Abstract
Background
Between 2007 and 2015, inpatient fluoroquinolone use declined in US Veterans Affairs (VA) hospitals. Whether fluoroquinolone use at discharge also declined, in particular since antibiotic stewardship programs became mandated at VA hospitals in 2014, is unknown.
Methods
In this retrospective cohort study of hospitalizations with infection between January 1, 2014, and December 31, 2017, at 125 VA hospitals, we assessed inpatient and discharge fluoroquinolone (ciprofloxacin, levofloxacin, moxifloxacin) use as (a) proportion of hospitalizations with a fluoroquinolone prescribed and (b) fluoroquinolone-days per 1000 hospitalizations. After adjusting for illness severity, comorbidities, and age, we used multilevel logit and negative binomial models to assess for hospital-level variation and longitudinal prescribing trends.
Results
Of 560219 hospitalizations meeting inclusion criteria as hospitalizations with infection, 37.4% (209602/560219) had a fluoroquinolone prescribed either during hospitalization (32.5%, 182337/560219) or at discharge (19.6%, 110003/560219). Hospitals varied appreciably in inpatient, discharge, and total fluoroquinolone use, with 71% of hospitals in the highest prescribing quartile located in the Southern United States. Nearly all measures of fluoroquinolone use decreased between 2014 and 2017, with the largest decreases found in inpatient fluoroquinolone and ciprofloxacin use. In contrast, there was minimal decline in fluoroquinolone use at discharge, which accounted for a growing percentage of hospitalization-related fluoroquinolone-days (52.0% in 2014; 61.3% by 2017).
Conclusions
Between 2014 and 2017, fluoroquinolone use decreased in VA hospitals, largely driven by decreased inpatient fluoroquinolone (especially ciprofloxacin) use. Fluoroquinolone prescribing at discharge, as well as levofloxacin prescribing overall, is a growing target for stewardship.
Keywords: antibiotic stewardship, fluoroquinolones, infection, transitions of care, veterans
Between 2014 and 2017, inpatient fluoroquinolone—particularly ciprofloxacin—prescribing declined in US Veterans Affairs hospitals. In contrast, fluoroquinolone prescribing at discharge has been relatively stable with small increases in levofloxacin prescribing at discharge.
Fluoroquinolone use is linked to Clostridioides difficile infection (CDI), antibiotic resistance, and severe adverse events [1, 2]. Despite these potential harms, fluoroquinolones are one of the most commonly prescribed, and overprescribed, antibiotic classes [3–5]. Fortunately, reducing fluoroquinolone prescribing can improve care. For example, between 1998 and 2014, decreased fluoroquinolone prescribing in the United Kingdom reduced CDI [6]. Similarly, local stewardship interventions are most effective at improving patient outcomes when they reduce fluoroquinolone prescribing [7]. Thus, national guidelines from the Centers for Disease Control and Prevention, Infectious Diseases Society of America, and Society for Healthcare Epidemiology of America all recommend reducing fluoroquinolone use as a top stewardship priority [8, 9].
In recognition of the harms of inappropriate antibiotic prescribing, the Veterans Health Administration (VHA) created the Antimicrobial Stewardship Task Force in 2011 to develop a national strategic plan to improve antibiotic use [10]. This was followed in 2014 by VHA Directive 1031, which established a policy for implementation and maintenance of antimicrobial stewardship programs at all VHA medical facilities [10]. Similar programs did not become mandatory in non-VHA hospitals until 2017 [11]. Possibly due to this early adoption of antibiotic stewardship programs, inpatient fluoroquinolone prescribing in VHA hospitals fell between 2007 and 2015, as did rates of hospital-onset CDI [10].
Despite reductions in inpatient prescribing, it is unknown whether fluoroquinolone prescribing at hospital discharge has similarly declined over time. Because fluoroquinolones are broad-spectrum and able to be administered orally, they are the most common antibiotic prescribed at discharge for a wide range of infectious conditions [12]. Discharge prescriptions account for up to two-thirds of fluoroquinolone use related to hospitalization with infection [5, 13]. Furthermore, a recent observational study suggested that stewardship interventions targeting inpatient fluoroquinolone prescribing may not decrease—and in some cases may increase—fluoroquinolone prescribing at discharge [4]. Thus, we aimed to determine whether discharge fluoroquinolone use in VHA hospitals has changed since 2014 and whether inpatient fluoroquinolone use has continued to decline.
METHODS
Study Setting and Cohort Identification
This retrospective cohort study identified veterans hospitalized in general or intensive care units with an infection between January 2014 and December 2017. Patients were eligible for inclusion if hospitalized at 1 of the 125 Veterans Affairs (VA) hospitals that reported at least 400 hospitalizations (~100 annual hospitalizations) from 2014 to 2017. “Hospitalizations with infection” were defined using the Centers for Disease Control and Prevention’s electronic health record–based definition, which has been validated for VA data [14]. This included any hospitalized patient with at least 1 blood culture drawn who received systemic antibiotics for at least 3 days, including duration after discharge. Patients under the age of 18 were excluded.
