Abstract
Mitochondrial damage is implicated as a major contributing factor for a number of noncommunicable chronic diseases such as cardiovascular diseases, cancer, obesity, and insulin resistance/type 2 diabetes. Here, we discuss the role of mitochondria in maintaining cellular and whole-organism homeostasis, the mechanisms that promote mitochondrial dysfunction, and the role of this phenomenon in noncommunicable chronic diseases. We also review the state of the art regarding the preclinical evidence associated with the regulation of mitochondrial function and the development of current mitochondria-targeted therapeutics to treat noncommunicable chronic diseases. Finally, we give an integrated vision of how mitochondrial damage is implicated in these metabolic diseases.
Keywords: mitochondria, obesity, insulin resistance, cancer, cardiovascular diseases
Graphical Abstract
Graphical Abstract.
Essential Points.
Noncommunicable chronic diseases are complex and multifactorial conditions that collectively pose a huge challenge for public health systems
Mitochondria are an integrative platform that support indispensable cell functions, including metabolism and cellular signaling
Several noncommunicable chronic diseases have in common the early development of a marked mitochondrial dysfunction
Definition of mitochondrial dysfunction and its contribution to cell function is imperative to understand the pathophysiology of noncommunicable chronic diseases
Understanding the molecular mechanisms involved in the effect of caloric restriction and exercises will pave the way toward new mitochondrial-targeted therapies to combat noncommunicable chronic diseases
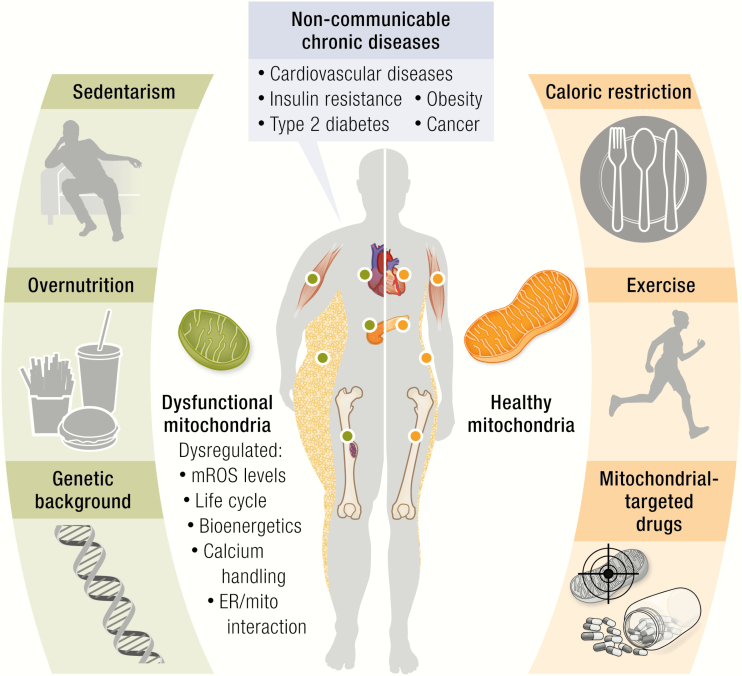
Noncommunicable chronic diseases (NCDs) are complex and multifactorial conditions that collectively pose a huge challenge for public health systems in both the industrialized and the developing world. NCDs are, by definition, noninfectious, nontransmissible disorders often associated with low-grade inflammation, among which the most common are obesity, insulin resistance (IR), diabetes, cancer, cardiovascular diseases (CVDs), among others.
According to the World Health Organization, in 2016, 6 of the “top 10” causes of death worldwide were NCDs. These included ischemic heart disease and stroke (representing a combined 15.2 million deaths); chronic obstructive pulmonary disease; Alzheimer’s disease and other dementias; trachea, bronchus, and lung cancers, and type 2 diabetes mellitus (T2DM). The latter (T2DM) exemplifies the alarming increase in the prevalence of many of these conditions, killing 1.6 million people in 2016 compared with fewer than 1 million in 2000. It has been estimated that the economic burden of NCDs for low- and middle-income countries will be more than US$7 trillion over the period 2011-2025 (1); thus, the challenge of NCDs is not unique to the developed world.
The urgent need to address this global health epidemic has prompted both scientific and social initiatives to combat NCDs. It is broadly understood that unhealthy eating behaviors and sedentary lifestyles are significant contributors, as being overweight or obese is highly associated with hypertension, IR, T2DM, cancer, nonalcoholic fatty liver disease (NAFLD), and CVD. As the world faces the epidemic of NCDs, basic scientific research has a critical role to play by identifying the cellular and molecular mechanisms that are involved in the pathophysiology and metabolic consequences of NCDs, thereby laying the groundwork for knowledge-based approaches to the prevention and treatment of these conditions.
A combination of genetic and environmental factors contributes to the development of NCDs, including low-grade inflammation and oxidative stress (1). Intriguingly, many of these factors have in common the ability to either damage mitochondria or interfere with mitochondrial repair (1), suggesting that mitochondria play a central role in the pathophysiology of NCDs.
Mitochondria are well placed in this respect: from a genetic standpoint, both mitochondrial and nuclear genomes must cooperate to maintain mitochondrial homeostasis and respond to changes in demand. Thus, mutations in either genomic compartment can have pathological consequences. From an environmental standpoint, mitochondria are central processing hubs for external nutrient input and energy output. In addition to generating adenosine triphosphate (ATP), they synthesize a number of important lipid species and provide metabolic substrates for post-translational modifications that are central to intra- and intercellular signaling (eg, acetylation, fumaration, succination, malonylation). They also act as central mediators of internal and external death signals.
Metabolic aberrations are at the core of many NCDs. Even before the mechanisms linking mitochondrial respiration to ATP generation were elucidated (eg, chemiosmotic theory in the 1960s), altered mitochondrial function was identified as a key feature of certain NCDs. Perhaps the most famous example is Otto Warburg’s observation in 1924 (2) that metabolism in cancer cells was very different from that of normal tissues, tending toward glycolysis of glucose to lactate even in the presence of oxygen, thereby linking respiratory defects to cancer. Over the last decade many lines of investigation have converged on mitochondrial dysfunction as a central feature in the development of a variety of NCDs (Table 1). Of particular note is the close association between mitochondrial dysfunction and obesity (40), with mitochondrial dysfunction often proposed as a critical factor mediating the progression of many obesity-related NCDs (Table 1) (41).
Table 1.
Tissue-specific mitochondrial dysfunction observed in noncommunicable diseases (NCDs) and the cellular phenotype change related to mitochondrial dysfunction.
| NCDs | Cell type | Mitochondrial dysfunction | Cellular change | References |
|---|---|---|---|---|
| Obesity | Adipocyte | Mitochondrial fragmentation | Increased proliferation and differentiation | (3,4) |
| mtROS overproduction | ||||
| Hepatocyte | Mitochondrial fragmentation | Metabolic shift and cell death | (5,6) | |
| ETC components reduction | ||||
| Impaired ΔΨmt and ATP synthesis | ||||
| Insulin resistance | Skeletal muscle cell | Mitochondrial fragmentation | Impaired insulin signaling, reduced glucose uptake and GLUT4 translocation | (7-13) |
| Mitochondrial Ca2+ mishandling | ||||
| mtROS overproduction | ||||
| MAM’s disruption | ||||
| Adipocyte | mtROS overproduction | Impaired GLUT4 trafficking and glucose uptake | (10,12) | |
| Hepatocyte | MAM’s disruption | Impaired insulin signaling and insulin resistance | (14,15) | |
| Mitochondrial fragmentation | ||||
| Type 2 diabetes mellitus | β-cells | Mitochondrial respiratory uncoupling | Reduced insulin secretion | (16-24) |
| Impaired ATP synthesis | ||||
| Altered mitophagy | ||||
| Exaggerated fission or fusion | ||||
| Cancer | Primary tumor cell | PCG-1α reduction | Adaptative metabolic shift, increased biosynthetic activity, proliferation, tumorigenesis and aggressiveness | (25-29) |
| Enhanced mitophagy | ||||
| Excessive glycolytic rate | ||||
| moderate mtDNA mutations | ||||
| Mitochondrial metabolic hyperactivity | ||||
| Circulatory and invasive tumor cell | PCG-1α overactivity | Metabolic shift, cellular migration and invasion | (30-34) | |
| Enhanced mitochondrial Ca2+ uptake | ||||
| mtROS overproduction | ||||
| Cardiovascular disease | Smooth muscle cell | mtDNA damage | Increased proliferation | (35) |
| Cardiomyocyte | Mitochondrial fragmentation | Hypertrophy, impaired contractility, energetic crisis, calcium overload and cell death | (13,36-39) | |
| MAM disruption | ||||
| Mitochondrial Ca2+ mishandling | ||||
| mtROS overproduction | ||||
| Decreased OCR |
Abbreviations: ATP, adenosine triphosphate; ETC, electron transport chain; mROS, mitochondrial reactive oxygen species; MAM, mitochondrial associated membrane; mtDNA, mitochondrial DNA; OCR, oxygen consumption rate.
The present review addresses the relevance of mitochondrial function to cell homeostasis and adaptation, examining the mechanisms and consequences of mitochondrial dysfunction in the development of NCDs, specifically obesity, IR, T2DM, cancer, and CVD. We focus on these conditions because of their shared characteristics of altered metabolism at the mitochondrial level and their close association with the obesity pandemic. We want to stress that mitochondrial dysfunction is not simply “broken” or damaged organelles but includes states in which there is a mismatch between mitochondrial activities and cellular demands. Normal human physiology requires continuous adaptation to changes in nutritional input (fasting/feeding, lipolysis/lipogenesis) versus energy expenditure (sleeping/waking, physical activity/sedentary behavior). The highly plastic nature of mitochondria allows them to adapt to these constant changes in order to maintain homeostasis or mediate cell death. The definition of mitochondrial dysfunction is in essence the failure of mitochondria to adapt appropriately or sufficiently and this is what lies at the core of many NCDs. The possible therapeutic opportunities that this knowledge provides will also be discussed.
Mitochondrial Physiology
General information
Mitochondria are bound by a double membrane. The inner mitochondrial membrane (IMM) contains multiprotein complexes of the electron transport chain (ETC) that shuttle electrons from nicotinamide adenine dinucleotide with hydrogen (NADH) and flavine adenine dinucleotide (FADH2) to molecular oxygen, ultimately forming water. In the process, protons are pumped from the internal matrix into the intermembrane space, storing potential energy in the form of an electrochemical gradient that can be used by complex V to generate ATP. The proton motive force (∆p) consists of both a pH gradient (∆pH) and an electrical gradient (mitochondrial membrane potential, ΔΨmt). In addition to oxidative phosphorylation, the IMM and the outer mitochondrial membrane (OMM) contain a diversity of channels and enzymes that along with proteins in the matrix carry out a variety of cellular functions including the synthesis of nucleotides, heme groups, and a subset of essential lipids, among others (42). These organelles are also at the center of cellular life or death decisions. Mitochondria continuously adapt in response to extra- and intracellular stimuli through transient changes in localization or metabolic activity as well as through more pronounced structural modifications, such as changes in morphology or content (43,44). The following section will discuss the basic concepts of structural and functional mitochondrial adaptations.
Mitochondrial dynamics: When the shape illuminates the function
Mitochondria are not static and can exist in a variety of ever-changing shapes, even within the same cell. “Bullet”-, “spaghetti”-, and “donut”-like, and a “mitochondrial reticulum” have all been used to describe mitochondrial architecture (45). These structures are the result of continuous cycles of mitochondrial transport, fragmentation, and fusion events, collectively termed mitochondrial dynamics (Fig. 1) (45). Defects in the fission machinery result in an elongated network. Conversely, reduced activity of profusion proteins leads to a more fragmented state (46,47). The primary functions of mitochondrial dynamics are metabolic adaptation and quality control, with fusion increasing oxidative capacity and fission facilitating degradation of damaged organelles and their replacement (48).
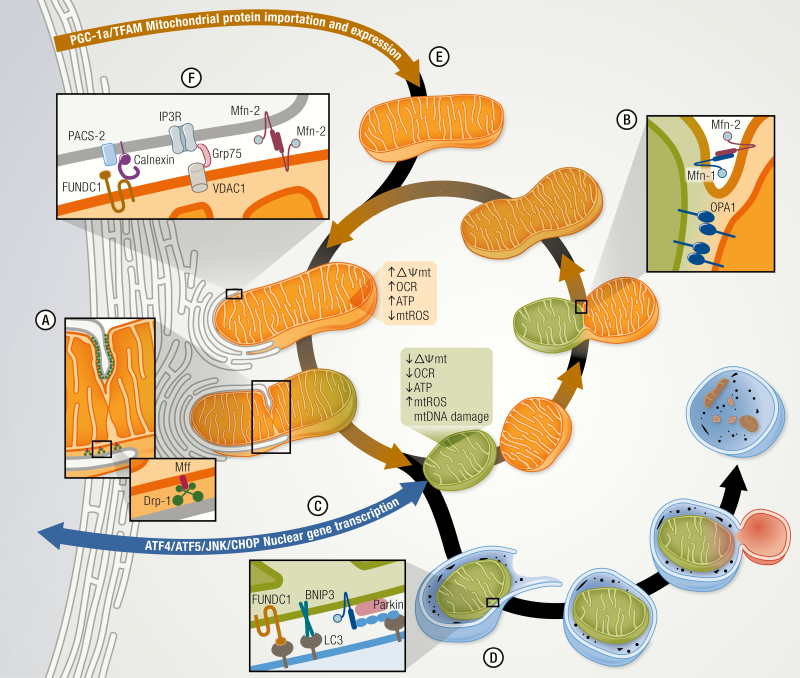
Figure 1.
Mitochondrial plasticity regulates the mitochondrial quality control and function. A mitochondrion is a malleable organelle that constantly changes its structure and shape to adapt its function and allow the normal replacement of anomalous mitochondria. (A) Mitochondrial fission is regulated by Drp-1, which binds to outer mitochondrial membrane (OMM) protein adaptor like Mff, which together with the endoplasmic reticulum, (ER) constrict and isolate the impaired mitochondria. On the other hand, mitochondrial fusion (B) is orchestrated by dynamin-related GTPases mitofusin (Mfn) 1/2 and optic atrophy type 1 (OPA1) promoting the OMM and inner mitochondrial membrane (IMM) fusion respectively. This process allows the function of an anomalous mitochondrion to be enhanced and averaged through combination with a healthy mitochondrion. (C) The stressed mitochondria also communicate with the nucleus through the mitochondrial unfolded protein response (UPRmt) and activate ATF4/5 and JNK/CHOP signaling pathways to induce the transcription of nuclear genes associated with mitochondrial survival and quality control. (D) Alternatively, impaired mitochondria may also be eliminated by mitophagy, triggered by the direct association of BNIP3 or FUNDC1 with LC3 or by marking mitochondria through the parkin-mediated polyubiquitination of OMM proteins. (E) Nuclear encoded mitochondrial proteins and mtDNA transcription are induced by PGC-1α that enhance mitochondrial biogenesis and maintain the mitochondrial mass. (F) ER–mitochondria communication through mitochondria-associated membranes (MAMs) allow molecules to be exchanged between these organelles and regulate the mitochondrial metabolism, bioenergetics, and signaling processes. MAMs are composed of ER proteins like PACS-2, Calnexin, and Mfn-2 and by mitochondrial proteins like VDAC, Mfn-2, and FUNDC1 among others.
Fusion of the mitochondrial network depends on the activity of large, membrane-localized GTPases. In the early 2000s, evidence pointed to an essential role for Mitofusin (Mfn-1/2) proteins in the tethering and fusion of the OMM (46,49,50). In mammals, overexpression of either Mfn-1 or Mfn-2 results in a more interconnected mitochondrial morphology and increased perinuclear clustering. Conversely, their downregulation increases mitochondrial fragmentation (46,49,50). Mfn molecules localized to the OMM of 1 mitochondrion can interact with Mfn from a different mitochondrion, forming homo- or heterocomplexes to tether mitochondria and facilitate GTP-dependent fusion with the opposing OMM (Fig. 1) (46,51,52). Mice with single deletions of either Mfn-1 or Mfn-2 alone are viable whereas the double deletion is lethal (49), demonstrating functional redundancy. Despite this, complementation experiments suggest that Mfn-1 may play a more dominant role in the fusion process (46,51,53).
Elegant microscopy experiments reveal that the process of IMM fusion is independent and distinct from that of OMM fusion (54,55). Optic atrophy 1 (OPA1), a GTPase on the external surface of the IMM (56) is involved in IMM fusion (57, 58). It also helps shape mitochondrial cristae morphology, promoting tightening of crista junctions (57-59). This, in turn, facilitates ETC super complex formation and increases oxidative phosphorylation (60,61), thereby mediating a mechanistic link between mitochondrial form and function (62,63).
