−1.5, 1.7) | 0.5 (−1.3, 2.3) |
| Q3 (12.8%) | −0.2 (−1.0, 0.6) | −0.2 (−1.1, 0.7) | −0.2 (−0.8, 0.4) | −0.3 (−0.9, 0.4) | 0.9 (−0.7, 2.5) | 1.1 (−0.6, 2.9) |
| Q4 (16.6%) | −0.9 (−1.6, −0.1)* | −0.9 (−1.7, 0.0)* | 0.1 (−0.5, 0.7) | 0.1 (−0.5, 0.7) | 0.0 (−1.6, 1.5) | 0.2 (−1.5, 1.8) |
| p, trend | 0.03 | 0.04 | 0.92 | 0.91 | 0.88 | 0.79 |
| Movement indexc | ||||||
| Q1 (mean = 10%) | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 (12.3%) | 0.1 (0.8, 1.1) | 0.2 (−0.8, 1.2) | 0.0 (−0.7, 0.7) | 0.1 (−0.6, 0.8) | −0.8 (−2.6, 1.0) | −0.5 (−2.5, 1.4) |
| Q3 (14.3%) | −0.3 (−1.1, 0.5) | −0.4 (−1.4, 0.5) | −0.2 (−0.9, 0.4) | −0.1 (−0.8, 0.5) | 0.0 (−1.7, 1.7) | 0.3 (−1.6, 2.2) |
| Q4 (18.2%) | −0.5 (−1.4, 0.3) | −0.6 (−1.5, 0.3) | 0.0 (−0.6, 0.6) | 0.0 (−0.7, 0.6) | −0.8 (−2.4, 0.9) | −0.6 (−2.4, 1.2) |
| p, trend | 0.10 | 0.08 | 0.83 | 0.72 | 0.56 | 0.62 |
aAdjusted for age, maternal education, smoking, and screen time.
bFragmentation index refers to the percentage of 1-minute periods of sleep out of total number of sleep bouts of any length.
cMovement index refers to percentage of minutes with count> 0 out of the total number of minutes in sleep period.
*p < 0.05. **p < 0.001.
Associations between sleep and DNA methylation in girls
In girls, sleep duration demonstrated a U-shaped association with LINE-1 methylation; participants in Q1 had 2.0% higher LINE-1 methylation than girls in Q3 ([0.4%, 3.5%]; p = 0.01), and participants in Q4 similarly had a 1.3% higher LINE-1 methylation than the reference quartile ([−0.1, 2.6]; p = 0.08). Further evidence for a U-shaped association was demonstrated by a statistically significant quadratic term (p = 0.03) when sleep duration was modeled continuously. Longer sleep duration was associated with higher H19 methylation (p, trend across quartiles = 0.047). Sleep fragmentation showed inverse associations with methylation of both PPARA and HSD11B2. As with boys, girls with more sleep fragmentation (Q4 vs. Q1) had 1.1% lower PPARA methylation ([−1.9 to −0.3] p = 0.007). Girls with more sleep fragmentation (Q4 vs. Q1) also had 0.6% lower HSD11B2 methylation ([−1.2%, 0%] p = 0.05). The movement index was not associated with methylation of any of the three genes, except for a nonlinear association with PPARA (Table 3).
Table 3.
