Abstract
The apicomplexan Toxoplasma gondii induces strong protective immunity dependent upon recognition by Toll-like receptors (TLR)11 and 12 operating in conjunction with MyD88 in the murine host. However, TLR11 and 12 proteins are not present in humans, inspiring us to investigate MyD88-independent pathways of resistance. Using bicistronic IL-12-YFP reporter mice on MyD88+/+ and MyD88-/- genetic backgrounds, we show that CD11c+MHCII+F4/80- dendritic cells, F4/80+ macrophages, and Ly6G+ neutrophils were the dominant cellular sources of IL-12 in both wild type and MyD88 deficient mice after parasite challenge. Parasite dense granule protein GRA24 induces p38 MAPK activation and subsequent IL-12 production in host macrophages. We show that Toxoplasma triggers an early and late p38 MAPK phosphorylation response in MyD88+/+ and MyD88-/- bone marrow-derived macrophages. Using the uracil auxotrophic Type I T. gondii strain cps1-1, we demonstrate that the late response does not require active parasite proliferation, but strictly depends upon GRA24. By i. p. inoculation with cps1-1 and cps1-1:Δgra24, we identified unique subsets of chemokines and cytokines that were up and downregulated by GRA24. Finally, we demonstrate that cps1-1 triggers a strong host-protective GRA24-dependent Th1 response in the absence of MyD88. Our data identify GRA24 as a major mediator of p38 MAPK activation, IL-12 induction and protective immunity that operates independently of the TLR/MyD88 cascade.
Author summary
Toxoplasma gondii is a protozoan parasite that infects over 1 billion people worldwide. Infection with the parasite is normally asymptomatic and Toxoplasma co-exists with its host in the form of latent cysts in brain and muscle tissue. The balance between immune recognition and immune evasion is likely a key factor in the outcome of this host-parasite interaction. It is therefore important to understand how Toxoplasma triggers immunity, and in particular how the protective cytokine IL-12 is induced during infection. While Toll-like receptor (TLR)/MyD88 signaling is important in mouse resistance to Toxoplasma, this pathway is likely less important in human infection. Here, we report that the parasite dense granule protein GRA24 triggers p38 MAPK activation and IL-12 production independently of TLR/MyD88 signaling. We identify additional cytokines and chemokines that are regulated by GRA24 during in vivo infection. Our data demonstrate that GRA24 initiates a protective MyD88-independent immune response during in vivo infection. The GRA24 molecule provides an example of a parasite molecule whose function is induction of a host protective immune response. From the standpoint of Toxoplasma, this likely reflects an evolutionary adaptation to ensure host survival and simultaneously enable latency to maximize the chance of transmission.
Introduction
The intracellular protozoan Toxoplasma gondii is a globally distributed parasite that infects humans, companion animals, livestock and wildlife. The parasite is estimated to infect 25–30% of the human population worldwide [1]. The course of infection is characterized by two phases. In the acute phase initiated in the intestine after oral infection, the parasites disseminate widely through tissues as rapidly dividing and highly invasive tachyzoites. This is followed by a chronic, or latent, phase associated with differentiation to slowly dividing bradyzoites that form long-lived cysts in tissues of the skeletal muscle and central nervous system [2]. Infection at this stage is usually asymptomatic. Nevertheless, in immunodeficient populations cysts may reactivate resulting in uncontrolled parasite replication that can rapidly culminate in death [3]. Toxoplasma may also cross the placenta during pregnancy causing life-threatening disease both before and after birth [4].
Toxoplasma is highly effective at stimulating a protective immune response, an outcome that accounts for the normally asymptomatic nature of infection [5–7]. While clearly aiding the host, the parasite also benefits from this protective response since host survival enables establishment of latent infection. The immune response to T. gondii revolves around early production of IL-12 by cells such as CD8α dendritic cells (DC) in the spleen as well as CD103+CD11b- and CD103-CD11b- DC in the intestinal mucosa [8–10]. The central role played by IL-12 in resistance is dramatically highlighted by the extreme susceptibility of IL-12 knockout (KO) mice to T. gondii infection [11]. Production of IL-12 drives early NK cell production of IFN-γ and generation of IFN-γ-producing Th1 cells. IFN-γ ultimately controls the parasite through its ability to induce anti-Toxoplasma effector molecules such as the immunity-related GTPase (IRG) family and guanylate-binding proteins (GBP) that destroy the parasitophorous vacuole harboring intracellular tachyzoites [12–16].
The molecular and cellular basis for recognition and subsequent IL-12 production in response to T. gondii and other microbial pathogens has been extensively studied in mouse models. Early on, it was understood that the Toll-like receptor (TLR) adaptor molecule MyD88 is important in triggering IL-12 and promoting host resistance to Toxoplasma [17,18]. Cell-specific knockout studies revealed CD11c+ DC as the major source of MyD88-dependent resistance rather than macrophages or neutrophils that can also contribute to protective IL-12 [19–21]. Amongst TLR, both TLR11 and TLR12 are major receptors involved in recognition and resistance. Together, these receptors are activated by profilin, a parasite protein required for host cell invasion that also serves as a classical pathogen-associated molecular pattern igniting innate immunity [22–27]. Other TLR, for example cell surface TLR2 and 4 and the endosomal nucleic acid receptors TLR7 and 9, have been suggested to play secondary roles in resistance to Toxoplasma [24,28,29].
While a great deal is known about initiation of immunity to T. gondii in mouse models, the major innate immune receptors and signaling molecules involved in human resistance to this intracellular protozoan remains an open question. This is because in humans TLR11 is present only as a pseudogene and the TLR12 gene is entirely absent [30]. Moreover, MyD88-deficient individuals remain resistant to all but a select few pyogenic bacterial infections [31,32]. Thus, while TLR/MyD88 signaling plays a major role in rodent resistance to Toxoplasma and other infections, this signaling axis appears less crucial for defense in humans.
Previously, we reported the presence of MyD88-independent pathways of immunity during Toxoplasma infection [33]. While MyD88 KO mice ultimately do not survive infection, the days immediately preceding death are characterized by emergence of IFN-γ-producing Th1 effectors. More strikingly, vaccination of MyD88-/- mice with cps1-1, an avirulent Toxoplasma uracil auxotroph, results in protective immunity to lethal challenge [33]. In parallel, we discovered that the Toxoplasma Type I strain RH triggers an unusual autophosphorylation pathway of p38 mitogen-activated protein kinase (MAPK) activation, and that this response drives IL-12 production in mouse bone marrow-derived macrophages [34]. This finding was later extended by others who identified the parasite dense granule secretory protein GRA24 as the activator of p38 MAPK [35,36]. Thus, GRA24 is targeted by the parasite into the host cell where it binds and triggers allosteric autoactivation of p38 MAPK. This results in nuclear translocation and changes in host gene transcription, including upregulation of genes controlling IL-12 transcription.
In the present study, we investigate the role of GRA24 in MyD88-independent triggering of immunity both in vitro and in vivo. Using genetically engineered cps1-1 GRA24 KO parasites, we show that this molecule drives p38 phosphorylation and IL-12 production independently of MyD88 signaling. In addition, we identify a novel subset of cytokines and chemokines that are controlled by GRA24 during in vivo infection. Furthermore, we show that GRA24 triggers a strong host defense response during induction of immunity by cps1-1. Our results provide a striking molecular example illustrating the evolutionary adaptation of Toxoplasma to actively trigger inflammatory cytokine production to promote host survival, parasite latency, and transmission.
Results
Toxoplasma triggers MyD88-independent IL-12 production during in vivo and in vitro infection
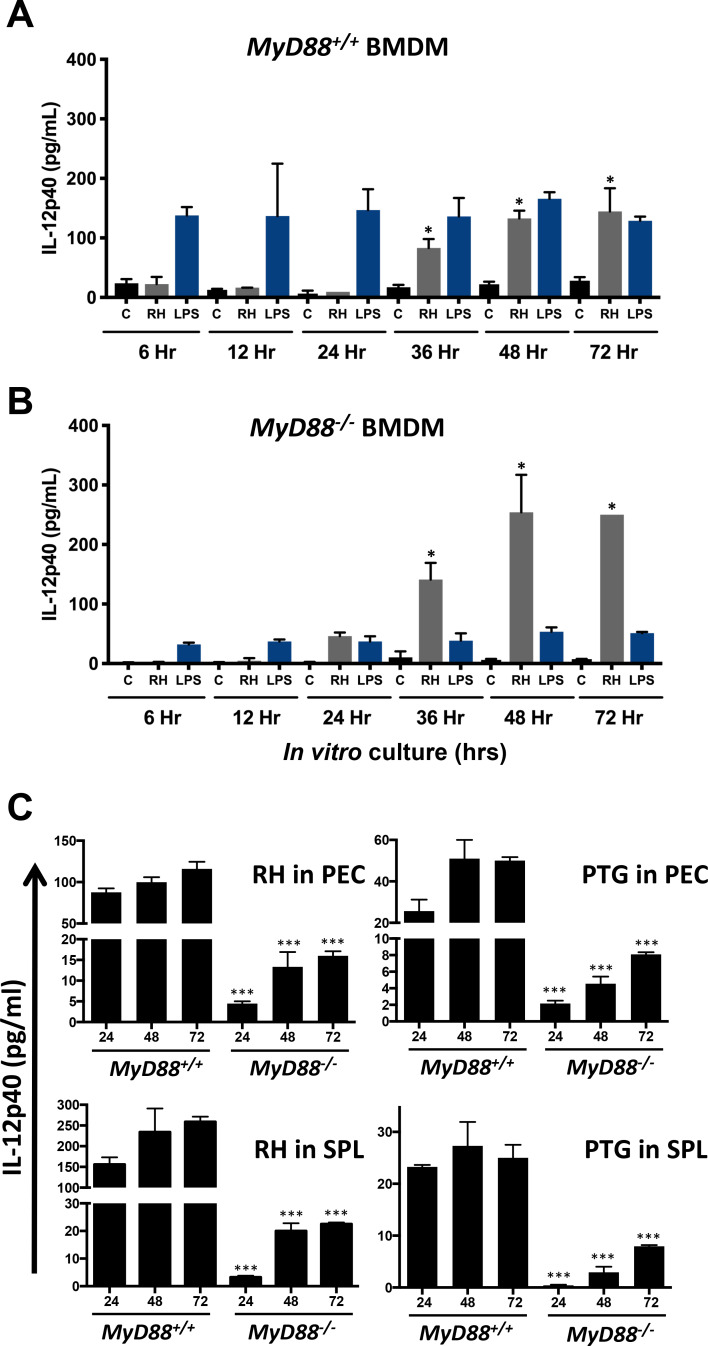
To understand the MyD88-independent IL-12 response during T. gondii infection, we generated bone marrow-derived macrophages (BMDM) from MyD88+/+ and MyD88-/- mice then stimulated the cells with Type I RH tachyzoites and lipopolysaccharide (LPS). We found increasing amounts of IL-12p40 production as infection progressed in MyD88+/+ (Fig 1A) and MyD88-/- (Fig 1B) BMDM infected with RH compared to media control. Nevertheless, we note that the cytokine was not produced at detectable levels until 24–36 hr after infection. In contrast, the response to LPS, which signals through TLR4 and MyD88, was largely abrogated in MyD88 KO BMDM, and in WT cells the maximum response was achieved within 6 hr of stimulation (Fig 1A and 1B). The parasites initiate egress at approximately 48 hr post-infection, likely accounting for lack of IL-12 increase between 48 and 72 hr. Although IL-12p40 levels were somewhat higher in MyD88-/- BMDM compared to MyD88+/+ BMDM infected with RH in this experiment, over biological replicates (n = 3) this result was not consistent. We examined BMDM IL-12p40 production following infection with Type II strain tachyzoites that have been reported to induce a more robust IL-12 response compared to Type I strains [37]. While there were some minor differences, we found overall that IL-12p40 production was very similar following infection with RH and Type II PTG in both MyD88+/+ (S1A Fig) and MyD88-/- (S1B Fig) BMDM. Caspase-8 has recently been shown to contribute to IL-12 production and antimicrobial defense to Toxoplasma independent of RIPK3 [38]. Therefore, we wanted to determine if RIPK3-independent caspase-8 signaling contributed to parasite-induced IL-12 in BMDM. We infected RIPK3-/- and RIPK3-/-Caspase8-/- BMDM with either RH or PTG tachyzoites and measured IL-12p40 in the supernatants. The results showed RIPK3-/- and RIPK3-/-Caspase8-/- BMDM infected with either RH or PTG produced similar amounts of IL-12p40 production compared to WT BMDM (S2A and S2B Fig). We conclude that caspase-8 is not involved in the response to T. gondii triggered in BMDM.