Data Source
Data from the VA Patient Database (VAPD), containing all inpatient VA hospitalizations from January 1, 2014, through December 31, 2017, were used in this study [14]. The VAPD was initially created to study patient physiology throughout acute hospitalization and thus contains data on acute hospitalizations from all parts of the nationwide VA health care system, including clinical data, intensive care unit indicators, and facility, patient, and hospitalization characteristics. This database also includes information on inpatient antibiotic administration originally identified from the Bar Code Medication Administration domain in the VA Corporate Data Warehouse (CDW) [14]. Discharge antibiotics were identified from outpatient prescriptions—obtained from the outpatient medications domain in CDW—written within 1 calendar day of hospital discharge.
Primary Outcomes: Fluoroquinolone Use
Fluoroquinolone antibiotics included intravenous or oral ciprofloxacin, levofloxacin, and moxifloxacin—the top 3 fluoroquinolones prescribed in the United States. As done previously [4], fluoroquinolone use was quantified by 2 methods. First, we determined the number of patients who received a fluoroquinolone per 1000 hospitalizations with infection. This was further divided into the number of patients who were prescribed a fluoroquinolone while hospitalized or at discharge, including outpatient fluoroquinolone prescription within 1 day of discharge. Second, we determined the number of inpatient or postdischarge days of fluoroquinolones prescribed per 1000 hospitalizations with infection. Inpatient days included the number of hospital days in which a fluoroquinolone was administered. Postdischarge days included the number of days indicated on discharge prescription.
Risk Adjustment
Patient-level adjustments included illness severity, comorbidities, and age. Illness severity was calculated using the validated, multivariable VHA intensive care unit severity score (predicted probability of 30-day mortality), which incorporates age, admission diagnosis category, 31 comorbid conditions, and 11 laboratory values collected within 1 day of admission. The score performs similarly to the APACHE IV, with a C-statistic of 0.845 [15]. In addition, we adjusted for 31 individual comorbidities, defined using the Van Walraven’s Elixhauser comorbidity score [16]. Finally, we adjusted for patient age (categorized as 18–44, 45–64, 65–74, 75–84, >85 years). Hospital-level variables were hospital region (Midwest, Northeast, South, West), hospital size (median number of annual hospitalizations), teaching hospital status (yes/no), and hospital complexity (defined by critical care capabilities, with higher scores representing higher complexity). The analytic code is available online (https://github.com/CCMRcodes/Fluoroquinolone), as is the code necessary to replicate the VAPD data source (https://github.com/CCMRcodes/VAPD).
Statistical Analysis
Descriptive statistics were used to describe annual and quarterly (eg, January–March) fluoroquinolone prescribing as well as fluoroquinolone prescribing by location, whether inpatient or at discharge. To examine facility characteristics, hospitals were categorized by quartile of adjusted fluoroquinolone use, defined as total inpatient plus discharge fluoroquinolone-days divided by 1000 hospitalizations with infection. Absolute (ARRs) and relative risk reductions (RRRs) comparing adjusted proportions of patients prescribed a fluoroquinolone between 2014 and 2017 were calculated.
We estimated risk-adjusted fluoroquinolone use among hospitalizations with infection using multilevel logit models. Risk-adjusted fluoroquinolone-days per 1000 hospitalizations with infection were estimated using negative binomial multilevel models—to account for overdispersion—with hospitalizations nested within hospitals. All models include a fixed effect for year.
For each outcome, we quantified the variation in fluoroquinolone use across hospitals using the intraclass correlation coefficient (ICC), as well as median odds ratios (MORs) or median rate ratios (MRRs) for dichotomous and continuous outcomes, respectively [17]. The MOR represents the increased odds of fluoroquinolone receipt that a patient with median baseline risk would have if moving to a hospital with greater risk. That is, given pairs of randomly selected hospitals, the MOR is the median value of all odds ratios between the hospital with higher fluoroquinolone use and the hospital with lower fluoroquinolone use [18]. The larger the MOR, the more important the hospital-level effects are in driving differences in outcome. MRR is similar, but for continuous outcomes [19]. SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA), and Stata, version 15.1 (StataCorp, College Station, TX, USA), were used for data analysis.
Ethics Statement
This study was approved by the VA Ann Arbor Healthcare System Institutional Review Board.