Associations between sleep measured and DNA methylation among Mexico City girls
| Sleep measures | PPARA, % methylated unadjusted | PPARA, % methylated adjusteda | HSD11B2, % methylated unadjusted | HSD11B2, % methylated adjusteda | H19, % methylated unadjusted | H19, % methylated adjusteda |
|---|---|---|---|---|---|---|
| Sleep duration | ||||||
| Q1 (mean = 7.5 hours) | −0.2 (−1.0, 0.7) | −0.1 (−1.0, 0.8) | −0.5 (−1.1, 0.1) | −0.6 (−1.2, 0.1) | −0.6 (−1.9, 0.7) | −0.9 (−2.2, 0.5) |
| Q2 (8.3 hours) | −0.6 (−1.5, 0.2) | −0.6 (−1.5, 0.3) | −0.2 (−0.8, 0.3) | −0.2 (−0.8, 0.4) | −1.0 (−2.2, 0.2) | −0.9 (−2.2, 0.3) |
| Q3 (8.9 hours) | Reference | Reference | Reference | Reference | Reference | Reference |
| Q4 (9.8 hours) | −0.6 (−1.5, 0.2) | −0.5 (−1.4, 0.3) | −0.1 (−0.6, 0.5) | −0.1 (−0.6, 0.5) | 0.1 (−1.1, 1.2) | 0.1 (−1.0, 1.3) |
| p, trend | 0.47 | 0.53 | 0.08 | 0.10 | 0.11 | 0.047 |
| Fragmentation indexb | ||||||
| Q1 (mean = 7.2%) | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 (10.4%) | −0.3 (−1.1, 0.5) | −0.2 (−1.0, 0.6) | −0.3 (−0.8, 0.3) | −0.2 (−0.7, 0.4) | −0.4 (−1.6, 0.8) | −0.4 (−1.5, 0.8) |
| Q3 (12.8%) | −1.0 (−1.8, −0.2)* | −0.8 (−1.6, 0.1) | −0.3 (−0.8, 0.2) | −0.4 (−1.0. 0.1) | 0.5 (−0.7, 1.6) | 0.9 (−0.3, 2.1) |
| Q4 (16.6%) | −0.8 (−1.7, 0.0) | −0.9 (−1.8, 0.1) | −0.7 (−1.2, −0.1)* | −0.6 (−1.2, 0.0) | 0.7 (−0.5, 2.0) | 0.7 (−0.6, 2.0) |
| p, trend | 0.02 | 0.03 | 0.04 | 0.03 | 0.14 | 0.11 |
| Movement indexc | ||||||
| Q1 (mean = 10%) | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 (12.3%) | −0.2 (−1.0, 0.5) | −0.2 (−0.9, 0.6) | −0.3 (−0.8, 0.2) | −0.4 (−0.9, 0.1) | −0.8 (−1.9, 0.3) | −0.9 (−1.9, 0.2) |
| Q3 (14.3%) | −0.7 (−1.6, 0.1) | −0.7 (−1.6, 0.1) | −0.2 (−0.8, 0.3) | −0.2 (−0.7, 0.4) | 0.3 (−0.9, 1.4) | 0.4 (−0.8, 1.6) |
| Q4 (18.2%) | −0.4 (−1.2, 0.5) | −0.4 (−1.2, 0.5) | −0.1 (−0.7, 0.4) | −0.1 (−0.7, 0.5) | 0.4 (−0.9, 1.6) | 0.3 (−0.9, 1.5) |
| p, trend | 0.19 | 0.19 | 0.62 | 0.79 | 0.36 | 0.36 |
aAdjusted for age, maternal education, smoking, and screen time.
bFragmentation index refers to the percentage of 1-minute periods of sleep out of total number of sleep bouts of any length.
cMovement index refers to percentage of minutes with count> 0 out of the total number of minutes in sleep period.
*p < 0.05. **p < 0.001.
In sensitivity analyses, there was no evidence that outliers were driving the sex-specific findings (data not shown). Similarly, estimates of association were unaltered upon addition of proportion of leukocytes and neutrophils in each cell.
Discussion
This sample from a well-characterized cohort of Mexico City adolescents demonstrates associations between actigraphy-assessed sleep and blood leukocyte DNA methylation of three genes known to be involved with metabolism and obesity. These associations depended on the aspect of sleep examined—duration versus sleep fragmentation—and were sex-specific. In particular, boys with longer and shorter sleep duration had lower LINE-1 methylation than boys in the reference category, while girls with longer and shorter sleep had higher LINE-1 methylation. Longer sleep duration was associated with higher H19 methylation among girls. Sleep fragmentation among girls only showed inverse associations with PPARA methylation and with HSD11B2 methylation.