Fig 1. Toxoplasma gondii triggers production of MyD88-independent IL-12 during in vivo and in vitro infection.
Bone marrow-derived macrophages (BMDM) from wild-type (A, MyD88+/+) and MyD88 knockout (B, MyD88-/-) mice were cultured in medium (C), infected with RH strain tachyzoites (1:1 ratio of parasite to cells) or stimulated with LPS (100 ng/ml). At the indicated time points, supernatants were collected for cytokine ELISA. (C) MyD88+/+ and MyD88-/- mice (n = 2 per group) were infected by intraperitoneal inoculation with 103 RH or PTG strain tachyzoites. Four days later, peritoneal exudate cells (PEC) and splenocytes (SPL) were isolated and cultured with no further stimulation. Supernatants were collected for cytokine ELISA at the indicated time points (hr). Data shown are the means ± SD of cells cultured in triplicate. Statistical significance was assessed using Student’s t test (* p < 0.05, *** p < 0.001). These experiments were performed three times with the similar result.
We also assessed IL-12p40 production in peritoneal exudate cells (PEC) and splenocytes (SPL) in MyD88+/+ and MyD88-/- mice that were infected by intraperitoneal (i. p.) inoculation with either Type I RH or Type II PTG tachyzoites. There was a strong IL-12p40 response in the MyD88+/+ PEC and SPL infected with both RH and PTG parasite strains (Fig 1C). The IL-12 response was less robust in PEC and SPL from infected MyD88 KO mice. Nevertheless, there was clear IL-12 production elicited by both parasite strains independent of MyD88 (Fig 1C). Together, our results identify an alternative MyD88-independent pathway of IL-12p40 production triggered by Type I and Type II strains of Toxoplasma during both in vitro and in vivo infection.
Dendritic cells, neutrophils and macrophages are sources of IL-12 production in T. gondii infected MyD88 deficient mice
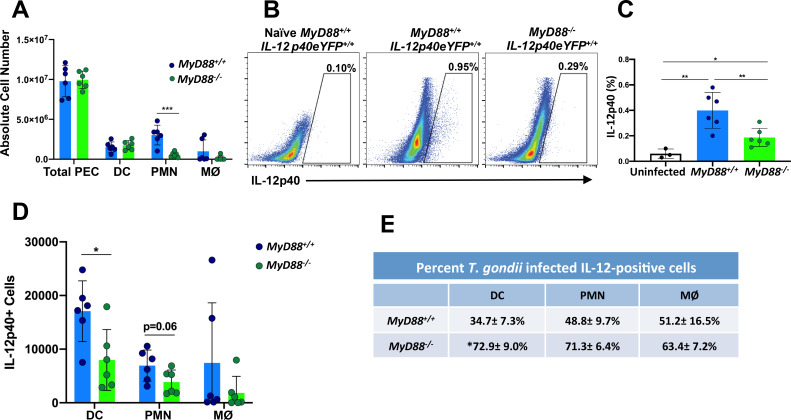
To understand the cells involved in IL-12 production in MyD88 deficient mice during T. gondii infection, we utilized IL-12eYFP reporter mice, a bicistronic reporter strain in which cells expressing IL-12 also express eYFP [39]. We crossed MyD88+/+IL-12p40eYFP/eYFP with MyD88-/- animals to generate strains expressing IL-12eYFP on MyD88+/+ and MyD88-/- backgrounds. The reporter mouse strains were infected with RH tachyzoites by i. p. inoculation and we then used flow cytometry to measure percentages and total cell numbers in the peritoneal cavity five days post infection. Our data show similar numbers of cells in the peritoneal cavity of MyD88+/+IL-12p40eYFP/eYFP and MyD88-/-IL-12p40eYFP/eYFP infected mice at this time point (Fig 2A). There were also similar numbers of DC in both mouse strains, but there was a 3–5 fold decrease in neutrophils (PMN) and macrophages (MØ) in the absence of MyD88 (Fig 2A). In line with the data shown in Fig 1C, IL-12-positive cells in MyD88-/- mice were present at approximately 30% of the levels in the IL-12 reporter MyD88+/+ animals (Fig 2B and 2C). In Fig 2D we examined the relative contributions of DC, PMN and MØ to the IL-12-positive populations in MyD88+/+ and MyD88-/- mice. While each cell type contributed to the IL-12-positive population, DC contributed the largest portion in the WT strain. In contrast, the proportions were more evenly divided in the KO mice (Fig 2D). By staining for intracellular tachyzoites we determined the percent infection in each of the IL-12-positive populations (Fig 2E). In the MyD88+/+ reporter strain, most of the IL-12-positive DC, PMN and MØ were uninfected. Interestingly, the majority of IL-12-positive cells were infected on the MyD88-/- background, regardless of identity (Fig 2E). Collectively, these data show that the cell types producing IL-12p40 in both MyD88 sufficient and MyD88 deficient mice are dendritic cells, neutrophils and macrophages. In addition, the MyD88-independent IL-12 that is produced derives predominantly from infected cells.
Fig 2. Multiple cell types produce MyD88-independent IL-12 during in vivo infection.
IL-12 reporter mice on a wild type (MyD88+/+IL-12p40eYFP/eYFP) and MyD88 knockout (MyD88-/-IL-12p40eYFP/eYFP) background were infected by intraperitoneal injection with 103 RH strain tachyzoites. On day 5 post-inoculation, peritoneal exudate cells were collected for ex vivo analysis. (A) Number of PEC, dendritic cells (DC), neutrophils (PMN) and macrophages (MØ) present in the peritoneal cavity. (B) Percentage of IL-12eYFP positive cells in the peritoneal cavity of one representative infected MyD88+/+IL-12p40eYFP/eYFP, one representative infected MyD88-/-IL-12p40eYFP/eYFP, and one representative noninfected MyD88+/+IL-12p40eYFP/eYFP mouse. (C) Percent IL-12 positive cells in the peritoneal cavities of WT and MyD88 KO IL-12 reporter mice. (D) Number of IL-12 positive cells amongst DC, PMN and MØ isolated from MyD88+/+ and MyD88-/- reporter mice. (E) Percent of infected cells that are positive for IL-12. In these experiments, DC were defined as MHCII+, CD11c+, F4/80- cells; PMN were defined as Ly6G+ cells; MØ were defined as F4/80+ cells. Values are the means ± SEM of 2 pooled independent experiments, n = 6 per group. Each symbol represents an individual mouse. Statistical significance was assessed using Student's t test (* p < 0.05, ** p < 0.01, *** p < 0.001) (A, C and D). Mann-Whitney test was used to determine statistical significance of IL-12-positive MyD88 WT vs. KO DC in E. These experiments were performed three times with similar results.
Parasite GRA24 plays a key role in MyD88-independent p38 MAPK-dependent IL-12 production
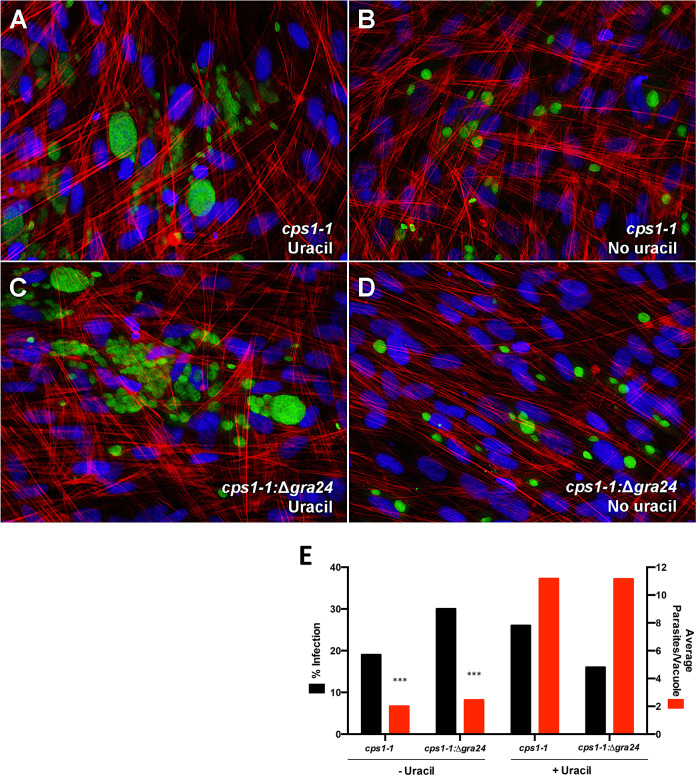
T. gondii dense granule protein GRA24 has been shown to trigger p38 MAPK-dependent IL-12 [35]. To examine the contribution of GRA24 to MyD88-independent IL-12, we assessed IL-12 production by the uracil auxotroph strain ΔompdcΔup (designated as cps1-1) and the corresponding GRA24 knockout strain ΔompdcupΔgra24 (designated as cps1-1: Δgra24) [40,41]. Inclusion or exclusion of exogenous uracil enabled us to determine the influence of tachyzoite proliferation on IL-12 production and MAPK activation. Fig 3 shows images of cps1-1 (panel A) and cps1-1:Δgra24 (panel C) on fibroblasts in the presence of uracil. At this time point (48 hr post-infection), cells were heavily infected and parasite egress was readily observed with uracil supplementation. In parallel cultures without uracil (Fig 3B and 3D), growth of both strains was restricted to ≤ 2 parasite divisions. Percent infection and average parasites per vacuole were measured in the cps1-1 and cps1-1:Δgra24 infected fibroblasts in the presence and absence of exogenous uracil (Fig 3E). We found that presence or absence of GRA24 had no significant effect on infection or replication rate whether or not exogenous uracil was provided.
Fig 3. Both cps1-1 and cps1-1:Δgra24 tachyzoites require supplemental uracil for sustained replication.
