Abstract
Background
Chagas disease, caused by the parasite Trypanosoma cruzi, once considered a disease confined to Mexico, Central America, and South America, is now an emerging global public health problem. An estimated 300 000 immigrants in the United States are chronically infected with T. cruzi. However, awareness of Chagas disease among the medical community in the United States is poor.
Methods
We review our experience managing 60 patients with Chagas disease in hospitals throughout the New York City metropolitan area and describe screening, clinical manifestations, EKG findings, imaging, and treatment.
Results
The most common country of origin of our patients was El Salvador (n = 24, 40%), and the most common detection method was by routine blood donor screening (n = 21, 35%). Nearly half of the patients were asymptomatic (n = 29, 48%). Twenty-seven patients were treated with either benznidazole or nifurtimox, of whom 7 did not complete therapy due to side effects or were lost to follow-up. Ten patients had advanced heart failure requiring device implantation or organ transplantation.
Conclusions
Based on our experience, we recommend that targeted screening be used to identify at-risk, asymptomatic patients before progression to clinical disease. Evaluation should include an electrocardiogram, echocardiogram, and chest x-ray, as well as gastrointestinal imaging if relevant symptoms are present. Patients should be treated if appropriate, but providers should be aware of adverse effects that may prevent patients from completing treatment.
Keywords: Chagas disease, heart transplant, New York City, nonendemic countries, Trypanosoma cruzi
Chagas disease, caused by infection with the parasite Trypanosoma cruzi, was once considered a disease confined to poverty-stricken rural areas of Mexico, Central America, and South America. The World Health Organization currently estimates that Chagas disease affects 6–7 million people and causes 10000 deaths each year [1, 2]. The countries with the highest prevalence of Chagas disease are Bolivia, followed by Argentina, Paraguay, Ecuador, El Salvador, and Guatemala [3]. In endemic countries, most infections are acquired through vectorborne transmission by triatomine bugs [4]. Oral transmission can also occur when food or liquids contaminated with feces from infected triatomine bugs are consumed and is associated with outbreaks of acute Chagas disease [5]. In nonendemic countries, T. cruzi can be transmitted through blood transfusion, organ transplantation, congenitally from mother to child, and, in rare cases, laboratory accidents [6, 7, 8, 9].
In the majority of cases, the acute phase of infection is never identified, and affected individuals are diagnosed with the indeterminate form of chronic Chagas disease. Although most chronically infected individuals are asymptomatic and unaware of their infection, they remain potential sources of transmission for the remainder of their life [10]. Roughly 20%–30% of chronically infected individuals progress to develop cardiomyopathy, and 10%–15% develop gastrointestinal involvement [11, 12]. During the chronic phase, diagnosis is based on positive results from testing with 2 different serologic tests, for example, an enzyme-linked immunosorbent assay (ELISA) or immunofluorescence assay (IFA), preferably using 2 different antigen preparations [13, 14]. Published sensitivity and specificity of diagnostic tests range from 17% to 100% and 76% to 100% respectively, but these estimates must be interpreted cautiously due to geographic variation, heterogeneity in study designs, and the lack of a gold standard [15, 16]. Two drugs are available for treatment, benznidazole and nifurtimox, although guidelines for treatment are not well established. The BENEFIT trial, a randomized controlled trial of benznidazole vs placebo, showed no significant difference in clinical cardiac disease over 5.4 years of follow-up [17]. Antiparasitic treatment is generally not used in advanced heart failure, and these patients may require implantation of cardiac devices or organ transplantation [18].
In recent decades, the rate of emigration from Chagas-endemic areas to the United States, Canada, Europe, Australia, and Japan has increased markedly and has changed the epidemiology of Chagas disease in these countries [19]. It is estimated that 23 million immigrants from endemic regions live in the United States and 300 000 of these persons are chronically infected by T. cruzi [20]. A screening program of >4000 Latin American immigrants residing in the Los Angeles area found a Chagas disease prevalence of 1.24% [21]. The number of T. cruzi–infected immigrants and the possibility of transmission by nonvectorial mechanisms have turned Chagas disease into an emerging disease and a new public health problem in destination countries. Physicians caring for migrant patients need to become familiar with the diagnosis, complications, and indications for treatment of Chagas disease. This manuscript reviews the experience managing 60 patients with Chagas disease in hospitals throughout the New York City metropolitan area.
METHODS
We performed a retrospective study extracting demographic and clinical information by manual chart review of patients diagnosed with Chagas disease who were evaluated between 2005 and 2017 in major hospital systems in the New York City metropolitan area, defined as the 5 New York City boroughs and surrounding suburbs. Jacobi Medical Center (JMC) is a hospital in the New York City Health and Hospital Corporation and a major teaching facility of the Albert Einstein College of Medicine. It is the main facility treating Chagas disease patients through its Tropical Disease Clinic in the Bronx. Montefiore Medical Center (MMC) in the Bronx is the site of a large heart failure clinic and performs heart transplants, including transplants in Chagas disease patients with end-stage heart failure. The Northwell-Hofstra University Medical Center is located on Long Island in suburban New York City. Patients were referred to the authors at these medical centers by physicians, family members, or the New York Blood Center.