RESULTS
Between January 2014 and December 2017, there were 2212492 hospitalizations across 125 VA hospitals; 560219 (25.3%) met inclusion criteria as hospitalizations with infection. Included patients were predominantly older (66.0% were ≥65 years old) and male (95.5%). The top 5 primary diagnosis categories (accounting for 38.0% of hospitalizations) were septicemia, pneumonia, skin and subcutaneous tissue infection, chronic obstructive pulmonary disease, and urinary tract infection (Table 1). More than a third (37.4% [209602/560219]) of veterans hospitalized with an infection received a fluoroquinolone antibiotic either during hospitalization or at discharge, including 32.5% (182337/560219) who received a fluoroquinolone during hospitalization and 19.6% (110003/560219) who received a fluoroquinolone within 1 day of discharge. Levofloxacin was the most commonly prescribed fluoroquinolone (53.8%), followed by ciprofloxacin (43.2%). The most common indication for prescribing levofloxacin was pneumonia (19.3%), the most common indication for prescribing ciprofloxacin was urinary tract infection (11.1%), and the most common indication for prescribing moxifloxacin was pneumonia (25.3%) (Supplementary Table 1). Just under half (47.7%) of patients started on a fluoroquinolone during hospitalization were continued after discharge. However, a quarter (25.2%) of all fluoroquinolone prescriptions at discharge were new starts (ie, prescribed to patients who had not received a fluoroquinolone during hospitalization). Between January 2014 and December 2017, there were 2824 total fluoroquinolone-days per 1000 hospitalizations with infection, including 1201 inpatient days and 1623 postdischarge days.
Table 1.
Characteristics of Hospitalizations for Infection Between 2014 and 2017, n = 560 219
| Patient Characteristics | |
|---|---|
| Age, mean (SD), y | 68.6 (12.6) |
| Age ≥65 y, No. (%) | 370243 (66.1) |
| Age ≥65 y and hypertension, No. (%) | 140348 (25.1) |
| Sex, No. (%) | |
| Male | 534856 (95.5) |
| Female | 25363 (4.5) |
| Race, No. (%) | |
| White | 414059 (73.9) |
| Black | 104217 (18.6) |
| Other | 41943 (7.5) |
| Probability of 30-d mortality, mean (SD)a | 0.07 (0.1) |
| Top 10 primary diagnoses, No. (%) | |
| Septicemia | 60072 (10.8) |
| Pneumonia | 50023 (9.0) |
| Skin and subcutaneous tissue infection | 37427 (6.7) |
| Chronic obstructive pulmonary disease or bronchiectasis | 33784 (6.1) |
| Urinary tract infection | 30119 (5.4) |
| Respiratory failure, insufficiency, or arrest | 21377 (3.8) |
| Congestive heart failure | 15439 (2.8) |
| Complication of device, implant, or graft | 14533 (2.6) |
| Complications of surgical procedures or medical care | 14266 (2.6) |
| Diabetes mellitus with complications | 12749 (2.3) |
| Comorbidities | |
| Weighted Elixhauser comorbidity index, median (IQR) | 7 (1, 14) |
| Congestive heart failure, No. (%) | 109817 (19.6) |
| Cardiac arrhythmia, No. (%) | 133694 (23.9) |
| Chronic pulmonary disease, No. (%) | 163723 (29.2) |
| Hypertension,b No. (%) | 194575 (34.7) |
| Diabetes,b No. (%) | 195337 (34.9) |
| Renal failure, No. (%) | 118195 (21.1) |
| Liver disease, No. (%) | 49311 (8.8) |
| Metastatic cancer, No. (%) | 23041 (4.1) |
| Solid tumor without metastasis, No. (%) | 60138 (10.7) |
| Length of stay, median (IQR), d | 6 (4, 11) |
| Admission to intensive care, No. (%) | 133765 (23.9) |
| Fluoroquinolone prescriptions | |
| Prescribed a fluoroquinolone, No. (%) | 209602 (37.4) |
| Inpatientc | 182337 (32.5) |
| Intravenous | 88644 (15.8) |
| Oral | 123436 (22.0) |
| At discharge | 110003 (19.6) |
| Intravenous | 5 (0) |
| Oral | 109998 (19.6) |
| Prescribed ciprofloxacin, No. (%) | 90502 (16.2) |
| Inpatientc | 76450 (13.6) |
| Intravenous | 33171 (5.9) |
| Oral | 53029 (9.5) |
| At discharge | 45189 (8.1) |
| Intravenous | 2 (0) |
| Oral | 45189 (8.1) |
| Prescribed levofloxacin, No. (%) | 112676 (20.1) |
| Inpatientc | 99387 (17.7) |
| Intravenous | 51204 (9.1) |
| Oral | 63536 (11.3) |
| At discharge | 56654 (10.1) |
| Intravenous | 3 (0) |
| Oral | 56653 (10.1) |
| Prescribed moxifloxacin, No. (%) | 16494 (2.9) |
| Inpatientc | 14176 (2.5) |
| Intravenous | 6702 (1.2) |
| Oral | 9324 (1.2) |
| At discharge | 8359 (1.5) |
| Intravenous | 0 (0) |
| Oral | 8359 (1.5) |
| Total duration of fluoroquinolone use in patients prescribed a fluoroquinolone (n = 209 602), median (IQR), d | 6 (3, 9) |
| Inpatient duration | 2 (1, 4) |
| After discharge duration | 2 (0, 7) |
| Duration of fluoroquinolone use in patients prescribed a fluoroquinolone at discharge (n = 110 003), median (IQR), d | 8 (6, 11) |
| Inpatient duration | 2 (1, 3) |
| After discharge duration | 6 (4, 10) |
| Hospital characteristics | |
| Geographic region, No. (%) | |
| Midwest | 117934 (21.0) |
| Northeast | 70464 (12.6) |
| South | 260797 (46.6) |
| West | 111024 (19.8) |
| Intensive care unit level, No. (%) | |
| 1 (highest acuity) | 360038 (64.3) |
| 2 | 92751 (16.5) |
| 3 | 83889 (15.0) |
| 4 (lowest acuity) | 8471 (1.5) |
| No intensive care unit | 15070 (2.7) |
| Teaching hospital, No. (%) | 360267 (64.3) |
Abbreviation: d, days; IQR, interquartile range.
