Abstract
Background:
Immune checkpoint inhibitors targeting PD-1 have demonstrated clinical benefit in adults with cancer, but experience is limited in children. We conducted a Phase 1/2 study of nivolumab, a PD-1 blocking monoclonal antibody, to determine its safety, pharmacokinetics, and efficacy in children and young adults with recurrent or refractory, non-CNS solid tumors.
Methods:
ADVL1412 is identified as NCT02304458. Analyses were conducted per protocol. Part A enrolled patients 1–18 years with measurable disease regardless of histology and assessed safety and pharmacokinetics of nivolumab (3 mg/kg) administered intravenously every 14 days in 28 day cycles. Part B administered nivolumab intravenously (3 mg/kg) to patients aged 1–30 yrs with measurable disease in the following disease cohorts: rhabdomyosarcoma, Ewing sarcoma, osteosarcoma, neuroblastoma, Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), and melanoma. Response was measured by RECIST v1.1. PD-L1 expression and immune cell infiltration was assessed via immunohistochemistry of archival tumor tissue. Primary objectives were to determine the tolerability, systemic exposure, and dosage of nivolumab and the anti-tumor effects at the adult recommended dose chidren and young adults. The trial is ongoing; single-agent nivolumab strata are completed, enrolling between February 22, 2015 – December 31, 2018.
Findings:
Eighty-five patients were enrolled between February 22, 2015 and December 31, 2018 with a median follow up of 30 days (IQR 27–83 days). The most common overall toxicity was anemia (35 patients; 5 patients Grade 3 or 4) and non-hematologic toxicity was fatigue (28 patients; none Grade 3 or 4). Pleural or pericardial effusions developed in 11 patients, of which ten had tumor involving the lungs or chest at baseline. Responses were observed in patients with lymphoma (3/10 with HL,1/10 with NHL) which consistently demonstrated PD-L1 expression. Objective responses were not observed in other tumor types.
Interpretation:
Nivolumab is safe and well-tolerated in children with clinical activity in lymphoma. Nivolumab has no significant single-agent activity in the common pediatric solid tumors studied here.
Introduction
Outcomes for children and adolescents with cancer have improved over the last four decades (1), however, recurrent and refractory pediatric solid tumors remain largely incurable (2). Despite progress in identifying oncogenic drivers and encouraging results targeting such drivers in some rare disease subsets (3–5), cytotoxic chemotherapy remains the mainstay of treatment for most pediatric solid tumors. Immune therapies have demonstrated promising activity, including chimeric antigen receptor modified T cells (6–8) and blinatumomab (9) in relapsed/refractory pediatric B-ALL and dinutuximab in high-risk neuroblastoma (10) and when combined with chemotherapy in recurrent/refractory neuroblastoma (11).
Immune checkpoint inhibitors (ICIs) block tumor derived signals that inhibit immune responses, thus amplifying antitumor immunity. ICIs have demonstrated impressive benefit in numerous advanced cancers in adults (reviewed in (12)) and can induce tumor regression in children with solid tumors associated with germline mismatch repair deficiency(13,14). Nivolumab, a humanized IgG4 monoclonal PD-1 blocking antibody (15), administered administered every 2 weeks (240 mg or approximately 3 mg/kg) or every 4 weeks (480 mg or approximately 6 mg/kg) is FDA approved in adults and children older than 12 years of age with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) metastatic colon cancer and as second line therapy in adults with advanced melanoma, renal cell carcinoma, urothelial carcinoma, hepatocellular carcinoma, metastatic squamous cell non-small cell lung cancer, head and neck cancer, and small cell lung cancer, and relapsed or progressed classical Hodgkin lymphoma. We conducted a Phase 1/2 trial of single agent nivolumab in children and adolescents with recurrent or refractory solid tumors, excluding central nervous system (CNS) tumors. Primary objectives were to define the toxicity and pharmacokinetics of nivolumab monotherapy in children, establish a recommended phase 2 dose (RP2D), and evaluate clinical activity in common pediatric solid tumor cohorts.
Methods
Study Design and Participants
The study was conducted in two parts. Part A was a dose-confirmation phase in children (age of eligibility 1–18 years) with recurrent or refractory solid tumors with measurable or evaluable disease (by RECIST criteria) to determine the RPD2 dose of Nivolumab. In Part B, children and young adults (age of eligibility 1–30 years) with measurable disease (by RECIST criteria) received the RP2D of nivolumab to identify signals of activity and to generate further information regarding toxicity of the agent in the following disease specific cohorts. Study participants were required to have adequate organ function (neutrophils ≥ 750/mm3, transfusion independent platelet count for 7 days ≥ 75,000/mm3, bilirubin ≤ 1.5x the upper limit of normal for age, ALT ≤ 135 U/L, lipase ≤ upper limit of normal, no evidence of dypnea at rest and pulse oximetry > 92% on room air), recovered from the acute toxic effects of prior anti-cancer therapies, adequate performace status (Karnofsky ≥ 50% or Lansky ≥ 60) and at least 42 days from autologous stem cell transplant (SCT), stem cell infusion or cellular therapy. Initially patients with allogenic stem cell transplant were excluded, however, the protocol was amended to include patients at least 100 days from allogenic SCT and had no evidence of graft-versus-host-disease. Patients with known CNS metastases or CNS tumors, those requiring daily systemic corticosteroids or those who had received systemic corticosteroids within 7 days prior to enrollment were ineligible. Patients or their guardians were required to sign informed consent prior to enrollment and the study was approved by central and institutional IRB.