Patients were diagnosed with chronic Chagas disease on the basis of serologic testing results. At the JMC Parasitology Laboratory, the Hemagen Chagas’ Enzyme Immunoassay Kit was used (Hemagen Diagnostics, Boston, MA, USA; sensitivity, 88%–92%; specificity, 99%–100%) [15]. At MMC and Northwell, an ELISA was performed at a commercial lab (most commonly the Hemagen Chagas Kit at Quest Diagnostics, Inc.). At the Centers for Disease Control and Prevention (CDC), reference confirmatory testing was performed with the commercial Chagatest ELISA recombinante, version 3.0 (Wiener Laboratorios, Argentina; sensitivity, 94%–97%; specificity, 97%–99%) [15], and an in-house IFA based on fixed epimastigotes (sensitivity, 94%; specificity, 95%) [22] until 2014. Thereafter, the in-house IFA was replaced with an in-house trypomastigote excreted-secreted antigens (TESA) immunoblot (sensitivity, 100%; specificity, 100%) [23]. When possible, family members of patients were also screened. Heart transplant recipients were tested preemptively for T. cruzi DNA approximately every 3 to 6 months after transplant to monitor for reactivation. Specimens were sent to the CDC, which employed an algorithm using 3 real-time polymerase chain reaction (PCR) assays with different targets run in parallel (individual PCR assay: sensitivities, 6%–78%; specificities, 40%–100%) [24].
The decision to initiate treatment was consistent with the practice of other experts and consensus groups [25, 26, 27]. In general, treatment was offered to patients without the following criteria: age >50 years, pregnancy, renal or hepatic dysfunction, or advanced heart disease. Treatment decisions could be individualized and were at the discretion of the treating physician. All heart transplant recipients with reactivation Chagas disease were treated. Whether the patient received benznidazole or nifurtimox was based on drug availability per the CDC Investigational New Drug (IND) protocol. Patients receiving treatment were asked to follow up every 1–2 weeks to monitor for adverse events. Asymptomatic patients not on treatment were asked to follow up at least every 3–6 months, with closer follow-up if symptoms developed.
Patient demographic and clinical information was stored in a Microsoft Access (Seattle, WA, USA) database. This study was approved by the Institutional Review Board of the Albert Einstein College of Medicine and the Northwell-Hofstra University Medical Center.
RESULTS
Epidemiology
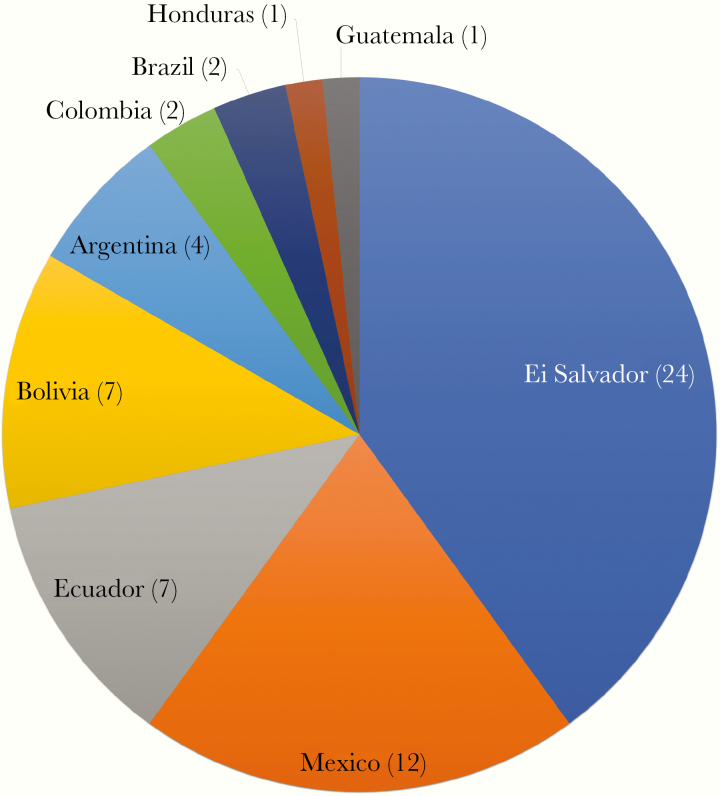
Sixty patients were included in the study, 30 males and 30 females. The mean age at diagnosis (SD) was 47 (15.65) years, ranging from 18 to 78 years. The plurality of patients (40%) were from El Salvador, followed by Mexico, Ecuador, Bolivia, Argentina, Brazil, Colombia, Guatemala, and Honduras (Figure 1). Of these, 5 patients were first-generation Americans born in the United States to immigrant parents, 3 from Bolivia and 2 from Argentina. There were 3 patients who did not fulfill all of the original criteria for confirmed infection but were nevertheless included because of compelling clinical findings compatible with Chagas disease and risk history of T. cruzi infection. One was a 28-year-old woman with a positive ELISA at both JMC and the CDC from El Salvador. The second patient was a 78-year-old woman from the State of Minas Gerais in Brazil who had a positive ELISA performed at Quest Laboratories and clinical findings of mega-esophagus and a right bundle-branch block (RBBB). The third patient was a 51-year-old woman born in Ecuador who had a positive ELISA performed at JMC and an RBBB.