aVeterans Health Administration intensive care unit severity score, which predicts mortality using age, admission diagnosis category, 31 comorbid conditions, and 11 laboratory values.
bIncludes comorbidities with and without complications.
cNumbers may add up to >100% as some patients received both intravenous and oral fluoroquinolone therapy.
Hospital-Level Variation
Even after adjustment for patient-level characteristics, hospitals varied appreciably in inpatient, postdischarge, and total fluoroquinolone use (Supplementary Table 2). The highest prescribing quartile of hospitals prescribed fluoroquinolones to an additional 19.0% of patients hospitalized with infection. Furthermore, the highest prescribing hospital quartile had nearly twice as many total fluoroquinolone-days as the lowest prescribing quartile (3654 total adjusted fluoroquinolone-days vs 2021 total adjusted fluoroquinolone-days per 1000 patients hospitalized with infection). The MORs, MRRs, and ICCs further suggest that there is significant variation in fluoroquinolone use across hospitals (Supplementary Table 3). For example, the MOR for any fluoroquinolone receipt (inpatient or at discharge) was 1.47 (95% confidence interval, 1.40–1.54)—indicating a 47.0% difference in odds of fluoroquinolone use between an average higher fluoroquinolone prescribing hospital vs an average lower fluoroquinolone prescribing hospital. Characteristics of hospitals by prescribing quartile are also shown in Supplementary Table 2. Most notably, 71.0% of hospitals in the highest fluoroquinolone prescribing quartile were located in the Southern United States, whereas hospital acuity and number of annual hospitalizations had little effect on facility-level prescribing.
Longitudinal Analysis
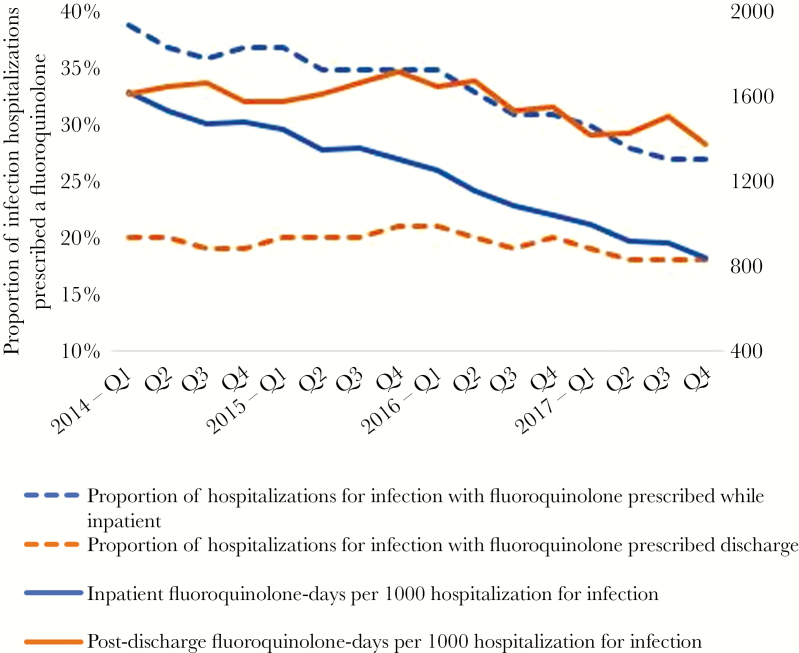
Unadjusted and adjusted fluoroquinolone prescribing by year is shown in Supplementary Tables 4 and 2, respectively. When adjusted rates of fluoroquinolone prescribing were analyzed longitudinally by year, nearly all measures of fluoroquinolone use decreased significantly between 2014 and 2017 (Figure 1, Table 2). There was a statistically significant decrease in the proportion of patients hospitalized with an infection who were prescribed any fluoroquinolone during hospitalization (from 2014 to 2017; RRR, 25.4%; ARR, 9.4%). Although there was a statistically significant decline in the proportion of patients prescribed a fluoroquinolone at discharge between 2014 and 2017, the decline was small (RRR, 7.4%; ARR, 1.4%). Similarly, the number of fluoroquinolone-days per 1000 infection hospitalizations decreased significantly between 2014 and 2017, largely due to a decrease in inpatient fluoroquinolone-days. In 2014, fluoroquinolone prescribing after discharge accounted for 52.0% of all fluoroquinolone-days (1634/3140); by 2017, fluoroquinolone prescribing after discharge accounted for 61.3% (1433/2339) of all fluoroquinolone-days. Among the 3 fluoroquinolones, moxifloxacin use was low and became lower. Ciprofloxacin saw the largest decreases in use—mostly due to a decrease in inpatient use. Conversely, levofloxacin use was largely stable over time, with small decreases in inpatient use and small increases in discharge use (Table 2).