Mitochondrial fragmentation, the opposing process, requires dynamin-related protein 1 (Drp-1), a member of the dynamin family of GTPases (47, 64) that resides in the cytosol. Upon activation, Drp-1 translocates to the OMM, binds adaptor proteins, and then assembles a collar-like structure to initiate GTP-dependent constriction of the lipid bilayers (Fig. 1) (47,64,65). A second GTPase, dynamin-2, is required for the final step of lipid fusion and organelle division (66). In mammals, several adaptor proteins capable of recruiting Drp-1 to the OMM have been identified, including mitochondrial fission 1 protein (Fis1), mitochondrial fission factor (Mff), and mitochondrial dynamics proteins, 49 kDa and 51 kDa (MiD49 and MiD51) (67-70). Deletion of these proteins often results in elongation and clustering of mitochondria around the nucleus (67,69,70). Recent studies using TAT fusion peptides to selectively disrupt adaptor interactions with Drp-1 suggest that Mff is required for mitochondrial fission under physiological conditions, whereas Fis1 mediates the interaction and subsequent fission under pathological stress (71).
Mitochondrial dynamics facilitate changes in metabolic activity. In general, when the activity of fission proteins is experimentally reduced to generate a more elongated network, it has been observed that cells are more metabolically active than those with smaller, fragmented mitochondria, even when total mitochondrial content is the same. On the contrary, artificially inhibiting fusion increases mitochondrial fragmentation while reducing both the rate of oxygen consumption and ΔΨmt (49, 72). These observations have often led to the impression that a larger, more fused mitochondrion is a “healthier” organelle. Caution should be taken though, as under basal, normally occurring conditions, this statement might be inaccurate. For instance, subsarcolemmal mitochondria on muscle fibers are more spherical and isolated units, while the intermyofibrillar counterparts are organized in a more complex network. Each population is matched to meet the metabolic and biosynthetic demands specific to its subcellular location (73,74). As such, both types of distinctively shaped mitochondria work synergistically to orchestrate cell metabolism. Both fission and fusion are needed to maintain cellular homeostasis. Fission in particular plays an essential role in repair processes. For instance, in adult muscle, damaged mitochondria are electrically and physically separated from the rest of the network, allowing the remaining mitochondria to resume their normal function (75) while the damaged organelle is repaired, replaced, or degraded. Suboptimal or damaged mitochondria are separated through fission events and can be eliminated by mitophagy or undergo fusion with healthy mitochondria to increase their function. The dynamic nature of the mitochondrial life cycle helps maintain a constant mitochondrial mass by establishing an equilibrium between mitochondrial biogenesis and degradation (Fig. 1) (48). These processes will be discussed later in greater detail.
The fission/fusion process participates in many cellular responses and adaptations. For instance, mitochondrial fragmentation can be an essential initiating signal in apoptosis (76). Mitochondrial elongation (fusion) can increase the rate of ATP synthesis and prevent mitochondrial degradation (77). Fusion can occur when cells are exposed to different oxidative substrates to stimulate oxidative phosphorylation (78). However, acute exposure to high glucose causes an immediate and transient increase in mitochondrial fragmentation and mitochondrial reactive oxygen species (mROS) production in cultured hepatocytes and myocytes that is blocked by inhibiting Drp-1 (3). Similarly, increasing OPA1 can protect mitochondria from damage caused by ETC inhibitors (61,79). These findings are physiologically relevant. For example, insulin action promotes mitochondrial fusion in cardiomyocytes in vivo, increasing mitochondrial metabolism via an OPA1-dependent mechanism (80). Taken together, there is a preponderance of evidence that mitochondrial morphology and function are intimately associated and mutually regulated.
Mitochondrial ROS: It depends on context and fuel
A hyperpolarized ∆p increases the potential for generating ROS, which can damage cellular content including DNA. Thus, since a more fused mitochondrial network is associated with higher metabolic activity, fusion also tends toward an increase in the capacity for ROS generation. However, not all ROS generation is pathological, and it is now widely recognized that ROS can function as an important cellular signaling molecule. Because increased mROS is often evoked as a primary mechanism through which dysfunctional mitochondria contribute to the pathology of NCDs, it is relevant to understand what regulates mitochondrial ROS production (81). Transfer of a single electron to molecular oxygen (O2) produces a highly reactive superoxide (O2•–) molecule. At physiological pH, O2•– is rapidly converted to peroxide (H2O2) with the help of superoxide dismutase in the cytoplasm and mitochondrial matrix (SOD1 and SOD2, respectively). Even though H2O2 is not actually a reactive radical, it is considered a ROS because in the presence of a transition metal (ie, Fe2+) it is converted to a highly reactive hydroxyl radical (HO•) via the Fenton reaction. At least 11 mitochondrial sites are known to produce O2•– and/or H2O2 (O2•–/H2O2) (82). Here we will only discuss ROS production at complex I, as it is thought to be the primary site in vivo, and particularly important with respect to NCDs. Furthermore, it provides an excellent example of how various factors that predispose individuals toward developing NCDs can influence mitochondrial ROS production.
Generation of O2•– is directly dependent on (1) ∆p (40), the local O2 concentration, and (41) the ratios of the redox donor–acceptor pairs NADH/NAD+ and CoQH2/CoQ (the coenzyme Q pool) (83). Under conditions where cellular demand for ATP keeps pace with its production, mitochondrial ∆p is dissipated by complex V and thus remains relatively depolarized. The NADH/NAD+ ratio is also reduced because of the flow of reducing equivalents from NADH into the ETC. Finally, respiration consumes O2, lowering its local concentration. This combination of conditions minimizes ROS generation from complex I. In contrast, there are 2 main modes of ROS generation at complex I. The first mode, forward electron transfer (FET), requires that the flavin mononucleotide (FMN) in complex I is in an almost fully reduced state. This only occurs when the NADH/NAD+ ratio is high. Any one of a variety of cellular perturbations that inhibit specific component of the ETC will raise the NADH/NAD+ ratio and increase ROS generation via this mechanism (84,85), including damage to or mutation of the complex, ischemia, loss of cytochrome c, or a low cellular demand for ATP. Conversely, conditions that lower the NADH/NAD+ ratio tend to suppress ROS production. In fact, the addition of complex I substrates augments NADH levels and increases H2O2 production in isolated mitochondria (86). Subsequent administration of ADP (to model an increase in demand for energy, eg, physical exercise) results in oxidation of NADH to NAD+ and reduced H2O2 levels (87). Thus, a careful balance between energy demand and supply is necessary to control mROS generation. Although responsive to the NADH/NAD+ ratio, the FET mode of ROS generation is relatively independent of ∆p, occurring at both hyper- and hypopolarized membrane potentials (83).
The second mode of ROS generation involves reverse electron transfer (RET) from CoQH2 through complex I, and reducing NAD+ to NADH (87,88). This mode of ROS generation can occur during oxidation of succinate or fatty acids (88). For instance, succinate accumulation and RET-dependent mROS generation has been observed during heart ischemia (89). Inhibition of succinate dehydrogenase attenuates mROS production via RET, thereby protecting the heart from mROS-induced damage (89). In contrast to ROS generation via FET, a hyperpolarized ∆p is essential for RET and very small depolarization in ∆p can abolish it, so as long as ATP demand remains high, a shift to fatty acid oxidation does not necessarily increase ROS production. It is reasonable to speculate the increase in mitochondrial fission that is often noted in metabolic disease could in part be a compensatory measure to reduce RET-mediated ROS.
It is important to note that mitochondrial fission often generates daughter organelles with an unequal distribution of ∆p, ETC component, mtDNA, etc. (90). Furthermore, deletion of Mfn-1/2 to prevent fusion evokes stochastic depolarization of a subset of mitochondria (49,72). Subsequently, daughter mitochondria with depolarized ∆p can be preferentially removed by mitophagy, whereas, the daughter mitochondria capable of maintaining or restoring ∆p may merge again with the mitochondrial network (91).
The mitochondrial uncoupling proteins (UCPs) provide an additional compensatory mechanism for reducing RET-mediated mROS generation. UCPs are sensitive to glutathionylation (92) and they have been implicated in many pathological conditions. For instance, overexpression of UPC2 in cancer cells confers a drug-resistant phenotype (93). Downregulation of UCP3 increases oxidative damage, impairs fatty acid oxidation, and compromises cardiac function (94), whereas overexpression of UCP3 in skeletal muscle protects against high-fat diet-induced IR (95). An acute increase in mROS production can provoke UCP2/3-dependent proton leak across the IMM (96,97) depolarizing mitochondria and thereby providing negative feedback to reduce RET-dependent mROS production (96,97) and maintaining mitochondrial–redox homeostasis.
Another important mode of mitochondrial ROS generation relevant to NCDs is ROS-induced ROS release (RIRR). This occurs when an initial burst of ROS production triggers a much larger feed-forward release from both the originating and neighboring mitochondria. The mechanisms of RIRR are reviewed in detail elsewhere (98). This is the mode of ROS release though to occur in response to ischemia reperfusion. The mitochondrial translocator protein is an integral OMM protein involved in gating RIRR and is involved in the propagation of cardiac arrhythmias following a heart attack and those associated with T2DM (99). Whether RIRR plays a role in NCDs requires further investigation.
Mitochondrial unfolded protein response: The whisper between nucleus and mitochondria
The human mitochondrial proteome is composed of nearly 1500 different proteins, only 13 of which are encoded by the maternally inherited mitochondrial genome (mtDNA) (100). The large number of nuclear-encoded mitochondrial proteins imposes a tremendous workload on the mitochondrial protein import system (101). It is therefore not surprising that mechanisms have evolved for mitochondrial–nuclear communication. For instance, mitochondrial stress can lead to remodeling of nuclear chromatin and global gene silencing (102). An important aspect of the mito-nuclear communication system is the mitochondrial unfolded protein response (UPRmt). The name “UPRmt” was originally coined based on its role to re-establish mitochondrial proteostasis. However, the UPRmt is now thought of as an integrated response to a variety of mitochondrial stressors, including ETC damage, and accumulation of misfolded proteins and mROS. Activation of the UPRmt can trigger compensatory adaptations (eg, changes in expression of nuclear encoded glycolytic enzymes) to sustain proper cell function (103).
Work primarily in yeast and worms has provided important insights into mechanisms mediating mito-nuclear communication (104,105). Although mammalian cells share some features, the specifics of the mammalian system are yet to be fully elucidated. The mammalian transcription factor ATF5 has been proposed to act in a fashion similar to that of ATFS-1 in Caenorhabditis elegans (105,106). Under basal conditions, ATF5 translocates to and accumulates in mitochondria. However, upon inhibition of ETC complex I or V, ATF5 moves to the nucleus, inducing the expression of hallmark UPRmt response genes (Fig. 1) (106). Other stresses, such as dissipation of ΔΨmt or inhibition of mitochondrial protein translation, results in the induction of a similar, but not identical, gene/protein signature committed to the re-establishment of mitochondrial homeostasis, via an eIF2a/ATF4-dependent pathway (107). Similar to the accumulation of unfolded proteins in the ER, the accumulation of misfolded proteins in mitochondria increases the expression of the stress-induced transcription factor C/EBP homologous protein (CHOP) (108). Accordingly, several genes induced by the UPRmt have CHOP binding sites in their promoters (108, 109). Both mROS and JNK2 activation are also involved in the response as their inhibition decreases expression of UPRmt response genes (108).
Although the mammalian UPRmt is not fully defined, there is evidence of its relevance in obesity and NCDs, with mROS generation acting as a mechanism for mediating mito-nuclear communication. In response to diet-induced obesity, ROS levels have been shown to increase in skeletal muscle, triggering the activation of AMP-activated protein kinase (AMPK) and PPAR gamma coactivator-1 alpha (PGC-1α) signaling pathways, leading to mitochondrial biogenesis and rewiring of systemic metabolism (110,111). Similar systemic changes in whole-body metabolism have been observed when mitochondrial translation was impaired by cardiomyocyte-specific deletion of mitochondrial aspartyl-tRNA synthetase (DARS2). Remarkably, expression of UPRmt-responsive genes, such as the mitokine FGF21, occurred even before evidence of respiratory chain deficiency, resulting in reduced body fat mass and glycemia (112). In contrast, deletion of DARS2 in skeletal muscle caused respiratory chain deficiency but did not activate the UPRmt or mediate system changes in metabolism, suggesting tissue specificity in both activation of the UPRmt and its downstream consequences. Taken together, the evidence indicates that upon mitochondrial oxidative or proteostatic stress, mitochondria engage a nuclear response, with consequences not only to the organelle and the cell in which it resides but that can have systemic effects on the whole organism.
Endoplasmic reticulum–mitochondria communication: A matter of distance
Mitochondria often interact directly with other organelles including the endoplasmic reticulum (ER). This was first described 60 years ago (113), and has subsequently received substantial attention because of its implications at the cellular and systemic level (114,115). Some of the first studies aimed at understanding the role of the ER–mitochondria interaction involved phospholipid metabolism (116). As reviewed elsewhere, synthesis of phosphatidylserine, phosphatidylethanolamine, and other phospholipids relies on lipid translocation between endoplasmic reticulum and mitochondria (117,118). After subcellular fractionation, crude mitochondrial extracts were observed to still retain enzymatic activities associated with ER lipid biosynthetic pathways, suggesting that some ER membrane was still attached to mitochondria (117,118). Those tightly bound membranes are currently known as mitochondria-associated membranes (MAMs). MAMs are involved in several important cellular processes including phospholipid synthesis, autophagosome formation (119,120), mitochondrial fragmentation (121), replication and distribution of mtDNA (122), and cell signaling (123,124), to name a few. ER–mitochondria communication is also intimately involved in mitochondrial activity and metabolism. Early studies from Denton et al. and McCormack et al. showed that several dehydrogenase enzymes involved in the Krebs cycle are activated by an increase in intramitochondrial Ca2+ (125,126). It is now known that release of Ca2+ from ER stores through IP3R Ca2+ channels generates Ca2+ “hot-spots,” promoting Ca2+ entry into the mitochondrial matrix, thereby increasing the production of reducing equivalents (NADH and FADH2) and ATP synthesis (126-130). Therefore, the close apposition of mitochondria to the ER is essential for the transfer of Ca2+ and metabolic regulation.
From a physical point of view, ER–mitochondria contact sites must be tightly regulated. The gap between ER and mitochondria is in the 10 to 30 nm range (Fig. 1) (131,132). This distance allows the accommodation and proper distribution of the protein entities that tether mitochondria to the ER. For efficient transfer of Ca2+, IP3R in the ER side and VDAC1 on the mitochondrial side are connected by the chaperone Grp75 (133,134). Physical binding of the organelles is maintained by additional proteins, such as Mfn-2, located on both the OMM and the ER (135), calnexin and PACS-2 on ER (136,137), and the recently described OMM protein FUNDC1, that interacts with calnexin (Fig. 1) (138).
Given the involvement of ER–mitochondria communication in mitochondrial metabolic activity, it is not surprising that it can also impact systemic metabolism. Genetic deletion of MAM constituent proteins interferes with proper liver and muscle insulin signaling in vivo. Conversely, increasing MAM formation has the opposite effect, protecting against palmitate-induced IR (7,14). Reduction of MAM formation in these tissues results in poor plasma glucose handling, highlighting the profound metabolic impact of MAM stability (7,8,14). In the opposite direction, mitochondria can regulate ER functions. For instance, studies in L6 myotubes show that palmitate-dependent mtDNA damage promotes ER stress and that protecting mtDNA from damage reduces markers of ER stress (139,140).
Regulation of mitochondrial content: Biogenesis and mitophagy
The processes discussed thus far are mechanisms associated with the response of the existing mitochondrial pool to different internal or external cues in order to sustain mitochondrial health and respond to energy demands. However, under certain circumstances total mitochondrial mass needs to change to meet cellular demands. This is achieved through 2 opposing mechanisms, namely mitochondrial biogenesis and autophagic, lysosomal degradation of mitochondria, also known as mitophagy (44,141).
Mitochondrial biogenesis involves the synthesis of new mitochondria from pre-existing mitochondria. It is a complex process that involves mtDNA replication, synthesis of mtDNA and nuclear DNA-encoded proteins, and the formation of new lipid membranes (142). Although a number of transcription factors are known to be involved, such as NRF-1/2, TFAM, and ERRα (142), the ultimate coordinator of mitochondrial biogenesis is PGC-1α. First described as a thermogenic factor in fat tissue (143), PGC-1α was soon after shown to induce the expression of ETC proteins and increase mitochondrial activity (144,145).