Procedures
Part A was a dose-confirmation phase in children (age of eligibility 1–18 years) with recurrent or refractory solid tumors with measurable or evaluable disease to determine the RP2D. Six patients were planned to receive nivolumab 3 mg/kg administered intravenously (IV) over 60 minutes on Day 1 and Day 15 of a 28 day cycle. If 2 of 6 patients (≥ 33%) experienced dose limiting toxicity (DLT) during Cycle 1, dose de-escalation was planned to 1 mg/kg. If <2 of 6 patients in part A experienced DLT, then 3 mg/kg would be considered the RP2D tested in Part B, as long as pharmacokinetic analysis ensured that a Cmin of ≥10 mcg/ml occurred in at least 5 of 6 patients where Cmin was defined as the Cycle 1, Day 15 pre-dose concentration, to confirm similar drug exposure to that observed in adults treated with the standard dose of 240 mg/dose administered every 14 days. Accumulation was calculated as the ratio of the AUC0–168hr for Cycle 2 versus Cycle 1. An additional six patients were also planned to enroll in Part A to complete a pharmacokinetic cohort.
In Part B, children and young adults (age of eligibility 1–30 years) with measurable disease received the RP2D of nivolumab to identify signals of activity and to generate further information regarding toxicity of the agent in the following disease specific cohorts: rhabdomyosarcoma (RMS), Ewing sarcoma (ES), osteosarcoma (OS), neuroblastoma (NBL), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL). A cohort with non-measurable neuroblastoma detected only by MIBG scintigraphy was also enrolled. Given the activity of nivolumab in adults with melanoma (15), patients less than 18 years old with recurrent, measurable melanoma were eligible for enrollment in Part B as part of a non-statistically powered cohort.
In Part A, patients were evaluable for toxicity if they received at least one dose of study drug and completed toxicity monitoring or experienced a dose limiting toxicity (DLT, Appendix p5) during Cycle 1. Patients in Part A and Part B were monitored for toxicity through the entire treatment period. For Part B, occurrence of a toxicity that met the definition of a DLT impacted subsequent treatment for that patient but did not inform selection of the RP2D. NCI Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 was used for description and grading of all toxicities. Adverse events (AEs) were deemed unrelated, unlikely, possibly, probably, or definitely related to nivolumab by the treating physician with central confirmation. Review of symptoms, physical examination, and laboratory assessments (electrolyes, complete blood count, liver enzymes, bilirubin, creatinine and C-reactive protein) were conducted weekly during cycle 1, then prior to each cycle and as clinically indicated. Patients were able to continue on study for up to 2 years unless they demonstrated disease progression (clinical or radiographic), experienced an adverse event requiring removal from therapy, refused further therapy or were non-compliant with therapy or refused further participation. Dose interruptions were allowed for some adverse events as based on protocol definitions (see Appendix p5 and online protocol). Dose modifications were allowed for toxicity and are outlined in detailed in the accompanying protocol. In general, protocol therapy was continued for Grade 1 toxicities, with the exception of pneumonitis and cardiac toxicities in which case therapy was held for Grade 1 toxicities. Grade 2 toxicities required holding all protocol therapy. If toxicity recovered to Grade 1 or less within 7 days, protocol therapy could continue. If not, protocol therapy was discontinued. Protocol therapy was discontinued for all toxicities of Grade 3 or 4.
Radiographic disease assessments were obtained after Cycles 1 and 2, then after every other Cycle. Type of imaging (computed tomography, magnetic resonance imaging, positron emissions tomography scans, and meta-iodobenzylguanidine scan) was determined by the treating physician and tumor type. Patients with measureable disease at baseline were evaluable for response if they received at least one dose of study drug and had a disease re-evaluation performed or clinical progression of disease was documented by the treating physician. Response was evaluated using revised Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, except inpatients with NBL evaluable only by MIBG scintigraphy, where response was assessed using Curie scoring as previously described (16,17). Due to the possibility of a delayed response following initial tumor progression, patients who experienced tumor growth greater than 20% but less than 40% were allowed to remain on study for up to 12 weeks with more frequent disease monitoring as long as the patient showed no rapid disease progression or deterioration in performance status, had not experienced a DLT and/or were otherwise demonstrating clinical benefit. Complete or partial responses required central radiographic review.
PD-L1 expression, assessed by immunohistochemistry (IHC) on tumor tissue obtained at initial diagnosis or subsequent biopsy, was evaluated centrally using the Dako PD-L1 IHC 28–8 pharmDx assay in formalin-fixed, paraffin-embedded (FFPE) tumor samples as previously described (18). The percentage of tumor cells demonstrating plasma membrane PD-L1 staining at 0, 1+, 2+ or 3+ intensity was quantified in a minimum of 100 evaluable tumor cells. The presence of tumor associated immune cells, as well as the presence of PD-L1 membrane staining on associated lymphocytes or macrophages were also assessed qualitatively.
Complete details on study design and execution (frequency and type of laboratory and adverse event monitoring and radiographic assessments, details on allowed dose reductions/interruptions and criteria for removal from study, statistical analyses) are available in the accompanying online clinical protocol. Study sites and amendments are available on page 21 and 22 of the appendix.
Outcomes
Primary objectives were to determine: i) the tolerability and describe the toxicities of nivolumab at the adult recommended dose of 3mg/kg in chidren and young adults with relapsed or refractory solid tumors; ii) systemic exposure of nivolumab in children and compare to that in adults; iii) the maximal tolerated dose of nivolumab in children; iv) the anti-tumor effects of nivolumab in selected childhood solid tumors in seven expansion cohorts as outlined above. Secondary objectives included exploring the presence of infiltrating lymphocytes and expression of PD-L1 in patient tumor specimens. The study is ongoing and some protocol pre-specified secondary analyses (nivolumab effect on T cell phenotype and function, nivolumab effect on circulating cytokines) will be addressed in future manuscripts.
Statistical Analysis
Part A was a Phase 1 dose finding study using the Rolling 6 study design. Part A evaluated a single dose level (3 mg/kg). If 1 or fewer of 6 evaluable patients experienced DLT and at least 5/6 of patients achieved a Cmin of at least 10 mcg/ml, the 3 mg/kg dose level would be the RP2D and children are not experiencing significantly less exposure than adults treated at the same dose. If 2 or more of the 6 patients experienced DLT at the 3 mg/kg dose level, then the MTD has been exceeded and the 1 mg/kg dose level would be evaluated. If 1 or fewer of 6 patients experienced DLT at the 1 mg/kg dose level and at least 5/6 of patients achieve a Cmin of at least 10 mcg/ml, then this dose level would be the RP2D. The operating characteristics of the rolling 6 study design have been previously described (19).