Figure 1.
Country of origin of Chagas disease patients (n = 60). Five patients were first-generation Americans born in the United States to Latin American immigrant parents and are included in this figure under their parents’ country of origin.
Routine blood donor screening was the most common way patients with Chagas disease were first identified (n = 21, 35%). Seventeen patients were tested as part of evaluation for cardiac disease. Ten patients were screened due to a family history of Chagas disease. In 3 families, the index patient was detected upon blood donation screening. In 1 family, the index patient was among the 17 patients identified during evaluation of cardiac disease. Nine patients were diagnosed as part of screening for endemic infectious diseases based on their country of origin. Two patients were tested due to a report of positive testing as a child, and 1 due to complaint of gastrointestinal symptoms.
Clinical Manifestations and Evaluation
Nearly half of patients (n = 29, 48%) were asymptomatic. Twenty-seven (45%) patients had cardiac manifestations including heart failure and chest pain. Fourteen (23%) had gastrointestinal manifestations such as constipation, dysphagia, and abdominal pain. Patients reporting gastrointestinal symptoms were from El Salvador (n = 6), Ecuador (n = 3), Mexico (n = 2), Bolivia (n = ), and Brazil (n = 1). One patient had a suspected cardioembolic stroke from a cardiac apical aneurysm. Detailed symptoms are described in Table 1. Fourteen out of 29 (48%) asymptomatic patients had abnormal electrocardiogram (EKG) or imaging findings.
Table 1.
Clinical Manifestations of Chagas Disease (n = 60); Patients May Report >1 Symptom so Numbers Sum to >100%
| No. | % | |
|---|---|---|
| Asymptomatic | 29 | 48 |
| Cardiac | 27 | 45 |
| Heart failure | 16 | 27 |
| Chest pain | 10 | 17 |
| Palpitations | 8 | 13 |
| Dyspnea | 5 | 8 |
| Gastrointestinal | 14 | 23 |
| Constipation | 10 | 17 |
| Dysphagia | 6 | 10 |
| Abdominal pain | 5 | 8 |
| Heartburn | 1 | 2 |
| Neurologic | 1 | 2 |
| Stroke | 1 | 2 |
| Headache | 1 | 2 |
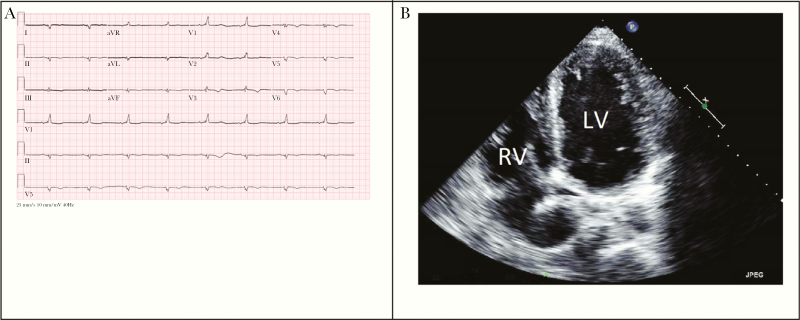
Out of 56 patients who had EKGs performed, 32 (57%) had abnormal findings. The most common abnormalities were RBBB (n = 12, 21%), sinus bradycardia (n = 12, 21%), and T-wave changes (n = 12, 21%). There were 52 patients who had an echocardiogram performed, of whom 28 (54%) were abnormal. The most common echocardiogram abnormalities were tricuspid regurgitation (n = 17, 33%) and ventricular wall motion abnormalities, including hypokinesis, akinesis, and regional wall motion abnormalities (n = 16, 31%). A representative EKG and echocardiogram are shown in Figure 2. Of the 41 patients who had a chest x-ray performed, 12 (29%) had cardiomegaly. Twenty-three patients had gastrointestinal imaging performed. The most common method of imaging was colonoscopy, followed by ultrasound, computed tomography, and esophagogastroduodenoscopy. Upper gastrointestinal series was performed in 4 patients. There was 1 patient with esophageal dilatation, 2 patients with small intestine dilatation, and 1 patient with megacolon. Both patients with esophageal dilatation and megacolon were from Brazil. Other findings included gastritis (n = 4) and gall bladder thickening (n = 3). Table 2 describes specific EKG, echocardiogram, and chest x-ray findings broken down by presence of symptoms.
Figure 2.
A, Representative electrocardiogram demonstrating sinus bradycardia, first-degree atrioventricular block, low voltage, T-wave changes, and right bundle branch block. B, Representative transthoracic echocardiogram 4-chamber view demonstrating severe left ventricular dilatation and moderate right ventricular dilatation. Abbreviations: LV, left ventricle; RV, right ventricle.
Table 2.