Figure 1.
Adjusted inpatient and postdischarge fluoroquinolone use between 2014 and 2017. Fluoroquinolone use was determined per quarter and is adjusted for predicted 30-day mortality, age, and 31 Elixhauser comorbidity indicators.
Table 2.
Adjusted Fluoroquinolone Use in Patients Hospitalized for Infection in VA Hospitals, by Yeara
| 2014 | 2015 | 2016 | 2017 | |
|---|---|---|---|---|
| Hospitalizations | ||||
| Total hospitalizations, No. | 560954 | 554112 | 550335 | 547091 |
| Infection hospitalizations, No. (% of total) | 146561 (26) | 141143 (25) | 137583 (25) | 134932 (24) |
| Proportion of hospitalizations for infection with fluoroquinolone prescribed inpatient or at discharge, No. (%) | ||||
| Any fluoroquinolone | 60825 (41.5) | 56481 (40.0) | 51154 (37.2) | 43886 (32.5) |
| Ciprofloxacin | 26323 (18.0) | 23018 (16.3) | 20813 (15.1) | 17938 (13.3) |
| Levofloxacin | 29972 (20.5) | 31910 (22.6) | 29206 (21.2) | 25286 (18.7) |
| Moxifloxacinb | 5545 (3.8) | 2608 (1.8) | 1886 (1.4) | 1241 (0.9) |
| Total (inpatient plus postdischarge) fluoroquinolone-days per 1000 hospitalizations for infection, d | ||||
| Any fluoroquinolone | 3140 | 3002 | 2733 | 2339 |
| Ciprofloxacin | 1523 | 1400 | 1274 | 1101 |
| Levofloxacin | 1319 | 1499 | 1461 | 1264 |
| Moxifloxacinb | 284 | 93 | 74 | 55 |
| Proportion of hospitalizations for infection with fluoroquinolone prescribed inpatient, No. (%) | ||||
| Any fluoroquinolone | 54553 (37.2) | 50130 (35.5) | 44501 (32.3) | 37477 (27.8) |
| Ciprofloxacin | 23027 (15.7) | 19829 (14.0) | 17411 (12.7) | 14636 (10.8) |
| Levofloxacin | 23348 (15.9) | 25114 (17.8) | 22579 (16.4) | 19074 (14.1) |
| Moxifloxacinb | 4647 (3.2) | 2194 (1.6) | 1550 (1.1) | 977 (0.7) |
| Inpatient fluoroquinolone-days per 1000 hospitalizations for infection, d | ||||
| Any fluoroquinolone | 1530 | 1368 | 1141 | 922 |
| Ciprofloxacin | 662 | 568 | 467 | 382 |
| Levofloxacin | 727 | 774 | 679 | 559 |
| Moxifloxacinb | 121 | 44 | 32 | 21 |
| Proportion of hospitalizations for infection with fluoroquinolone prescribed at discharge, No. (%) | ||||
| Any fluoroquinolone | 28812 (19.7) | 28297 (20.0) | 27755 (20.2) | 24574 (18.2) |
| Ciprofloxacin | 11894 (8.1) | 11145 (7.9) | 11130 (8.1) | 10227 (7.6) |
| Levofloxacin | 13352 (9.1) | 15598 (11.1) | 15497 (11.3) | 13634 (10.1) |
| Moxifloxacinb | 2498 (1.7) | 1175 (0.8) | 869 (0.6) | 608 (0.5) |
| Postdischarge fluoroquinolone-days per 1000 hospitalizations for infection, d | ||||
| Any fluoroquinolone | 1634 | 1649 | 1604 | 1433 |
| Ciprofloxacin | 939 | 912 | 892 | 811 |
| Levofloxacin | 621 | 751 | 791 | 707 |
| Moxifloxacinb | 151 | 47 | 38 | 33 |
Fluoroquinolone use is adjusted for predicted 30-day mortality, age, and 31 Elixhauser comorbidity indicators.
aAll but 2 outcomes (rows) had a statistically significant decline over time, with P < .001. Total levofloxacin-days per 1000 hospitalizations for infection (inpatient or postdischarge) showed a statistically significant decline over time, with P = .002. The proportion of hospitalizations for infection with levofloxacin prescribed at discharge showed a statistically significant increase over time (P < .001).
bAs there were not enough observations at the hospital level to report predicted probabilities from both the fixed and random parts of the moxifloxacin models, we report predicted probabilities from the fixed part of the model only.