To our knowledge, this is the first epidemiological study of adolescents to examine associations between sleep duration or quality and DNA methylation. Overall, the suggestion that sleep could play a role in epigenetic modification is in line with animal studies as well as some human studies [32]. In mice, sleep restriction has been linked to higher gene expression of DNA methyltransferase (Dnmt3a1 and Dnmt3a2), enzymes involved in DNA methylation [33]. In humans, lower DNA methylation of certain genes related to circadian rhythms—including PER1, PER2, PER3, BMAL1, and CSNK1E—have been noted in nurses and midwives performing night shift work compared to those on day shifts [34, 35]. In addition, an experimental study among young men subjected to complete sleep restriction reported differential DNA methylation in the liver and adipose tissue compared to a full night of sleep, such that genome-wide methylation (as well as transcriptome and metabolite) signatures in the liver were consistent with breakdown of lean muscle whereas signatures in adipose tissue were indicative of weight gain [36]. Finally, associations between obstructive sleep apnea and DNA methylation of genes related to inflammation have been reported in a few studies of children with obstructive sleep apnea [12]. In general, these prior studies are quite distinct from the present study as they mostly focused on the extremes of sleep (shift work, full sleep deprivation, obstructive sleep apnea), and focused primarily on adults. The present work on sleep and DNA methylation in adolescents highlights that DNA methylation may be responsive to variations in sleep duration and fragmentation that are observed in a typically developing adolescent population. Although the effect sizes were of small magnitude, they are on par with those of other environmental exposures, including arsenic exposure (e.g. highest to lowest levels of arsenic associated with 1.36% higher LINE-1), and the highly-replicated associations between cigarette smoking and DNA methylation [37]. Furthermore, even small changes have the potential to affect transcriptional activity. For example, a study of IGF2 DNA methylation in cord blood reported that a 1% difference in methylation was associated with 2-fold higher transcription of the gene [38]. Further work will be needed to evaluate whether the identified associations do in fact represent cause-and-effect relationships; whether these associations are more pronounced during the adolescent transition period, when dramatic changes often emerge in sleep timing and duration [2]; and also whether these changes occur at other genes not included in this study.
Associations with sleep duration
Only LINE-1 was associated with estimated sleep duration in both boys and girls. LINE-1 is a family of repetitive elements that comprise 17% of the human genome and is typically heavily methylated and repressed. Interestingly, the direction of associations between sleep duration and LINE-1 depended on sex; in boys, longer sleep duration was associated with lower LINE-1 methylation while in girls, both longer and shorter sleep duration was associated with higher LINE-1 methylation. This sex-specificity is not necessarily surprising, as it has been suggested in other literature that associations between sleep and adiposity-related markers depend on sex. For example, the association between short sleep duration and obesity may be stronger in males [39]. In addition, puberty itself could play a role, as DNA methylation patterns are likely responsive to pubertal transitions. Although the ages of males and females in our sample were roughly equivalent and the majority had entered the latter stages of puberty, males and females initiate and progress through puberty at different rates, with distinct hormonal processes involved. The U-shaped association observed in girls is also noteworthy; although there is a current debate on its causal nature [40], there is some epidemiological evidence of a U-shaped relationship between sleep and cardiometabolic health among adolescents [41, 42].
After adjustment for potential confounders, a statistically significant positive association emerged between H19 hypermethylation and longer sleep duration among girls only. H19 is a maternally imprinted gene involved in weight regulation, and its expression has been implicated in prevention of obesity in some studies [25]. Yet the role of H19 remains unclear, as findings on H19 DNA methylation and adiposity-related endpoints have been mixed. For example, among 75 Mexican–American children, those with lower in comparison to higher methylation of the H19 CpG site 4 had higher birthweights [43]. In contrast, another study in US children found that higher methylation of the IGF2/H19 domain was associated with higher birthweight [44], and a study of adolescents also showed that higher methylation of this domain was associated with higher skinfold thickness and subcutaneous adiposity [45].