EKG, Echocardiogram, and Chest X-ray Findings in Chagas Disease; Patients May Have >1 Abnormality so Numbers Sum to >100%
| EKG Findings | ||||
|---|---|---|---|---|
| Asymptomatic | Symptomatic | Total | ||
| (n = 25) | (n = 31) | (n = 56) | % | |
| Normal | 16 | 8 | 24 | 43 |
| Right bundle branch block | 3 | 9 | 12 | 21 |
| Sinus bradycardia | 5 | 7 | 12 | 21 |
| T wave changes | 1 | 11 | 12 | 21 |
| Premature atrial or ventricular contractions | 2 | 9 | 11 | 20 |
| Low voltage | 1 | 6 | 7 | 13 |
| Left axis deviation | 0 | 6 | 6 | 11 |
| First-degree AV block | 0 | 5 | 5 | 9 |
| Left bundle branch block | 0 | 5 | 5 | 9 |
| Atrial fibrillation | 0 | 5 | 5 | 9 |
| Prolonged QT | 0 | 1 | 1 | 2 |
| Left ventricular hypertrophy | 1 | 0 | 1 | 2 |
| Left atrial enlargement | 0 | 1 | 1 | 2 |
| Echocardiogram Findings | ||||
| Asymptomatic | Symptomatic | Total | ||
| (n = 21) | (n = 31) | (n = 52) | % | |
| Normal | 16 | 8 | 24 | 46 |
| Ventricular abnormalities | ||||
| Wall motion abnormalities | 0 | 16 | 16 | 31 |
| Systolic dysfunction | 0 | 13 | 13 | 25 |
| Left ventricle dilatation | 1 | 9 | 10 | 19 |
| Left ventricle aneurysm | 0 | 5 | 5 | 10 |
| Right ventricle dilatation | 1 | 3 | 4 | 8 |
| Diastolic dysfunction | 2 | 2 | 4 | 8 |
| Atrial abnormalities | ||||
| Left atrial dilatation/enlargement | 2 | 9 | 11 | 21 |
| Right atrial dilatation | 1 | 2 | 3 | 6 |
| Atrial septal aneurysm | 1 | 1 | 2 | 4 |
| Increased left atrial pressure | 0 | 2 | 2 | 4 |
| Valvular abnormalities | ||||
| Tricuspid regurgitation | 1 | 16 | 17 | 33 |
| Mitral regurgitation | 2 | 13 | 15 | 29 |
| Aortic regurgitation | 1 | 7 | 8 | 15 |
| Pulmonary regurgitation | 0 | 4 | 4 | 8 |
| Mitral stenosis | 0 | 1 | 1 | 2 |
| Pulmonary hypertension | 0 | 4 | 4 | 8 |
| Pericardial effusion | 0 | 4 | 4 | 8 |
| Chest X-ray Findings | ||||
| Asymptomatic | Symptomatic | Total | ||
| (n = 14) | (n = 27) | (n = 41) | % | |
| Cardiomegaly | 1 | 11 | 12 | 29 |
Abbreviations: AV, atrioventricular; EKG, electrocardiogram; QT, interval from start of the Q wave to the end of the T wave.
Treatment
In total, 27 patients were treated with either benznidazole or nifurtimox. Adverse effects were common, with 21 (78%) patients reporting at least 1 symptom. Despite the presence of adverse effects, 15 patients (71%) completed the full treatment course. Of the 17 patients treated with benznidazole, 5 (29%) did not complete therapy due to rash (n = 5) with or without severe transaminitis (n = 2). Of the 10 patients treated with nifurtimox, 2 (20%) did not complete therapy. One patient developed nausea and abdominal pain, and 1 patient was lost to follow-up. Characteristics and adverse effects among patients receiving antitrypanosomal treatment are described in detail in Table 3.
Table 3.