Degradation of mitochondria involves the coordinated action of both the general autophagy machinery and the specific signaling from mitochondria. Mitophagy is a “selective” form of autophagy, in the sense that mitochondria are specifically marked for processing through the autophagic machinery (146). The most well-studied mechanism for mitophagy involves the PINK1/Parkin pathway. When mitochondrial membrane potential is lost, the PTEN induced kinase 1 (PINK1) is stabilized at the OMM where it recruits and phosphorylates the E3 ligase protein Parkin, which, in turn, polyubiquitinates proteins in the OMM ((147-149). Adaptor proteins recognize these ubiquitinated tags and direct the mitochondria for degradation via autophagosomes (150). In addition, proteins, such as BNIP3, Nix/BNIP3L, and FUNDC1, can elicit autophagy-mediated degradation of mitochondria independent of Parkin or ubiquitination (Fig. 1) (151-153).
Mitochondrial generation and degradation are usually intimately associated and occur simultaneously. For instance, myoblast differentiation is accompanied by an increase in both PGC-1α and in markers of mitophagy (154,155). In vivo, mitochondrial biogenesis in skeletal muscle in response to exercise occurs in parallel with an increased expression of autophagy-related genes (156,157). Both genetic and pharmacological reduction of either PGC-1α or autophagic activity results in the inhibition of the opposing process (156,158). Mechanistically, protein kinase AMPK (159) or the protein PARIS (160,161) have been shown to coordinately modulate mitochondrial biogenesis and mitophagy.
Retrograde mitochondrial signaling: Beyond ATP
Mitochondria are well known for their central role in generating ATP, but as previously mentioned, these organelles also have biosynthetic and intracellular signaling functions (Fig. 2) (162). These mitochondrial processes act together with the rest of the cell to maintain homeostasis or adapt to environmental changes. For instance, the Krebs cycle in the mitochondrial matrix generates metabolic intermediates (eg, acetyl-coenzyme A) that can be used to generate new cellular molecules (biosynthetic activity), as well as FADH2 and NADH to provide reducing equivalents to feed the ETC for ATP synthesis (bioenergetic activity). ETC activity, in turn, is coupled to the generation of mROS, which can activate redox-sensitive proteins in the cytoplasm (signaling activity) modulating function of the whole cell (48).
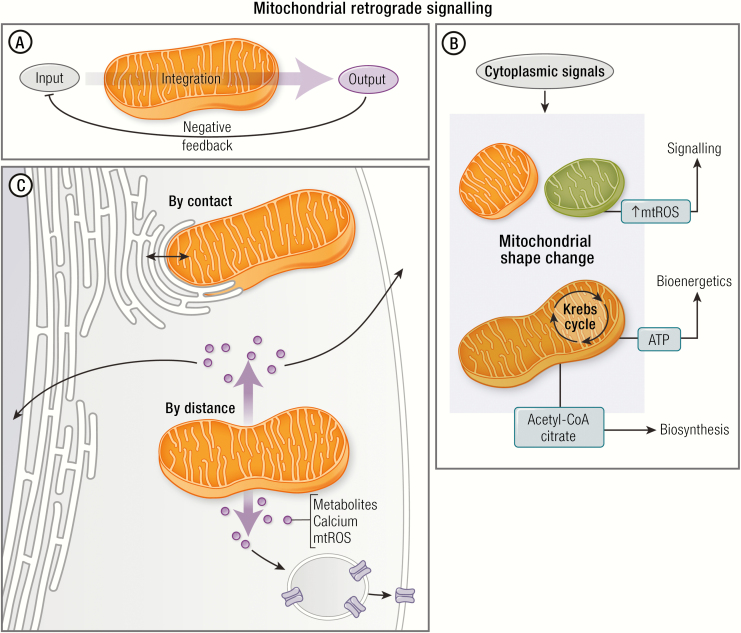
Figure 2.
Mitochondrial retrograde signaling. (A) Mitochondria can be considered as an integration node. This organelle receives signals from the cytoplasm (Input), integrates the information, and generates responses (output). This response may regulate several cellular functions, generating a negative feedback on the input. (B) Cytoplasmic signals induce morphological and functional changes in mitochondria that regulate several intracellular functions such as signaling (eg, mROS generation), bioenergetics (ATP synthesis), and biosynthetic (eg, acetyl-CoA) roles. These roles are necessary for the whole cell function. (C) There are 2 mechanisms by which mitochondria regulate whole cell function: (1) by contact (eg, ER–mitochondria interaction), and (2) by distance (eg, mROS activate transcription factors to modulate nuclear gene expression). Communication by distance would include several molecules, such as small metabolites, peptides, and ions. These molecules would regulate nuclear gene expression, vesicle trafficking, and cell death, among others.
A current challenge in the field is to identify specific mitochondrial processes driving specific pathological conditions. Currently, most interventions targeting mitochondria affect every other organelle function. For example, inhibition of the ETC with specific drugs (eg, Antimycin A) reduces oxygen consumption rate (OCR) but also reduces ATP synthesis (bioenergetic), damps tricarboxylic acid (TCA) activity (biosynthetic intermediate), and increases mROS production (signaling). Therefore, interpretation of experimental results from these approaches can be difficult and need to be taken in the context of the whole system.
There is overwhelming evidence that mitochondrial activity impacts intracellular signaling pathways (163,164). It is also clear that multiple mechanisms are involved (Fig 2). Certainly, as previously discussed, production of mROS and the subsequent redox modification of cytoplasmic proteins are the mechanisms most frequently considered relative to NCDs (98). As an example, mROS has been shown to impact cell proliferation and differentiation via activation of redox-sensitive proteins such as HSP-70 (165). Mitochondria are also key players in a metabolic–genomic regulatory axis because they regulate levels of key metabolites (NAD+, acetyl-CoA, alpha-ketoglutarate, ATP, among others) used in epigenetic chromatin modification (Fig. 2) (166,167). Another emerging example are mitochondria-derived peptides (168). The most recently described is the MOTS-c peptide that is derived from an open reading frame in the mitochondrial 12S rRNA-c gene (169). MOTS-c activates AMPK and regulates nuclear gene expression in response to metabolic stress (170). These mechanisms by which mitochondria control the cell functions beyond the ATP production are collectively known as retrograde signaling (Fig. 2) (164).
Although the retrograde signal was initially described in yeast this signaling pathway is now receiving greater attention in mammalian cells. Crucial questions remain. How do mitochondria communicate stress signals to another cellular compartment? Which transduction mechanisms are involved? What cytoplasm factors respond to and/or regulate mitochondrial signaling? Current omics technology, high-resolution microscopy, and base-machine learning technology create a formidable stage to study the retrograde signaling of mitochondria in mammals and its role in the pathology, prevention, and treatment of NCDs.
Regulation of mitochondrial enzymatic activity
Mitochondrial activity exhibits several enzymatic regulation nodes. Some of these nodes are controlled by metabolic intermediates such as ADP/ATP, NAD+/NADH, acetyl-CoA, among others (171). The levels of these metabolic intermediates are in turn influenced by the energetic state of the cell, providing a tight relationship between energy demand and mitochondrial metabolism.
ADP is thought to be one of the primary molecules controlling the rate of oxidative phosphorylation (172). The addition of adenosine diphosphate (ADP) increases the OCR of isolated mitochondria and permeabilized cells. Furthermore, declines in ADP levels decreases OCR even in presence of excess ETC substrates (172). Thus, ADP produced by ATP hydrolysis during cellular metabolism (energy demand) can signal an increase in OCR (energy supply).
Another point of control is the mitochondrial Ca2+-sensitive dehydrogenases that supply NADH and FADH2 to the ETC (173). Activation of these dehydrogenases increases ATP synthesis. Among these is pyruvate dehydrogenase (PDH), the rate-limiting enzyme of glucose oxidation that is inhibited when phosphorylated by pyruvate dehydrogenase kinase (PDK), or in the presence of acetyl-CoA or NADH (171). An increase in mitochondrial Ca2+ activates pyruvate dehydrogenase phosphatase 1 (PDP1c), the phosphatase responsible for dephosphorylating PDH and restoring its activity, and increasing glucose oxidation (173). Inhibiting mitochondrial Ca2+ uptake leads to hyperphosphorylation of PDH, and reduction of both glucose oxidation and OCR (174, 175). There are many additional posttranslational modifications known to regulate mitochondrial metabolism that have been reviewed in detail elsewhere (176,177).
Mitochondrial dysfunction: What is the gold standard to define it?
Classically, mitochondrial dysfunction has been defined as the inability of mitochondria to sustain ATP synthesis sufficient to satisfy cellular demands (178). However, as discussed above, mitochondrial dysfunction exhibits many faces, causes, and consequences. For this reason, diagnosis of mitochondrial dysfunction needs to integrate measures of the organelle’s function and structure with the availability of substrates and reducing equivalents. Ultimately, these parameters need to be placed in the context of human physiology. Despite this complexity, functional readouts from isolated mitochondria can still yield important insights and provide a first step toward integration with other cellular parameters.
OCR and ATP production are fundamental functions of mitochondria. Brand and Nichols propose that OCR is the parameter that can provide the most information (179). OCR can yield insights into the processes of substrate transport, substrate metabolism, proton pumping, electron delivery through the ETC, as well as others. Measuring ΔΨmt can provide indirect information regarding proton motive force, although in many ways it can be less sensitive than OCR (179). For instance, deletion of complex I subunit NDUFAF3 reduces OCR and ETC activity without changing the mitochondrial membrane potential (180). This is because ΔΨmt can be generated and maintained via the reverse activity of the F0F1 ATP synthase even in the absence of oxidative phosphorylation and ETC activity (181). Thus, ΔΨmt cannot always be taken as an indication of mitochondrial metabolic activity. Measures of mROS generation, ATP production, mitochondrial mass, morphology, and distribution are likewise not exempt from false-positive or negative errors and need to be interpreted with appropriate caution (179).
Recently, Fisher-Wellman et al. designed and validated an elegant multiplexed assay platform that provides a comprehensive phenotyping of mitochondrial functions under conditions that model in vivo changes in energy supply and demand (182). They provide detailed rationales for each of their assays, offering an excellent resource for anyone undertaking similar analyses. This important approach, however, uses isolated mitochondria and is therefore devoid of information regarding the contribution of the intact mitochondrial network or its interaction with other organelles. An additional issue is that mitochondrial isolation from whole organs yields mitochondria from a variety of cell types which could mask important changes in a specific population. Despite these limitations, when the technique was applied to mitochondria isolated from the hearts of mice on a high-fat diet, there was a generalized decrease in oxygen flux, regardless of substrate, and an increase in proton leak as well as increased H2O2 production (183). Although these changes were small, they demonstrate the power of the approach and validate the presence of fundamental changes in cardiac mitochondria in the setting of obesity. Although the approach proposed by Fisher-Wellman et al. does not incorporate measures of mitochondrial Ca2+ retention or the mitochondrial permeability transition pore activity (mPTP), it represents a major advancement in the assessment of mitochondrial function, complementing other comprehensive approaches (184-186). Future studies should focus on developing the same kind of workflow to define cell type-specific mitochondrial adaptation in intact living cells and tissues.
Other methodological approaches have been developed that avoid the invasiveness, the lack of biological context and tissue destruction. Near infrared spectroscopy gives information regarding OCR and its reduction by cytochrome C oxidase in human and murine skeletal muscles (187). Magnetic resonance spectroscopy can also be used to measure the OCR and ATP synthesis in vivo, providing measures of mitochondrial coupling in skeletal muscle (188). These 2 techniques avoid artefacts associated with tissue extraction but so far have only been applied to skeletal muscle.
Mitochondrial (dys)function in NCDs
Remarkably, a common feature of many NCDs is a bioenergetic crisis characterized by a reduction in mitochondrial function. In this section, we will discuss the role of mitochondrial dysfunction in NCD pathophysiology.
Mitochondrial dysfunction in obesity
The mitochondrial network is an important node that integrates nutrient supply with the energy requirements of the cell. In obesity, nutrient oversupply generates mitochondrial alterations leading to a dysfunctional organelle. While mitochondrial fusion is associated with higher oxidative capacity and ATP production, mitochondrial fission is associated with an opposite response. Changes in mitochondrial morphology have been documented in a variety of organs in the setting of metabolic disease (189). Importantly, fission/fusion events depend partly on the balance between nutrient supply and demand. Starvation and higher metabolic demand (eg, during exercise) are associated with increased network fusion (190,191). The higher ΔΨmt favored by fusion increases the potential for ROS generation, yet fasting or exercise would tend to decrease the NADH/NAD+ ratio, lowering the potential for mROS generation (83). In turn, nutrient overload (eg, high glucose) promotes mitochondria fragmentation that occurs in conjunction with only a transient increase in mROS generation (3); however, this coupled with a sedentary lifestyle that lowers ATP demand, raising the NADH/NAD+ ratio could lead to increase in mROS generation. It may be that increased mitochondrial fragmentation in metabolic disease is a compensatory response to limit this by reducing OCR. Compelling evidence indicates that oxidative stress plays a causal role in the development of obesity-related diseases, but the exact ROS source is still under investigation. Due to the strong association between mitochondrial function and nutrient availability, mROS may play a central role in adipogenesis. Recently, Tormos et al. demonstrated in primary human mesenchymal stem cells undergoing differentiation into adipocytes that complex III-derived ROS production promotes adipocyte differentiation via mTORC1 (4).
NAFLD is a liver disease strongly associated with being either overweight or obese. The disease can progress to inflammatory nonalcoholic steatohepatitis, liver fibrosis, and cirrhosis, driven finally to liver failure (192). Importantly, no drugs/therapies are approved for NAFLD treatment. It has been suggested that a metabolic shift, characterized by a reduction of mitochondrial lipid oxidation, is responsible for NAFLD development. Although there is elevated mitochondrial function in response to the higher lipid availability in NAFLD, this adaptation is insufficient to prevent lipotoxicity in the long term. Teodoro et al. showed in a choline-deficient NAFLD model that mitochondrial function exhibits a biphasic response (5). They observed an increment in the ETC components in the short term; however, the long term was characterized by a reduction of these components, alongside a drop in ΔΨmt and ATP synthesis (5). Moreover, mitochondrial network remodeling appears to be crucial for NAFLD development. For instance, in primary mouse hepatocytes, mitochondrial fission plays a vital role in the progression of NAFLD (6). An elegant work of Hammerschmidt et al. recently showed that prevention of mitochondrial fission by deletion of Mff rescues NAFLD in mice fed a high-fat diet (15). In spite of the strong relationship between mitochondrial fission and NAFLD, the mechanism that connects these events remains poorly understood.
Are mitochondrial perturbations upstream of insulin resistance?
Current evidence indicates that in addition to increasing glucose uptake, insulin also stimulates mitochondrial activity in adipocytes (193), and skeletal and cardiac muscle (80,194). Conversely, mitochondrial damage can lead to impaired insulin signaling (9,10). Canonical insulin signaling that promotes glucose uptake involves sequential activation of a phosphorylation cascade. This signaling initiates when insulin binds to its receptor, leading to the activation of several proteins, including IRS1, PI3K, and Akt (proximal components of insulin signaling) (195,196). Deficiencies in this pathway have been used as molecular markers of IR. However, the removal of plasma membrane cholesterol restores insulin-stimulated glucose uptake in adult skeletal muscle without recovery of Akt phosphorylation in obese mice (197). Also, the reduction of Akt phosphorylation is not consistently observed across different models of IR (198). These results suggest that a mechanism independent of Akt could be responsible for the development of IR (199).
As mentioned, optimal mitochondrial function is ensured by a quality control system which is tightly associated with fusion–fission events. There are studies that point to a role for mitochondrial dynamics in IR development. For instance, Sebastián et al. showed that Mfn2 deficiency increases H2O2 production leading to IR in skeletal muscle and liver (8). In addition, del Campo et al. showed in L6 myotubes that deletion of Mfn2 and OPA1 (to promote mitochondrial fission) reduced insulin-dependent GLUT-4 translocation and impaired mitochondrial Ca2+ uptake. Interestingly, inhibition of mitochondrial Ca2+ uptake recapitulated IR without affecting mitochondrial dynamics (9). These results suggest that mitochondrial Ca2+ mishandling is downstream of mitochondrial fragmentation and provides signals that promote IR.
On the other hand, Taddeo et al. described that opening of mPTP is required to develop IR in skeletal muscle. In vitro, inhibition of mPTP with cyclosporin A prevented insulin resistance evoked by C2-ceramide or palmitate in L6 myotubes. Moreover, mice lacking cyclophilin D were protected against diet-induced IR in skeletal muscle. Interestingly, although no changes were observed in the mitochondrial morphology, the ability of mitochondria to take up Ca2+ was reduced in the different models of insulin resistance (200).