Part B used a 10+10 Simon two-stage design for each disease cohort separately. To determine whether Nivolumab would be of sufficient interest for further evaluation in a disease category, Part B defined a true response rate of 5% of insufficient interest for further evaluation, while a response rate is 25% as sufficient for further evaluation. A Simon’s optimal two stage design was used (type 1 error of 0.07 and 88% power to detect the alternative hypothesis) wherein each cohort would enroll 10 patients in Stage 1. If no responses were observed, the stratum was terminated and nivolumab deemed ineffective. If 1 or more subjects demonstrated objective response in Stage 1, the stratum would proceed to Stage 2 and enter an additional 10 patients. If 3 or more responses were observed in 20 evaluable patients, the treatment would be considered sufficiently active to warrant further study.
For all parts of the study, any patient who receives at least one dose of the study drug and who experienced a dose-limiting toxicity was considered evaluable for adverse events. In addition, for Parts A, during Cycle 1, patients must have the appropriate toxicity monitoring studies performed to be considered evaluable for dose limiting toxicity. In Part A, patients who did not have DLT and did not receive at least 85% of the prescribed dose within the first 28 days (the DLT observation period) for reasons other than toxicities (e.g. progressive disease) would not be considered evaluable for toxicity and would be replaced. The rolling 6 study design was used for analysis of primary and secondary outcomes (20).
Any patient who is enrolled and meets eligibility criteria for Parts B and received at least one dose of protocol therapy would be considered evaluable for response provided: (1) the patient demonstrates progressive disease or death while on protocol therapy or (2) the patient is observed on protocol therapy for at least one cycle and the tumor is not removed surgically prior to the time complete response or partial response is confirmed, or (3) the patient demonstrates a complete or partial response as confirmed according to protocol criteria. Two objective status determinations are required to confirm best response. All other patients will be considered non-responders. All patients considered to have a response (CR or PR) must have imaging studies reviewed centrally. The central review was the final reviewed assessment of response. Patients inevaluable for response would be considered for replacement. The Simon two-stage study design was used for analysis of primary and secondary outcomes (21).
The rolling 6 study design uses interim analyses to determine whether to increase the dose level, decrease the dose level, or define the MTD/RP2D. Interim analyses occur after a cohort of 6 (or 3 in some cases) evaluable patients are assessed. The Simon two-stage design used interim analysis to determine whether or not to continue enrolling patients into the second stage of the study. 10 patients were assessed for response in the first stage for each disease cohort. If at least 1 responder was observed, then the 2nd stage opened for enrollment. Otherwise, the disease cohort would close to enrollment and concluded to have insufficient evidence for response. Categorical variables are summarized by frequencies (%) and continuous variables are summarized by medians with interquartile range. SAS v 9.4 was used for statistical analysis. This trial is registered as NCT02304458.
Role of the funding source
The funders contributed to the study design, data collection, data analysis and interpretation, and writing the report in collaboration with the authors. All authors had full access to the data included in the study. The corresponding author had full access to all of the data and had final responsibility for the decision to submit for publication.
Results
Enrollment was initiated on February 22, 2015, and data cut off for analysis was December 31, 2018. Eighty-five eligible patients were enrolled and 75 patients were fully evaluable for toxicity. Median follow up was 30 days (IQR 27–83 days). Patient characteristics are shown in Table 1. Two patients remained on protocol therapy at the data cutoff date. Part A enrolled 13 patients (median age 10 yrs, 6 (46%) 13 female) with the following diagnoses: embryonal rhabdomyosarcoma (n=1), epithelioid sarcoma (n=2), Ewing sarcoma (n=1), neuroblastoma (n=4), osteosarcoma (n=3), undifferentiated sarcoma (n=1) and unspecified sarcoma (n=1). Twelve were evaluable for toxicity (one was removed from study prior to receiving study drug by the treating physician due to progressive disease) and none experienced a DLT.
Table 1.
Patient characteristics for eligible patients (Part A and B, N = 85)
| Characteristic | Number (%) (Part A) N=13 | Number (%) (Part B) N=72 | Number (%) (All) N=85 |
|---|---|---|---|
| Age (years) | |||
| Median | 10 | 15 | 14 |
| Range | 5 – 15 | 1 – 27 | 1 – 27 |
| Sex | |||
| Male | 7 (54) | 44 (61) | 51 (60) |
| Female | 6 (46) | 28 (39) | 34 (40) |
| Race | |||
| White | 13 (100) | 51 (71) | 64 (75) |
| Asian | 0 (0) | 5 (7) | 5 (6) |
| American Indian or Alaska Native | 0 (0) | 0 (0) | 0 (0) |
| Black or African American | 0 (0) | 8 (11) | 8 (10) |
| Multiple Races | 0 (0) | 2 (3) | 2 (2) |
| Unknown | 0 (0) | 6 (8) | 6 (7) |
| Ethnicity | |||
| Non-Hispanic | 10 (77) | 60 (83) | 70 (82) |
| Hispanic | 3 (13) | 10 (14) | 13 (15) |
| Unknown | 0 (0) | 2 (3) | 2 (2) |
| Diagnosis | |||
| Epithelioid sarcoma | 2 (15.4) | 2 (2.4) | |
| Ewing sarcoma | 1 (7.7) | 10 (13.9) | 11 (12.9) |
| Hodgkin lymphoma | 12 (16.7) | 12 (14.2) | |
| Non-Hodgkin lymphoma | |||
| Diffuse large B-cell | 3 (4.2) | 3 (3.5) | |
| Non-Hodgkin, NOS | 1 (1.4) | 1 (1.2) | |
| Burkitt lymphoma | 3 (4.2) | 3 (3.5) | |
| Mediastinal large B-cell lymphoma | 3 (4.2) | 3 (3.5) | |
| Melanoma | 1 (1.4) | 1 (1.2) | |
| Neuroblastoma, NOS | 4 (30.8) | 18 (25)* | 22 (25.9) |
| Osteosarcoma, NOS | 3 (23.1) | 10 (13.9) | 13 (15.3) |
| Rhabdomyosarcoma, NOS | 1 (7.7) | 11 (15.3) | 12 (14.2) |
| Sarcoma, NOS | 1 (7.7) | 1 (1.2) | |
| Undifferentiated sarcoma | 1 (7.7) | 1 (1.2) | |
| Prior Therapy | |||
| Chemotherapy Regimens | N=13 | N=71 | N=84 |
| Median | 2 | 3 | 2.5 |
| Range | 1–6 | 1–16 | 1–16 |
| Radiation Therapy | N=10 | N=46 | N=56 |
| Median | 1 | 1 | 1 |
| Range | 1–3 | 1–5 | 1–5 |
Cohort B1(neuroblastoma measurable disease) and Cohort B8 (neuroblastoma detectable by MIBG only) patients are combined in this table.