Characteristics and Adverse Effects of Patients Receiving Antitrypanosomal Therapy (n = 27)
| Benznidazole (n = 17) | Nifurtimox (n = 10) | Overall (n = 27) | |
|---|---|---|---|
| Age, median (range), y | 36 (26–52) | 31 (23–44) | 36 (23–52) |
| Female, No. (%) | 9 (53) | 4 (40) | 13 (48) |
| Asymptomatic, No. (%) | 12 (71) | 5 (50) | 17 (63) |
| Completed treatment, No. (%) | 12 (71) | 8 (80) | 20 (74) |
| Experienced adverse effects, No. (%) | 13 (76) | 8 (80) | 21 (78) |
| Gastrointestinal | 6 (35) | 7 (70) | 13 (48) |
| Abdominal pain | 1 (6) | 6 (60) | 7 (26) |
| Anorexia | 0 (0) | 2 (20) | 2 (7) |
| Heartburn | 0 (0) | 0 (0) | 0 (0) |
| Nausea | 2 (12) | 4 (40) | 6 (22) |
| Transaminitis | 4 (24) | 0 (0) | 4 (15) |
| Dermatologic | 7 (41) | 2 (20) | 9 (33) |
| Rash | 7 (41) | 2 (20) | 9 (33) |
| Edema | 2 (12) | 0 (0) | 2 (7) |
| Pruritis | 0 (0) | 1 (10) | 1 (4) |
| Neurologic/ neuropsychiatric | 3 (18) | 5 (50) | 8 (30) |
| Headache | 0 (0) | 4 (40) | 4 (15) |
| Sleep disturbance | 2 (12) | 2 (20) | 4 (15) |
| Memory loss | 0 (0) | 1 (10) | 1 (4) |
| Anxiety | 0 (0) | 1 (10) | 1 (4) |
| Peripheral neuropathy | 2 (12) | 1 (10) | 3 (11) |
| Lightheadedness | 0 (0) | 2 (20) | 2 (7) |
| Myalgias | 0 (0) | 2 (20) | 2 (7) |
| Palpitations | 0 (0) | 1 (10) | 1 (4) |
| Fatigue | 0 (0) | 1 (10) | 1 (4) |
| Lost to follow-up | 0 (0) | 1 (10) | 1 (4) |
Ten patients had advanced heart disease requiring a pacemaker (n = 1), implantable cardioverter defibrillator (n = 3), or left ventricular assist device (LVAD) (n = 6). One LVAD recipient died while waiting for transplant, and the autopsy showed cardiomyocyte hypertrophy and fibrosis that were attributed to chronic Chagas disease. Six of the 10 patients received a heart transplant. Of these, 2 patients had reactivation of T. cruzi infection after transplant, as determined by T. cruzi PCR positivity, and were treated with benznidazole. One patient had graft rejection, and 1 had graft vasculopathy. Four patients were alive at 2, 2.5, 4, and 7 years post-transplant. One died due to postoperative complications, and 1 died from unrelated trauma.
DISCUSSION
To our knowledge, this is the largest case series describing the clinical experience of Chagas disease in the United States. Over a period of 12 years, we have cared for 60 patients. Most of the patients were referred to the tropical medicine clinic at Jacobi Medical Center, 1 of the few centers in New York City with experts in Chagas disease. Therefore the patients described in this series likely represent a significant portion of patients who have been diagnosed with Chagas disease in the region.
Similar to other studies from the United States, the most common country of origin was El Salvador, representing 40% of patients. In a Los Angeles–based screening program, Salvadorans were 6.2 times more likely than other Latin American immigrants to test positive for Chagas disease [21]. Salvadorans also accounted for 72% of all Latin American patients who received benznidazole under the CDC IND protocol [28]. In contrast, Bolivia is frequently the most common country of origin in other nonendemic countries [29]. New York City is home to nearly 1 million Latin American immigrants, who account for the highest proportion (32%) of the city’s foreign-born individuals. Of these, the largest number were born in the Dominican Republic, a country that is not endemic for Chagas disease. The largest number of foreign-born individuals in New York City from a Chagas-endemic country come from Mexico (186 298), followed by Ecuador (137 791), Colombia (65 678), El Salvador (32 903), and Honduras (28 552) [30, 31]. In our series, 5 patients were born in the United States to immigrant parents, demonstrating the possibility of mother-to-child vertical transmission within nonendemic countries, although transmission through other mechanisms due to shared epidemiologic risk cannot be ruled out.
Nearly half of our patients were asymptomatic. Despite the lack of overt symptoms, 48% of asymptomatic patients had abnormal EKG or imaging findings, underscoring the need for screening to identify patients before progression to clinical disease. Blood donation was the most common reason for screening and would capture individuals who are infected but asymptomatic. In addition, our experience highlights the importance of targeted screening in immigrants from endemic countries, particularly if cardiac or gastrointestinal symptoms are present. When a new diagnosis of Chagas disease is made, family members who share the same exposure history or were born to an infected mother should be tested as well [32]. Family-based screening has also been used in other medical centers to effectively identify new cases, yielding a Chagas disease prevalence of 7.4% in a nonendemic setting [33] and as high as 42% in endemic settings [34].
In keeping with expert opinion [25, 26, 27], we recommend that the evaluation of Chagas disease include an EKG, echocardiogram, and chest x-ray. These studies may be able to detect subclinical cardiac involvement, such as sinus bradycardia, RBBB, and wall motion abnormalities. Among our patients, 57% had abnormal EKGs and 54% had abnormal echocardiograms, compared with 31.5% and 5.6%, respectively, in a study of 485 Chagas disease patients in Spain [35]. Additionally, 27% of our patients developed heart failure, compared with only 2.6% in the Spanish study. The higher prevalence of cardiac disease and abnormal cardiac evaluation in our study can be explained by recruitment bias introduced from the fact that 1 of the treatment sites is a center for heart failure and transplantation.
Gastrointestinal disease is more common in patients from the southern cone of South America (Brazil, Argentina, Bolivia, Chile, and Paraguay) [36]. However, in our series patients endorsing gastrointestinal symptoms were predominantly from Mexico and Central America, possibly reflective of the fact that subjective symptoms are nonspecific for Chagasic gastrointestinal disease. Imaging findings of dilated esophagus and megacolon, findings more specific for Chagasic gastrointestinal disease, were present in 2 patients from Brazil. Therefore, to evaluate for Chagasic gastrointestinal disease, we recommend that imaging be performed if any relevant complaints are present.