Associations with sleep fragmentation
Overall, a higher number of significant associations (p < 0.05) were observed with sleep quality—specifically sleep fragmentation—than with sleep duration. Notably, the association between higher sleep fragmentation and lower PPARA methylation was the strongest association observed in girls. It was associated in the same direction among boys but did not reach statistical significance. PPARA is a gene involved in fatty acid oxidation, and is typically most active in the liver. A recent study of 17 healthy young men noted upregulation of a gene in the same family, PPARG, in the skeletal muscle after a night of sleep deprivation [36]. A similar recent study of the effects of circadian misalignment in 14 young men reported that the genes that were highly enriched during the misaligned condition were related to fatty acid metabolism and PPAR signaling, suggesting that metabolism of fat may be favored over metabolism of glucose during circadian misalignment [46]. Some epidemiological evidence also may shed light on a potential link between fatty acid oxidation and sleep fragmentation specifically. Among adults from the Netherlands, higher actigraphically assessed sleep fragmentation was associated with lower intake of carbohydrates, especially simple rather than complex carbohydrates [47]. In turn, lower intake of carbohydrates in favor of higher fat consumption would likely induce PPARA-regulated fatty acid oxidation [23].
In the present study, sleep fragmentation was also associated with HSD11B2 methylation in a linear fashion among girls only. HSD11B2 is a gene involved in converting cortisol to the inactive form of cortisone, and its expression in peripheral blood mononuclear cells has been associated with lower risk of hypertension [24]. However, reported findings are again mixed with regards to cardiometabolic health, as higher enzymatic activity of HSD11B2, proxied by the ratio of urinary free cortisone to urinary free cortisol, has been positively associated with obesity [48].
The present study has multiple strengths, including the use of actigraphy in the estimation of sleep duration and quality, and the application of robust statistical methods that account for within-gene correlations of DNA methylation values. The fact that the data derived from a well-characterized cohort also meant we were able to account for potential confounders. Among limitations of the study, the first is that we were unable to examine tissue-specific DNA methylation as only whole blood was collected. Second, given the cross-sectional design, we were not able to ascertain whether sleep preceded changes in DNA methylation or vice versa. Third, a limited sample size was available for sex-stratified analysis, which likely reduced power to detect associations. Moreover, no additional information regarding self-reported sleep of the adolescents (e.g. snoring, perceived sleep quality) was collected, nor was sleep laboratory polysomnography conducted. Nonetheless, actigraphy provides well-validated estimates of sleep and wake patterns, and carries several advantages: feasibility for administration in a large sample; assessment under home rather than laboratory conditions; and ability to collect data from each participant for seven nights (in most cases) rather than just one night of study.
In summary, we have demonstrated associations between short and fragmented sleep and DNA methylation of genes related to metabolism in a sample of adolescents. These findings represent a first step towards understanding epigenetic pathways that may link sleep and metabolic health outcomes in adolescents. This is an opportune time to focus research efforts to better understand longitudinal relationships; epigenetics may provide a key for understanding mechanisms. More research is needed to establish cause-and-effect relationships, examine a wider array of metabolically related genes, and evaluate whether these associations are unique to the adolescent transition period.
Funding
E.C.J. reports support from National Heart, Lung, and Blood Institute grant 5T32HL110952-05 during the conduct of the study. R.D.C. reports grants from Foundation for the National Institutes of Health during the conduct of the study; his institution and collaborators have received grant support from the American Sleep Medicine Foundation; grant support from the National Multiple Sclerosis Society; financial and non-financial support from Michigan Medicine, other from International Pediatric Sleep Association, personal fees and non-financial support from American Academy of Sleep Medicine, personal fees and non-financial support from American Academy of Dental Sleep Medicine, personal fees from UpToDate, personal fees from Cambridge University Press, and non-financial support from Association of Professional Sleep Societies, all outside the submitted work.
Conflict of interest statement. R.D.C. developed questionnaires for childhood sleep problems (PSQ, PSQ-SRBD Scale), copyright owned by University of Michigan with royalties paid from Zansors, and patents or patents pending, for technology, tools, or agents relevant to diagnosis and treatment of sleep disorders. None of the other authors has any disclosures to make.
Supplementary Material
Acknowledgments
We gratefully acknowledge the American British Cowdray Medical Center (ABC) in Mexico for providing research facilities.