Part B enrolled 72 patients were enrolled (median age 15 yrs, 28 (39%) of 72 female) (Table 1). Prior to treatment with single-agent nivolumab, 71 of these patients had been treated with a median of 3 previous chemotherapy regimens (range 1–16), and 46 had received radiation therapy with a median of 1 radiotherapy regimen (range 1–5). Sixty-three were evaluable for toxicity. Seven patients did not receive full dosage of nivolumab (n=5 due to disease progression and n=2 due to patient/physician preference) and two patients did not undergo all required laboratory evaluations.
In Part A, nivolumab 3mg/kg was well tolerated and was confirmed as the pediatric RP2D; no dose de-escalation was required and no DLTs were observed. In Part B, five patients experienced protocol defined DLTs: grade 3 elevated lipase greater than 7 days (n=1), grade 4 neutropenia (n=1), grade 3 pain at tumor site (n=1), grade 3 upper gastrointestinal hemorrhage (n=1), and grade 2 enterocolitis infection (n=1) (Appendix p6). Also in Part B, two patients required dose modifications, one due to Grade 2 wheezing that resolved and the patient was able to continue on therapy after holding one dose and one due to Grade 2 transaminitis that eventually necessitated coming off protocol. A complete listing of adverse events possibly, probably or definitely attributed to nivolumab is listed in the Appendix on pages 6–16. The most common toxicities attributable to therapy among all patients evaluable for toxicity (n=69) were hematologic: anemia (24 (51%) of 69, 5 grade ≥ 3) decreased WBC (24 (35%) of 69, 3 grade ≥ 3), decreased lymphocytes (22 (32%) of 69; 10 grade ≥ 3), and decreased platelets (14 (20%) of 69, 2 grade ≥ 3). The most common non-hematologic toxicity was fatigue in 28 (41%) of 69 patients but none was ≥ grade 3. Of particular interest are immune-related adverse events (irAEs, Table 2), the most common of which was hepatic toxicity (AST 22 (32%) of 69 or ALT 18 (26%) of 69). Less than 5% of irAE’s were grade 3 or higher. Pleural and/or pericardial effusions developed in 11 (14.7%) of 85 evaluable patients, with 3 patients developing both (Table 3). Effusions developed during the first cycle of nivolumab in seven patients, during the second cycle in two patients, and after discontinuation of nivolumab in three patients; severity ranged from moderate (grade 2 or lower, n = 7) to severe (grade 3, n = 3) and life-threatening (grade 4, n = 1). Evaluation of the pleural fluid in at least one patient supported an inflammatory effusion as there was no evidence of malignant cells. In total, 7 patients on part B discontinued protocol therapy due to adverse events (2 patients with prolonged elevation of liver enzymes, 1 patient with prolonged elevated lipase, 1 patient with prolonged fever, 1 patient with GI bleed, 1 patient with infection, 1 patient with autoimmune disorder including thyroiditis, elevated creatine kinase, elevated creatinine). There were 37 patient deaths on study or during the follow up period, none were attributable to study therapy.
Table 2.
All hematologic and non-Hematologic immune related adverse events possibly, probably or definitely related to protocol therapy observed in patients evaluable for toxicity (Part A and B, N=75)
| CTCAE v5 term | All Grades No. (%)* | No. Patients by Grade | ||||
|---|---|---|---|---|---|---|
| Selected Toxicity | N events | N patients (%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Aspartate aminotransferase increased | 32 | 22 (32) | 19 | 2 | 1 | 0 |
| Alanine aminotransferase increased | 25 | 18 (26) | 16 | 1 | 1 | 0 |
| Lipase increased | 15 | 6 (9) | 3 | 0 | 2 | 1 |
| Hypothyroidism | 12 | 10 (14) | 7 | 3 | 0 | 0 |
| Rash maculo papulars | 9 | 8 (12) | 7 | 1 | 0 | 0 |
| Diarrhea | 9 | 6 (9) | 6 | 0 | 0 | 0 |
| Hyperthyroidism | 7 | 6 (9) | 4 | 2 | 0 | 0 |
| Serum amylase increased | 5 | 3 (4) | 3 | 0 | 0 | 0 |
| Pleural effusion | 4 | 4 (6) | 1 | 1 | 2 | 0 |
| Pericardial effusion | 3 | 3 (4) | 0 | 3 | 0 | 0 |
| Pancreatitis | 2 | 2 (3) | 0 | 2 | 0 | 0 |
| Rash acneiform | 1 | 1 (1) | 1 | 0 | 0 | 0 |
| Esophagitis | 1 | 1 (1) | 0 | 1 | 0 | 0 |
| Autoimmune disorder | 1 | 1 (1) | 0 | 0 | 1 | 0 |
| Gastritis | 1 | 1 (1) | 0 | 0 | 1 | 0 |
Table 3.