Tachyzoites of strains cps1-1 or cps1-1:Δgra24 were inoculated onto human foreskin fibroblast monolayers in the presence (A and C) or absence (B and D) of exogenous uracil. Two days after infection, monolayers were fixed and stained with FITC conjugated anti-Toxoplasma antibody (green), phalloidin-Texas red to stain host cell actin, and DAPI (blue) to stain nuclei. Images were collected under 40x magnification. (E) Percent infection and the average parasites per vacuole were quantified in cps1-1 and cps1-1:Δgra24 infected fibroblasts in the presence and absence of exogenous uracil. This experiment was repeated twice with the same result. Data shown are the mean counts of three independent experiments. Statistical significance was assessed using Mann-Whitney test comparing cps1-1 (+/-) exogenous uracil and cps1-1:Δgra24 (+/-) exogenous uracil (E) (*** p < 0.001).
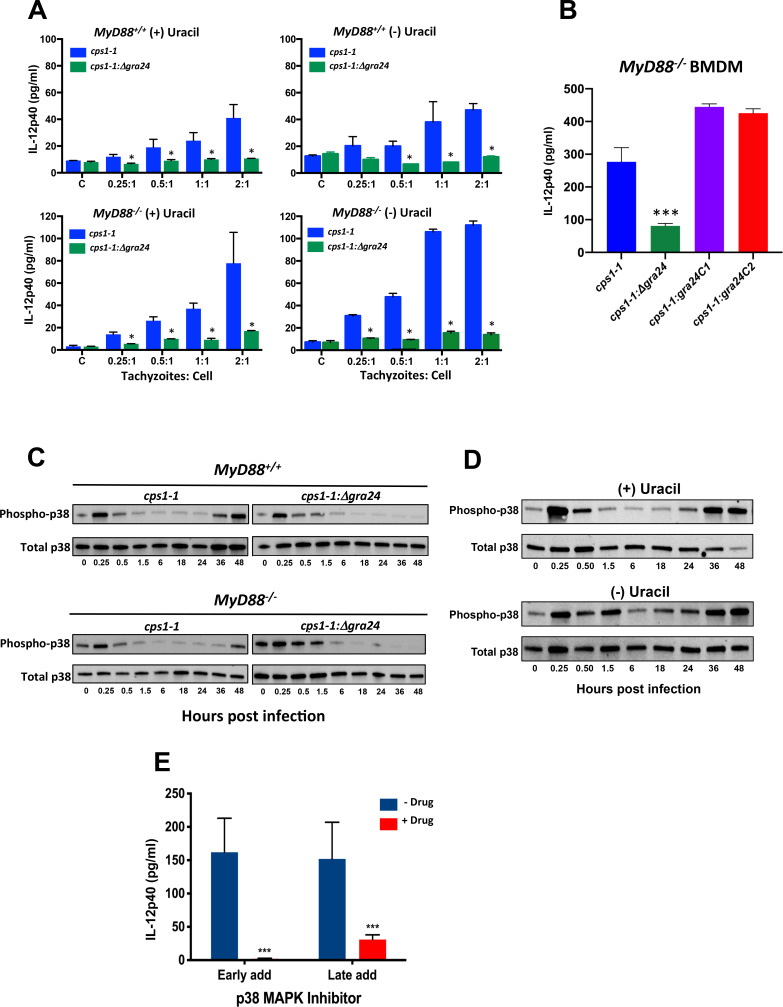
MyD88+/+ and MyD88-/- BMDM were inoculated with either cps1-1 and cps1-1:Δgra24 tachyzoites and supernatants were collected 48 hr later for cytokine assay (Fig 4A). In the presence of exogenous uracil, proliferating cps1-1 parasites triggered IL-12p40 in both WT and KO BMDM (Fig 4A, left panels). Strikingly, these responses failed to occur using GRA24-negative parasites. Because parasite-induced IL-12 was not produced until 24–36 hr post-infection, it was possible the response was tied to tachyzoite proliferation. Accordingly, the experiment was conducted in the absence of exogenous uracil. Even when proliferation was restricted, cps1-1 tachyzoites maintained the ability to induce IL-12 in dependence upon GRA24 (Fig 4A, right panels). We also generated two independent GRA24 complementation mutants, designated cps1-1:gra24C1 and cps1-1:gra24C2, and measured IL-12p40 production in BMDM. As expected, restoration of GRA24 expression in cps1-1:Δgra24 parasites also reestablished production of IL-12 (Fig 4B).
Fig 4. GRA24 controls MyD88-independent p38 MAPK-dependent IL-12 production.
(A) MyD88+/+ and MyD88-/- BMDM were infected with cps1-1 or cps1-1:Δgra24 tachyzoites at the indicated parasite to cell ratios. Cultures were initiated with (+) and without (-) exogenous uracil and supernatants were collected 48 hr after infection. C, control non-infected cells. (B) MyD88-/- BMDM were inoculated with cps1-1, cps1-1:Δgra24, cps1-1:gra24C1 or cps1-1:gra24C2 tachyzoites at a 2:1 parasite to cell ratio. Supernatants were collected 48 hr post infection for IL-12 measurement. Production of IL-12 was significantly reduced in cells infected with cps1-1:Δgra24 in pairwise comparisons with cps1-1 and the two complementation mutants. (C) cps1-1 or cps1-1:Δgra24 tachyzoites were inoculated onto MyD88+/+ and MyD88-/- BMDM at a ratio of 2:1. Cultures were briefly centrifuged to initiate parasite and cell contact, then lysates were prepared for Western blot assay at the indicated time points after infection (hr). (D) WT BMDM were infected with cps1-1 parasites in the presence and absence of uracil, then lysates were prepared for Western blot analysis as in (C). (E) WT BMDM were infected with cps1-1 tachyzoites in the presence and absence of p38 MAPK inhibitor SB202190 (10 μM). Supernatants were collected 36 hr after infection for cytokine ELISA. The inhibitor was added either 1 hr prior to infection (“Early add”) or 10 hr after infection (“Late add”). Data shown are the means ± SD of cells cultured in triplicate. Statistical significance was evaluated using Student’s t test (* p < 0.05, *** p < 0.001). These collective experiments were repeated with the similar result two times.
We next examined the influence of GRA24 on p38 MAPK activation in the context of cps1-1 infection of BMDM. Interestingly, infection triggered an early wave of p38 phosphorylation (≤ 15 min post infection) followed by dephosphorylation and a later wave that occurred 36 hr after infection (Fig 4C). Both responses occurred independently of MyD88, but the second wave was strictly dependent upon GRA24 (Fig 4C). Type I Toxoplasma is known to activate both STAT3 and ERK1/2 [42–44]. Accordingly, we examined the influence of MyD88 and GRA24 on these responses. We found neither host MyD88 nor parasite GRA24 influenced ERK1/2 phosphorylation (S3A Fig) or STAT3 phosphorylation (S3B Fig). The experiment in Fig 4C was carried out in the presence of uracil, and we therefore wondered if the second wave of p38 activation was driven by parasite proliferation and possibly host cell lysis and reinfection of new cells. Accordingly, we examined cps1-1-driven p38 MAPK activation under proliferation permissive (+ uracil) and proliferation non-permissive (- uracil) conditions (Fig 4D). Even when parasite replication was prevented by exclusion of uracil, we saw the same two-wave pattern of p38 MAPK phosphorylation.
Production of GRA24-dependent IL-12 correlated most closely with the late wave of p38 phosphorylation, which was also dependent upon GRA24. To formally demonstrate that late p38 phosphorylation was the key event in cps1-1-induced IL-12 production, we employed timed addition of the small molecule p38 MAPK inhibitor SB202190 [45]. Addition of the inhibitor 1 hr prior to infection (“Early add”) completely prevented IL-12 production during infection (Fig 4E), as measured in 48 hr supernatants. Addition of the inhibitor 10 hr after infection (“Late add”) also almost completely blocked the IL-12 response. Thus, the second wave of p38 activation stimulated by GRA24 underlies delayed IL-12 production in Toxoplasma infected BMDM.
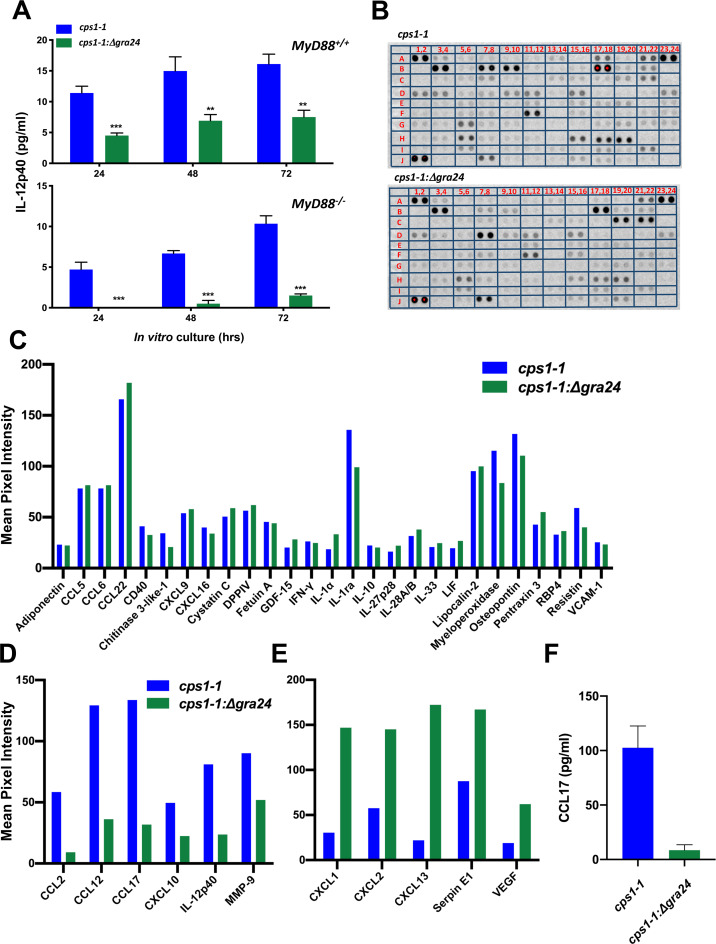
GRA24 regulates IL-12p40 and other cytokines and chemokines during in vivo infection
To determine if GRA24 controls production of IL-12p40 during in vivo infection, MyD88+/+ and MyD88-/- mice were infected with either cps1-1 or cps1-1:Δgra24 by i. p. injection. Four days post infection, PEC were harvested and cultured without further stimulation. IL-12p40 was measured in the supernatants at 24 hr, 48 hr and 72 hr. In line with the BMDM responses, cps1-1 infected MyD88+/+ PEC produced approximately twice as much IL-12p40 compared to the cps1-1:Δgra24 infected mice (Fig 5A). Furthermore, there was a near complete ablation of IL-12 production in MyD88 KO mice infected with the GRA24 deletion mutant (Fig 5A). To determine if GRA24 controls expression of other chemokines and cytokines, we used a proteomic array to screen a total of 111 cytokines, chemokines and related immune mediators released by PEC isolated from WT mice infected with cps1-1 and cps1-1:Δgra24. Fig 5B shows that overall, a large collection of cytokines and chemokines were up-regulated in PEC isolated from mice infected with each parasite strain. A schematic showing the coordinates of each cytokine and chemokine on the proteomic array is shown in S4 Fig. A collection of immune-related factors were up-regulated similarly during infection with the two parasite strains. Fig 5C shows factors up-regulated 20-fold or greater above background by both parasite strains. A subset of cytokines and chemokines were clearly up-regulated in dependence upon GRA24, including IL-12p40 and CCL17 (Fig 5D). Interestingly, we also identified a subset of immune-related mediators whose expression was negatively regulated by GRA24 including CXCL1 and CXCL2 (Fig 5E). As independent confirmation of these results, we used ELISA to measure CCL17. The result shows CCL17 production is clearly dependent upon GRA24 (Fig 5F). Together, these results demonstrate that GRA24 positively and negatively regulates distinct sets of cytokines and chemokines during in vivo T. gondii infection.