We and others showed that mROS promotes IR in skeletal muscle cells and adipocytes (11,12). We recently demonstrated that mROS is sufficient to produce IR without affecting either the proximal component of insulin signaling or mitochondrial respiration (10). Of interest, mROS can activate mitochondrial fragmentation (201). Some authors have proposed mROS generation as a physiological signal to prevent nutrient overload (11). This signal would be crucial at limiting further substrate uptake when fuel supply exceeds metabolic demand (eg, overnutrition and sedentarism). In fact, antioxidant treatment of IR has shown contradictory results (202,203). It is worth noting that the subcellular ROS source may determine the effect of these molecules. For instance, cytoplasmic NADPH oxidase 2-dependent ROS production is necessary to promote GLUT4 translocation in response to insulin (204) and physical exercise (205) in muscle cells. Thus, the development of mitochondria-targeted antioxidants to prevent mROS-mediated damage could have important therapeutic implications.
Hammerschmidt et al. showed that the sphingolipid ceramide 16:0 physically interacts with the adaptor protein Mff to promote mitochondrial fission and hepatic IR (15). Interestingly, downregulation of Mff prevented ceramide-dependent IR. Of note, mitochondrial fission is often associated with a ΔΨmt depolarization which can reduce the ability of mitochondria to take up and buffer Ca2+ (43). However, the authors did not evaluate the mechanism by which mitochondrial fragmentation generates IR. This will be an important question to focus on in the future.
The ER–mitochondria interaction has also been suggested as critical for maintaining insulin sensitivity. Disruption of the ER–mitochondria interaction dampens insulin action in neonatal cardiomyocytes and in skeletal muscle cells (7,13). Tubbs et al. elegantly showed that disruption of ER–mitochondria interactions is a common characteristic in various mouse models of obesity and T2DM (7). Of interest, an experimental increase in ER–mitochondria interactions prevented palmitate-induced IR in human myotubes. However, no correlation was observed between insulin sensitivity and ER–mitochondria interactions in myotubes from lean, obese subjects and subjects with T2DM (R2 = 0.11, P = .04). However, it is important to note that this author used insulin-dependent Akt phosphorylation as a molecular marker of insulin signaling, instead of a more specific readout such as GLUT-4 translocation or glucose uptake. Although conflicting findings have been published regarding the role of ER–mitochondria interactions in insulin sensitivity in the liver (14,206), these are united regarding the concept that a proper interaction between these 2 organelles is crucial to maintain insulin sensitivity in hepatocytes.
Much of the evidence connecting mitochondrial dysfunction with IR has been obtained in the setting of established IR, making it difficult to determine whether mitochondrial dysfunction is cause or consequence. Recently, Fazakerley et al. (10) reported that 2 hours of mitochondria-targeted paraquat (mPQ) treatment, which promotes mROS production by redox cycling at the flavin site complex I of ETC (207), leads to IR in 3T3-L1 adipocytes, L6-myotubes, and adult skeletal muscle (10). Of interest, mPQ treatment did not affect mitochondrial respiration or insulin-dependent phosphorylation of either Akt or TBC1D4 (10). This suggests that acute mROS generation is sufficient to promote IR independent of AKT/TBC1D4 signaling. However, whether mPQ elicits effects other than increased mROS generation (eg, mitochondria fission) to prevent insulin-dependent GLUT4 translocation requires further investigation.
A pattern of mitochondrial alterations (eg, mROS, mitochondrial fission, impaired ER–mitochondria interaction, mitochondrial Ca2+ mishandling) appears to be conserved in the insulin target tissues, such as hepatocytes, adipocytes, skeletal myocytes, and cardiomyocytes under the IR state. However, the important question that remains is whether the features of the mitochondrial dysfunction are connected or converge on a common signal that promotes IR. Further efforts are necessary to dissect the molecular mechanisms by which mitochondrial damage generates IR across different cellular and animal models of IR.
Impact of mitochondria function on insulin release
Impaired or overtly absent insulin secretion is a common feature of type 1 DM and long-lasting T2DM (208-210). Mitochondrial function is vital for proper insulin release from pancreatic β-cells. A simplistic overview of this connection is as follows: after its uptake through GLUT2, glucose is rapidly metabolized by glycolysis to generate pyruvate, which is later incorporated to the TCA. Increased Krebs cycle activity leads to a rise in mitochondrial NADH levels, mitochondrial membrane hyperpolarization, and, ultimately, an increase in ATP synthesis. Once it is translocated to the cytosol, ATP binds to and closes nucleotide-sensitive KATP/SUR1 channel complexes, leading to β-cell membrane depolarization and generation of cytosolic Ca2+ elevations. Finally, an increase in cytosolic Ca2+ leads to the fusion of insulin-containing granules to the plasma membrane, releasing their content outside the cell (211,212).
This linear flux of events has been challenged and complemented over time. Contributing roles for insulin secretion have been associated with the NADH cytosolic–mitochondrial shuttle (213), mitochondrial Ca2+ signaling (214,215), mitochondria-originated ROS (216,217), TCA metabolic intermediates (218), and so on. As noted, most of these signals are related in some way to mitochondrial function, emphasizing the role of this organelle in β-cell function.
The idea that mitochondria are essential for insulin release is supported by the identification of UCP2 as a quantitative trait loci for obesity and human insulin-dependent T2DM (219). In vitro studies later demonstrated that overexpression of this uncoupling protein leads to ΔΨmt hyperpolarization in response to a glucose challenge in rat islets. In turn, this decreased mitochondrial response resulted in impaired ATP synthesis, and, therefore, diminished insulin secretion (16,17,18). Accordingly, islet levels of UCP2 were increased in obese mice and biopsies from T2DM patients, alongside reduced ATP levels (17,19). The presence of an A/G polymorphism at -866 position on UCP2 promoter has been associated with reduced glucose-induced insulin secretion in human islets, in a manner dependent on A-allele dosage (220). Mechanistically, –866A/A is preferentially bound by the β-cell transcription factor PAX6, which promotes its expression over their G-allele counterparts (221). Therefore, it seems possible that upregulation of UCP2 in β-cells could be responsible, at least in part, for the reduced insulin secretion in genetically susceptible subjects carrying the polymorphism. Additional studies identify this polymorphism as having a population-specific role relative to predisposition for obesity and T2DM (221-223).
A Korean population study revealed a similar effect of PARK2 gene variant (encoding Parkin protein) on insulin release (20). The presence of the G allele on rs10455889 and rs9365294 SNPs was associated with increased fasting glucose and reduced circulating insulin in males (20). In vitro studies demonstrated that reduced Parkin levels in INS-1 β-cells results in reduced glucose-induced ATP synthesis, reverberating on insulin secretion (20).
Mitochondrial morphology impacts insulin secretion from pancreatic β-cells. For instance, Drp-1 deletion results in impaired glucose-stimulated insulin secretion, despite generating a more connected and fused mitochondrial network (21-23). Altered metabolite delivery into mitochondria resulting in reduced ATP synthesis, or mishandling of Ca2+ mitochondrial signaling have been proposed as possible mechanisms for the reduced insulin release (22, 23). Notably, β-cell–specific deletion of OPA1 recapitulates the aforementioned results (24). Mitochondrial dynamics in tissues other than the pancreas also influences insulin secretion from β-cells. Deletion of the profusion protein Mfn1 in POMC neurons results in reduced glucose-induced insulin secretion from pancreatic islets (224). Remarkably, deletion of OPA1 or Mfn2 in the same neurons did not alter insulin secretion (224). Together, these observations point to a crucial role for mitochondrial dynamics in insulin secretion. The question is, however, which morphological state of the mitochondrial network is better suited for glucose sensing and the subsequent insulin release in diabetic patients. As both exaggerated fragmentation and elongation result in altered insulin secretion, it seems that an equilibrium between these opposing processes is the most plausible answer.
The (dys)function of mitochondria in cancer
Since the seminal work published by Warburg (1956) (225), it is well known that cancer cells in culture catabolize glucose by lactic fermentation even in presence of oxygen. This suggests a potential role for mitochondrial metabolism in cancer. In fact, mutation of key mitochondrial enzymes is associated with cancer progression in humans (226). Thus, mitochondrial metabolism is frequently considered an important player in the development and progression of cancer.
PGC1-α, which promotes mitochondrial biogenesis has been proposed as a tumor suppressor (227). In fact, the hypoxia inducible factor-1 alpha (HIF-1α) has been shown to reduce PGC1-α levels leading to a higher glycolytic rate in tumorigenic cells (25). Importantly, mitochondria still maintain their biosynthetic activity in cancer cells, thus providing metabolic intermediates for cell proliferation and tumorigenesis (228).
PGC-1α expression may also play a key role in malignancy and survival of some types of cancer. In hepatocellular carcinoma, PGC-1α promotes cellular migration and invasion (30). The metabolic switch induced by PGC-1α is sufficient to enhance mitochondrial function and cell motility needed to develop an invasive phenotype in circulating cancer cells (31). Conversely, when circulating cancer cells achieve their target tissue, they undergo a metabolic switch to aerobic glycolysis that ensures the cell survival (229).
Recently, Tosatto et al. showed a role for mitochondrial Ca2+ uptake in breast cancer progression. In triple-negative breast cancer—the most aggressive breast tumor subtype—mitochondrial Ca2+ uptake and mROS are required for tumor growth and metastasis in vivo. Mitochondrial Ca2+ accumulation could promote mROS production which is necessary for HIF-1α activation (32). In line with these results, Cárdenas et al. reported, in several tumorigenic cancer cell lines and in vivo tumors, that inhibition of Ca2+ transfer from ER to mitochondria reduces mitochondrial Ca2+ levels, generating a bioenergetic crisis and reducing cell viability (33). In the mitochondrial matrix there are several Ca2+ sensitive enzymes—such as isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase—in the TCA cycle. It is possible that constitutive mitochondrial Ca2+ accumulation is required to maintain sufficiently high TCA cycle activity. In fact, TCA are the main source of biosynthetic intermediates that are required to sustain the high rate of cancer cell proliferation (34). Interestingly, Cárdenas et al. provided evidence that cancer cell death induced by the reduction of mitochondrial Ca2+ uptake was prevented by exogenous nucleoside supply (33).
It has been described that mtDNA also plays a key role in cancer development. An extensive description of mtDNA copy number was performed in several types of human cancer biopsies. As a common feature, and despite the large variability among tissues, several cancers displayed a reduction in mtDNA (230) and mtRNA copy numbers (231). Of interest, mtRNA expression correlated positively with the overall survival of the patients (231). Despite these results, the mechanisms that link mtDNA copy with cancer phenotype requires further investigation.
mtDNA mutations have also been strongly associated with oncogenesis and metastasis. The characteristic way of how mtDNA is replicated favors heteroplasmic point mutations in both strands of mtDNA (232). In fact, a large proportion of cancers have ~60% of these somatic mtDNA mutations (26). The role of mtDNA mutation in carcinogenesis has been supported by clinical studies. In patient-derived prostate cancer biopsies, a correlation between cancer aggressiveness and mtDNA mutations was observed (27). Moreover, mtDNA replacement of nonmetastatic cancer cells with mtDNA from a highly metastatic cancer cell increased their malignancy and metastatic potential (28). Based on these data and recent findings in cells with a tunable grade of mtDNA point mutations, it is possible that mitochondrial dysfunction derived by mtDNA mutation stimulates oncogenesis and metastatic potential (232). Nevertheless, an excessive grade of mtDNA mutation and mitochondrial dysfunction deeply alter cell viability and reduce the aggressiveness of cancer cells (232).
Based on single cell RNAseq, Xiao et al. showed that malignant cells are enriched for the expression of genes that confer metabolic plasticity (29). The authors observed that both glycolysis and Krebs cycle were enhanced in cancer cells. These data could explain why cancer cells can adapt to their environment so rapidly and change their phenotype favoring growth and invasiveness.
Altogether, these results suggest that a variety of mitochondrial functions can contribute from different angles to the development of a pathological state. Therefore, it will be important to fully understand the relative contribution of each to tumor development and progression.
Mitochondrial dysfunction in cardiovascular diseases
CVDs are the main cause of death worldwide. Initial observations showed that mitochondrial alterations are characteristic of dilated cardiomyopathy in humans and mice (233,234). Also, mitochondrial damage plays a central role in vascular pathologies. For instance, mitochondrial DNA damage promotes atherosclerosis by increasing smooth muscle cell proliferation (35). To date, it is widely believed that mitochondrial damage is involved in the pathophysiology of CVD (114)
Heart muscle exhibits a high metabolic demand, and this may explain the central role mitochondrial metabolism plays in its pathophysiology. Pennanen et al. showed that norepinephrine promoted mitochondrial fragmentation which correlated with cardiomyocyte hypertrophy. Importantly, while a dominant-negative Drp-1 prevented the norepinephrine-dependent hypertrophy, the downregulation of Mfn2 was sufficient to promote cardiomyocyte hypertrophy (36). Norepinephrine also disrupted ER–mitochondria interactions, impaired mitochondrial Ca2+ handling, increased mROS, and reduced mitochondrial efficiency, thereby driving cardiomyocytes to an energetic crisis (13). Interestingly, Mfn1/2 deletion in adult heart reduces fractional shortening, increases left ventricular end-diastolic volume and promotes dilated cardiomyopathy (37). Deletion of Drp-1 in adult heart likewise leads to heart failure, highlighting the need for both fission and fusion processes. Finally, Opa1+/– mice are more sensitive to pathological cardiac remodeling induced by aortic constriction and to ischemia–reperfusion (I/R) damage (38).
Recently, Parra et al. showed both in vitro and in vivo that reduction of the regulator of calcineurin 1 (RCAN1) promotes mitochondrial fission by a calcineurin–Drp-1 dependent mechanism. In RCAN-1 depleted cardiomyocytes, mitochondria exhibited reduced capacity for Ca2+ uptake, and decreased OCR. In addition, RCAN1 knockout mice were more susceptible to ischemia/reperfusion damage. Interestingly, pharmacological inhibition of Drp-1 restored mitochondrial function and conferred protection against ischemia/reperfusion damage (39). These results highlight the role of mitochondrial metabolism in the development of heart diseases and suggest Drp-1 as a therapeutic target.
Key Experiments and Unaddressed Questions
Important questions require further investigation.
Is mitochondrial dysfunction a cause or consequence of NCDs? This is a contentious point because most studies have assessed mitochondrial parameters in the diseased state rather than across its progression.
What specific mitochondrial properties promote NCDs development? Mitochondrial damage has many faces including impairment of mitochondrial dynamics, ER–mitochondria interactions, mROS production, mitochondrial Ca2+ handling, mitochondria respiration, among others. Thus, a big challenge is to determine which alterations are involved in NCDs, which are cause or consequence, and how they interact under pathological conditions.
How does mitochondrial dysfunction lead to NCDs? Do all mitochondrial changes associated with NCDs share a common, downstream pathological mechanism?
Emerging omics technology and machine learning approaches generate an outstanding opportunity to explore the effect of specific mitochondrial alterations in whole cell/organism physiology to help address these questions.
Mitochondrial Dysfunction: Can it Be Reduced or Reverted?
Caloric restriction modulates mitochondrial function
Reduction of mitochondrial respiratory capacity is reported as a common feature of several NCDs. For instance, short-term high-fat diet promotes adipose tissue mitochondrial uncoupling. This uncoupled respiration generates local hypoxia and IR of the adipose tissue (235). Furthermore, obese, IR and diabetic subjects present reduction of their respiratory capacity on different tissues such as skeletal muscle (236-238), liver (237), and adipose tissue (239). The decrease in the OCR has also been observed in some CVDs such as atherosclerosis (240) and hypertension (241-243). Similarly, cancer has been proposed as a metabolic disease (244,245). However, because of the high diversity of cancer cell types in a tumor and the number of different tumor types, it is not possible to conclude that mitochondrial dysfunction is a common feature of all cancers (246). Given the common feature of mitochondrial dysfunction in NCDs, can similar approaches be taken to improve mitochondrial function across this diversity of pathologies? In the following paragraph we discuss mechanisms by which caloric restriction (CR) may improve mitochondrial function and its application to NCDs.
As previously mentioned, mitochondria are important nodes that integrate environmental clues associated with nutrient availability. CR is well known as a potent and reliable strategy to reduce the incidence of NCDs among different species including mice and nonhuman primates (247-249). In Rhesus monkeys, for instance, CR increases lifespan and also reduces the prevalence of obesity, CVD, cancer, and T2DM (247,248,250).