Incidence, timing and attribution of pleural and pericardial effusions
| Patient Diagnosis | Location | Cycle Reported | Grad e | Attribution | Received Steroids | Intrathoracic Disease at Enrollment |
|---|---|---|---|---|---|---|
| Epithelioid sarcoma | Pleural | 2 | 3 | possible | No | paraesophageal mass |
| RMS, embryonal | Pleural | 1 | 3 | unlikely | No | pulmonary metastasis |
| Osteosarcoma | Pleural | 1 | 3 | unrelated | No | Pulmonary metastasis |
| Neuroblastoma | Pleural | 100 day follow up | 3 | possible | No | pulmonary metastasis |
| RMS, NOS | Pleural | 100 day follow up | 2 | unrelated | No | None at enrollment |
| RMS, alveolar | Pleural | 1 | 1 | possible | Yes | Pulmonary metastasis |
| ES | Pleural/pericardial | 2/2 | 2/2 | unlikely/possible | Yes | Mediastinal and chest wall mass |
| ES | Pleural/pericardial | 1/100 day follow up | 2/2 | possible/possible | No | Pleural metastasis |
| PMLCL | Pleural/pericardial | 1/1 | 2/2 | unlikely/possible | Yes | pleural and mediastinal metastasis |
| ES | Pericardial | 1 | 2 | unlikely | No | Pleural metastasis |
| Melanoma | Pleural | 100 day follow up | 4 | unrelated | No | pulmonary metastasis |
RMS: rhabdomyosarcoma; ES Ewing sarcoma, OS: osteosarcoma; NBL: neuroblastoma; PMLCL: primary medistinal large cell lymphoma
Twelve patients in Part A and 64 patients in Part B were evaluable for PK and results are summarized in Table S4 (Appendix p17 – 18). The median Cmin for the first six evaluable patients enrolled in Part A was 19.0 mcg/mL, and 11 of 12 patients achieved Cmin ≥10 mcg/mL (range, 9.7–32.0). Mean Cmax (standard deviation) was 65.2 (16.6) mcg/ml in Part A and 66.3 (43.1) mcg/ml in Part B, median half-life was 285 hours (range, 154 – 444 hours) in Part A and 379 hours (range, 92 – 2772 hours) in Part B. Clearance (Clp) was 0.17 ± 0.07 in Part A and and 0.16 ± 0.08 ml/h/kg in Part B. The Clp was similar across cohorts in Part B. For Part A, the weight adjusted Clp was 0.18 ± 0.09 ml/h/kg for males, 0.16 ± 0.05 ml/h/kg for females, 0.20 ± 0.08 ml/h/kg for patients <12 years old at enrollment and 0.15 ± 0.06 ml/h/kg for patients ≥12 years old. For Part B, the weight adjusted Clp was 0.16 ± 0.09 ml/h/kg for males, 0.14 ± 0.07 ml/h/kg for females, 0.15 ± 0.07 ml/h/kg for patients <12 years old at enrollment and 0.16 ± 0.09 ml/h/kg for patients ≥12 years. An accumulation ratio of 1.7 ± 0.4 was consistent with a half-life of 13.2 days.
Twelve patients with HL were enrolled, but two patients did not start therapy due to change in disease status following enrollment and therefore were not evaluable for response. In ten patients with HL evaluable for response, 1 patient had a complete response, 2 patients had a partial response, 5 patients had stable disease (median 12.8 cycles with range 2–36 cycles) and 2 patients had a mixed response, with decrease in size of target lesions but appearance of new lesions while on study. Two patients were removed from study due to physician preference. Patients with HL completed a median (range) of 4.5 (1 – 36) cycles. Despite achieving the number of responses required to expand the HL cohort in the planned Simon’s two stage design, the HL cohort was not expanded in this trial in order to evaluate nivolumab in a combination regimen for patients with relpased HL in a separate trial (NCT02927769, AHOD1712).
Ten patients with NHL enrolled and evaluable for response: mediastinal large B-cell lymphoma (LBCL, n=3), diffuse large B cell lymphoma (DLBCL, n=3), Burkitt lymphoma (n=3), and B-cell lymphoblastic lymphoma (n=1). One patient with mediastinal LBCL had a metabolic complete response by PET and partial response by RECIST and remained on therapy for 11 cycles. The toxicity profile in patients with lymphoma was not different from that of other histologies (Appendix p19 Table S5 and p 20 Table S6). The NHL cohort was not expanded due to inadequate accrual to complete the expansion cohort.
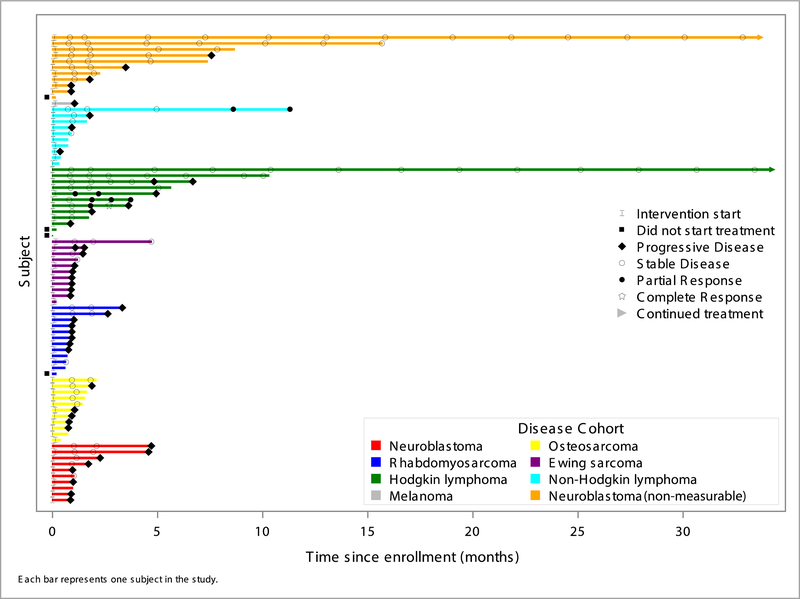
As shown in Figure 1, no objective responses were observed in patients with non-lymphoma, solid tumors evaluated in this trial. Stable disease was reported as best response in a minority of patients in each sarcoma cohort and the neuroblastoma cohort. Among patients enrolled in the NBL-MIBG cohort, 4 patients were deemed to have stable disease (via central review) following two cycles of therapy. One patient with poorly differentiated melanoma (negative for BRAF V600E) was enrolled and did not respond. This patient had metastatic disease at the time of enrollment and had progressed despite three surgical resections and prior therapy with interferon-alpha and dendritic cell therapy.