When appropriate, patients were treated with either benznidazole or nifurtimox based on drug availability through the CDC. After the study period, benznidazole was FDA approved and, as of May 2018, has been commercially available [28]. Benznidazole is now generally considered first-line therapy due to better tolerability, although both benznidazole and nifurtimox are associated with high rates of adverse effects [27]. Benznidazole most commonly produces dermatologic effects, such as rash, edema, and itching. Gastrointestinal (eg, nausea, abdominal pain, anorexia) and neurologic (eg, headache, neuropathy, insomnia) symptoms are also frequently reported [37, 38, 39]. Nifurtimox most commonly causes gastrointestinal and neurologic symptoms; dermatologic symptoms are uncommon [40, 41]. In our series, the prevalence of adverse effects was high overall (78%), and the effects reported for benznidazole and nifurtimox were similar to those described in the literature. The majority of adverse effects were minor and did not require discontinuation of therapy. The discontinuation rate was 29% among patients treated with benznidazole (compared with 13%–30% reported in the literature) [17, 37, 38, 39] and 20% among patients treated with nifurtimox (compared with 20%–44% in the literature) [41, 42], although the number of treated patients was small.
Patients with advanced heart disease were not offered antiparasitic treatment, as treatment would not alter clinical outcomes. In some cases, Chagasic heart disease was severely advanced to the point of requiring implantable devices or transplantation. Limited evidence suggests that post-transplant outcomes in Chagas disease patients are comparable to the outcomes of patients with heart failure from other causes [13], but further evaluation is warranted. Similar to observations from other US transplant centers [42], Chagas reactivation was a significant concern in our heart transplant recipients. Risk of reactivation is highest in the first 2 years post-transplant, and highest for transplantation of hearts compared with other solid organs [43]. In accordance with expert consensus, our transplant patients were monitored routinely with PCR, and those with detectable parasitemia were treated. Prophylactic treatment is not recommended [44–45]. Donor-derived infection, that is, transmission from a T. cruzi–infected donor to an uninfected recipient, was not present in this case series, though it has been described in the United States [46–48]. Current consensus guidelines recommend targeted screening of donors born in endemic countries. Transplantation of kidney or liver from a T. cruzi–infected donor is acceptable with informed consent, frequent parasitological monitoring post-transplant, and immediate treatment if infection is detected. Heart transplants from infected donors should be avoided [46].
In an era of increased migration, Chagas disease is emerging as an important public health issue worldwide. Latin Americans represent the largest foreign-born population in the New York City metropolitan area and in the United States [30]. However, the medical community in the United States has little awareness or experience with Chagas disease [49–50]. Most infected persons are asymptomatic and go undiagnosed, but Chagas disease can progress to severe heart disease and even death. Increased awareness of risk factors and clinical manifestations of Chagas disease is necessary to identify and treat patients early.
Limitations
This is a retrospective study that describes patients limited to the practice of the authors and may not be representative of other settings. Additionally, we describe only patients who presented to our hospitals, including some referred by family members, private physicians, and the New York Blood Center, which excludes those with limited access to care. One patient was lost to follow-up. Symptoms reported or test results may not be related to Chagas disease, and tests may have been performed for reasons other than evaluation of Chagas disease. Due to the fact that patients were identified by referral, we are unable to determine prevalence in our hospitals or communities. A prior study found the prevalence of Chagas disease to be 0.008% among the New York City metropolitan area blood donor population, which was the most common source of referral for our patients [51].
Acknowledgments
We wish to thank the many physicians who referred their patients to us and the laboratory personnel both at the Parasitology Laboratory of Jacobi Medical Center and the Parasitic Diseases Branch, Centers for Disease Control and Prevention. We also wish to thank Dr. Susan Montgomery for her help with reviewing the manuscript. This article is dedicated to the memory of Dr. Herbert B. Tanowitz.
Financial support. None.
Potential conflicts of interest. The authors have no conflicts to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. C.Z., O.Q., and F.E. contributed to data analysis and manuscript writing. E.K.R., Y.A.P., M.B.O., F.E., D.R.-B., C.R.S., L.F.H., F.S.M., O.S., J.S., and S.R.P. contributed to data collection. Y.A.P. and C.M.C. reviewed and contributed ideas significant to the manuscript. H.T. contributed to the study design, data analysis, and manuscript writing.
References
- 1. World Health Organization. Chagas disease (American trypanosomiasis) fact sheet.2017 Available at: http://www.who.int/mediacentre/factsheets/fs340/en/. Accessed 15 August 2017.
