Abstract
Background
Overfeeding is associated with obesity and insulin dysregulation (ID), which are both risk factors for equine metabolic syndrome. How chronic overfeeding affects development of these factors is poorly understood.
Objectives
To examine the influence of long‐term high‐energy diet provision on body condition and ID.
Animals
Eleven Shetland pony mares.
Methods
In a 3‐phase study, the high‐energy group (n = 7) was fed 200% of net energy (NE) requirements (hay; concentrate: 36% sugar and starch, 13% fat) for 24 weeks, followed by 17 weeks hay‐only feeding before resuming the high‐energy diet (n = 4) for an additional 29 weeks. Mares were weighed weekly. Oral glucose tolerance tests were performed 3 to 4 times per dietary period. Results were compared with those of a control group (phase 1, n = 4; phases 2 and 3, n = 6) that received 100% NE requirements, using a general linear mixed model with post hoc Bonferroni testing.
Results
The mean body weight of the high‐energy group increased by 27% per high‐energy feeding period. During both feeding periods, area under the curve (AUC) for plasma glucose concentration decreased (P < .01), whereas AUC for plasma insulin concentration increased. Mean basal plasma glucose concentration and peak plasma insulin concentrations were higher (P < .05) in the high‐energy group than in the control group.
Conclusion and Clinical Importance
Feeding a high‐energy diet to healthy nonobese Shetland pony mares led to more efficient glucose metabolism within 5 weeks, followed by significant hyperinsulinemia and obesity. Hyperinsulinemic status was reversed during 17 weeks of hay‐only feeding, regardless of body condition, but returned rapidly after restarting the high‐energy diet.
Keywords: glucose metabolism, horse, insulin dysregulation, obesity
Abbreviations
- BCS
body condition score
- BW
body weight
- CAC
Coat‐a‐Count
- CLIA
chemiluminescence immunometric assay
- DM
dry matter
- EMS
equine metabolic syndrome
- FSIGTT
frequently sampled intravenous glucose tolerance test
- HE
high energy
- ID
insulin dysregulation
- IR
insulin resistance
- NE
net energy
- OGTT
oral glucose tolerance test
- RIA
radioimmunoassay
1. INTRODUCTION
Insulin dysregulation (ID) is considered the central feature of equine metabolic syndrome (EMS).1, 2 Overfeeding and obesity are assumed to be contributory factors in the development or exacerbation of ID, but the relationship between chronic overfeeding and the development of ID and obesity is not completely understood. In addition to an association of obesity with ID, ID may contribute to the development of obesity.3, 4 What is known about the development of ID in horses is based exclusively on short‐term follow‐up studies examining the influence of overfeeding, changes in dietary content, or both on ID status.
Recent studies showed that when energy intake matched requirements, nonobese Standardbred horses (body condition score [BCS] < 7/9) that consumed a sugar‐ and starch‐rich concentrate feed for 6 weeks became more prone to ID, whereas horses that consumed a concentrate with high concentrations of fat (14%) did not. 5 Increasing energy intake to twice the energy requirement, thereby inducing weight gain (up to 20%), led to a similar outcome in initially nonobese horses (BCS < 7/9). Thus, ID increased in horses fed a concentrate rich in sugar and starch, 6 but not in those fed a fat‐rich diet.7, 8, 9 These findings suggest that a limited amount of weight gain alone does not trigger ID.
In addition, the effect of dietary energy source on the development of ID seems to be less important when horses are already obese (BCS ≥7/9) and showing ID. No distinct effect of dietary changes (from forage only to forage plus supplements rich in starch and sugar or fiber and fat) on ID over an 8‐week period were found in groups of moderately obese and obese Thoroughbred horses, 10 but the nonobese group seemed to show the most marked changes in ID on the starch and sugar‐rich diet.
In contrast, horses and ponies consuming a ration containing a once daily glucose load (1.5 g/kg body weight [BW]) exhibited improved insulin sensitivity, despite becoming obese. 9 Conversely twice daily cereal‐rich meals (nonstructural carbohydrates, 3.34 g/kg) decreased insulin sensitivity, 11 indicating a possible difference in effect of glycemic source or glycemic load on ID status. Moreover, postprandial insulin but not glucose responses to sugar‐ and starch‐rich meals also are influenced markedly by breed, with Andalusian and mixed breed ponies showing a more exaggerated response than Standardbred horses. 12
The objective of our 2‐year study was to determine the influence of a long‐term high‐energy (HE) diet on the development of ID and obesity in a group of young Shetland pony mares. It was hypothesized that ID would appear soon after initiating the diet, and that prolonged feeding of the diet would lead to progression of ID. Body weight was expected to increase gradually over time and it was assumed that once mares were obese, a change to a roughage‐only diet matching the energy requirements would not affect the established ID status, as measured by an oral glucose tolerance test (OGTT).