Figure 1.
Response to single agent nivolumab in relapsed pediatric solid tumor cohorts. Cohort B1(neuroblastoma measurable disease) and Cohort B8 (neuroblastoma non-measurable) patients are reported separtely in this figure.
Seventy five percent of patients (64/85) had archival tumor evaluated for PD-L1 expression on tumor cells and on tumor infiltrating leukocytes via IHC. All tissues tested were available prior to start of anti-PD-1 therapy, although this was not an eligibility criterion. Using a conservative cutoff of 1% PD-L1 expression on tumor cells to define positivity, only 7–50% (7 of 47 total tested patients) of non-lymphoma tumor cohorts expressed PD-L1 on their tumor cells (Table 4). By comparison, in the HL cohort, 9 of 9 patients tested had PD-L1 expression on their tumor cells, at levels ranging from 30 to 100% of cells. In the NHL cohort, PD-L1 expression was evaluated in 8 of 10 patients and ranged from 1–100% of tumor cells. The patient with 100% PD-L1 tumor staining experienced a partial response. A patient with DLBCL demonstrated 99% tumor positivity for PD-L1 and infiltrating PD-L1+ lymphocytes but did not demonstrate a response to nivolumab. Fifty-six subjects (88% of 64,) with available tissue demonstrated infiltrating immune cells. PD-L1 membrane staining was most commonly observed on tumor associated macrophages, although 9 (16%) of 58 patients, across various histologies (NHL = 3, NBL = 2, sarcoma = 4), demonstrated predominant infiltration of PD-L1+ lymphocytes. Together, these results demonstrate an overall paucity of PD-L1 expression on the common childhood solid tumors evaluated here, excluding lymphomas, and a predominance of PD-L1 expressing macrophages in the tumor microenvironment.
Table 4.
PD-L1 expression on tumor cells and infiltrating immune cells.
| Histology | Tumor PD-L1 testing Number of patients (%) | PD-L1+tumor$ no. (%) | Range of % PD-L1+positive tumor cells | Tumors with Infiltrating Immune Cells no. (%) | Predominant PD-L1+ Immune Cell |
|---|---|---|---|---|---|
| HL | 9/12 (75) | 9/9 (100) | 30–100 | 9/9 (100) | Macrophages |
| NHL | 8/10 (80) | 7/8 (88) | 1–100 | 8/8 (100) | Macrophages or Lymphocytes |
| NBL | 15/22 (68) | 1/15 (7) | 1 | 14/15 (93) | Macrophages or Lymphocytes |
| OS | 9/13 (69) | 2/9 (22) | 1–3 | 7/9 (78) | Macrophages or Lymphocytes |
| RMS | 9/12 (75) | 1/9 (11) | 1 | 8/9 (89) | Macrophages or Lymphocytes |
| ES | 10/11 (91) | 1/10 (10) | 1 | 8/10 (80) | Macrophages |
| Malignant melanoma | 0/1 (0) | - | - | - | - |
| Other Sarcoma* | 4/4 (100) | 2/4 (50) | 5–70 | 4/4 (100) | Macrophages |
positive tumors are those showing PD-L1 on ≥ 1% of tumor cells
Malignant spindle cell and epithelioid neoplasm with focally rhabdoid features (1), treated spindle and pleomorphic sarcoma (1), proximal type epithelial sarcoma (1), and metastatic epithelioid sarcoma (1).
Discussion
In this pediatric phase I/II trial, nivolumab was found to be well tolerated in children and to demonstrate similar pharmacokinetics as reported in adults receiving the standard dose of 240 mg every 14 days (22). Similar to findings in adults (23), no clinically relevant effects on nivolumab clearance were observed across the age spectrum of pediatrics and across the wide range of diseases enrolled on this trial. Based on this data, the recommended phase 2 dose and schedule of nivolumab for pediatric patients is 3mg/kg administered every 14 days. Grade 3 or 4 adverse events considered to be drug-related occurred in 36% (27/75) of patients and were consistent with previously reported immune-related AEs observed with PD-1 blockade in adults. We observed greater frequency of Grade 3/4 haematologic toxicity in our cohort compared with that reported in adults, which we suspect is due to our heavily pre-treated patient population who received numerous myelotoxic chemotherapy and/or radiotherapy regimens before enrollment. It was notable that pleural and/or pericardial effusions developed in 13 patients, 11 of which were evaluable for toxicity. Ten of these patients had neoplastic involvement of the lungs or chest at the time of treatment, raising the prospect that this was attributable to nivolumab induced tumor associated inflammation. Although management of nivolumab associated effusions could not be systematically assessed in this study, several patients were treated with supportive care and corticosteroids and demonstrated symptomatic improvement in the effusion.