It has been proposed that CR improves mitochondrial function by modulating molecular targets related to energy sensing (Fig. 3). One of the most important energy sensors is the AMPK protein, which is upregulated in different experimental models of CR (251-253). AMPK is an important promoter of mitochondrial biogenesis through direct phosphorylation of PGC-1α at Thr177/Ser538 inducing the expression of several nuclear genes involved in mitochondrial biogenesis and adaptations (254). AMPK mRNA levels are decreased in both obese and IR humans alongside a reduction of the NAD+-dependent deacetylase SIRT1 (255).
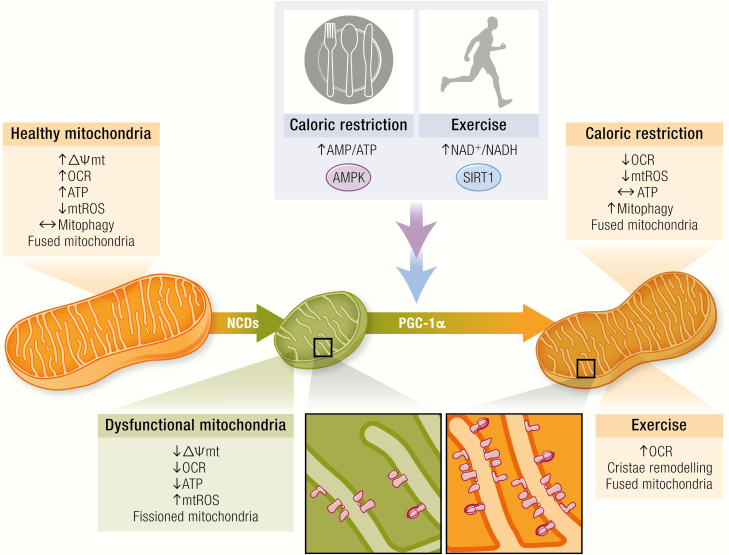
Figure 3.
Caloric restriction and exercise restore mitochondrial dysfunction. NCDs induce an alteration of functional and structural features of mitochondria. The energetic stress challenge induced by caloric restriction and exercise create a negative balance by altering the AMP/ATP and NAD+/NADH ratios. These changes are sensed by 2 main proteins, AMPK and SIRT1, that are able to phosphorylate and deacetylate correspondingly the mitochondrial biogenesis regulator PGC-1α. Alteration of energy homeostasis increases mitophagy, changes mitochondrial morphology (more elongated) allowing mROS production to be reduced, antioxidant capacity to be increased, and, on a differential manner, oxygen consumption rate (OCR) to be altered to ensure more efficient ATP to be maintained during caloric restriction or ATP synthesis for muscular work. These ATP-driven differences are allowed by more coupled electron transport chain assembly induced by changes in mitochondrial mass and a fused pattern encouraged by initial energetic sensors.
In addition to the previously discussed role of the NADH/NAD+ ratio in mROS generation, NADH and NAD+ are important regulators of many enzymes and transcription factors. CR-dependent increases in NAD+ are sufficient to promote sirtuin activation of PGC-1α (Fig. 3) (256,257). CR also induces mitochondrial biogenesis and improves mitochondrial function by a PGC-1α-dependent mechanism (258-260). However, skeletal muscle-specific overexpression of PGC-1α did not promote adaptations typical of CR and instead increased the susceptibility to high-fat diet-induced IR (261). These results suggest that under CR, PGC-1α may play a cell- or organ-specific role.
In vitro experiments have shown that CR modulates not just mitochondrial content but also its efficiency. López-Lluch et al. showed in vitro and in vivo that despite finding no change in ATP levels, CR elevated mitochondrial mass and reduced both mitochondrial respiration and mROS production (262). This maintenance of ATP levels implies a more efficient or “coupled” ETC under caloric restriction. Remarkably, these adaptations have been consistently observed in human and murine models under CR (263-265). Importantly, caloric restriction has been associated with a more connected mitochondrial network and increased mitophagy, both of which are necessary to maintain a healthy mitochondrial network (77).
Like any intervention, CR has limitations. For instance, in the long run, CR increases the risk of developing some pathologies such as osteoporosis, slow wound healing, and sensitivity to cold environments, among others (266).
Physical exercise and mitochondrial function
Physical inactivity is associated with the prevalence of NCDs (Fig. 3). Like CR, physical exercise generates a negative energy balance that improves mitochondrial function (Fig. 3). These 2 strategies trigger similar intracellular molecular pathways. Pioneering work by John Holloszy, more than 50 years ago, showed that sustained, strenuous physical exercise doubled mitochondrial content and activity in skeletal muscle (Fig. 3) (267). It also increased mitochondrial coupling, suggesting important changes in efficiency and function. Since then, experimental evidence has repeatedly shown the profound effect of physical exercise on mitochondrial function/structure, although the mechanisms involved are not completely understood (268).
Mitochondrial responses to physical exercise can be divided in 2 types: acute and chronic adaptations. Acute adaptations involve changes in mitochondrial function during a bout of exercise. Of interest, the change of mitochondrial function during acute exercise are extremely hard to determine. As we previously mentioned, noninvasive methods such as 31P magnetic resonance spectroscopy and near-infrared spectroscopy have been used to estimate mitochondrial function (187, 269). Nevertheless, these techniques are constrained by the enzymatic equilibrium and the subcellular distribution of the molecules.
Currently, most of the evidence available has been obtained from in vitro models of physical exercise, for example, skeletal muscle fibers contraction or permeabilized cells. In vitro muscle fiber activation increases mitochondrial OCR (127) and promotes mitochondria fusion (270), without changing mitochondrial content. This is a relevant model because (1) it avoids exposing the cells to supraphysiological concentrations of mitochondrial substrates; (2) it prevents the artefacts associated with mitochondrial isolation protocols and maintains mitochondrial environment intact (ie, mitochondria–ER interaction). On the other hand, permeabilized muscle allows exposing mitochondria to different metabolites whose concentration changes during exercise. This is an interesting approach, especially because it can be applied to human muscle biopsies. For instance, initial work from Walsh et al. (271). reported that sequential addition of ADP, creatine (Cr), and phosphocreatine (PCr) modulates mitochondrial function. The authors showed for the first time that PCr reduces ADP-stimulated respiration whereas Cr has the opposite effect. Of interest during transition from rest to high-intensity exercise, decreases of PCr/Cr ratio increase ADP-dependent mitochondrial respiration (271). Indeed, the addition of creatine or phosphocreatine in assay media completely changes the interpretation of how chronic exercise training affects mitochondrial function (272). Despite these experimental observations, mitochondrial response during acute exercise will also depend on (1) vasculature, (2) O2/nutrient availability, (3) cardiac output, (4) mitochondrial mass, and (5) total effective surface of capillaries (273). In consequence, conclusions from these in vitro experiments need to be interpreted in the appropriate physiological context.
Chronic adaptations occur in response to regular physical exercise training. These adaptations include increases in mitochondrial mass (274), higher oxidative phosphorylation (275), cristae remodeling in the IMM (276). The specifics of mitochondrial adaptation can depend on the duration, intensity, modality, and frequency of physical exercise. Remarkably, ROS, generated in the cytoplasm, is emerging as an essential signal in mitochondrial adaptation to exercise in heart and skeletal muscle (268,277,278).
Similar to CR, physical exercise promotes the activation of the molecular energy sensor AMPK (254). Acetylation of PGC-1α by GCN5 suppresses its activity, whereas deacetylation by SIRT1 increases its activity. PGC-1α is deacetylated in response to either fasting or endurance exercise. During fasting, deacetylation by SIRT1 controls PGC-1α activity (279), whereas the exact mechanism of exercise-induced deacetylation of PGC-1α remains controversial and may also rely on suppression of GCN5-mediated acetylation (280). Interestingly, supplementation with nicotinamide mononucleotide—a NAD+ precursor that promotes SIRT1 activation—improves mitochondrial function in obese mice (281). In humans, physical exercise increases expression of nicotinamide phosphoribosyltransferase, the rate-limiting enzyme of the NAD+ salvage pathway (282). In line with the idea that this mechanism contributes to mitochondrial biogenesis, nicotinamide phosphoribosyltransferase transgenic mice show higher mitochondrial respiration in response to voluntary wheel training (283). To better understand the molecular mechanisms triggered in response to physical exercise Hoffman et al. undertook a global phosphoproteomic analysis of physical exercise in human skeletal muscle. Remarkably, the majority of the exercise-regulated phosphoinositides and kinases identified had not been previously associated with physical exercise (284). These remarkable findings raise many important questions.
How these global changes are integrated with ROS signals?
How different are the global responses to different types of exercise?
How do different tissues respond to physical exercise?
Are responses attenuated/altered in NCDs?
How does the genetic background impact the global response to exercise?
Which molecular mechanisms are key to the beneficial effects of exercise?
Do different stimuli, such as caloric restriction and physical exercise converge on common pathways? Address these questions will pave the way toward new mitochondria-targeted therapies to combat NCDs.
Current Challenges and Futures Perspectives: From the Bench to Clinical Trials
As discussed, mitochondria play a leading role in many NCDs. Substantial efforts over the last 3 decades to have been directed toward the development of specific mitochondria-targeted therapies. Several mitochondria-related molecules have been identified as possible interventional tools. However, there are many obstacles to advancing mitochondria-targeted drugs toward clinical trials. These concerns include drug pharmacokinetics, biodistribution, and off-target effects, among others (285). The following section will touch on some of the current therapies that are being tested in clinical trials with positive outcomes (286).
The synthetic tetrapeptide SS-31 (also called elamipretide or Bedavia) represents a novel generation of drugs. SS-31 targets cardiolipin, a mitochondria-specific lipid that resides in the IMM (287). SS-31 protects mitochondria form insults by preventing cardiolipin peroxidation, promoting cytochrome C retention and the appropriate supercomplex maintenance at the IMM (287). This peptide has been successfully used in animal models of IR (11) or CVD (288). Notably, a recent study showed that elamipretide also has an acute and cardiolipin-independent effect over mitochondria in cardiac ventricular samples from healthy and heart failure patients. This drug increases complex I-dependent oxygen flux and promotes supercomplex formation in the IMM (289). Randomized clinical trials to treat human pathologies where mitochondria play a critical role have been carried out. In patients with heart failure, elamipretide significantly improved ventricular function without adverse effects in high doses (0.25 mg/kg/hour), placing this molecule as a good candidate for heart failure treatment (290).
Mitoquinone (MitoQ) is another molecule that has been extensively studied. MitoQ is a lipophilic cation consisting of a triphenylphosphonium moiety covalently attached to a ubiquinone (antioxidant CoQ10). It accumulates on mitochondria up to 1000-fold driven by mitochondrial membrane potential and has been shown to reduce mROS levels (291,292). This feature makes it a perfect candidate in those pathologies with an excess generation of mROS. For instance, MitoQ treatment improves mitochondrial function and lipid metabolism in skeletal muscle of obese rats (293). In the same line, as other mitochondrial antioxidants (11), MitoQ rescued insulin sensitivity in different models of IR (294). MitoQ has also shown positive effects in the cardiovascular system. For example, 4 weeks of oral MitoQ treatment prevented the development of arterial stiffness in aged rats (295). In a transverse aortic constriction-induced heart failure model, oral treatment with MitoQ improved mitochondrial function, restored Ψmt, oxygen consumption and β-oxidation (296). A randomized controlled-trial showed that long-term treatment with MitoQ in chronic heart failure patients improved the symptoms and significantly reduced cardiac events, decreasing cardiovascular deaths by 43% (297). Despite the fact that MitoQ has been used in in preclinical cancer models (298,299), to our knowledge, there are not current trials on MitoQ to cancer treatment in humans.
Mitochondria-targeted therapies under current study for their potential role on cancer progression are centered on some metabolic modulators of the TCA cycle. These include isocitrate dehydrogenase (IDH1/2), PDH, and PDK1 inhibitors. Treatment with AG-221 (enasidenib), a selective IDH2 inhibitor in IDH2 mutant patients, results in suppression of the onco-metabolites (R)-2-hidroxyglutarate production in tumor xenograph models (300). In contrast to chemotherapy, AG-221 seems to increase myeloid differentiation and the population of functional and mature neutrophils (300). Early-phase trial AG-221-treated patients have a response rate of 40% (301). AG-221 is currently under clinical trial phase I/II on subjects with advanced hematologic malignancies with IDH2 mutations (Clinical Trial identifier: NCT01915498). Modulation of glucose-related metabolism by dichloroacetate (DCA) or CPI-613 has also shown interesting and promising results. DCA acts as a PDK1 inhibitor, and it has been demonstrated that it modulates proliferation in colorectal cancer cells by increasing proteasomal degradation, without changes in apoptosis (302). A recent DCA human trial in patients with solid tumors showed a mild decrease in glucose uptake, and reported toxicity issues on some of the patients (303). On the other hand, CPI-613 inhibits both PDH and alpha ketoglutarate dehydrogenase: the former through activation of PDK, while the latter is mediated by a rapid change on redox signal in mitochondrial tumor (304). In vitro CPI-613 treatment on cancer stem cells showed a reduction on sphere-forming capacity and tumorigenicity, with an important reduction in CD133+ and DC117 cell frequency, suggesting a relevant role in improving treatment for ovarian cancer (305). CPI-613 has also been tested on patients suffering metastatic pancreatic adenocarcinoma within a phase I trial, where the combination with folfirinox chemotherapy was well tolerated in most of the patients and showed favorable outcomes (306).
Another promising area of mitochondrial therapeutics is directly centered on ETC. In this regard, metformin, a drug originally used for treating T2DM, is one of the most widely reported ETC-targeting drugs tested in the context of cancer due to its safety and apparent harmlessness. Metformin works by directly blocking complex I (NADH dehydrogenase), reducing oxygen consumption and ATP production, while increasing the AMP/ATP ratio that leads to AMPK activation and reduction of mTOR signaling pathways (a more compressive review can be found in reference (307). Metformin has been tested on different types of cancer, such as endometrial cancer (308), metastatic pancreatic cancer (309), colorectal cancer (310), and breast cancer (311), among others. Overall, metformin treatment is well tolerated and shows mixed results regarding survival and clinical benefit, depending on the patient’s characteristics and pharmacological interactions.
Undoubtedly, mitochondrial therapies are promising and represent a fresh perspective for treatment of long-lasting diseases. However, they are still at early stages of development.
Conclusion
NCDs are the most important cause of death worldwide and they generate extremely high economic cost (1). Importantly, the major part of these diseases is generated by an environmental–genetic interaction; therefore, they are partly preventable (1). In this review we discussed the concept of mitochondrial dysfunction and its emerging role as a common root for NCDs. Because of the many faces of mitochondrial dysfunction, it is challenging to definitively identify how these various mitochondrial properties interact to promote NCDs. To date, most evidence suggests a common pattern of mitochondrial alterations although the contribution of each to disease progression may vary. In consequence, the therapeutic control of specific mitochondrial alterations is a crucial step in mitochondrial physiology and its application may depend on the pathological context. New tools for analysis and therapeutic application are being aggressively pursued.
Acknowledgments
Financial Support : This work was supported by grants to S.L. from the Agencia Nacional de Investigacion y Desarrollo (ANID), Chile: FONDECYT 1161156, FONDECYT 1200490 and FONDAP 15130011). We are also thankful for the PhD fellowships from CONICYT Chile to P.S.A., P.E.M. Grants to B.A.R. from the American Heart Association (19TPA34920001), the National Institutes of Health (RO1HD101006), and the Australian National Health and Medical Research Council (APP1142403). Grant to M.C. from Proyecto de Enlace ENL 18/19, VID, Universidad de Chile. Grant to A.D.V. from Australian Research Council (DP180103482).