Fig 5. GRA24 controls the up-regulation of IL-12p40 and other cytokines and chemokines during T. gondii infection.
(A) MyD88+/+ and MyD88-/- mice (n = 3 per group) were infected by intraperitoneal injection of 106 cps1-1 or cps1-1:Δgra24 tachyzoites. Four days later, cells were harvested from the peritoneal cavity and cultured without further stimulation for the indicated times. (B) MyD88+/+ mice (n = 2 per group) were infected by i. p. inoculation with 106 cps1-1 or cps1-1:Δgra24 tachyzoites. Four days later, peritoneal exudate cells were harvested and cultured for 72 hr. The supernatants were harvested and prepared for cytokine proteome array. (C) Cytokine mean pixel intensity of the proteomic cytokine array in (B) that were equivalent between MyD88+/+ mice infected with cps1-1 and cps1-1:Δgra24, and that were greater than 20 fold up-regulated above background. (D) Cytokines up-regulated in MyD88+/+ mice infected with cps1-1 relative to cps1-1:Δgra24 in the cytokine proteome array. (E) Cytokines in the proteomic array that were up-regulated in cps1-1:Δgra24 relative to cps1-1 in infected MyD88+/+ mice. (F) MyD88+/+ mice (n = 2 per group) were infected, cells were collected and cultured as in (B). The supernatants were harvested and CCL17 was measured by ELISA. Data shown are the means ± SD of cells cultured in triplicate. Statistical significance was assessed using Student’s t test (** p < 0.001, *** p < 0.001). These collective experiments were repeated 2–3 times.
GRA24 plays a role in protective immunity independent of the MyD88 adaptor protein
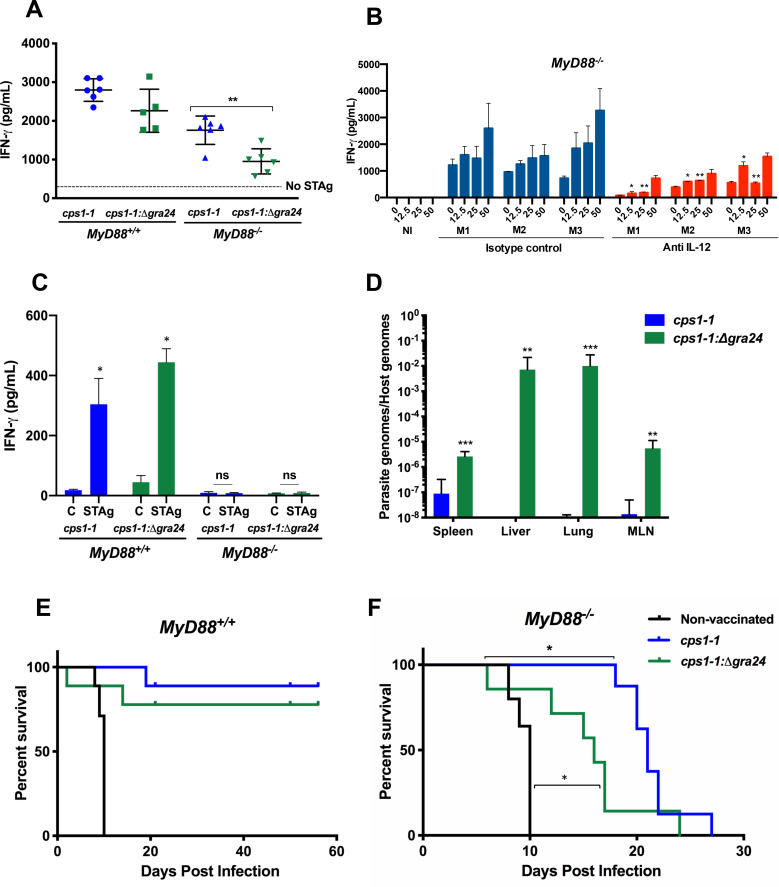
Vaccination with cps1-1 induces a strong Th1 response and protective immunity in both MyD88+/+ and MyD88-/- mice [33]. We wanted to determine the role of GRA24 in the response. Accordingly, we employed a vaccination protocol involving two sequential inoculations with cps1-1 and cps1-1:Δgra24 parasite strains. Two weeks after the final inoculation, a splenocyte cytokine recall assay was performed using soluble tachyzoite antigen (STAg). The results (Fig 6A) revealed that vaccination of WT mice with either cps1-1 or cps1-1:Δgra24 tachyzoites induced a strong IFN-γ response. However, the splenic recall response in cps1-1:Δgra24 vaccinated MyD88 KO mice was significantly weaker than the corresponding cps1-1 response (Fig 6A). Production of IFN-γ during T. gondii infection is strongly dependent upon IL-12 in wild type mice [46]. To determine if the IFN-γ recall response in cps1-1 vaccinated MyD88-/- mice was dependent upon IL-12, we treated MyD88-/- mice with a depleting anti-IL-12p40 mAb or rat immunoglobulin isotype control over the course of an i. p. infection. One week following infection, splenocytes were subjected to an in vitro recall assay with STAg. The results (Fig 6B) show that in vivo depletion of IL-12 substantially reduces the IFN-γ response relative to the Ab control-treated mice. We also examined the IFN-γ response in mesenteric lymph nodes in the vaccinated mice. Interestingly, production of IFN-γ was completely dependent upon MyD88 at this location (Fig 6C).
Fig 6. GRA24 induces protective immunity in the absence of MyD88.
(A) MyD88+/+ and MyD88-/- animals (n = 5–6 mice per group) were i. p. inoculated at Day 0 and Day 14 with 106 cps1-1 or cps1-1:Δgra24 tachyzoites, then at day 28 splenocytes were isolated and subjected to in vitro stimulation with soluble tachyzoite antigen (STAg; 50 μg/ml). Supernatants were collected at 72 hr for cytokine ELISA. Each point represents a single mouse. (B) MyD88-/- mice (n = 3 mice per group) were administered IL-12 neutralizing antibody or isotype control antibody during the course of infection initiated by inoculation with 5 x 106 cps1-1 tachyzoites. At Day 10 post-infection, splenocytes were isolated and stimulated with STAg at the indicated concentrations (μg/ml), then 72 hr later supernatants were collected for IFN-γ assay. NI, splenocytes from noninfected mice. M1, M2, M3 are individual MyD88-/- mice treated with the isotype control and M4, M5, M6 are individual MyD88-/- mice treated with the IL-12 neutralizing antibody. (C) Mesenteric lymph node cells were pooled from vaccinated mice, and subjected to STAg re-stimulation and cytokine assay as in (A). C, control cells cultured in medium alone. (D) MyD88-/-IL-12p40eYFP/eYFP mice (n = 7 per group) were vaccinated by i. p. inoculation with cps1-1 or cps1-1:Δgra24 tachyzoites. Two weeks after the final inoculation, mice were challenged with 103 RH tachyzoites. Nine days post challenge tissues were harvested from each individual mouse and processed independently for parasite quantitation by qPCR. In E and F, MyD88+/+ and MyD88-/- animals were vaccinated by i. p. inoculation with cps1-1 or cps1-1:Δgra24 tachyzoites as in (D). Two weeks after the final inoculation, mice were challenged by subcutaneous injection with 103 RH strain tachyzoites, and survival was monitored. Data shown are the means ± SD of cells cultured in triplicate (A, B and C). Statistical significance was evaluated using Student’s t test (A, C, D). A Mann-Whitney test was used to measure statistical significance between isotype controls and the corresponding anti-IL-12 concentrations (B). Log rank Mantel-Cox test was used to measure the statistical significance between the survival curves. (E and F). The asterisks in F indicate MyD88-/- mice vaccinated with the cps1-1:Δgra24 strain were significantly more susceptible than cps1-1 vaccinated mice, and that animals vaccinated with the cps1-1:Δgra24 strain were significantly more resistant than the nonvaccinated group. For the results shown, * p < 0.05, ** p < 0.01, and *** p < 0.001. These collective experiments were repeated with the same result two to three times.
To determine if GRA24 was responsible for protective immunity elicited by cps1-1, we determined how cps1-1 vaccination with or without GRA24 impacted systemic parasite burden in MyD88 deficient mice following challenge infection with virulent RH strain tachyzoites. Nine days after challenge, spleen, liver, lungs and MLN were collected and parasite burden was measured by quantitative PCR measurement of the Toxoplasma B1 gene relative to the host argininosuccinate lyase gene. As shown in Fig 6D, there was a dramatic increase in parasite burden in all four organs in the cps1-1:Δgra24 vaccinated mice compared to the cps1-1 vaccinated mice.
To further elucidate the impact of GRA24 during vaccination in induction of protective immunity, we monitored survival in lethally challenged animals. Non-vaccinated MyD88+/+ and MyD88-/- mice died by day 10 after challenge (Fig 6E and 6F). In contrast, MyD88+/+ mice vaccinated with cps1-1 and cps1-1:Δgra24 were resistant to challenge (Fig 6E). While WT mice vaccinated with cps1-1:Δgra24 appeared slightly less resistant, this result was not statistically significant. MyD88-/- mice vaccinated with cps1-1 were resistant to RH inoculation, with animals beginning to succumb at approximately 3 weeks post-challenge. In striking contrast MyD88 KO animals vaccinated with the GRA24 deletion mutant were significantly more susceptible to challenge (Fig 6F). We also note that this group of mice appeared more resistant than non-vaccinated counterparts, indicating the presence of protective mechanisms that involve neither GRA24 nor MyD88. Regardless, these collective results demonstrate that GRA24 elicits MyD88 independent immunity, thereby limiting systemic dissemination and enhancing host survival.
Discussion
The TLR/MyD88 signaling module in mice underlies resistance to a large number of pathogens, including T. gondii. However, the emerging realization that this axis of immunity is likely less critical for anti-microbial defense in humans has prompted significant interest in identifying MyD88-independent pathways of resistance. Here, we identify an MyD88-independent pathway of immunity to T. gondii that relies upon host p38 MAPK phosphorylation initiated by parasite dense granule protein GRA24. Activation of p38 MAPK by GRA24 triggers production of IL-12 during in vitro and in vivo models of infection. We also identified a novel subset of cytokines and chemokines whose expression during in vivo infection was upregulated by GRA24, and a unique subset that was down-regulated by this dense granule protein. In a vaccination model employing the uracil auxotrophic Toxoplasma strain cps1-1, we found that GRA24 drives an MyD88-independent Type I cytokine response associated with production of IFN-γ and protection from lethal challenge infection.