Some patients with lymphoma experienced clinical benefit with single agent nivolumab. Of the 10 patients with HL treated, 3 experienced an objective response (1CR, 2PR) and 5 demonstrated stable disease. Despite this encouraging activity, the response rate appears lower than the 66–87% ORR reported in adults with HL (24, 25). The median age of patients with HL in our cohort was 16 (range 11–27 years) compared to a median of 35 years in the study by Ansell et al. and a mean of 39 years in the study by Younes (24, 25). Younes et al. reported on 80 adults with classical HL (median age 39 years) (25). Ansell et al., reported on 23 patients with HL (median age 35 years) with 96% (22 of 23) reported to have nodular sclerosing subtype of classical HL (24). In this cohort, all patients had classical HL and 6 were reported to be nodular sclerosing subtype. Overexpression of PD-L1, associated with PD-L1 amplification, is believed to contribute to the high response rate of HL to ICI (24, 26). Although PD-L1 mutational status was not evaluated in this study, 100% of HL tumors analyzed demonstrated high levels of (2+ or 3+) PD-L1 expression as previously reported in adult trials. Thus, differences in histologic subtypes treated or PD-L1 expression levels compared to prior reports in adults do not seem to account for the lower response rate observed. Anti-PD1 monotherapy was previously reported to demonstrate a 41% ORR in a Phase II trial in PMBCL (27). In this study, of 10 evaluable NHL patients, we observed 1 partial response in a patient with PMBCL and in the responding patient, 100% of NHL cells demonstrated 3+ PD-L1 expression. Future work is needed to determine whether children with HL or PMBCL experience a lower proportion of response compared to adults and whether this reflects age associated distinctions in immunobiology associated with HL or PMBCL.
In this study there was no evidence for single agent activity of nivolumab in non-lymphoma pediatric solid tumors, although some pediatric tumor histologies such as Wilms tumor, malignant rhabdoid tumor and germ cell tumors were not evaluated in this study. Biologic correlates used to predict response to ICI are imperfect although high expression of PD-L1 on tumor cells or infiltrating T cells and high tumor mutational burden have been reported to correlate with response (28–30). Our data, consistent with previously published reports (31–33), demonstrate low PD-L1 expression levels on the histologic tumor types enrolled here and a paucity of infiltrating T cells. Given the current concept that response to PD-1/PD-L1 blockade in non-lymphoma tumors reflects reinvigoration of T cells recognizing neoantigens derived from tumor mutations, the absence of response may reflect the relatively low mutational burden in sporadic pediatric solid tumors (28). Our study did not assess tumor mutational burden limiting assessment of its impact in the pediatric tumors studied here although prior studies have demonstrated only ~5% of sporadic pediatric solid tumors are classified as “hypermutant”, as defined by more than 10 mutations/mB (28, 29), a minimum level that has been suggested to be associated with response to ICI. Of note, children with hypermutant tumors associated with germline mutations in mismatch repair genes show significant responses to PD-1 blockade (13, 14), providing evidence that pediatric patients are capable of mounting PD-1 induced antitumor effects in the presence of adequate antigen to drive T cell responses. Future studies are needed to determine whether selected cohorts of children with hypermutant sporadic solid tumors would demonstrate clinical benefit following PD-1 blockade.
In summary, these results demonstrate that nivolumab (3 mg/kg every 14 days) is tolerable in children. Activity was observed in HL and in one patient with NHL, but single agent activity was not observed for the common, non-lymphoma, non-CNS pediatric solid tumors studied here. Future studies evaluating nivolumab, or other PD-1/PD-L1 blockers, alone or in combination with other immunomodulators, may be warranted in selected populations of patients with pediatric solid tumors whose tumors manifest higher mutational burden and/or other biomarkers that provide evidence for increased tumor immunogenicity.
Supplementary Material
Research In Context.
Evidence before this study
We searched PubMed to retrieve data regarding the safety and efficacy of PD-1/PD-L1 blockers for treatment of pediatric cancer between its inception and July 17, 2019. The search contained key terms including “checkpoint inhibitor” “nivolumab” “PD-L1” “PD-1” “children” “pediatric” with specific attention to Phase 1 or 2 trials. No language restrictions were applied. This search highlighted use of PD-1/PD-L1 blockers, including nivolumab, in a wide range of adult tumors often with impressive clinical benefit. In addition, reports of expression of PD-L1 on common childhood tumor histologies have been published. No Phase 1 or 2 trials have been published safety or efficacy of PD-1 targeting in sporadic pediatric cancers.
Added value of this study
This report details, to our knowledge, the first experience demonstrating safety and assessing the efficacy of nivolumab in children and young adults with relapsed or refractory non-CNS pediatric solid tumors. The data demonstrate that nivolumab is well tolerated in the pediatric age group and that pharmacokinetics and pharmacodynamics are comparable to those observed in adult cohorts. In expanded Phase 2 cohorts, no objective responses were observed outside of patients with recurrent lymphoma. This was consistent with an observed low expression of PD-L1 on solid tumors of childhood, which stands in contrast to pediatric lymphomas which express substantial levels of PD-L1.
Implications of all the available evidence
Nivolumab demonstrates similar immune adverse events and pharmacodynamic profiles in children as in adult patients. As a single agent, Nivolumab has promising activity for children and young adults with lymphoma and further study of this agent is warranted in this group, but has no significant activity in the sporadic pediatric solid tumor histotypes evaluated in this trial. The favorable safety profile raises the prospect that Nivolumab could be safely combined with other agents, which may enhance its therapeutic value in these populations.
Acknowledgements:
The research reported is supported by Bristol-Myers Squibb, the Children’s Oncology Group, the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award number UM1 CA228823, and the Cookies for Kids’ Cancer Foundation. This work was also supported in part by a St. Baldrick’s–Stand Up to Cancer (SU2C) Dream Team translational research grant (SU2C-AACR-DT-27–17) (to C.L.M.). SU2C is a division of the Entertainment Industry Foundation, and research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C. Dr. Reid was supported in part by Grant Number P30 CA015083 from the National Cancer Institute (NCI). We thank the patients and their families for making this study possible.