Address where work was conducted: Michael S. Aldrich Sleep Disorders Center, 1500 East Medical Drive, Ann Arbor, MI 48109.
References
- 1. Wheaton AG, et al. Short sleep duration among middle school and high school students — United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(3):85–90. doi: 10.15585/mmwr.mm6703a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Owens J; Adolescent Sleep Working Group; Committee on Adolescence Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;134(3):e921–e932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fatima Y, et al. Longitudinal impact of sleep on overweight and obesity in children and adolescents: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2015;16(2):137–149. [DOI] [PubMed] [Google Scholar]
- 4. Fatima Y, et al. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17(11):1154–1166. [DOI] [PubMed] [Google Scholar]
- 5. Dashti HS, et al. Habitual sleep duration is associated with BMI and macronutrient intake and may be modified by CLOCK genetic variants. Am J Clin Nutr. 2015;101(1):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmid SM, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90(6):1476–1482. [DOI] [PubMed] [Google Scholar]
- 7. Reutrakul S, et al. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. 2018;84:56–66. [DOI] [PubMed] [Google Scholar]
- 8. Loche E, et al. Early nutrition, epigenetics, and cardiovascular disease. Curr Opin Lipidol. 2016;27(5):449–458. [DOI] [PubMed] [Google Scholar]
- 9. Trivedi MS, et al. Short-term sleep deprivation leads to decreased systemic redox metabolites and altered epigenetic status. PLoS One. 2017;12(7):e0181978. doi: 10.1371/journal.pone.0181978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cedernaes J, et al. Acute sleep loss induces tissue-specific epigenetic and transcriptional alterations to circadian clock genes in men. J Clin Endocrinol Metab. 2015;100(9):E1255–E1261. [DOI] [PubMed] [Google Scholar]
- 11. Trzepizur W, et al. Murine models of sleep apnea: functional implications of altered macrophage polarity and epigenetic modifications in adipose and vascular tissues. Metabolism. 2018;84:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perikleous E, et al. DNA Methylation in pediatric obstructive sleep apnea: an overview of preliminary findings. Front Pediatr. 2018;6:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis RC, et al. Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere. 2013;93(10):2390–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ettinger AS, et al. Effect of calcium supplementation on bone resorption in pregnancy and the early postpartum: a randomized controlled trial in Mexican women. Nutr J. 2014;13(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chung S, Youn S, Lee C, et al. Environmental noise and sleep disturbance: night-to-night variability of sleep/wake pattern. Sleep Med Res. 2016;7(2):78–81. doi: 10.17241/smr.2016.00122 [DOI] [Google Scholar]
- 16. Sadeh A, et al. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. [DOI] [PubMed] [Google Scholar]
- 17. Goodrich JM, et al. Quality control and statistical modeling for environmental epigenetics: a study on in utero lead exposure and DNA methylation at birth. Epigenetics. 2015;10(1):19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montrose L, et al. Maternal levels of endocrine disrupting chemicals in the first trimester of pregnancy are associated with infant cord blood DNA methylation. Epigenetics. 2018;13(3):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goodrich JM, et al. Adolescent epigenetic profiles and environmental exposures from early life through peri-adolescence. Environ Epigenet. 2016;2(3):dvw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Y, et al. Association of blood leukocyte DNA methylation at LINE-1 and growth-related candidate genes with pubertal onset and progression. Epigenetics. 2018;13(12):1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. LaRocca J, et al. The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes. Environ Res. 2014;133:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, et al. Lead exposure during early human development and DNA methylation of imprinted gene regulatory elements in adulthood. Environ Health Perspect. 2016;124(5):666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Contreras AV, et al. PPAR-α as a key nutritional and environmental sensor for metabolic adaptation. Adv Nutr. 2013;4(4):439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friso S, et al. Epigenetic control of 11 beta-hydroxysteroid dehydrogenase 2 gene promoter is related to human hypertension. Atherosclerosis. 2008;199(2):323–327. [DOI] [PubMed] [Google Scholar]