Evidence for an unusual pathway of T. gondii-driven p38 MAPK autophosphorylation during in vitro infection of mouse macrophages was first uncovered several years ago [34]. GRA24 was subsequently identified as the critical parasite effector protein using in silico methods to predict proteins capable of both entering the parasite secretory pathway and targeting the host cell nucleus [35,47,48]. GRA24 is unusual in that it has no homology to genes in other apicomplexa. Interestingly, the closely related apicomplexan Neospora caninum uses a distinct pathway to phosphorylate host p38 MAPK dependent upon G-protein-coupled receptor signaling [49]. In contrast to our results, p38 MAPK activation by N. caninum is associated with IL-12 down-regulation and evasion of host immunity.
Like other dense granule proteins, GRA24 is composed of intrinsically disordered regions that are predicted to favor its activity as a host-directed effector molecule [50]. The protein is exported across the parasitophorous vacuole membrane using a translocation pathway involving parasite proteins MYR1 and ASP5 [51–53]. GRA24 subsequently binds to p38α MAPK, triggering allosteric autoactivation, nuclear translocation and upregulation of transcription factors such as Egr-1 and c-fos [35,54]. Despite a relatively clear picture of GRA24 function inside the host cell, our understanding of the importance of this parasite molecule in MyD88-independent pathways of Th1-induction and protective immunity has been relatively limited.
Our in vitro infection experiments revealed a bi-phasic pattern of p38 MAPK activation in which the kinase was activated in a GRA24-independent manner within minutes of infection, followed by a second GRA24-dependent activation occurring 24–36 hr later. The second wave occurred even when replication and egress was prevented by excluding exogenous uracil, a result that argues against cell lysis and secondary infection as the stimulus for GRA24-dependent p38 MAPK phosphorylation. While we do not understand the underlying basis for this pattern, it may be noteworthy that GRA24 appears in the host cell nucleus only at later points in infection [35]. Regardless, it appears that the delayed wave of GRA24-dependent p38 MAPK phosphorylation is the critical driver of IL-12. First, IL-12 is only produced at later time points (36–48 hr) of BMDM infection, in contrast to LPS that triggers a response within the first 6 hr of stimulation. Second, addition of a pharmacological p38 MAPK inhibitor during the eclipse phase of parasite-induced p38 phosphorylation (10 hr post-infection) potently blocked subsequent IL-12 secretion.
The cause and significance of the early MyD88-independent p38 MAPK activation event is unclear at present. However, it is interesting to note that Toxoplasma-triggered STAT3 and STAT6 activation follows a similar pattern, with an early ROP16-independent phase followed by a later phase dependent upon this parasite kinase [42]. In this case, early STAT3 activation is dependent upon a FAK-Src-STAT3 signaling pathway associated with invasion [55]. We are currently examining whether this pathway also triggers early p38 MAPK activation.
We were able to induce an MyD88-independent protective immune response that depended upon GRA24 activity in a vaccine strain of the parasite. Yet, we also observed that even in the absence of this dense granule protein there remained a significant MyD88-independent protective response. The latter result provides strong evidence for the existence of other pathways of protection that rely on neither host TLR/MyD88 nor parasite GRA24. A recent study employing human cells revealed that peripheral blood monocytes and dendritic cells produce IL-12 only after phagocytosis of live tachyzoites, a process not generally associated with involvement of TLR or MyD88 [56]. It has also been shown that TLR11-independent inflammasome activation triggers Th1 immunity during Toxoplasma infection and the neutrophils can provide an early source on IFN-γ independently of TLR signaling [57,58]. It is possible that these phenomena contribute to MyD88-independent activation of immunity in the mouse model employed here. It has also been reported that the T. gondii dense granule protein GRA15 possesses proinflammatory cytokine-inducing activity. GRA15 is trafficked to the parasitophorous vacuole membrane where it activates NFκB signaling independently of MyD88. This in turn leads to induction of IL-12 [59]. However, GRA15 from Type I parasite strains (including the cps1-1 strain used here) does not display this activity and is therefore unlikely to account for cps1-1-induced, GRA24-independent immunity in MyD88-/- mice.
Dense granule proteins GRA24 and GRA15 are members of a growing family of Toxoplasma host-directed effectors that target signaling to remodel cell function [60]. Parasite GRA6 activates host transcription factor NFAT4 through binding to calcineurin activator calcium-modulating ligand (CAMLG) [61]. GRA6-dependent NFAT4 activation results in production of the chemokines CCL2 and CXCL2 which together promote recruitment of inflammatory monocytes and neutrophils. Because these cells are targets of infection, they may play a role in parasite dissemination. By interacting with herpesvirus-associated ubiquitin-specific protease (HAUSP) and the phosphate PP2A-B55, GRA16 alters steady-state levels of host p53, which may be important in host cell survival under conditions of stress associated with intracellular infection [47]. Through secretion of dense granule protein TgIST, Toxoplasma potently suppresses STAT1-induced gene expression. In the infected cell, this results in generalized nonresponsiveness to the anti-microbial effects of IFN-γ [62,63].
Rhoptries contain host-directed effector molecules that are released early during invasion. Rhoptry protein ROP16 is a host-directed kinase that is injected into the host cytoplasm during invasion where it activates both STAT3 and STAT6 [42,64]. In macrophages, this results in deviation to an M2 phenotype and down-regulation of IL-12 [65]. The Toxoplasma rhoptry protein TgWIP is secreted into the host cell where it interacts with the WAVE regulatory complex and SHP2 phosphatase. This impacts actin dynamics in dendritic cells, enhancing host cell motility that in turn promotes parasite dissemination [66]. Together, these studies form the basis for our expanding understanding of T. gondii as a microbial parasite equipped with a sophisticated toolbox used during infection to deal with the host immune system.
GRA24 is an effector molecule that the parasite deploys to activate signaling leading to IL-12. In one sense this appears counter-intuitive given that this cytokine is critical in activation of immunity leading to parasite elimination [5,46,67]. However, mice lacking IL-12 succumb extremely rapidly to infection before parasite latency is established [11]. Our findings support the model that GRA24 induces MyD88-independent IL-12 production to promote survival of the host in acute infection and parasite survival and transmission by promoting latency. The ability to establish a balance between excessive immunity and inadequate immunity is likely a major factor accounting for the evolutionary success of Toxoplasma.
Materials and methods
Ethics statement
All experiments performed in this study were conducted in accordance with the Guide for the Care and Use of Laboratory Animals. Protocols were approved by the Institutional Animal Care and Use Committee at the University of New Mexico (Animal Welfare Assurance Number A4023-01). All efforts were made to reduce animal suffering during this study.
Mice
Female and male mice (6–12 weeks of age) were used in these studies. Female C57BL/6 mice (5 weeks of age) were purchased from Taconic Biosciences Inc. (Rensselaer, NY). Female and male (5 weeks of age) B6.1292(SPJ)-MyD88tm1.1Defr/J (MyD88-/-) and B6.129-IL12btm1.1Lky/J (IL-12p40eYFP/eYFP) mouse strains were purchased from The Jackson Laboratory (Bar Harbor, ME). The MyD88-/- and IL-12p40eYFP/eYFP mouse strains were crossed in house to generate a MyD88-/-IL-12p40eYFP strain. The MyD88-/-, IL-12p40eYFP and MyD88-/-IL-12p40eYFPstrains were bred in-house in the University of New Mexico Department of Biology Animal Research Facility. Mouse genotyping was performed by Transnetyx, Inc. (Cordova, TN).
Parasites
Confluent human foreskin fibroblast monolayers (HFF; ATCC, Manassas, VA) were infected with T. gondii Type I (RH) or Type II (PTG) strains and passaged weekly into confluent HFF monolayers. HFF were grown to confluence in Dulbecco’s Modified Eagle’s Medium (DMEM) (VWR, Randor, PA) supplemented with 1 mM glutamine and 10% bovine growth serum (FBS; HyClone, Logan, UT), 100 U/ml penicillin (ThermoFisher Scientific, Waltham, MA) and 0.1 mg/ml streptomycin (ThermoFisher Scientific). Construction of the uracil auxotroph strains ΔompdcΔup (designated as cps1-1) and ΔompdcΔupΔgra24 (designated as cps1-1: Δgra24) has previously been described [40,41].
The cps1-1: Δgra24 strain was complemented with a C-terminal HA-tagged copy of GRA24 to independently isolate complemented strains cps1-1:gra24C1 and cps1-1:gra24C2. The gra24C targeting plasmid was constructed by recombining 5 PCR fragments using forward (F) and reverse (R) primers to synthesize the 5’ target region (F = TTGGGTAACGCCAGGGTTTTCCCAGTCACGACGGTTTAAACCTAGGACAGATG-TCCTCATCTCAGCGTCC; R = GGTGCGACGATTCGCTGCTG), the N-terminal region (F = GGTTGATGAGAGCGGTCCAGC; R = GGGATTATTGTGCCGGGTTGGC), the middle region (F = GGTTCAGCACATGGTAGGCCAAC; R = TCACTCAGGCAC-GCTCTGTATGTC), the C-terminal region (F = TCGGTGTCTCGCTGACATACAGAG; R = GACTTTGTCATCGTCGTCCTTGTAGTCCTCGAGATTACCCTTAGTGGGTGGTTT-AACGATGC), and the 3’ target region (F = CTCGAGGACTACAAGGACGACGATGAC-AAAGTCAAGCTCTACCCATACGATGTTCCAGATTACGCTGCTTAGCCTAGGGTTCAAAGTGCCCTATTGGTACTGGCA; R = GTGAGCGGATAACAATTTCACACAGGAAACA-GCGCGGCCGCACTGCGCTGATACCCGTCGTG) of GRA24. Complemented strains cps1-1:gra24C1 and cps1-1:gra24C2 were isolated after selection in 6-thioxanthine and uracil as previously described [40], and insertion of HA-tagged GRA24 at the GRA24 locus was confirmed using primer pairs (F = CAGCCAACAACGACACTCAGCG; R = GTTGGCCTACCATGTGCTGAACC), and (F = ACCCATACGATGTTCCAGATTACGC; R = CAGGCAACGCCGTGATCCAC).
The mutant parasites were maintained in DMEM (VWR) supplemented with 300 μM uracil, 1% FBS (ThermoFisher Scientific), 100 U/ml penicillin (ThermoFisher Scientific) and 0.1 mg/ml streptomycin (ThermoFisher Scientific). Parasite propagation was maintained by passaging weekly onto a confluent HFF monolayer. To verify that all uracil auxotroph T. gondii strains maintained dependence upon exogenous uracil, the parasites were passaged into growth medium without supplemental uracil once a month. Parasites were tested for Mycoplasma contamination every 3 months employing the MycoProbe detection assay (R & D Systems, Minneapolis, MN).
Infections and vaccination
Toxoplasma was administered to mice by intraperitoneal injection of tachyzoites into mice 6–12 weeks of age. Animals were vaccinated by two intraperitoneal injections (106 tachyzoites) of cps1-1 or cps1-1:Δgra24 administered two weeks apart. Two weeks after the final vaccination mice were challenged by subcutaneous injection of 103 RH strain tachyzoites.