Funding: Bristol-Meyers Squibb, Children’s Oncology Group, National Institutes of Health, Cookies for Kids Cancer Foundation.
C.L.M. is a founder, equity holder and consultant for Lyell Immunopharma, holds equity in Allogene and Apricity Health, and is a consultant for Roche, Nekktar, Lyell Immunopharma and Apricity Health. M.S.M is employed by Epizyme and holds stock at AstraZeneca.
Footnotes
Conflicts of Interest: All other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kara L. Davis, Stanford University, Stanford, CA, USA
Elizabeth Fox, Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Melinda S. Merchant, National Institutes of Health, Bethesda, MD, USA
Joel M. Reid, Mayo Clinic, Rochester, MN, USA
Rachel A. Kudgus, Mayo Clinic, Rochester, MN, USA
Xiaowei Liu, Children’s Oncology Group, Monrovia, CA, USA.
Charles G. Minard, Baylor College of Medicine, Houston, TX, USA
Stephan Voss, Dana Farber Cancer Institute, Boston, MA, USA.
Stacey L. Berg, Baylor College of Medicine, Houston, TX, USA
Brenda J. Weigel, University of Minnesota, Minneapolis, MN, USA
Crystal L. Mackall, Stanford University, Stanford, CA, USA
References
- 1.Smith MA, Altekruse SF, Adamson PC, Reaman GH, Seibel NL. Declining childhood and adolescent cancer mortality. Cancer. 2014;120(16):2497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho HW, Lee JW, Ma Y, Yoo KH, Sung KW, Koo HH. Treatment Outcomes in Children and Adolescents with Relapsed or Progressed Solid Tumors: a 20-year, Single-Center Study. J Korean Med Sci. 2018;33(41):e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378(8):731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosse YP, Voss SD, Lim MS, Rolland D, Minard CG, Fox E, et al. Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children’s Oncology Group Study. J Clin Oncol. 2017;35(28):3215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laetsch TW, DuBois SG, Mascarenhas L, Turpin B, Federman N, Albert CM, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/Phase II Study of Blinatumomab in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. J Clin Oncol. 2016;34(36):4381–9. [DOI] [PubMed] [Google Scholar]
- 10.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mody R, Naranjo A, Van Ryn C, Yu AL, London WB, Shulkin BL, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol. 2017;18(7):946–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J Clin Oncol. 2016;34(19):2206–11. [DOI] [PubMed] [Google Scholar]
- 14.Larouche V, Atkinson J, Albrecht S, Laframboise R, Jabado N, Tabori U, et al. Sustained complete response of recurrent glioblastoma to combined checkpoint inhibition in a young patient with constitutional mismatch repair deficiency. Pediatr Blood Cancer. 2018;65(12):e27389. [DOI] [PubMed] [Google Scholar]
- 15.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perel Y, Conway J, Kletzel M, Goldman J, Weiss S, Feyler A, et al. Clinical impact and prognostic value of metaiodobenzylguanidine imaging in children with metastatic neuroblastoma. J Pediatr Hematol Oncol. 1999;21(1):13–8. [DOI] [PubMed] [Google Scholar]
- 17.Ady N, Zucker JM, Asselain B, Edeline V, Bonnin F, Michon J, et al. A new 123I-MIBG whole body scan scoring method--application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer. 1995;31A(2):256–61. [DOI] [PubMed] [Google Scholar]
- 18.Phillips T, Simmons P, Inzunza HD, Cogswell J, Novotny J Jr., Taylor C, et al. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl Immunohistochem Mol Morphol. 2015;23(8):541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onar-Thomas A, Xiong Z. A simulation-based comparison of the traditional method, Rolling-6 design and a frequentist version of the continual reassessment method with special attention to trial duration in pediatric Phase I oncology trials. Contemp Clin Trials. 2010. May;31(3):259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett JS, Jayaraman B, Patel D, Skolnik JM. A SAS-based solution to evaluate study design efficiency of phase I pediatric oncology trials via discrete event simulation. Comput Methods Programs Biomed. 2008. June; 90(3):240–50. [DOI] [PubMed] [Google Scholar]
- 21.Simon R Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989. March;10(1):1–10 [DOI] [PubMed] [Google Scholar]
- 22.Kushner BH, Kramer K, Modak S, Cheung NK. Sensitivity of surveillance studies for detecting asymptomatic and unsuspected relapse of high-risk neuroblastoma. J Clin Oncol. 2009;27(7):1041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto N, Nokihara H, Yamada Y, Shibata T, Tamura Y, Seki Y, et al. Phase I study of Nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Invest New Drugs. 2017;35(2):207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y. Model-Based Population Pharmacokinetic Analysis of Nivolumab in Patients With Solid Tumors. CPT Pharmacometrics Syst Pharmacol. 2017;6(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J Clin Oncol. 2016;34(23):2690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zinzani PL, Ribrag V, Moskowitz CH, Michot JM, Kuruvilla J, Balakumaran A, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood. 2017;130(3):267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell BB, Light N, Fabrizio D, Zatzman M, Fuligni F, de Borja R, et al. Comprehensive Analysis of Hypermutation in Human Cancer. Cell. 2017;171(5):1042–56 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majzner RG, Simon JS, Grosso JF, Martinez D, Pawel BR, Santi M, et al. Assessment of programmed death-ligand 1 expression and tumor-associated immune cells in pediatric cancer tissues. Cancer. 2017;123(19):3807–15. [DOI] [PubMed] [Google Scholar]
- 32.Aoki T, Hino M, Koh K, Kyushiki M, Kishimoto H, Arakawa Y, et al. Low Frequency of Programmed Death Ligand 1 Expression in Pediatric Cancers. Pediatr Blood Cancer. 2016;63(8):1461–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Dam LS, de Zwart VM, Meyer-Wentrup FA. The role of programmed cell death-1 (PD-1) and its ligands in pediatric cancer. Pediatr Blood Cancer. 2015;62(2):190–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.