- 2.World Health Organization. Chagas disease (American trypanosomiasis) fact sheet (revised in June 2010). Wkly Epidemiol Rec 2010; 85:334–6. [PubMed] [Google Scholar]
- 3.World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 2015; 90:33–44. [PubMed] [Google Scholar]
- 4. de Fuentes-Vicente JA, Gutiérrez-Cabrera AE, Flores-Villegas AL, et al. What makes an effective Chagas disease vector? Factors underlying Trypanosoma cruzi-triatomine interactions. Acta Trop 2018; 183:23–31. [DOI] [PubMed] [Google Scholar]
- 5. Shikanai-Yasuda MA, Carvalho NB. Oral transmission of Chagas disease. Clin Infect Dis 2012; 54:845–52. [DOI] [PubMed] [Google Scholar]
- 6. Tanowitz HB, Machado FS, Jelicks LA, et al. Perspectives on Trypanosoma cruzi-induced heart disease (Chagas disease). Prog Cardiovasc Dis 2009; 51:524–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bern C, Martin DL, Gilman RH. Acute and congenital Chagas disease. Adv Parasitol 2011; 75:19–47. [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Blood donor screening for chagas disease—United States, 2006–2007. MMWR Morb Mortal Wkly Rep 2007; 56:141–3. [PubMed] [Google Scholar]
- 9. Antinori S, Galimberti L, Bianco R, et al. Chagas disease in Europe: a review for the internist in the globalized world. Eur J Intern Med 2017; 43:6–15. [DOI] [PubMed] [Google Scholar]
- 10. Kirchhoff LV. Epidemiology of American trypanosomiasis (Chagas disease). Adv Parasitol 2011; 75:1–18. [DOI] [PubMed] [Google Scholar]
- 11. Rassi A Jr, Rassi A, Marcondes de Rezende J. American trypanosomiasis (Chagas disease). Infect Dis Clin North Am 2012; 26:275–91. [DOI] [PubMed] [Google Scholar]
- 12. de Oliveira RB, Troncon LE, Dantas RO, Menghelli UG. Gastrointestinal manifestations of Chagas’ disease. Am J Gastroenterol 1998; 93:884–9. [DOI] [PubMed] [Google Scholar]
- 13. Bern C, Messenger L, Whitman J, Maguire J. Chagas disease in the United States: a public health approach. Clin Microbiol Rev 2019; 33:e00023–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pane S, Giancola ML, Piselli P, et al. Serological evaluation for Chagas disease in migrants from Latin American countries resident in Rome, Italy. BMC Infect Dis 2018; 18:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whitman JD, Bulman CA, Gunderson EL, et al. Chagas disease serological test performance in U.S. blood donor specimens. J Clin Microbiol 2019; 57:e01217-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Afonso AM, Ebell MH, Tarleton RL. A systematic review of high quality diagnostic tests for Chagas disease. PLoS Negl Trop Dis 2012; 6:e1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morillo CA, Marin-Neto JA, Avezum A, et al. ; BENEFIT Investigators Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med 2015; 373:1295–306. [DOI] [PubMed] [Google Scholar]
- 18. Benatti RD, Al-Kindi SG, Bacal F, Oliveira GH. Heart transplant outcomes in patients with Chagas cardiomyopathy in the United States. Clin Transplant 2018; 32:e13279. [DOI] [PubMed] [Google Scholar]
- 19. Tanowitz HB, Weiss LM, Montgomery SP. Chagas disease has now gone global. PLoS Negl Trop Dis 2011; 5:e1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis 2009; 49:e52–4. [DOI] [PubMed] [Google Scholar]
- 21. Meymandi SK, Forsyth CJ, Soverow J, et al. Prevalence of Chagas disease in the Latin American-born population of Los Angeles. Clin Infect Dis 2017; 64:1182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cassandra D, Steurer F, Czaicki N, Todd C. Determination of the sensitivity and specificity of three serodiagnostic assays for Chagas disease by latent class analysis [abstract]. Am J Trop Med Hyg 2011; 85:256. [Google Scholar]
- 23. Umezawa ES, Nascimento MS, Kesper N Jr, et al. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas’ disease. J Clin Microbiol 1996; 34:2143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qvarnstrom Y, Schijman AG, Veron V, et al. Sensitive and specific detection of Trypanosoma cruzi DNA in clinical specimens using a multi-target real-time PCR approach. PLoS Negl Trop Dis 2012; 6:e1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bern C, Montgomery SP, Herwaldt BL, et al. Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA 2007; 298:2171–81. [DOI] [PubMed] [Google Scholar]
- 26. Dias JC, Ramos AN Jr, Gontijo ED, et al. 2nd Brazilian consensus on Chagas disease. Rev Soc Bras Med Trop. 2016; 49(Suppl 1:3–60. [DOI] [PubMed] [Google Scholar]
- 27. Meymandi S, Hernandez S, Park S, et al. Treatment of Chagas disease in the United States. Curr Treat Options Infect Dis 2018; 10:373–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herwaldt BL, Dougherty CP, Allen CK, et al. Characteristics of patients for whom benznidazole was released through the CDC-sponsored investigational new drug program for treatment of Chagas disease - United States, 2011-2018. MMWR Morb Mortal Wkly Rep 2018; 67:803–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salvador F, Treviño B, Sulleiro E, et al. Trypanosoma cruzi infection in a non-endemic country: epidemiological and clinical profile. Clin Microbiol Infect 2014; 20:706–12. [DOI] [PubMed] [Google Scholar]
- 30. City of New York Department of City Planning. 2013. The newest New Yorkers: Characteristics of the city’s foreign-born population. Available at: https://www1.nyc.gov/assets/planning/download/pdf/data-maps/nyc-population/nny2013/nny_2013.pdf. Accessed 12 April 2020.