Cell culture
Bone marrow was isolated from the tibias of donor mouse strains C57BL/6, MyD88-/-, RIPK3-/- and RIPK3-/-Caspase8-/-. A 10 ml syringe loaded with a 27 Ga needle was used to flush the bone marrow out of the tibias into a 50 ml conical tube. The media containing the bone marrow was subsequently passed through an 18 Ga needle. The bone marrow suspension was plated into five petri dishes, and cells were allowed to differentiate into bone marrow derived macrophages (BMDM) in DMEM (VWR) supplemented with 10% BGS (HyClone), nonessential amino acids (ThermoFisher Scientific), 100 U/mL penicillin (ThermoFisher Scientific), 0.1 mg/mL streptomycin (ThermoFisher Scientific) and L929 culture supernatant as previously described [42]. BMDM were harvested after five days of differentiation for use in experiments.
Peritoneal exudate cells (PEC) were collected by filling the peritoneal cavity with sterile PBS then using a 21 Ga needle to collect the PBS containing PEC. The cells were re-suspended in cDMEM consisting of DMEM (VWR) supplemented with 10% bovine growth serum (HyClone), 1% sodium pyruvate (ThermoFisher Scientific), 1% Non-Essential Amino Acids (ThermoFisher Scientific), 3% HEPES (ThermoFisher Scientific), 0.0001% 2-mercaptoethanol (Sigma, St. Louis, MO), 100 U/ml penicillin (ThermoFisher Scientific) and 0.1 mg/ml streptomycin (ThermoFisher Scientific). Splenic single cell suspensions were prepared by homogenizing spleens, then lysing red blood cells with RBC ACK lysis buffer (ThermoFisher Scientific) as previously described [33]. Removal of cell debris was accomplished by filtration through a 40 μm cell strainer. Cells were washed in PBS and re-suspended cDMEM. Mesenteric lymph node (MLN) single cell suspensions were prepared by crushing the tissue through a 40 μm cell strainer. The cells were re-suspended in cDMEM. Cells were stimulated with soluble tachyzoite lysate antigen (STAg) and supernatants were collected for cytokine ELISA 72 hr later. STAg was prepared from RH strain tachyzoites by sonication of parasites at 0°C in the presence of protease inhibitors, followed by centrifugation at 10,000 x g. Supernatant was collected and dialyzed into PBS and stored in aliquots at -80°C.
In Vivo IL-12 depletion
Mice were i. p. injected with 0.5 mg anti-mouse IL-12p40 mAb (BioXCell, New Hampshire, Catalog #BE0051) or normal rat gamma globulin (Jackson Immunoresearch, West Grove PA, Catalog #012000002) at Day 0. On Day 1, mice were i. p. inoculated with 5 x 106 cps1-1 or cps1-1:Δgra24 tachyzoites. On Days 3 and 6, mice received further injections with anti-IL-12 mAb or control Ab (0.5 mg per mouse). On Day 11 splenocytes were isolated for in vitro cytokine assay.
Flow cytometry
Single cell suspensions of peritoneal exudate cells were stained with primary antibodies including anti-CD11c eFluor610 (Catalog # 61011482, eBioscience, San Diego, CA), anti-MHCII AlexaFluor 647 (Catalog # 107618, BioLegend, San Diego, CA), anti-F4/80 Brilliant Violet 711 (Catalog # 123147, BioLegend), and anti-Ly-6G PE/Cy7 (Catalog #127618, BioLegend) for 20 min at 4°C in FACS buffer (1% BGS/ 0.01% NaN3 in PBS). Cells were then fixed in 3.7% formaldehyde (MilliporeSigma, Burlington, MA) for 15 min and then permeabilized in permeabilization buffer (0.1% saponin in PBS). Intracellular T. gondii tachyzoites were detected by staining with monoclonal anti-T. gondii (TP3) AlexaFluor 700 (Catalog # NB1102570, Novus Biologicals, Centennial, CO) for 45 min at 4°C in permeabilization buffer. All samples were run on a four laser (violet, blue, yellow, red) Attune NxT flow cytometer (ThermoFisher Scientific) and the data were analyzed using FlowJo v.10 software (FlowJo, Ashland, OR).
Cytokine ELISA
The production of IL-12p40 and IFN-γ were measured using a murine IL-12/IL-23p40 ELISA Kit (Invitrogen, Carlsbad, CA) and a IFN-γ ELISA Kit (Invitrogen) according to the manufacturer’s instruction. Briefly, 96 well ELISA plates (Corning Costar, Corning, NY) were coated with anti-cytokine capture antibody in coating buffer (Invitrogen) and incubated overnight at 4°C. The plates were washed 3 times in PBS containing 0.05% Tween-20 (PBST) then blocked with diluent (Invitrogen) for 1 hr at room temperature. After washing the plates once in PBST, sample supernatants as well as recombinant standard was added and incubated overnight at 4°C. The plates were washed 5 times in PBST then anti-cytokine biotin detection antibody was added and plates were incubated at room temperature for 1 hr. The plates were washed 5 times in PBST, avidin-horseradish peroxidase was added and plates were incubated for 30 min at room temperature. The plates were washed 5 times in PBST and 3,3’,5,5’-Tetramethylbenzidine (TMB) was added and plates incubated for 20 min. The reaction was quenched with 2 M H2SO4 and plates were read at 450 nm on an iMark ELISA reader (Bio-Rad, Hercules, CA).
Western blot analysis
BMDM were plated overnight in a 24-well tissue culture plate, then parasites were added at a 3: 1 ratio of tachyzoites to cells. The plates were centrifuged at 63 x g for 3 min to initiate contact between parasites and cells. Lysates were collected using 200 μl SDS lysis buffer and passaged 3 times through a 27 Ga needle to shear DNA. The samples were boiled for 5 min and subjected to Western blot analysis using primary anti- phospho-p38 antibody (Catalog #4511, Cell Signaling, Danvers, Massachusetts), anti-phosphoERK1/2 (Catalog #4370, Cell Signaling), and anti-phosphoSTAT3 (Catalog #4074, Cell Signaling). Samples were also probed for total p38 (Catalog #9218, Cell Signaling), total ERK1/2 (Catalog #4695, Cell Signaling) and total STAT3 (Catalog #4904, Cell Signaling). Lysates were loaded into a 10% acrylamide protein gel (Bio-Rad). Proteins were transferred onto a nitrocellulose membrane then blocked with Tris-buffered PBS containing 5% nonfat dry milk and 0.05% Tween 20 for 2 hr. The nitrocellulose membranes were washed 3 times before the primary antibody was added in Tris-buffered PBS containing 5% BSA and 0.05% Tween 20 for overnight incubation on a rocking platform at 4°C. The nitrocellulose membranes were washed 6 times, then horseradish peroxidase-linked anti-rabbit antibody was added and membranes were incubated for 2 hr at room temperature on a rocking platform. A chemiluminescent substrate system (ThermoFisher Scientific) was used to detect the bound antibodies.
Cytokine proteome array
Cytokine and chemokine expression was measured using the Murine Proteome Profiler Mouse XL Cytokine Array (R&D Systems, Minneapolis, MN). To obtain samples, C57BL/6 mice were i. p. inoculated with either cps1-1 or cps1-1:Δgra24 (106 tachyzoites per mouse). Four days post infection the peritoneal exudate cells were collected by filling the peritoneal cavity with sterile PBS then using a 21 Ga needle to collect the cell suspension. The PECs were plated in cDMEM at 2 x 106 cells per well and were incubated for 72 hr. Following incubation, the supernatants were collected and the Murine Proteome Profiler Mouse XL Cytokine Array was used according to the manufacturer’s directions. In brief, nitrocellulose membranes were blocked for 1 hr then sample supernatants were added to the membranes. After overnight incubation, membranes were washed and a detection antibody cocktail (R&D Systems) was added. The membranes were incubated with the detection antibody for 1 hr, washed, and streptavidin-horseradish peroxidase was added. After 30 min incubation, membranes were washed three times (10 min per wash) and spots visualized by addition of enhanced chemiluminescence reagent. The membranes were imaged on a Chemi Touch Imaging System (Bio-Rad), and semi-quantitative analysis was accomplished using ImageJ software.
qPCR for parasite load
DNA was extracted from tissues using the DNeasy Blood and Tissue Kit (Qiagen Inc. Valencia, CA) following the manufacturer’s instruction. Quantitative PCR was performed on the indicated tissue targeting the conserved Toxoplasma B1 gene and the murine argininosuccinate lyase (ASL) gene [21]. The extracted DNA from each tissue was normalized to 125 ng of DNA for each 20 μl reaction. A 5 μM primer working solution containing the forward and reverse primers for B1 (forward 5’-GGA-GGA-CTG-GCA-ACC-TGG-TGT-CG-3’, reverse 5’-TTG-TTT-CAC-CCG-GAC-CGT-TTA-GCA-3’) and a 5 μM working solution containing the forward and reverse primers for ASL (forward 5’-TCT-TCG-TTA-GCT-GGC-AAC-TCA-CCT-3’, reverse 5’-ATG-ACC-CAG-CAG-CTA-AGC-AGA-TCA-3’) was made. 2.5 μl of primer solution of B1 or ASL was added to 10 μl SYBR green (Bio-Rad), 2.5 μl molecular biology grade water and 4 μl of template for a total volume of 20 μl. The reactions were carried out using a Bio-Rad CFX96 Real Time System C1000 Touch thermal cycler with the following thermal cycling conditions: 98°C for 5 min, 95°C for 5 sec, 60°C for 30 sec followed by 40 cycles of 95°C at 5 sec and 60°C for 30 sec. A melting curve analysis was performed to ensure specificity of amplification. Quantification of the number of B1 and ASL gene copy numbers was accomplished by using a quantitative standard curve. Bio-Rad CFX manager version 3 software was used to quantify B1 and ASL gene copy number. Non-infected tissues were used as negative controls and molecular grade water was used as a negative template control.
Immunofluorescence
HFF were grown to confluency on glass coverslips for five days. The confluent HFFs were infected with tachyzoites in 1% HFM in the presence or absence of uracil (0.3 mM). After 48 hrs, coverslips were washed in PBS, then fixed with 3.7% PFA for 20 min. The cells were washed in permeabilization buffer (PB; 0.1% saponin in PBS) then blocked in PB containing 5% normal mouse serum (Invitrogen) for 1 hour. After blocking, the cells were washed with PBS and stained by the addition of goat anti-Toxoplasma FITC-conjugated antibody (Catalog #PA17253, Invitrogen) and Texas Red-X phalloidin (Catalog #T7471, Invitrogen) diluted in PB containing 5% bovine serum albumin (VWR) for 1 hr. The cells were washed 3 times in PB and 4 times in PBS. Finally, the coverslips were dried then mounted onto slides using DAPI containing mounting media (Catalog #P36962, Invitrogen). Imaging was accomplished with a BX53 fluorescence microscope (Olympus America, Inc, Waltham, MA) and DP manager software (Olympus).
Quantification of Immunofluorescence staining
For the quantification of percent infection, 100–150 cells were counted per field of view (n = 10 fields per treatment). Infected cells were counted and percent infection was calculated. To quantify average number of tachyzoites per vacuole, number of parasites in each parasitophorous vacuole (20–30 per field of view) was counted and average number of tachyzoites per vacuole was calculated.
Statistical analyses
Student t-test was used to assess the significant difference between groups. A p value <0.05 was considered significant. A two way ANOVA with a Tukey’s multiple comparisons test was used to compare three groups across multiple time points. A Mann-Whitney test was used to assess statistical significance in the in vivo IL-12 depletion experiment. Survival after challenge with a virulent RH strain was assessed using a Kaplan-Meier curve and the statistical significant difference between groups was calculated using the Log-Rank test with the use of GraphPad Prism Software. All experiments were repeated 2–3 times.