DISCUSSION
In this national study of nearly half a million patients hospitalized with infections at VA hospitals, nearly all measures of hospitalization-related fluoroquinolone use decreased between 2014 and 2017. Though the VA continued to see large decreases in inpatient fluoroquinolone prescribing (particularly ciprofloxacin use), fluoroquinolone prescribing at discharge remained relatively stable, with levofloxacin prescribing at discharge slightly increasing over time. As a result, the percentage of fluoroquinolone-days related to hospitalization that were prescribed at discharge grew from 52% to 61%. Hospitals varied widely in fluoroquinolone prescribing, with 71.0% of the highest fluoroquinolone prescribing hospitals located in the Southern United States.
We found that inpatient fluoroquinolone use has declined in VA hospitals since inpatient antibiotic stewardship programs became mandatory in 2014 [10]. This continues the trend seen from 2007 and 2015, when fluoroquinolones fell as a percentage of all antibiotic use from 18.0% to 13.0% [10]. Though national VA antibiotic stewardship policies may partially explain this decrease, studies of non-VA hospitals have found similar decreases in inpatient fluoroquinolone use [20]. Thus, decreasing fluoroquinolone use may reflect the national movement away from prescribing fluoroquinolones unless alternative agents are unavailable. For example, since 2008, the Food and Drug Administration (FDA) has issued 6 safety warnings related to fluoroquinolones, and national stewardship guidelines recommend reducing fluoroquinolone use as a top stewardship priority [8, 9]. Despite improvements, additional reductions are necessary. Recent reports suggest a correlation between fluoroquinolone prescribing and aortic aneurysm, rupture, and dissection, which led the FDA to issue a warning in 2018 that clinicians “should avoid prescribing fluoroquinolone antibiotics to…patients with peripheral atherosclerotic vascular disease, hypertension…and elderly patients” [1, 21]. In this study, two-thirds of patients were over the age of 65, one-third had hypertension, and a quarter both were elderly and had hypertension. Further interventions are needed to promote prescribing alternative agents for these patients when possible.
To our knowledge, our study is the first to find that—despite reductions in inpatient fluoroquinolone use—fluoroquinolone use at discharge has been largely stable since 2014. We confirm previous findings that fluoroquinolone use at discharge eclipses inpatient fluoroquinolone use [4, 5, 22], but also report that the contribution of discharge prescriptions to fluoroquinolone use has grown over time: from 52% of total fluoroquinolone-days in 2014 to 61% in 2017. As prior studies found that up to 70% of fluoroquinolones prescribed at discharge are inappropriate, excessive, or unnecessary [3, 4, 23], fluoroquinolone prescribing at discharge represents a prime target for stewardship. One potential explanation for the lack of improvement in postdischarge fluoroquinolone prescribing is that many stewardship programs do not monitor or target fluoroquinolone prescribing at discharge (eg, audit and feedback, restriction) [4, 24]. Interestingly, fluoroquinolone-days per 1000 patients hospitalized with infection decreased more than the proportion of patients discharged on a fluoroquinolone. This decrease may reflect the national movement toward prescribing shorter courses of therapy for patients with infections like community-acquired pneumonia [25] rather than the effect of interventions targeting fluoroquinolone prescribing. Reducing duration of therapy may therefore be 1 effective method for reducing fluoroquinolone use at discharge. Regardless, the fact that inpatient but not discharge fluoroquinolone use has seen substantial decreases may partially explain why hospital-onset CDI is decreasing but community-onset CDI is not [26].
We also add to the existing literature by evaluating individual fluoroquinolones. This allowed us to note that ciprofloxacin use in particular has decreased more than other fluoroquinolones. Ciprofloxacin is used to treat many infections, most commonly urinary tract infection. In 2016, the FDA warned against fluoroquinolone use for uncomplicated urinary tract infection based on studies demonstrating that harms often outweighed benefits [27]. Furthermore, urinary pathogens are easy to isolate and test for antibiotic sensitivities, providing information to clinicians who can then safely narrow antibiotic treatment. In contrast, levofloxacin use was largely stable over time, with a small increase in its use at discharge. The most common indication for inpatient antibiotic use—and levofloxacin use—is pneumonia [28]. In contrast to urinary tract infection, it is difficult to isolate causal pathogens in most patients hospitalized with pneumonia, of whom <10% may have a bacterial organism identified [13]. Thus, there is limited clinical information to allow antibiotics to be narrowed for many patients with pneumonia, making levofloxacin an appealing antibiotic choice. In addition, national pneumonia guidelines still recommend fluoroquinolones as potential first-line therapy [29].
Finally, we found large interhospital variation in fluoroquinolone use, and many high-prescribing hospitals located in the Southern US have also been found to have the highest rates of inpatient antibiotic use and inappropriate outpatient antibiotic prescriptions [30, 31]. Many other medications, such as opiates and benzodiazepines, are also more commonly prescribed or overprescribed in the Southern states [32, 33], potentially implying that regional culture may have a role to play in prescribing practices.