- 31. Mayor’s Office of Immigrant Affairs. 2018. State of our immigrant city. Available at: https://www1.nyc.gov/assets/immigrants/downloads/pdf/moia_annual_report_2018_final.pdf. Accessed 12 April 2020.
- 32. Mongeau-Martin G, Ndao M, Libman M, et al. A family cluster of Chagas disease detected through selective screening of blood donors: a case report and brief review. Can J Infect Dis Med Microbiol 2015; 26:157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hernandez S, Forsyth CJ, Flores CA, Meymandi SK. Prevalence of Chagas disease among family members of previously diagnosed patients in Los Angeles, California. Clin Infect Dis 2019; 69:1226–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zulantay I, Apt W, Ramos D, et al. The epidemiological relevance of family study in Chagas disease. PLoS Negl Trop Dis 2013; 7:e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sánchez-Montalvá A, Salvador F, Rodríguez-Palomares J, et al. Chagas cardiomyopathy: usefulness of EKG and echocardiogram in a non-endemic country. PLoS One 2016; 11:e0157597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pinazo MJ, Cañas E, Elizalde JI, et al. Diagnosis, management and treatment of chronic Chagas’ gastrointestinal disease in areas where Trypanosoma cruzi infection is not endemic. Gastroenterol Hepatol 2010; 33:191–200. [DOI] [PubMed] [Google Scholar]
- 37. Pinazo MJ, Muñoz J, Posada E, et al. Tolerance of benznidazole in treatment of Chagas’ disease in adults. Antimicrob Agents Chemother 2010; 54:4896–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Olivera MJ, Cucunubá ZM, Valencia-Hernández CA, et al. Risk factors for treatment interruption and severe adverse effects to benznidazole in adult patients with Chagas disease. PLoS One 2017; 12:e0185033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller DA, Hernandez S, Rodriguez De Armas L, et al. Tolerance of benznidazole in a United States Chagas disease clinic. Clin Infect Dis 2015; 60:1237–40. [DOI] [PubMed] [Google Scholar]
- 40. Jackson Y, Alirol E, Getaz L, et al. Tolerance and safety of nifurtimox in patients with chronic Chagas disease. Clin Infect Dis 2010; 51:e69–75. [DOI] [PubMed] [Google Scholar]
- 41. Forsyth CJ, Hernandez S, Olmedo W, et al. Safety profile of nifurtimox for treatment of Chagas disease in the United States. Clin Infect Dis 2016; 63:1056–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gray EB, La Hoz RM, Green JS, et al. Reactivation of Chagas disease among heart transplant recipients in the United States, 2012-2016. Transpl Infect Dis 2018; 20:e12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pierrotti LC, Carvalho NB, Amorin JP, et al. Chagas disease recommendations for solid-organ transplant recipients and donors. Transplantation 2018; 102(2S Suppl 2):S1–7. [DOI] [PubMed] [Google Scholar]
- 44. Pinazo MJ, Miranda B, Rodríguez-Villar C, et al. Recommendations for management of Chagas disease in organ and hematopoietic tissue transplantation programs in nonendemic areas. Transplant Rev (Orlando) 2011; 25:91–101. [DOI] [PubMed] [Google Scholar]
- 45. La Hoz RM, Morris M; Infectious Diseases Community of Practice of the American Society of Transplantation. Tissue and blood protozoa including toxoplasmosis, Chagas disease, leishmaniasis, Babesia, Acanthamoeba, Balamuthia, and Naegleria in solid organ transplant recipients—Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13546. [DOI] [PubMed] [Google Scholar]
- 46. Centers for Disease Control and Prevention. Chagas disease after organ transplantation—United States, 2001. MMWR Morb Mortal Wkly Rep 2002; 51:210–2. [PubMed] [Google Scholar]
- 47. Centers for Disease Control and Prevention. Chagas disease after organ transplantation—Los Angeles, California, 2006. MMWR Morb Mortal Wkly Rep 2006; 55:798–800. [PubMed] [Google Scholar]
- 48. Kun H, Moore A, Mascola L, et al. Transmission of Trypanosoma cruzi by heart transplantation. Clin Infect Dis 2009; 48:1534–40. [DOI] [PubMed] [Google Scholar]
- 49. Forsyth CJ, Hernandez S, Flores CA, et al. “It’s like a phantom disease”: patient perspectives on access to treatment for Chagas disease in the United States. Am J Trop Med Hyg 2018; 98:735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stimpert KK, Montgomery SP. Physician awareness of Chagas disease, USA. Emerg Infect Dis 2010; 16:871–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zaniello BA, Kessler DA, Vine KM, et al. Seroprevalence of Chagas infection in the donor population. PLoS Negl Trop Dis 2012; 6:e1771. [DOI] [PMC free article] [PubMed] [Google Scholar]