Supporting information
BMDM were generated from C57BL/6 (A) and MyD88-/- (B) mice and infected with either Type I RH or Type II PTG tachyzoites at a 1:1 ratio of parasites to cells. Supernatants were collected for cytokine ELISA at the indicated time points. NI, non-infected BMDM, supernatants were collected at 24 hr. Data shown are the means ± SD of cells cultured in triplicate. Statistical significance was analyzed using two way ANOVA with Tukey's multiple comparisons test (*** p > 0.001). This experiment was repeated two times and yielded similar results.
(TIF)
BMDM were generated from C57BL/6 (WT), RIPK3-/-, RIPK-/-caspase8-/- mice and infected with either Type I RH (A) or Type II PTG (B) tachyzoites at a 1:1 ratio of parasites to cells. Supernatants were collected for cytokine ELISA at the indicated time points. NI, non-infected BMDM, supernatants were collected at 24 hr. Data shown are the means ± SD of cells cultured in triplicate, n = 2 mice per group. Statistical significance was assessed using two way ANOVA with Tukey's multiple comparisons test (** p > 0.01).
(TIF)
BMDM from MyD88+/+ and MyD88-/- mice were infected with cps1-1 or cps1-1:Δgra24 tachyzoites at a 1:1 ratio of parasites to cells. Cell lysates were prepared for Western blot analysis at the indicated time points.
(TIF)
Diagram indicating the coordinates of each cytokine and chemokine included on the cytokine proteome array.
(TIF)
Acknowledgments
We thank Dr. I. Brodsky (University of Pennsylvania) for generously providing tibias from RIPK3-/- and RIPK3-/-Caspase8-/- mice. We also thank Dr. E. Casadei for expert discussion and advice, and we gratefully acknowledge the services of the staff of the Animal Research Facility in the UNM Department of Biology.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by grants from the National Institute of Allergy and Infectious Diseases (EYD, AI139628 and AI119708; DJB, AI131630 and AI137118) and the National Institute of General Medical Sciences (EYD; GM110907). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Robert-Gangneux F, Darde ML (2012) Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 25: 264–296. 10.1128/CMR.05013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubey JP (2013) The history and life-cycle of Toxoplasma gondii In: Weiss LM, Kim K, editors. Toxoplasma gondii The model apicomplexan: Perspective and methods. 2nd ed San Diego: Academic Press; pp. 1–17. [Google Scholar]
- 3.McLeod RM, van Tubbergen C, Montoya JG, Petersen E (2013) Human Toxoplasma infection In: Weiss LM, Kim K, editors. Toxoplasma gondii The Model Apicomplexan: Perspectives and Methods. 2nd ed San Diego: Academic Press; pp. 100–159. [Google Scholar]
- 4.Pfaff AW, Liesenfeld O, Candolfi E (2007) Congenital toxoplasmosis In: Ajioka JW, Soldati D, editors. Toxoplasma molecular and cellular biology. Norfolk: Horizon Bioscience; pp. 93–110. [Google Scholar]
- 5.Denkers EY, Gazzinelli RT (1998) Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev 11: 569–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupont CD, Christian DA, Hunter CA (2012) Immune response and immunopathology during toxoplasmosis. Semin Immunopathol 34: 793–813. 10.1007/s00281-012-0339-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarovinsky F (2014) Innate immunity to Toxoplasma gondii infection. Nat Rev Immunol 14: 109–121. 10.1038/nri3598 [DOI] [PubMed] [Google Scholar]
- 8.Cohen SB, Denkers EY (2015) Impact of Toxoplasma gondii on Dendritic Cell Subset Function in the Intestinal Mucosa. J Immunol 195: 2754–2762. 10.4049/jimmunol.1501137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, Sher A, Ploegh HL, Murphy TL, Sibley LD, Murphy KM (2011) CD8alpha(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity 35: 249–259. S1074-7613(11)00313-X [pii] 10.1016/j.immuni.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A (1997) In vivo microbial stimulation induces rapid CD40L-independent production of IL-12 by dendritic cells and their re-distribution to T cell areas. J Exp Med 186: 1819–1829. 10.1084/jem.186.11.1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yap G, Pesin M, Sher A (2000) IL-12 is required for the maintenance of IFN-g production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol 165: 628–631. 10.4049/jimmunol.165.2.628 [DOI] [PubMed] [Google Scholar]
- 12.Degrandi D, Kravets E, Konermann C, Beuter-Gunia C, Klumpers V, Lahme S, Wischmann E, Mausberg AK, Beer-Hammer S, Pfeffer K (2013) Murine guanylate binding protein 2 (mGBP2) controls Toxoplasma gondii replication. Proc Natl Acad Sci U S A 110: 294–299. 10.1073/pnas.1205635110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haldar AK, Saka HA, Piro AS, Dunn JD, Henry SC, Taylor GA, Frickel EM, Valdivia RH, Coers J (2013) IRG and GBP host resistance factors target aberrant, "non-self" vacuoles characterized by the missing of "self" IRGM proteins. PLoS Pathog 9: e1003414 10.1371/journal.ppat.1003414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC (2005) Disruption of Toxoplasma gondii Parasitophorous Vacuoles by the Mouse p47-Resistance GTPases. PLoS Pathog 1: e24 10.1371/journal.ppat.0010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto M, Okuyama M, Ma JS, Kimura T, Kamiyama N, Saiga H, Ohshima J, Sasai M, Kayama H, Okamoto T, Huang DC, Soldati-Favre D, Horie K, Takeda J, Takeda K (2012) A Cluster of Interferon-gamma-Inducible p65 GTPases Plays a Critical Role in Host Defense against Toxoplasma gondii. Immunity. 10.1016/j.immuni.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 16.Zhao YO, Khaminets A, Hunn JP, Howard JC (2009) Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog 5: e1000288 10.1371/journal.ppat.1000288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A (2002) Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol 168: 5997–6001. 10.4049/jimmunol.168.12.5997 [DOI] [PubMed] [Google Scholar]
- 18.Gazzinelli RT, Denkers EY (2006) Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol 6: 895–906. 10.1038/nri1978 [DOI] [PubMed] [Google Scholar]
- 19.Hou B, Benson A, Kuzmich L, DeFranco AL, Yarovinsky F (2011) Critical coordination of innate immune defense against Toxoplasma gondii by dendritic cells responding via their Toll-like receptors. Proc Natl Acad Sci U S A 108: 278–283. 1011549108 [pii] 10.1073/pnas.1011549108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bliss SK, Butcher BA, Denkers EY (2000) Rapid recruitment of neutrophils with prestored IL-12 during microbial infection. J Immunol 165: 4515–4521. 10.4049/jimmunol.165.8.4515 [DOI] [PubMed] [Google Scholar]
- 21.Cohen SB, Maurer KJ, Egan CE, Oghumu S, Satoskar AR, Denkers EY (2013) CXCR3-dependent CD4(+) T cells are required to activate inflammatory monocytes for defense against intestinal infection. PLoS Pathog 9: e1003706 10.1371/journal.ppat.1003706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pifer R, Benson A, Sturge CR, Yarovinsky F (2011) UNC93B1 is essential for TLR11 activation and IL-12 dependent host resistance to Toxoplasma Gondii. J Biol Chem 286: 3307–3314. 10.1074/jbc.M110.171025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarovinsky F, Kanzler H, Hieny S, Coffman RL, Sher A (2006) Toll-like receptor recognition regulates immunodominance in an antimicrobial CD4+ T cell response. Immunity 25: 655–664. 10.1016/j.immuni.2006.07.015 [DOI] [PubMed] [Google Scholar]
- 24.Andrade WA, Souza MD, Ramos-Martinez E, Nagpal K, Dutra MS, Melo MB, Bartholomeu DC, Ghosh S, Golenbock DT, Gazzinelli RT (2013) Combined Action of Nucleic Acid-Sensing Toll-like Receptors and TLR11/TLR12 Heterodimers Imparts Resistance to Toxoplasma gondii in Mice. Cell Host Microbe 13: 42–53. 10.1016/j.chom.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raetz M, Kibardin A, Sturge CR, Pifer R, Li H, Burstein E, Ozato K, Larin S, Yarovinsky F (2013) Cooperation of TLR12 and TLR11 in the IRF8-dependent IL-12 response to Toxoplasma gondii profilin. J Immunol 191: 4818–4827. 10.4049/jimmunol.1301301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yarovinsky F, Zhang D, Anderson JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A (2005) TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308: 1626–1629. 10.1126/science.1109893 [DOI] [PubMed] [Google Scholar]
- 27.Plattner F, Yarovinsky F, Romero S, Didry D, Carlier MF, Sher A, Soldati-Favre D (2008) Toxoplasma profilin is essential for host cell invasion and TLR dependent induction of interleukin-12. Cell Host and Microbe 14: 77–87. [DOI] [PubMed] [Google Scholar]
- 28.Debierre-Grockiego F, Campos MA, Azzouz N, Schmidt J, Bieker U, Resende MG, Mansur DS, Weingart R, Schmidt RR, Golenbock DT, Gazzinelli RT, Schwarz RT (2007) Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol 179: 1129–1137. 10.4049/jimmunol.179.2.1129 [DOI] [PubMed] [Google Scholar]
- 29.Mun H-S, Aosai F, Norose K, Chen M, Piao L-X, Takeuchi O, Akira S, Ishikura H, Yano A (2003) TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Internat Parasitol 15: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 30.Gazzinelli RT, Mendonca-Neto R, Lilue J, Howard J, Sher A (2014) Innate resistance against Toxoplasma gondii: an evolutionary tale of mice, cats, and men. Cell Host Microbe 15: 132–138. 10.1016/j.chom.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, Vasconcelos J, Sirvent N, Guedes M, Vitor AB, Herrero-Mata MJ, Arostegui JI, Rodrigo C, Alsina L, Ruiz-Ortiz E, Juan M, Fortuny C, Yague J, Anton J, Pascal M, Chang HH, Janniere L, Rose Y, Garty BZ, Chapel H, Issekutz A, Marodi L, Rodriguez-Gallego C, Banchereau J, Abel L, Li X, Chaussabel D, Puel A, Casanova JL (2008) Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321: 691–696. 10.1126/science.1158298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Bernuth H, Picard C, Puel A, Casanova JL (2012) Experimental and natural infections in MyD88- and IRAK-4-deficient mice and humans. Eur J Immunol 42: 3126–3135. 10.1002/eji.201242683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sukhumavasi W, Egan CE, Warren AL, Taylor GA, Fox BA, Bzik DJ, Denkers EY (2008) TLR adaptor MyD88 is essential for pathogen control during oral toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. J Immunol 181: 3464–3473. 10.4049/jimmunol.181.5.3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim L, Del Rio L, Butcher BA, Mogensen TH, Paludan S, Flavell RA, Denkers EY (2005) p38 MAPK autophosphorylation drives macrophage IL-12 production during intracellular infection. J Immunol 174: 4178–4184. 10.4049/jimmunol.174.7.4178 [DOI] [PubMed] [Google Scholar]