Our study has limitations. First, while we adjusted for illness severity and patient comorbidities, we could not adjust for all factors, such as allergies and antibiotic resistance. Second, we did not assess fluoroquinolone appropriateness or compensatory increases in other potentially inappropriate antibiotic classes. Third, though the results included 125 hospitals across the United States, all hospitals were VA hospitals, and thus results may not be generalizable to non-VA populations. Fourth, we used VA outpatient pharmacy claims to identify antibiotic prescriptions, which may underestimate out-of-system medication use. However, it is likely that veterans had their medications filled through the VA system due to potentially lower costs than an outside pharmacy, and it is not expected that such patterns would change over time. Fifth, we did not evaluate fluoroquinolone prescribing at discharge to nursing homes or other long-term care facilities and thus may have underestimated fluoroquinolone use at discharge. Study strengths include a large national assessment of fluoroquinolone use, including, to our knowledge, the largest assessment of fluoroquinolone prescribing at hospital discharge. Furthermore, we included all patients with suspected infection, rather than limiting to specific disease groups, enabling a broader assessment.
Our study has implications for antibiotic stewardship programs. First, the percentage of hospitalization-related fluoroquinolone-days that occur postdischarge has grown, indicating that efforts to reduce fluoroquinolone use should focus on discharge prescriptions. Second, stewardship efforts to reduce total antibiotic duration—such as for pneumonia—may have the secondary benefit of reducing postdischarge fluoroquinolone use. Third, stewardship teams should consider providing nonfluoroquinolone antibiotic recommendations for de-escalation in patients with pneumonia and negative or no respiratory culture data, especially those at risk for fluoroquinolone-related harm (eg, the elderly or those with hypertension). Further research is needed to identify optimal therapy for such patients, to determine why some hospitals already prescribe fluoroquinolones at much lower rates than others, and to determine whether fluoroquinolone use at discharge is associated with community-onset CDI.
In summary, between 2014 and 2017, fluoroquinolone use decreased in VA hospitals, largely driven by a decrease in inpatient fluoroquinolone—especially ciprofloxacin—use. Fluoroquinolone prescribing at discharge, and levofloxacin prescribing in particular, is a growing target for stewardship. Further studies should evaluate whether interventions to reduce total antibiotic duration may be the most effective at decreasing postdischarge fluoroquinolone use.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors would like to thank Theodore J. Iwashyna of the VA Center for Clinical Management Research, who led the initial development of the VAPD, and David Ratz, also of the VA Center for Clinical Management Research, for his expert programming.
Financial support. This work was supported by a Locally Initiated Project grant from the Center for Clinical Management Research at the VA Ann Arbor Healthcare System. In addition, this work builds on prior work supported by grants from the Department of Veteran Affairs, Veterans Health Administration, Health Services Research and Development Services (11–109), the Office of Clinical Analytics and Reporting, and US Centers for Disease Control and Prevention, subcontract to Epicenters U54K000172 (2015–2016). Dr. Prescott was supported by grant number K08 GM115859 from the National Institutes of Health.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Role of the funding source. The funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. X.W. and S.S. had full access to all the data in the study, and V.V. and H.P. had final responsibility for the decision to submit for publication.
References
- 1. US Food and Drug Administration. Drug Safety Communication: FDA Warns About Increased Risk of Ruptures or Tears in the Aorta Blood Vessel With Fluoroquinolone Antibiotics in Certain Patients. Silver Spring, MD: US Food and Drug Administration; 2018.