Glossary
Abbreviations
- ADP
adenosine diphosphate
- AMPK
AMP-activated protein kinase
- ATP
adenosine triphosphate
- COQ
coenzyme Q
- CR
caloric restriction
- CVD
cardiovascular disease
- DCA
dichloroacetate
- Drp-1
dynamin-related protein 1
- ER
endoplasmic reticulum
- ETC
electron transport chain
- FADH2
flavine adenine dinucleotide
- FET
forward electron transfer
- FMN
flavin mononucleotide
- IDH
isocitrate dehydrogenase
- IMM
inner mitochondrial membrane
- IR
insulin resistance
- MAM
mitochondria-associated membrane
- Mfn
Mitofusin
- MitoQ
Mitoquinone
- mPQ
mitochondria-targeted paraquat
- NADH
nicotinamide adenine dinucleotide with hydrogen
- NAFLD
nonalcoholic fatty liver disease
- NCD
noncommunicable chronic disease
- OCR
oxygen consumption rate
- OMM
outer mitochondrial membrane
- OPA1
Optic atrophy 1
- PDH
pyruvate dehydrogenase
- PDK
pyruvate dehydrogenase kinase
- RCAN1
regulator of calcineurin 1
- RET
reverse electron transfer
- RIRR
ROS-induced ROS release
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- T2DM
type 2 diabetes
- TCA
tricarboxylic acid
- UCP
uncoupling protein
- UPRmt
mitochondrial unfolded protein response
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Peña-Oyarzun D, Bravo-Sagua R, Diaz-Vega A, et al. Autophagy and oxidative stress in non-communicable diseases: a matter of the inflammatory state? Free Radic Biol Med. 2018;124:61-78. [DOI] [PubMed] [Google Scholar]
- 2. Warburg O, Posener K, Negelein E. üeber den Stoffwechsel der Tumoren. Biochem Z. 1924;152:319-344. [Google Scholar]
- 3. Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103(8):2653-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tormos KV, Anso E, Hamanaka RB, et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011;14(4):537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teodoro JS, Rolo AP, Duarte FV, Simões AM, Palmeira CM. Differential alterations in mitochondrial function induced by a choline-deficient diet: understanding fatty liver disease progression. Mitochondrion. 2008;8(5-6):367-376. [DOI] [PubMed] [Google Scholar]
- 6. Galloway CA, Lee H, Brookes PS, Yoon Y. Decreasing mitochondrial fission alleviates hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2014;307(6):G632-G641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tubbs E, Chanon S, Robert M, et al. Disruption of mitochondria-associated endoplasmic reticulum membrane (MAM) integrity contributes to muscle insulin resistance in mice and humans. Diabetes. 2018;67(4):636-650. [DOI] [PubMed] [Google Scholar]
- 8. Sebastián D, Hernández-Alvarez MI, Segalés J, et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U S A. 2012;109(4):5523-5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. del Campo A, Parra V, Vásquez-Trincado C, et al. Mitochondrial fragmentation impairs insulin-dependent glucose uptake by modulating Akt activity through mitochondrial Ca2+ uptake. Am J Physiol Endocrinol Metab. 2014;306(1):E1-E13. [DOI] [PubMed] [Google Scholar]
- 10. Fazakerley DJ, Minard AY, Krycer JR, et al. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J Biol Chem. 2018;293(19):7315-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119(3):573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoehn KL, Salmon AB, Hohnen-Behrens C, et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci U S A. 2009;106(42):17787-17792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gutiérrez T, Parra V, Troncoso R, et al. Alteration in mitochondrial Ca(2+) uptake disrupts insulin signaling in hypertrophic cardiomyocytes. Cell Commun Signal. 2014;12:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tubbs E, Theurey P, Vial G, et al. Mitochondria-associated endoplasmic reticulum membrane (MAM) integrity is required for insulin signaling and is implicated in hepatic insulin resistance. Diabetes. 2014;63(10):3279-3294. [DOI] [PubMed] [Google Scholar]
- 15. Hammerschmidt P, Ostkotte D, Nolte H, et al. CerS6-derived sphingolipids interact with Mff and promote mitochondrial fragmentation in obesity. Cell 2019; 177:1536-1552 e1523 [DOI] [PubMed] [Google Scholar]
- 16. Hong Y, Fink BD, Dillon JS, Sivitz WI. Effects of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. Endocrinology. 2001;142(1):249-256. [DOI] [PubMed] [Google Scholar]
- 17. Chan CB, MacDonald PE, Saleh MC, Johns DC, Marbàn E, Wheeler MB. Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes. 1999;48(7):1482-1486. [DOI] [PubMed] [Google Scholar]
- 18. Chan CB, De Leo D, Joseph JW, et al. Increased uncoupling protein-2 levels in beta-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes. 2001;50(6):1302-1310. [DOI] [PubMed] [Google Scholar]
- 19. Anello M, Lupi R, Spampinato D, et al. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005;48(2):282-289. [DOI] [PubMed] [Google Scholar]
- 20. Jin HS, Kim J, Lee SJ, et al. The PARK2 gene is involved in the maintenance of pancreatic β-cell functions related to insulin production and secretion. Mol Cell Endocrinol. 2014;382(1):178-189. [DOI] [PubMed] [Google Scholar]
- 21. Reinhardt F, Schultz J, Waterstradt R, Baltrusch S. Drp1 guarding of the mitochondrial network is important for glucose-stimulated insulin secretion in pancreatic beta cells. Biochem Biophys Res Commun. 2016;474(4):646-651. [DOI] [PubMed] [Google Scholar]
- 22. Kabra UD, Pfuhlmann K, Migliorini A, et al. Direct substrate delivery into mitochondrial fission-deficient pancreatic islets rescues insulin secretion. Diabetes. 2017;66(12):1247-1257. [DOI] [PubMed] [Google Scholar]
- 23. Hennings TG, Chopra DG, DeLeon ER, et al. In vivo deletion of β-cell Drp1 impairs insulin secretion without affecting islet oxygen consumption. Endocrinology. 2018;159:3245-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Z, Wakabayashi N, Wakabayashi J, et al. The dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in pancreatic beta cells. Mol Biol Cell. 2011;22(13):2235-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cai FF, Xu C, Pan X, et al. Prognostic value of plasma levels of HIF-1a and PGC-1a in breast cancer. Oncotarget. 2016;7(47):77793-77806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stewart JB, Alaei-Mahabadi B, Sabarinathan R, et al. Simultaneous DNA and RNA mapping of somatic mitochondrial mutations across diverse human cancers. Plos Genet. 2015;11:e1005333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hopkins JF, Sabelnykova VY, Weischenfeldt J, et al. Mitochondrial mutations drive prostate cancer aggression. Nat Commun. 2017;8(1):656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishikawa K, Takenaga K, Akimoto M, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320(5876):661-664. [DOI] [PubMed] [Google Scholar]
- 29. Xiao Z, Dai Z, Locasale JW. Metabolic landscape of the tumor microenvironment at single cell resolution. Nat Commun. 2019;10(1):3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tohme S, Yazdani HO, Liu Y, et al. Hypoxia mediates mitochondrial biogenesis in hepatocellular carcinoma to promote tumor growth through HMGB1 and TLR9 interaction. Hepatology. 2017;66(1):182-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. LeBleu VS, O’Connell JT, Gonzalez Herrera KN, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 2014;16(10):992-1003, 1001-1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tosatto A, Sommaggio R, Kummerow C, et al. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1α. EMBO Mol Med. 2016;8(5):569-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cárdenas C, Müller M, McNeal A, et al. Selective vulnerability of cancer cells by inhibition of Ca(2+) transfer from endoplasmic reticulum to mitochondria. Cell Rep. 2016;14(10):2313-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keibler MA, Wasylenko TM, Kelleher JK, Iliopoulos O, Vander Heiden MG, Stephanopoulos G. Metabolic requirements for cancer cell proliferation. Cancer Metab. 2016;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu E, Calvert PA, Mercer JR, et al. Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans. Circulation. 2013;128(7):702-712. [DOI] [PubMed] [Google Scholar]
- 36. Pennanen C, Parra V, López-Crisosto C, et al. Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin signaling pathway. J Cell Sci. 2014;127(Pt 12):2659-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hall AR, Burke N, Dongworth RK, et al. Hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction. Cell Death Dis. 2016;7:e2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Le Page S, Niro M, Fauconnier J, et al. Increase in cardiac ischemia-reperfusion injuries in Opa1+/- mouse model. Plos One. 2016;11:e0164066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parra V, Altamirano F, Hernández-Fuentes CP, et al. Down syndrome critical region 1 gene, rcan1, helps maintain a more fused mitochondrial network. Circ Res. 2018;122(36):e20-e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Woo CY, Jang JE, Lee SE, Koh EH, Lee KU. Mitochondrial dysfunction in adipocytes as a primary cause of adipose tissue inflammation. Diabetes Metab J. 2019;43(3):247-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pinti MV, Fink GK, Hathaway QA, Durr AJ, Kunovac A, Hollander JM. Mitochondrial dysfunction in type 2 diabetes mellitus: an organ-based analysis. Am J Physiol Endocrinol Metab. 2019;316(2):E268-E285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol. 2018;20(7):745-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eisner V, Picard M, Hajnóczky G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat Cell Biol. 2018;20(7):755-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pickles S, Vigié P, Youle RJ. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol. 2018;28(4):R170-R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79-99. [DOI] [PubMed] [Google Scholar]
- 46. Eura Y, Ishihara N, Yokota S, Mihara K. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem. 2003;134(3):333-344. [DOI] [PubMed] [Google Scholar]
- 47. Pitts KR, Yoon Y, Krueger EW, McNiven MA. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol Biol Cell. 1999;10(12):4403-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17(4):491-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160(2):189-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114(Pt 5):867-874. [DOI] [PubMed] [Google Scholar]
- 51. Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117(Pt 26):6535-6546. [DOI] [PubMed] [Google Scholar]
- 52. Santel A, Frank S, Gaume B, Herrler M, Youle RJ, Fuller MT. Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci. 2003;116(Pt 13):2763-2774. [DOI] [PubMed] [Google Scholar]
- 53. Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101(45):15927-15932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meeusen S, McCaffery JM, Nunnari J. Mitochondrial fusion intermediates revealed in vitro. Science. 2004;305(5691):1747-1752. [DOI] [PubMed] [Google Scholar]
- 55. Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20(15):3525-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Olichon A, Emorine LJ, Descoins E, et al. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 2002;523(1-3):171-176. [DOI] [PubMed] [Google Scholar]
- 57. Meeusen S, DeVay R, Block J, et al. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006;127(2):383-395. [DOI] [PubMed] [Google Scholar]
- 58. Olichon A, Baricault L, Gas N, et al. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278(10):7743-7746. [DOI] [PubMed] [Google Scholar]
- 59. Frezza C, Cipolat S, Martins de Brito O, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126(1):177-189. [DOI] [PubMed] [Google Scholar]
- 60. Cogliati S, Frezza C, Soriano ME, et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155(1):160-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Varanita T, Soriano ME, Romanello V, et al. The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 2015;21(6):834-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Del Dotto V, Mishra P, Vidoni S, et al. OPA1 isoforms in the hierarchical organization of mitochondrial functions. Cell Rep. 2017;19(12):2557-2571. [DOI] [PubMed] [Google Scholar]
- 63. Patten DA, Wong J, Khacho M, et al. OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J. 2014;33(22):2676-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12(8):2245-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ingerman E, Perkins EM, Marino M, et al. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170:1021-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee JE, Westrate LM, Wu H, Page C, Voeltz GK. Multiple dynamin family members collaborate to drive mitochondrial division. Nature. 2016;540(7631):139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2008;19(6):2402-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Losón OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24(5):659-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Otera H, Wang C, Cleland MM, et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010;191(6):1141-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Palmer CS, Osellame LD, Laine D, Koutsopoulos OS, Frazier AE, Ryan MT. MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep. 2011;12(6):565-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kornfeld OS, Qvit N, Haileselassie B, Shamloo M, Bernardi P, Mochly-Rosen D. Interaction of mitochondrial fission factor with dynamin related protein 1 governs physiological mitochondrial function in vivo. Sci Rep. 2018;8(1):14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280(28):26185-26192. [DOI] [PubMed] [Google Scholar]
- 73. Díaz-Vegas A, Eisner V, Jaimovich E. Skeletal muscle excitation-metabolism coupling. Arch Biochem Biophys. 2019;664:89-94. [DOI] [PubMed] [Google Scholar]
- 74. Glancy B, Hartnell LM, Malide D, et al. Mitochondrial reticulum for cellular energy distribution in muscle. Nature. 2015;523(7562):617-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Glancy B, Hartnell LM, Combs CA, et al. Power grid protection of the muscle mitochondrial reticulum. Cell Rep. 2018;23(9):2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6(8): 657-663. [DOI] [PubMed] [Google Scholar]
- 77. Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13(5):589-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mishra P, Carelli V, Manfredi G, Chan DC. Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab. 2014;19(4):630-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Quintana-Cabrera R, Quirin C, Glytsou C, et al. The cristae modulator Optic atrophy 1 requires mitochondrial ATP synthase oligomers to safeguard mitochondrial function. Nat Commun. 2018;9(1):3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Parra V, Verdejo HE, Iglewski M, et al. Insulin stimulates mitochondrial fusion and function in cardiomyocytes via the Akt-mTOR-NFκB-Opa-1 signaling pathway. Diabetes. 2014;63(1):75-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rigoulet M, Yoboue ED, Devin A. Mitochondrial ROS generation and its regulation: mechanisms involved in H(2)O(2) signaling. Antioxid Redox Signal. 2011;14(3):459-468. [DOI] [PubMed] [Google Scholar]
- 82. Wong HS, Dighe PA, Mezera V, Monternier PA, Brand MD. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J Biol Chem. 2017;292(41):16804-16809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A. 2006;103(20):7607-7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pryde KR, Hirst J. Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J Biol Chem. 2011;286(20):18056-18065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86(5):1101-1107. [DOI] [PubMed] [Google Scholar]
- 87. Chance B, Hollunger G. The interaction of energy and electron transfer reactions in mitochondria. I. General properties and nature of the products of succinate-linked reduction of pyridine nucleotide. J Biol Chem. 1961;236:1534-1543. [PubMed] [Google Scholar]
- 88. Selivanov VA, Votyakova TV, Pivtoraiko VN, et al. Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. Plos Comput Biol. 2011;7(3):e1001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chouchani ET, Pell VR, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27(2):433-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Legros F, Lombès A, Frachon P, Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell. 2002;13(12):4343-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mailloux RJ, Seifert EL, Bouillaud F, Aguer C, Collins S, Harper ME. Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. J Biol Chem. 2011;286(24):21865-21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mailloux RJ, Adjeitey CN, Harper ME. Genipin-induced inhibition of uncoupling protein-2 sensitizes drug-resistant cancer cells to cytotoxic agents. Plos One. 2010;5(10):e13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Edwards KS, Ashraf S, Lomax TM, et al. Uncoupling protein 3 deficiency impairs myocardial fatty acid oxidation and contractile recovery following ischemia/reperfusion. Basic Res Cardiol. 2018;113(6):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Choi CS, Fillmore JJ, Kim JK, et al. Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest. 2007;117(7):1995-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Echtay KS, Roussel D, St-Pierre J, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415(6867):96-99. [DOI] [PubMed] [Google Scholar]
- 97. Echtay KS, Murphy MP, Smith RA, Talbot DA, Brand MD. Superoxide activates mitochondrial uncoupling protein 2 from the matrix side. Studies using targeted antioxidants. J Biol Chem. 2002;277(49):47129-47135. [DOI] [PubMed] [Google Scholar]
- 98. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94(3):909-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ilkan Z, Akar FG. The mitochondrial translocator protein and the emerging link between oxidative stress and arrhythmias in the diabetic heart. Front Physiol. 2018;9:1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pfanner N, Warscheid B, Wiedemann N. Mitochondrial proteins: from biogenesis to functional networks. Nat Rev Mol Cell Biol. 2019;20(5):267-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wiedemann N, Pfanner N. Mitochondrial machineries for protein import and assembly. Annu Rev Biochem. 2017;86:685-714. [DOI] [PubMed] [Google Scholar]
- 102. Tian Y, Garcia G, Bian Q, et al. Mitochondrial stress induces chromatin reorganization to promote longevity and UPR(mt). Cell. 2016;165(5):1197-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Shpilka T, Haynes CM. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol Cell Biol. 2018;19(2):109-120. [DOI] [PubMed] [Google Scholar]