- 35.Braun L, Brenier-Pinchart MP, Yogavel M, Curt-Varesano A, Curt-Bertini RL, Hussain T, Kieffer-Jaquinod S, Coute Y, Pelloux H, Tardieux I, Sharma A, Belrhali H, Bougdour A, Hakimi MA (2013) A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J Exp Med 210: 2071–2086. 10.1084/jem.20130103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellegrini E, Palencia A, Braun L, Kapp U, Bougdour A, Belrhali H, Bowler MW, Hakimi MA (2017) Structural Basis for the Subversion of MAP Kinase Signaling by an Intrinsically Disordered Parasite Secreted Agonist. Structure 25: 16–26. 10.1016/j.str.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robben PM, Mordue DG, Truscott SM, Takeda K, Akira S, Sibley LD (2004) Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J Immunol 172: 3686–3694. 10.4049/jimmunol.172.6.3686 [DOI] [PubMed] [Google Scholar]
- 38.DeLaney AA, Berry CT, Christian DA, Hart A, Bjanes E, Wynosky-Dolfi MA, Li X, Tummers B, Udalova IA, Chen YH, Hershberg U, Freedman BD, Hunter CA, Brodsky IE (2019) Caspase-8 promotes c-Rel-dependent inflammatory cytokine expression and resistance against Toxoplasma gondii. Proc Natl Acad Sci U S A. 10.1073/pnas.1820529116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinhardt RL, Hong S, Kang SJ, Wang ZE, Locksley RM (2006) Visualization of IL-12/23p40 in vivo reveals immunostimulatory dendritic cell migrants that promote Th1 differentiation. J Immunol 177: 1618–1627. 10.4049/jimmunol.177.3.1618 [DOI] [PubMed] [Google Scholar]
- 40.Fox BA, Bzik DJ (2010) Avirulent uracil auxotrophs based on disruption of orotidine-5'-monophosphate decarboxylase elicit protective immunity to Toxoplasma gondii. Infect Immun 78: 3744–3752. 10.1128/IAI.00287-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox BA, Sanders KL, Rommereim LM, Guevara RB, Bzik DJ (2016) Secretion of Rhoptry and Dense Granule Effector Proteins by Nonreplicating Toxoplasma gondii Uracil Auxotrophs Controls the Development of Antitumor Immunity. PLoS Genet 12: e1006189 10.1371/journal.pgen.1006189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butcher BA, Fox BA, Rommereim LM, Kim SG, Maurer KJ, Yarovinsky F, Herbert DR, Bzik DJ, Denkers EY (2011) Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog 7: e1002236 10.1371/journal.ppat.1002236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butcher BA, Kim L, Panopoulos A, Watowich SS, Murray PJ, Denkers EY (2005) Cutting Edge: IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-a in host macrophages. J Immunol 174: 3148–3152. 10.4049/jimmunol.174.6.3148 [DOI] [PubMed] [Google Scholar]
- 44.Kim L, Denkers EY (2006) Toxoplasma gondii triggers Gi-dependent phosphatidylinositol 3-kinase signaling required for inhibition of host cell apoptosis. J Cell Sci 119: 2119–2126. 10.1242/jcs.02934 [DOI] [PubMed] [Google Scholar]
- 45.Cuenda A, Alessi DR (2000) Use of kinase inhibitors to dissect signaling pathways. Methods Mol Biol 99: 161–175. 10.1385/1-59259-054-3:161 [DOI] [PubMed] [Google Scholar]
- 46.Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A (1994) Parasite-induced IL-12 stimulates early IFN-g synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol 153: 2533–2543. [PubMed] [Google Scholar]
- 47.Bougdour A, Durandau E, Brenier-Pinchart MP, Ortet P, Barakat M, Kieffer S, Curt-Varesano A, Curt-Bertini RL, Bastien O, Coute Y, Pelloux H, Hakimi MA (2013) Host cell subversion by Toxoplasma GRA16, an exported dense granule protein that targets the host cell nucleus and alters gene expression. Cell Host Microbe 13: 489–500. 10.1016/j.chom.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 48.Bougdour A, Tardieux I, Hakimi MA (2014) Toxoplasma exports dense granule proteins beyond the vacuole to the host cell nucleus and rewires the host genome expression. Cell Microbiol 16: 334–343. 10.1111/cmi.12255 [DOI] [PubMed] [Google Scholar]
- 49.Mota CM, Oliveira AC, Davoli-Ferreira M, Silva MV, Santiago FM, Nadipuram SM, Vashisht AA, Wohlschlegel JA, Bradley PJ, Silva JS, Mineo JR, Mineo TW (2016) Neospora caninum Activates p38 MAPK as an Evasion Mechanism against Innate Immunity. Front Microbiol 7: 1456 10.3389/fmicb.2016.01456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hakimi MA, Bougdour A (2015) Toxoplasma's ways of manipulating the host transcriptome via secreted effectors. Curr Opin Microbiol 26: 24–31. 10.1016/j.mib.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 51.Coffey MJ, Sleebs BE, Uboldi AD, Garnham A, Franco M, Marino ND, Panas MW, Ferguson DJ, Enciso M, O'Neill MT, Lopaticki S, Stewart RJ, Dewson G, Smyth GK, Smith BJ, Masters SL, Boothroyd JC, Boddey JA, Tonkin CJ (2015) An aspartyl protease defines a novel pathway for export of Toxoplasma proteins into the host cell. Elife 4 10.7554/eLife.10809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curt-Varesano A, Braun L, Ranquet C, Hakimi MA, Bougdour A (2016) The aspartyl protease TgASP5 mediates the export of the Toxoplasma GRA16 and GRA24 effectors into host cells. Cell Microbiol 18: 151–167. 10.1111/cmi.12498 [DOI] [PubMed] [Google Scholar]
- 53.Franco M, Panas MW, Marino ND, Lee MC, Buchholz KR, Kelly FD, Bednarski JJ, Sleckman BP, Pourmand N, Boothroyd JC (2016) A Novel Secreted Protein, MYR1, Is Central to Toxoplasma's Manipulation of Host Cells. MBio 7: e02231–02215. 10.1128/mBio.02231-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baba M, Batanova T, Kitoh K, Takashima Y (2017) Adhesion of Toxoplasma gondii tachyzoite-infected vehicle leukocytes to capillary endothelial cells triggers timely parasite egression. Sci Rep 7: 5675 10.1038/s41598-017-05956-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Portillo JC, Muniz-Feliciano L, Lopez Corcino Y, Lee SJ, Van Grol J, Parsons SJ, Schiemman WP, Subauste CS (2017) Toxoplasma gondii induces FAK-Src-STAT3 signaling during infection of host cells that prevents parasite targeting by autophagy. PLoS Pathog 13: e1006671 10.1371/journal.ppat.1006671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tosh KW, Mittereder L, Bonne-Annee S, Hieny S, Nutman TB, Singer SM, Sher A, Jankovic D (2016) The IL-12 Response of Primary Human Dendritic Cells and Monocytes to Toxoplasma gondii Is Stimulated by Phagocytosis of Live Parasites Rather Than Host Cell Invasion. J Immunol 196: 345–356. 10.4049/jimmunol.1501558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lopez-Yglesias AH, Camanzo E, Martin AT, Araujo AM, Yarovinsky F (2019) TLR11-independent inflammasome activation is critical for CD4+ T cell-derived IFN-gamma production and host resistance to Toxoplasma gondii. PLoS Pathog 15: e1007872 10.1371/journal.ppat.1007872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sturge CR, Benson A, Raetz M, Wilhelm CL, Mirpuri J, Vitetta ES, Yarovinsky F (2013) TLR-independent neutrophil-derived IFN-gamma is important for host resistance to intracellular pathogens. Proc Natl Acad Sci U S A 110: 10711–10716. 10.1073/pnas.1307868110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KD, Saeij JP (2011) Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med 208: 195–212. 10.1084/jem.20100717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hakimi MA, Olias P, Sibley LD (2017) Toxoplasma Effectors Targeting Host Signaling and Transcription. Clin Microbiol Rev 30: 615–645. 10.1128/CMR.00005-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma JS, Sasai M, Ohshima J, Lee Y, Bando H, Takeda K, Yamamoto M (2014) Selective and strain-specific NFAT4 activation by the Toxoplasma gondii polymorphic dense granule protein GRA6. J Exp Med 211: 2013–2032. 10.1084/jem.20131272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gay G, Braun L, Brenier-Pinchart MP, Vollaire J, Josserand V, Bertini RL, Varesano A, Touquet B, De Bock PJ, Coute Y, Tardieux I, Bougdour A, Hakimi MA (2016) Toxoplasma gondii TgIST co-opts host chromatin repressors dampening STAT1-dependent gene regulation and IFN-gamma-mediated host defenses. J Exp Med. 10.1084/jem.20160340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olias P, Etheridge RD, Zhang Y, Holtzman MJ, Sibley LD (2016) Toxoplasma Effector Recruits the Mi-2/NuRD Complex to Repress STAT1 Transcription and Block IFN-gamma-Dependent Gene Expression. Cell Host Microbe 20: 72–82. 10.1016/j.chom.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC (2007) Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445: 324–327. 10.1038/nature05395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jensen KD, Wang Y, Wojno ED, Shastri AJ, Hu K, Cornel L, Boedec E, Ong YC, Chien YH, Hunter CA, Boothroyd JC, Saeij JP (2011) Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host Microbe 9: 472–483. 10.1016/j.chom.2011.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sangare LO, Olafsson EB, Wang Y, Yang N, Julien L, Camejo A, Pesavento P, Sidik SM, Lourido S, Barragan A, Saeij JPJ (2019) In Vivo CRISPR Screen Identifies TgWIP as a Toxoplasma Modulator of Dendritic Cell Migration. Cell Host Microbe 26: 478–492 e478. 10.1016/j.chom.2019.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gazzinelli RT, Hieny S, Wynn T, Wolf S, Sher A (1993) IL-12 is required for the T-cell independent induction of IFN-g by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA 90: 6115–6119. 10.1073/pnas.90.13.6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BMDM were generated from C57BL/6 (A) and MyD88-/- (B) mice and infected with either Type I RH or Type II PTG tachyzoites at a 1:1 ratio of parasites to cells. Supernatants were collected for cytokine ELISA at the indicated time points. NI, non-infected BMDM, supernatants were collected at 24 hr. Data shown are the means ± SD of cells cultured in triplicate. Statistical significance was analyzed using two way ANOVA with Tukey's multiple comparisons test (*** p > 0.001). This experiment was repeated two times and yielded similar results.
(TIF)
BMDM were generated from C57BL/6 (WT), RIPK3-/-, RIPK-/-caspase8-/- mice and infected with either Type I RH (A) or Type II PTG (B) tachyzoites at a 1:1 ratio of parasites to cells. Supernatants were collected for cytokine ELISA at the indicated time points. NI, non-infected BMDM, supernatants were collected at 24 hr. Data shown are the means ± SD of cells cultured in triplicate, n = 2 mice per group. Statistical significance was assessed using two way ANOVA with Tukey's multiple comparisons test (** p > 0.01).
(TIF)
BMDM from MyD88+/+ and MyD88-/- mice were infected with cps1-1 or cps1-1:Δgra24 tachyzoites at a 1:1 ratio of parasites to cells. Cell lysates were prepared for Western blot analysis at the indicated time points.
(TIF)
Diagram indicating the coordinates of each cytokine and chemokine included on the cytokine proteome array.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.