- 2. Werner NL, Hecker MT, Sethi AK, Donskey CJ. Unnecessary use of fluoroquinolone antibiotics in hospitalized patients. BMC Infect Dis 2011; 11:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scarpato SJ, Timko DR, Cluzet VC, et al. . An evaluation of antibiotic prescribing practices upon hospital discharge. Infect Control Hosp Epidemiol 2016; 38:353–5. [DOI] [PubMed] [Google Scholar]
- 4. Vaughn VM, Gandhi T, Conlon A, Chopra V, Malani AN, Flanders SA. The association of antibiotic stewardship with fluoroquinolone prescribing in michigan hospitals: a multi-hospital cohort study. Clin Infect Dis 2019; 69(8): 1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suzuki H, Perencevich EN, Alexander B, et al. . Inpatient fluoroquinolone stewardship improves the quantity and quality of fluoroquinolone-prescribing at hospital discharge: a retrospective analysis among 122 Veterans Health Administration hospitals. Clin Infect Dis. In press. [DOI] [PubMed] [Google Scholar]
- 6. Dingle KE, Didelot X, Quan TP, et al. . Effects of control interventions on Clostridium difficile infection in England: an observational study. Lancet Infect Dis 2017; 17:411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feazel LM, Malhotra A, Perencevich EN, Kaboli P, Diekema DJ, Schweizer ML. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother 2014; 69:1748–54. [DOI] [PubMed] [Google Scholar]
- 8. Pollack LA, Srinivasan A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin Infect Dis 2014; 59(suppl_3):S97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barlam TF, Cosgrove SE, Abbo LM, et al. . Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelly AA, Jones MM, Echevarria KL, et al. . A report of the efforts of the Veterans Health Administration National Antimicrobial Stewardship Initiative. Infect Control Hosp Epidemiol 2017; 38:513–20. [DOI] [PubMed] [Google Scholar]
- 11. Joint Commission. Approved: new antimicrobial stewardship standard. Jt Comm Perspect 2016; 36:1, 3–4, 8. [PubMed] [Google Scholar]
- 12. Yogo N, Haas MK, Knepper BC, Burman WJ, Mehler PS, Jenkins TC. Antibiotic prescribing at the transition from hospitalization to discharge: a target for antibiotic stewardship. Infect Control Hosp Epidemiol 2015; 36:474–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vaughn V, Flanders S, Snyder A, et al. . Excess antibiotic duration and adverse-events in patients hospitalized with pneumonia: a multi-hospital cohort study. Ann Intern Med 2019; 171(3):153–63. [DOI] [PubMed] [Google Scholar]
- 14. Wang XQ, Vincent BM, Wiitala WL, et al. . Veterans Affairs patient database (VAPD 2014–2017): building nationwide granular data for clinical discovery. BMC Med Res Methodol 2019; 19:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Render ML, Deddens J, Freyberg R, et al. . Veterans Affairs intensive care unit risk adjustment model: validation, updating, recalibration*. Crit Care Med 2008; 36:1031–42. [DOI] [PubMed] [Google Scholar]
- 16. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 2009; 47:626–33. [DOI] [PubMed] [Google Scholar]
- 17. Merlo J, Chaix B, Ohlsson H, et al. . A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 2006; 60:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol 2005; 161:81–8. [DOI] [PubMed] [Google Scholar]
- 19. Austin PC, Stryhn H, Leckie G, Merlo J. Measures of clustering and heterogeneity in multilevel Poisson regression analyses of rates/count data. Stat Med 2018; 37:572–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yarrington M, Anderson DJ, Ashley ED, et al. . 855. Impact of FDA black box warning on fluoroquinolone and alternative antibiotic use in Southeastern US hospitals. Open Forum Infect Dis 2018; 5(Suppl 1):S18–S. [DOI] [PubMed] [Google Scholar]
- 21. Lee CC, Lee MT, Chen YS, et al. . Risk of aortic dissection and aortic aneurysm in patients taking oral fluoroquinolone. JAMA Intern Med 2015; 175:1839–47. [DOI] [PubMed] [Google Scholar]
- 22. Feller J, Lund BC, Perencevich EN, et al. . Post-discharge oral antimicrobial use among hospitalized patients across an integrated national healthcare network. Clin Microbiol Infect 2020; 26:327–32. [DOI] [PubMed] [Google Scholar]
- 23. Suzuki H, Perencevich E, Alexander B, et al. . Fluoroquinolone Use at Hospital Discharge: A New Target for Antimicrobial Stewardship Efforts. Boston: Society for Healthcare Epidemiology of America; 2019. [Google Scholar]
- 24. Vaughn VM, Petty LA, Flanders SA, et al. . A deeper dive into antibiotic stewardship needs: a multihospital survey. Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spellberg B. The new antibiotic mantra—“shorter is better.” JAMA Intern Med 2016; 176:1254–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention. Data summary of HAIs in the US: assessing progress 2006-2016. Available at: https://www.cdc.gov/hai/data/archive/data-summary-assessing-progress.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fhai%2Fsurveillance%2Fdata-reports%2Fdata-summary-assessing-progress.html. Accessed 1 October 2019.
- 27. Manges K, Groves PS, Farag A, Peterson R, Harton J, Greysen SR. A mixed methods study examining teamwork shared mental models of interprofessional teams during hospital discharge. BMJ Qual Saf. In press. [DOI] [PubMed] [Google Scholar]
- 28. Fridkin S, Baggs J, Fagan R, et al. . Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014; 63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 29. Metlay JP, Waterer GW, Long AC, et al. . Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Steinman MA, Kaplan CM. Geographic variation in outpatient antibiotic prescribing among older adults. Arch Intern Med 2012; 172:1465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yogo N, Shihadeh K, Young H, et al. . Intervention to reduce broad-spectrum antibiotics and treatment durations prescribed at the time of hospital discharge: a novel stewardship approach. Infect Control Hosp Epidemiol 2017; 38:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maust DT, Lin LA, Blow FC, Marcus SC. County and physician variation in benzodiazepine prescribing to Medicare beneficiaries by primary care physicians in the USA. J Gen Intern Med 2018; 33:2180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Y, Baicker K, Newhouse JP. Geographic variation in the quality of prescribing. N Engl J Med 2010; 363:1985–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.