- 104. Jazwinski SM. The retrograde response: when mitochondrial quality control is not enough. Biochim Biophys Acta. 2013;1833(2):400-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science. 2012;337(6094):587-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fiorese CJ, Schulz AM, Lin YF, Rosin N, Pellegrino MW, Haynes CM. The transcription factor ATF5 mediates a mammalian mitochondrial UPR. Curr Biol. 2016;26(15):2037-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Quirós PM, Prado MA, Zamboni N, et al. Multi-omics analysis identifies ATF4 as a key regulator of the mitochondrial stress response in mammals. J Cell Biol. 2017;216:2027-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Horibe T, Hoogenraad NJ. The chop gene contains an element for the positive regulation of the mitochondrial unfolded protein response. Plos One. 2007;2(9):e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Aldridge JE, Horibe T, Hoogenraad NJ. Discovery of genes activated by the mitochondrial unfolded protein response (mtUPR) and cognate promoter elements. Plos One. 2007;2(9):e874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jain SS, Paglialunga S, Vigna C, et al. High-fat diet-induced mitochondrial biogenesis is regulated by mitochondrial-derived reactive oxygen species activation of CaMKII. Diabetes. 2014;63(6):1907-1913. [DOI] [PubMed] [Google Scholar]
- 111. Shi SY, Lu SY, Sivasubramaniyam T, et al. DJ-1 links muscle ROS production with metabolic reprogramming and systemic energy homeostasis in mice. Nat Commun. 2015;6:7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dogan SA, Pujol C, Maiti P, et al. Tissue-specific loss of DARS2 activates stress responses independently of respiratory chain deficiency in the heart. Cell Metab. 2014;19(3):458-469. [DOI] [PubMed] [Google Scholar]
- 113. Copeland DE, Dalton AJ. An association between mitochondria and the endoplasmic reticulum in cells of the pseudobranch gland of a teleost. J Biophys Biochem Cytol. 1959;5(3):393-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lopez-Crisosto C, Pennanen C, Vasquez-Trincado C, et al. Sarcoplasmic reticulum-mitochondria communication in cardiovascular pathophysiology. Nat Rev Cardiol. 2017;14(6):342-360. [DOI] [PubMed] [Google Scholar]
- 115. Rieusset J. The role of endoplasmic reticulum-mitochondria contact sites in the control of glucose homeostasis: an update. Cell Death Dis. 2018;9(3):388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265(13):7248-7256. [PubMed] [Google Scholar]
- 117. Flis VV, Daum G. Lipid transport between the endoplasmic reticulum and mitochondria. Cold Spring Harb Perspect Biol 2013;5(6):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Petrungaro C, Kornmann B. Lipid exchange at ER-mitochondria contact sites: a puzzle falling into place with quite a few pieces missing. Curr Opin Cell Biol. 2019;57:71-76. [DOI] [PubMed] [Google Scholar]
- 119. Garofalo T, Matarrese P, Manganelli V, et al. Evidence for the involvement of lipid rafts localized at the ER-mitochondria associated membranes in autophagosome formation. Autophagy. 2016;12(6):917-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hamasaki M, Furuta N, Matsuda A, et al. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495(7441):389-393. [DOI] [PubMed] [Google Scholar]
- 121. Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334(6054):358-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lewis SC, Uchiyama LF, Nunnari J. ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science 2016; 353(6296):aaf5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Betz C, Stracka D, Prescianotto-Baschong C, Frieden M, Demaurex N, Hall MN. Feature Article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc Natl Acad Sci U S A. 2013;110(31):12526-12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bononi A, Bonora M, Marchi S, et al. Identification of PTEN at the ER and MAMs and its regulation of Ca2+ signaling and apoptosis in a protein phosphatase-dependent manner. Cell Death Differ. 2013;20:1631-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Denton RM, McCormack JG, Edgell NJ. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980;190(1):107-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. McCormack JG, Denton RM. Role of Ca2+ ions in the regulation of intramitochondrial metabolism in rat heart. Evidence from studies with isolated mitochondria that adrenaline activates the pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes by increasing the intramitochondrial concentration of Ca2+. Biochem J. 1984;218(1):235-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Díaz-Vegas AR, Cordova A, Valladares D, et al. Mitochondrial Calcium Increase Induced by RyR1 and IP3R channel activation after membrane depolarization regulates skeletal muscle metabolism. Front Physiol. 2018;9:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82(3):415-424. [DOI] [PubMed] [Google Scholar]
- 129. Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96(24):13807-13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262(5134):744-747. [DOI] [PubMed] [Google Scholar]
- 131. Csordás G, Renken C, Várnai P, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Csordás G, Várnai P, Golenár T, et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell. 2010;39:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Rapizzi E, Pinton P, Szabadkai G, et al. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol. 2002;159(4):613-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Szabadkai G, Bianchi K, Várnai P, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175(6):901-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456(7222):605-610. [DOI] [PubMed] [Google Scholar]
- 136. Myhill N, Lynes EM, Nanji JA, et al. The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell. 2008;19(7):2777-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Simmen T, Aslan JE, Blagoveshchenskaya AD, et al. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005;24(13):717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wu W, Lin C, Wu K, et al. FUNDC1 regulates mitochondrial dynamics at the ER-mitochondrial contact site under hypoxic conditions. EMBO J. 2016;35(106):1368-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yuzefovych LV, LeDoux SP, Wilson GL, Rachek LI. Mitochondrial DNA damage via augmented oxidative stress regulates endoplasmic reticulum stress and autophagy: crosstalk, links and signaling. PLoS One. 2013;8(12):e83349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One. 2013;8(1):e54059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ploumi C, Daskalaki I, Tavernarakis N. Mitochondrial biogenesis and clearance: a balancing act. FEBS J. 2017;284(2):183-195. [DOI] [PubMed] [Google Scholar]
- 142. Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23(9):459-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829-839. [DOI] [PubMed] [Google Scholar]
- 144. Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267-273. [DOI] [PubMed] [Google Scholar]
- 145. Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115-124. [DOI] [PubMed] [Google Scholar]
- 146. Gatica D, Lahiri V, Klionsky DJ. Cargo recognition and degradation by selective autophagy. Nat Cell Biol. 2018;20(3):233-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183(5):795-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Narendra DP, Jin SM, Tanaka A, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8(1):e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Shiba-Fukushima K, Imai Y, Yoshida S, et al. PINK1-mediated phosphorylation of the Parkin ubiquitin-like domain primes mitochondrial translocation of Parkin and regulates mitophagy. Sci Rep. 2012;2:1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Yamano K, Matsuda N, Tanaka K. The ubiquitin signal and autophagy: an orchestrated dance leading to mitochondrial degradation. EMBO Rep. 2016;17(3):300-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Chen M, Chen Z, Wang Y, et al. Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy. 2016;12(4):689-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, Gustafsson ÅB. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J Biol Chem. 2012;287(23):19094-19104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Rikka S, Quinsay MN, Thomas RL, et al. Bnip3 impairs mitochondrial bioenergetics and stimulates mitochondrial turnover. Cell Death Differ. 2011;18(4):721-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Fortini P, Ferretti C, Iorio E, et al. The fine tuning of metabolism, autophagy and differentiation during in vitro myogenesis. Cell Death Dis. 2016;7:e2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sin J, Andres AM, Taylor DJ, et al. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy. 2016;12(2):369-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ju JS, Jeon SI, Park JY, et al. Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition. J Physiol Sci. 2016;66(5):417-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Vainshtein A, Tryon LD, Pauly M, Hood DA. Role of PGC-1alpha during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am J Physiol Cell Physiol 2015;308(9):C710-719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Palikaras K, Lionaki E, Tavernarakis N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature. 2015;521(7553):525-528. [DOI] [PubMed] [Google Scholar]
- 159. Laker RC, Drake JC, Wilson RJ, et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun. 2017;8(1):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Shin JH, Ko HS, Kang H, et al. PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Stevens DA, Lee Y, Kang HC, et al. Parkin loss leads to PARIS-dependent declines in mitochondrial mass and respiration. Proc Natl Acad Sci U S A. 2015;112(7):11696-11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Chandel NS. Evolution of mitochondria as signaling organelles. Cell Metab. 2015;22(2):204-206. [DOI] [PubMed] [Google Scholar]
- 163. Hunt RJ, Bateman JM. Mitochondrial retrograde signaling in the nervous system. FEBS Lett. 2018;592(5):663-678. [DOI] [PubMed] [Google Scholar]
- 164. Liu Z, Butow RA. Mitochondrial retrograde signaling. Annu Rev Genet. 2006;40(1):159-185. [DOI] [PubMed] [Google Scholar]
- 165. Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48(2):158-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Aon MA, Cortassa S, Juhaszova M, Sollott SJ. Mitochondrial health, the epigenome and healthspan. Clin Sci (Lond). 2016;130(15):1285-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Kalea AZ, Drosatos K, Buxton JL. Nutriepigenetics and cardiovascular disease. Curr Opin Clin Nutr Metab Care. 2018;21(4):252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Yang Y, Gao H, Zhou H, et al. The role of mitochondria-derived peptides in cardiovascular disease: recent updates. Biomed Pharmacother. 2019;117:109075. [DOI] [PubMed] [Google Scholar]
- 169. Lee C, Zeng J, Drew BG, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21(3):443-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kim KH, Son JM, Benayoun BA, Lee C. The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metab 2018; 28(3):516-524 e517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Halim ND, Mcfate T, Mohyeldin A, et al. Phosphorylation status of pyruvate dehydrogenase distinguishes metabolic phenotypes of cultured rat brain astrocytes and neurons. Glia. 2010;58(10):1168-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Küster U, Bohnensack R, Kunz W. Control of oxidative phosphorylation by the extra-mitochondrial ATP/ADP ratio. Biochim Biophys Acta. 1976;440(2):391-402. [DOI] [PubMed] [Google Scholar]
- 173. Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta. 2009;1787(11):1309-1316. [DOI] [PubMed] [Google Scholar]
- 174. Pan X, Liu J, Nguyen T, et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15(22):1464-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Kwong JQ, Huo J, Bround MJ, et al. The mitochondrial calcium uniporter underlies metabolic fuel preference in skeletal muscle. JCI Insight 2018; 3(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Nunes-Nesi A, Araújo WL, Obata T, Fernie AR. Regulation of the mitochondrial tricarboxylic acid cycle. Curr Opin Plant Biol. 2013;16(3):335-343. [DOI] [PubMed] [Google Scholar]
- 177. Nicholls D. Mitochondrial bioenergetics, aging, and aging-related disease. Sci Aging Knowledge Environ 2002;2002(31):pe12. [DOI] [PubMed] [Google Scholar]
- 178. Kusminski CM, Scherer PE. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol Metab. 2012;23(9):435-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435(2):297-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Jaña F, Bustos G, Rivas J, et al. Complex I and II are required for normal mitochondrial Ca2+ homeostasis. Mitochondrion. 2019;49:73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Jonckheere AI, Smeitink JA, Rodenburg RJ. Mitochondrial ATP synthase: architecture, function and pathology. J Inherit Metab Dis. 2012;35(2):211-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Fisher-Wellman KH, Davidson MT, Narowski TM, Lin CT, Koves TR, Muoio DM. Mitochondrial diagnostics: a multiplexed assay platform for comprehensive assessment of mitochondrial energy fluxes. Cell Rep 2018;24(13):3593-3606.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Fisher-Wellman KH, Draper JA, Davidson MT, et al. Respiratory phenomics across multiple models of protein hyperacylation in cardiac mitochondria reveals a marginal impact on bioenergetics. Cell Rep 2019;26(6):1557-1572.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Willis WT, Jackman MR, Messer JI, Kuzmiak-Glancy S, Glancy B. A Simple Hydraulic Analog Model of Oxidative Phosphorylation. Med Sci Sports Exerc. 2016;48(6):990-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Lark DS, Torres MJ, Lin CT, Ryan TE, Anderson EJ, Neufer PD. Direct real-time quantification of mitochondrial oxidative phosphorylation efficiency in permeabilized skeletal muscle myofibers. Am J Physiol Cell Physiol. 2016;311(2):C239-C245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Guzun R, Timohhina N, Tepp K, et al. Systems bioenergetics of creatine kinase networks: physiological roles of creatine and phosphocreatine in regulation of cardiac cell function. Amino Acids. 2011;40(5):1333-1348. [DOI] [PubMed] [Google Scholar]
- 187. Willingham TB, McCully KK. In Vivo assessment of mitochondrial dysfunction in clinical populations using near-infrared spectroscopy. Front Physiol. 2017;8:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Marcinek DJ. Mitochondrial dysfunction measured in vivo. Acta Physiol Scand. 2004;182(4):343-352. [DOI] [PubMed] [Google Scholar]
- 189. Whitley BN, Engelhart EA, Hoppins S. Mitochondrial dynamics and their potential as a therapeutic target. Mitochondrion. 2019;49:269-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Axelrod CL, Fealy CE, Mulya A, Kirwan JP. Exercise training remodels human skeletal muscle mitochondrial fission and fusion machinery towards a pro-elongation phenotype. Acta Physiol (Oxf). 2019;225(4):e13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A. 2011;108(25):10190-10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16(7):411-428. [DOI] [PubMed] [Google Scholar]
- 193. Krycer JR, Elkington SD, Diaz-Vegas A, et al. Mitochondrial oxidants, but not respiration, are sensitive to glucose in adipocytes. J Biol Chem. 2020;295(1):99-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci U S A. 2003;100(13):7996-8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Humphrey SJ, Yang G, Yang P, et al. Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2. Cell Metab. 2013;17(6):1009-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Ng Y, Ramm G, Lopez JA, James DE. Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3-L1 adipocytes. Cell Metab. 2008;7(4):348-356. [DOI] [PubMed] [Google Scholar]
- 197. Llanos P, Contreras-Ferrat A, Georgiev T, et al. The cholesterol-lowering agent methyl-β-cyclodextrin promotes glucose uptake via GLUT4 in adult muscle fibers and reduces insulin resistance in obese mice. Am J Physiol Endocrinol Metab. 2015;308(4):E294-E305. [DOI] [PubMed] [Google Scholar]
- 198. Hoehn KL, Hohnen-Behrens C, Cederberg A, et al. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 2008;7(5):421-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Fazakerley DJ, Krycer JR, Kearney AL, Hocking SL, James DE. Muscle and adipose tissue insulin resistance: malady without mechanism? J Lipid Res. 2019;60(10):1720-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Taddeo EP, Laker RC, Breen DS, et al. Opening of the mitochondrial permeability transition pore links mitochondrial dysfunction to insulin resistance in skeletal muscle. Mol Metab. 2014;3(2):124-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Booty LM, Gawel JM, Cvetko F, et al. Selective disruption of mitochondrial thiol redox state in cells and in vivo. Cell Chem Biol 2019; 26(3):449-461 e448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Henriksen EJ. Exercise training and the antioxidant alpha-lipoic acid in the treatment of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2006;40(1):3-12. [DOI] [PubMed] [Google Scholar]
- 203. Bloch-Damti A, Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal. 2005;7(11-12):1553-1567. [DOI] [PubMed] [Google Scholar]
- 204. Contreras-Ferrat A, Llanos P, Vásquez C, et al. Insulin elicits a ROS-activated and an IP3-dependent Ca²⁺ release, which both impinge on GLUT4 translocation. J Cell Sci. 2014;127(9):1911-1923. [DOI] [PubMed] [Google Scholar]
- 205. Henríquez-Olguin C, Knudsen JR, Raun SH, et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat Commun. 2019;10(1):4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Arruda AP, Pers BM, Parlakgül G, Güney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20(12):1427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Robb EL, Gawel JM, Aksentijević D, et al. Selective superoxide generation within mitochondria by the targeted redox cycler MitoParaquat. Free Radic Biol Med. 2015;89:883-894. [DOI] [PubMed] [Google Scholar]
- 208. Steele C, Hagopian WA, Gitelman S, et al. Insulin secretion in type 1 diabetes. Diabetes. 2004;53(2):426-433. [DOI] [PubMed] [Google Scholar]
- 209. Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic ß-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59(11):2846-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151-S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Skelin Klemen M, Dolenšek J, Slak Rupnik M, Stožer A. The triggering pathway to insulin secretion: Functional similarities and differences between the human and the mouse β cells and their translational relevance. Islets. 2017;9(6):109-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75(1):155-179. [DOI] [PubMed] [Google Scholar]
- 213. Eto K, Tsubamoto Y, Terauchi Y, et al. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science. 1999;283(5404):981-985. [DOI] [PubMed] [Google Scholar]