- 25. Schmidt E, et al. LincRNA H19 protects from dietary obesity by constraining expression of monoallelic genes in brown fat. Nat Commun. 2018;9(1):3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Virani S, et al. Delivery type not associated with global methylation at birth. Clin Epigenetics. 2012;4(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoyo C, et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics. 2011;6(7):928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Busato F, et al. Quantitative DNA methylation analysis at single-nucleotide resolution by pyrosequencing®. Methods Mol Biol. 2018;1708:427–445. doi: 10.1007/978-1-4939-7481-8_22 [DOI] [PubMed] [Google Scholar]
- 29. Chavarro JE, et al. Validity of self-assessed sexual maturation against physician assessments and hormone levels. J Pediatr. 2017;186:172–178.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hernández B, et al. Validez y reproducibilidad de un cuestionario de actividad e inactividad fisica para escolares de la ciudad de Mexico. Salud Publica Mex. 2000;42(4):315–323. doi: 10.1590/S0036-36342000000400006 [DOI] [PubMed] [Google Scholar]
- 31. Jansen EC, et al. Adiposity in adolescents: the interplay of sleep duration and sleep variability. J Pediatr. 2018;203:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gaine ME, et al. Sleep deprivation and the epigenome. Front Neural Circuits. 2018;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Massart R, et al. The genome-wide landscape of DNA methylation and hydroxymethylation in response to sleep deprivation impacts on synaptic plasticity genes. Transl Psychiatry. 2014;4:e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bhatti P, et al. Nightshift work and genome-wide DNA methylation. Chronobiol Int. 2015;32(1):103–112. doi: 10.3109/07420528.2014.956362 [DOI] [PubMed] [Google Scholar]
- 35. Reszka E, et al. Circadian gene methylation in rotating-shift nurses: a cross-sectional study. Chronobiol Int. 2018;35(1):111–121. [DOI] [PubMed] [Google Scholar]
- 36. Cedernaes J, et al. Acute sleep loss results in tissue-specific alterations in genome-wide DNA methylation state and metabolic fuel utilization in humans. Sci Adv. 2018;4(8):eaar8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Breton CV, et al. Small-magnitude effect sizes in epigenetic end points are important in children’s environmental health studies: the children’s environmental health and disease prevention research center’s epigenetics working group. Environ Health Perspect. 2017;125(4):511–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murphy SK, et al. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene. 2012;494(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tatone-Tokuda F, et al. Sex differences in the association between sleep duration, diet and body mass index: a birth cohort study. J Sleep Res. 2012;21(4 PG-448–60):448–460. doi: 10.1111/j.1365-2869.2011.00989.x [DOI] [PubMed] [Google Scholar]
- 40. Kurina LM, et al. Sleep duration and all-cause mortality: a critical review of measurement and associations. Ann Epidemiol. 2013;23(6):361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Javaheri S, et al. Association of short and long sleep durations with insulin sensitivity in adolescents. J Pediatr. 2011;158(4):617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sivertsen B, et al. Sleep and body mass index in adolescence: results from a large population-based study of Norwegian adolescents aged 16 to 19 years. BMC Pediatr. 2014;14:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hernández-Valero MA, et al. Interplay between polymorphisms and methylation in the H19/IGF2 gene region may contribute to obesity in Mexican-American children. J Dev Orig Health Dis. 2013;4(6):499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perkins E, et al. Insulin-like growth factor 2/H19 methylation at birth and risk of overweight and obesity in children. J Pediatr. 2012;161(1):31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang RC, et al. DNA methylation of the IGF2/H19 imprinting control region and adiposity distribution in young adults. Clin Epigenetics. 2012;4(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wefers J, et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc Natl Acad Sci U S A. 2018;115(30):7789–7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dashti HS, et al. Actigraphic sleep fragmentation, efficiency and duration associate with dietary intake in the Rotterdam Study. J Sleep Res. 2016;25(4):404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Müssig K, et al. 11beta-hydroxysteroid dehydrogenase 2 activity is elevated in severe obesity and negatively associated with insulin sensitivity. Obesity (Silver Spring). 2008;16(6):1256–1260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.