- 214. Maechler P, Kennedy ED, Pozzan T, Wollheim CB. Mitochondrial activation directly triggers the exocytosis of insulin in permeabilized pancreatic beta-cells. EMBO J. 1997;16(13):3833-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. De Marchi U, Thevenet J, Hermant A, Dioum E, Wiederkehr A. Calcium co-regulates oxidative metabolism and ATP synthase-dependent respiration in pancreatic beta cells. J Biol Chem. 2014;289(13):9182-9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Pi J, Bai Y, Zhang Q, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56(7):1783-1791. [DOI] [PubMed] [Google Scholar]
- 217. Leloup C, Tourrel-Cuzin C, Magnan C, et al. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58(3):673-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18(2):162-185. [DOI] [PubMed] [Google Scholar]
- 219. Fleury C, Neverova M, Collins S, et al. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997;15(3):269-272. [DOI] [PubMed] [Google Scholar]
- 220. Sesti G, Cardellini M, Marini MA, et al. A common polymorphism in the promoter of UCP2 contributes to the variation in insulin secretion in glucose-tolerant subjects. Diabetes. 2003;52(5):1280-1283. [DOI] [PubMed] [Google Scholar]
- 221. Krempler F, Esterbauer H, Weitgasser R, et al. A functional polymorphism in the promoter of UCP2 enhances obesity risk but reduces type 2 diabetes risk in obese middle-aged humans. Diabetes. 2002;51(11):3331-3335. [DOI] [PubMed] [Google Scholar]
- 222. Esterbauer H, Schneitler C, Oberkofler H, et al. A common polymorphism in the promoter of UCP2 is associated with decreased risk of obesity in middle-aged humans. Nat Genet. 2001;28(2):178-183. [DOI] [PubMed] [Google Scholar]
- 223. Andersen G, Dalgaard LT, Justesen JM, et al. The frequent UCP2 -866G > A polymorphism protects against insulin resistance and is associated with obesity: a study of obesity and related metabolic traits among 17 636 Danes. Int J Obes (Lond). 2013;37(2):175-181. [DOI] [PubMed] [Google Scholar]
- 224. Ramirez S, Gomez-Valades AG, Schneeberger M, et al. Mitochondrial dynamics mediated by mitofusin 1 is required for POMC neuron glucose-sensing and insulin release control. Cell Metab 2017; 25(6):1390-1399 e1396 [DOI] [PubMed] [Google Scholar]
- 225. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314. [DOI] [PubMed] [Google Scholar]
- 226. Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5(11):857-866. [DOI] [PubMed] [Google Scholar]
- 227. Bost F, Kaminski L. The metabolic modulator PGC-1α in cancer. Am J Cancer Res. 2019;9(2):198-211. [PMC free article] [PubMed] [Google Scholar]
- 228. Cannino G, Ciscato F, Masgras I, Sánchez-Martín C, Rasola A. Metabolic plasticity of tumor cell mitochondria. Front Oncol. 2018;8:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Torrano V, Valcarcel-Jimenez L, Cortazar AR, et al. The metabolic co-regulator PGC1α suppresses prostate cancer metastasis. Nat Cell Biol. 2016;18(6):645-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Reznik E, Miller ML, Senbabaoglu Y, et al. Mitochondrial DNA copy number variation across human cancers. Elife 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Reznik E, Wang Q, La K, Schultz N, Sander C. Mitochondrial respiratory gene expression is suppressed in many cancers. Elife 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Gammage PA, Frezza C. Mitochondrial DNA: the overlooked oncogenome? BMC Biol. 2019;17(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Buchwald A, Till H, Unterberg C, Oberschmidt R, Figulla HR, Wiegand V. Alterations of the mitochondrial respiratory chain in human dilated cardiomyopathy. Eur Heart J. 1990;11(6):509-516. [DOI] [PubMed] [Google Scholar]
- 234. Graham BH, Waymire KG, Cottrell B, Trounce IA, MacGregor GR, Wallace DC. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat Genet. 1997;16(3):226-234. [DOI] [PubMed] [Google Scholar]
- 235. Lee YS, Kim JW, Osborne O, et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell. 2014;157(233):1339-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Rabøl R, Højberg PM, Almdal T, et al. Effect of hyperglycemia on mitochondrial respiration in type 2 diabetes. J Clin Endocrinol Metab. 2009;94(234):1372-1378. [DOI] [PubMed] [Google Scholar]
- 237. Holmström MH, Iglesias-Gutierrez E, Zierath JR, Garcia-Roves PM. Tissue-specific control of mitochondrial respiration in obesity-related insulin resistance and diabetes. Am J Physiol Endocrinol Metab. 2012;302(6):E731-E739. [DOI] [PubMed] [Google Scholar]
- 238. Antoun G, McMurray F, Thrush AB, et al. Impaired mitochondrial oxidative phosphorylation and supercomplex assembly in rectus abdominis muscle of diabetic obese individuals. Diabetologia. 2015;58(12):2861-2866. [DOI] [PubMed] [Google Scholar]
- 239. Wessels B, Honecker J, Schöttl T, et al. Adipose mitochondrial respiratory capacity in obesity is impaired independently of glycemic status of tissue donors. Obesity (Silver Spring). 2019;27(5):756-766. [DOI] [PubMed] [Google Scholar]
- 240. Yu EPK, Reinhold J, Yu H, et al. Mitochondrial respiration is reduced in atherosclerosis, promoting necrotic core formation and reducing relative fibrous cap thickness. Arterioscler Thromb Vasc Biol. 2017;37(12):2322-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Shimizu S, Ishibashi M, Kumagai S, et al. Decreased cardiac mitochondrial tetrahydrobiopterin in a rat model of pressure overload. Int J Mol Med. 2013;31(3):589-596. [DOI] [PubMed] [Google Scholar]
- 242. Tang Y, Mi C, Liu J, Gao F, Long J. Compromised mitochondrial remodeling in compensatory hypertrophied myocardium of spontaneously hypertensive rat. Cardiovasc Pathol. 2014;23(2):101-106. [DOI] [PubMed] [Google Scholar]
- 243. Walther T, Tschöpe C, Sterner-Kock A, et al. Accelerated mitochondrial adenosine diphosphate/adenosine triphosphate transport improves hypertension-induced heart disease. Circulation. 2007;115(3):333-344. [DOI] [PubMed] [Google Scholar]
- 244. Zadra G, Loda M. Metabolic vulnerabilities of prostate cancer: diagnostic and therapeutic opportunities. Cold Spring Harb Perspect Med 2018;8(10):a030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Asgari Y, Zabihinpour Z, Salehzadeh-Yazdi A, Schreiber F, Masoudi-Nejad A. Alterations in cancer cell metabolism: the Warburg effect and metabolic adaptation. Genomics. 2015;105(5-6):275-281. [DOI] [PubMed] [Google Scholar]
- 246. Viale A, Corti D, Draetta GF. Tumors and mitochondrial respiration: a neglected connection. Cancer Res. 2015;75(18):3685-3686. [DOI] [PubMed] [Google Scholar]
- 247. Mattison JA, Colman RJ, Beasley TM, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Mattison JA, Roth GS, Beasley TM, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641-654. [DOI] [PubMed] [Google Scholar]
- 250. Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Stenesen D, Suh JM, Seo J, et al. Adenosine nucleotide biosynthesis and AMPK regulate adult life span and mediate the longevity benefit of caloric restriction in flies. Cell Metab. 2013;17(1):101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Weir HJ, Yao P, Huynh FK, et al. Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metab 2017;26(6):884-896.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Wierman MB, Maqani N, Strickler E, Li M, Smith JS. Caloric restriction extends yeast chronological life span by optimizing the snf1 (AMPK) signaling pathway. Mol Cell Biol 2017;37(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017-12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Arab Sadeghabadi Z, Nourbakhsh M, Pasalar P, et al. Reduced gene expression of sirtuins and active AMPK levels in children and adolescents with obesity and insulin resistance. Obes Res Clin Pract. 2018;12(2):167-173. [DOI] [PubMed] [Google Scholar]
- 256. Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem. 2005;280(16):16456-16460. [DOI] [PubMed] [Google Scholar]
- 257. Verdin E. NAD⁺ in aging, metabolism, and neurodegeneration. Science. 2015;350(6265):1208-1213. [DOI] [PubMed] [Google Scholar]
- 258. Gali Ramamoorthy T, Laverny G, Schlagowski AI, et al. The transcriptional coregulator PGC-1β controls mitochondrial function and anti-oxidant defence in skeletal muscles. Nat Commun. 2015;6:10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Pardo R, Vilà M, Cervela L, et al. Calorie restriction prevents diet-induced insulin resistance independently of PGC-1-driven mitochondrial biogenesis in white adipose tissue. FASEB J. 2019;33(2):2343-2358. [DOI] [PubMed] [Google Scholar]
- 260. Waldman M, Cohen K, Yadin D, et al. Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving ‘SIRT1 and PGC-1α’. Cardiovasc Diabetol. 2018;17(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Wong KE, Mikus CR, Slentz DH, et al. Muscle-specific overexpression of PGC-1α does not augment metabolic improvements in response to exercise and caloric restriction. Diabetes. 2015;64(5):1532-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. López-Lluch G, Hunt N, Jones B, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103(6):1768-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. Am J Physiol Endocrinol Metab. 2005;289(3):E429-E438. [DOI] [PubMed] [Google Scholar]
- 264. Heilbronn LK, de Jonge L, Frisard MI, et al. ; Pennington CALERIE Team . Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295(13):1539-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, Ravussin E. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab 2018;27(4):805-815 e804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Dirks AJ, Leeuwenburgh C. Caloric restriction in humans: potential pitfalls and health concerns. Mech Ageing Dev. 2006;127(1):1-7. [DOI] [PubMed] [Google Scholar]
- 267. Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242(9):2278-2282. [PubMed] [Google Scholar]
- 268. Henríquez-Olguín C, Renani LB, Arab-Ceschia L, et al. Adaptations to high-intensity interval training in skeletal muscle require NADPH oxidase 2. Redox Biol. 2019;24(66):101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Layec G, Gifford JR, Trinity JD, et al. Accuracy and precision of quantitative 31P-MRS measurements of human skeletal muscle mitochondrial function. Am J Physiol Endocrinol Metab. 2016;311(2):E358-E366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Picard M, Gentil BJ, McManus MJ, et al. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J Appl Physiol (1985). 2013;115(10):1562-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Walsh B, Tonkonogi M, Söderlund K, Hultman E, Saks V, Sahlin K. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol. 2001;537(Pt 3):971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Ydfors M, Hughes MC, Laham R, Schlattner U, Norrbom J, Perry CG. Modelling in vivo creatine/phosphocreatine in vitro reveals divergent adaptations in human muscle mitochondrial respiratory control by ADP after acute and chronic exercise. J Physiol. 2016;594(11):3127-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Weibel ER, Hoppeler H. Exercise-induced maximal metabolic rate scales with muscle aerobic capacity. J Exp Biol. 2005;208(Pt 9):1635-1644. [DOI] [PubMed] [Google Scholar]
- 274. Lundby C, Jacobs RA. Adaptations of skeletal muscle mitochondria to exercise training. Exp Physiol. 2016;101(1):17-22. [DOI] [PubMed] [Google Scholar]
- 275. Nielsen J, Gejl KD, Hey-Mogensen M, et al. Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. J Physiol. 2017;595(9):2839-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Greggio C, Jha P, Kulkarni SS, et al. Enhanced respiratory chain supercomplex formation in response to exercise in human skeletal muscle. Cell Metab. 2017;25(2):301-311. [DOI] [PubMed] [Google Scholar]
- 277. Hancock M, Hafstad AD, Nabeebaccus AA, et al. Myocardial NADPH oxidase-4 regulates the physiological response to acute exercise. Elife 2018;7:e41044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Trewin AJ, Berry BJ, Wojtovich AP. Exercise and mitochondrial dynamics: keeping in shape with ROS and AMPK. Antioxidants (Basel) 2018;7(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Gerhart-Hines Z, Rodgers JT, Bare O, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26(7):1913-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Philp A, Chen A, Lan D, et al. Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) deacetylation following endurance exercise. J Biol Chem. 2011;286(75):30561-30570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Uddin GM, Youngson NA, Doyle BM, Sinclair DA, Morris MJ. Nicotinamide mononucleotide (NMN) supplementation ameliorates the impact of maternal obesity in mice: comparison with exercise. Sci Rep. 2017;7(1):15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. de Guia RM, Agerholm M, Nielsen TS, et al. Aerobic and resistance exercise training reverses age-dependent decline in NAD+ salvage capacity in human skeletal muscle. Physiol Rep. 2019;7(12):e14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Brouwers B, Stephens NA, Costford SR, et al. Elevated nicotinamide phosphoribosyl transferase in skeletal muscle augments exercise performance and mitochondrial respiratory capacity following exercise training. Front Physiol. 2018;9:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Hoffman NJ, Parker BL, Chaudhuri R, et al. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK Substrates. Cell Metab. 2015;22(5):922-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Bonora M, Wieckowski MR, Sinclair DA, Kroemer G, Pinton P, Galluzzi L. Targeting mitochondria for cardiovascular disorders: therapeutic potential and obstacles. Nat Rev Cardiol. 2019;16(1):33-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. El-Hattab AW, Zarante AM, Almannai M, Scaglia F. Therapies for mitochondrial diseases and current clinical trials. Mol Genet Metab. 2017;122(3):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014;171(8):2029-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Dai W, Shi J, Gupta RC, Sabbah HN, Hale SL, Kloner RA. Bendavia, a mitochondria-targeting peptide, improves postinfarction cardiac function, prevents adverse left ventricular remodeling, and restores mitochondria-related gene expression in rats. J Cardiovasc Pharmacol. 2014;64(6):543-553. [DOI] [PubMed] [Google Scholar]
- 289. Chatfield KC, Sparagna GC, Chau S, et al. Elamipretide improves mitochondrial function in the failing human heart. JACC Basic Transl Sci. 2019;4(2):147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Daubert MA, Yow E, Dunn G, et al. Novel mitochondria-targeting peptide in heart failure treatment: a randomized, placebo-controlled trial of elamipretide. Circ Heart Fail 2017;10(12). [DOI] [PubMed] [Google Scholar]
- 291. Subramanian S, Kalyanaraman B, Migrino RQ. Mitochondrially targeted antioxidants for the treatment of cardiovascular diseases. Recent Pat Cardiovasc Drug Discov. 2010;5(1):54-65. [DOI] [PubMed] [Google Scholar]
- 292. Kelso GF, Porteous CM, Coulter CV, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276(7):4588-4596. [DOI] [PubMed] [Google Scholar]
- 293. Coudray C, Fouret G, Lambert K, et al. A mitochondrial-targeted ubiquinone modulates muscle lipid profile and improves mitochondrial respiration in obesogenic diet-fed rats. Br J Nutr. 2016;115(7):1155-1166. [DOI] [PubMed] [Google Scholar]
- 294. Fazakerley DJ, Chaudhuri R, Yang P, et al. Mitochondrial CoQ deficiency is a common driver of mitochondrial oxidants and insulin resistance. Elife 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol (1985) 2018;124(5):1194-1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Ribeiro Junior RF, Dabkowski ER, Shekar KC, O Connell KA, Hecker PA, Murphy MP. MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic Biol Med. 2018;117:18-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Mortensen SA, Rosenfeldt F, Kumar A, et al. ; Q-SYMBIO Study Investigators . The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014;2(6):641-649. [DOI] [PubMed] [Google Scholar]
- 298. Sun C, Liu X, Di C, et al. MitoQ regulates autophagy by inducing a pseudo-mitochondrial membrane potential. Autophagy. 2017;13(4):730-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Biel TG, Rao VA. Mitochondrial dysfunction activates lysosomal-dependent mitophagy selectively in cancer cells. Oncotarget. 2018;9(1):995-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Yen K, Travins J, Wang F, et al. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 Mutations. Cancer Discov. 2017;7(5):478-493. [DOI] [PubMed] [Google Scholar]
- 301. Amatangelo MD, Quek L, Shih A, et al. Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response. Blood. 2017;130(6):732-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Delaney LM, Ho N, Morrison J, Farias NR, Mosser DD, Coomber BL. Dichloroacetate affects proliferation but not survival of human colorectal cancer cells. Apoptosis. 2015;20(1):63-74. [DOI] [PubMed] [Google Scholar]
- 303. Chu QS, Sangha R, Spratlin J, et al. A phase I open-labeled, single-arm, dose-escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors. Invest New Drugs. 2015;33(3):603-610. [DOI] [PubMed] [Google Scholar]
- 304. Stuart SD, Schauble A, Gupta S, et al. A strategically designed small molecule attacks alpha-ketoglutarate dehydrogenase in tumor cells through a redox process. Cancer Metab. 2014;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Bellio C, DiGloria C, Spriggs DR, Foster R, Growdon WB, Rueda BR. The metabolic inhibitor CPI-613 negates treatment enrichment of ovarian cancer stem cells. Cancers (Basel) 2019;11(11):1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Alistar A, Morris BB, Desnoyer R, et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017;18(6):770-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Saraei P, Asadi I, Kakar MA, Moradi-Kor N. The beneficial effects of metformin on cancer prevention and therapy: a comprehensive review of recent advances. Cancer Manag Res. 2019;11:3295-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Schuler KM, Rambally BS, DiFurio MJ, et al. Antiproliferative and metabolic effects of metformin in a preoperative window clinical trial for endometrial cancer. Cancer Med. 2015;4(2):161-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Kordes S, Pollak MN, Zwinderman AH, et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16(7):839-847. [DOI] [PubMed] [Google Scholar]
- 310. Miranda VC, Braghiroli MI, Faria LD, et al. Phase 2 trial of metformin combined with 5-fluorouracil in patients with refractory metastatic colorectal cancer. Clin Colorectal Cancer 2016; 15(4):321-328 e321 [DOI] [PubMed] [Google Scholar]
- 311. Martin-Castillo B, Pernas S, Dorca J, et al. A phase 2 trial of neoadjuvant metformin in combination with trastuzumab and chemotherapy in women with early HER2-positive breast cancer: the METTEN study. Oncotarget. 2018;9(86):35687-35704. [DOI] [PMC free article] [PubMed] [Google Scholar]