2. MATERIALS AND METHODS
2.1. Animals and experimental design
To investigate the effect of long‐term provision of an HE diet on ID and BW increase, a group of Shetland pony mares was studied over a 2‐year period. This breed was chosen because of the breed predisposition to developing ID. 11 All ponies were quarantined on the premises and fed a hay‐only diet for at least 4 weeks before entering the study. Eleven Shetland pony mares (7 HE ponies [mean ± SD age, 4.6 ± 1.6 years] and 4 control ponies [mean ± SD age, 4.5 ± 1.3 years]) entered the study in 2014. All mares had moderate BCS (range, 4‐6/9) at the onset of the study. 13 The HE group received a diet containing 200% of energy required for maintenance to induce weight gain, as previously described 6 with minor modifications (ie, the previous study used a feed evaluation system based on digestible energy). In our study, the control group was fed 100% of net energy (NE) requirements advised by the Centraal Veevoederbureau (approximately 0.348 megajoule NE × BW0.75). 14 The energy intake for the HE group was adapted to account for weight gain throughout the study (ie, concentrate ratios were recalculated weekly based on current BW). All mares received their diet for 24 weeks in 2014, followed by a hay‐only period of 17 weeks during the winter. The HE diet was resumed for an additional 29 weeks in 2015, during which 4/7 HE ponies and 6 control ponies (3/4 ponies continued; 3 new ponies added to the study [mean ± SD age, 5.3 ± 2.3 years]) were monitored. Only mares were used in this study, because it was part of a larger study examining the effects of obesity on embryonic development. Replacement of mares was related to the primary (reproductive) goal. Routine foot care, vaccination, and anthelmintic treatments were performed as required, and rectal temperature, heart rate, and gait were monitored daily to assess general health. The experimental protocol was approved by the Committee for Animal Welfare at Utrecht University.
2.2. Diet
The composition of the ration has been described previously. 15 In brief, the diet of both the control and HE groups consisted of a concentrate feed (36% sugar and starch, 13% fat), grass hay (9% sugar, 2% fat) and a feed supplement to ensure adequate provision of minerals, trace elements, and vitamins (Pavo Vital Complete; Pavo, Boxmeer, the Netherlands). The macronutrient composition of the concentrate and hay is presented in Table 1. Control ponies received 85% of NE intake in the form of hay, and the remaining 15% as concentrate. Ponies allocated to the HE group received 42.5% of NE intake in the form of hay and the remaining 57.5% as concentrate. All ponies received >1 kg dry matter (DM) forage per 100 kg BW per day. Net energy content of the hay was analyzed (BLGG AgroXpertus, Wageningen, the Netherlands) and hay intake was adapted to maintain similar energy partitioning throughout the study. The concentrate and hay were fed in the form of multiple meals at 08:00, 13:00, and 17:00 hours. In addition, all ponies had free access to water and a salt lick (KNZ; Hengelo, the Netherlands). During the first year (2014), control ponies were housed as a group but separated at mealtimes so that they could be fed individually, whereas HE ponies were housed and fed individually. Both groups were bedded on wood shavings. In between the experimental periods of 2014 and 2015 (winter 2014‐2015), all ponies were housed together as a group, bedded on straw, and supplied a hay‐only diet of at least 100% NE requirements. During the second year (2015), all ponies were housed and fed individually and bedded on shavings. Groups were allowed access to a sand paddock every other day to enable social contact and limited exercise during the dietary periods.
TABLE 1.
Macronutrient composition of the concentrate (PAVO, Heijen, the Netherlands) and hay (analysis provided by BLGG, AgroXpertus) fed to Shetland pony mares, as described by Siegers et al 15
| Item | Concentrate composition (g/kg) (as‐fed) | Hay |
|---|---|---|
| DM (g/kg) | 890 | 902 |
| Ash (g/kg DM) | 66 | 62 |
| CP (g/kg DM) | 117 | 70 |
| Crude fat (g/kg DM) | 127 | 16 |
| Starch (g/kg DM) | 330 | ‐ |
| Sugars (g/kg DM) | 31 | 92 |
| Crude fiber (g/kg DM) | 86.1 | 318 |
2.3. Measurements of adiposity
The BW of each pony was measured weekly during the dietary periods in both years, using a calibrated weighing scale (Epelsa BCN100M; Grupo Epelsa, Madrid, Spain). The BCS of each pony was graded by a single evaluator at the start and end of both study years, using a 9‐point scale. 13 Ultrasonographic fat depth measurements were performed on some of the ponies and were reported previously. 15
2.4. Oral glucose tolerance test
Oral glucose tolerance tests were performed in at least 1 control and 1 HE pony on the same day during anestrus or, when the ponies were cycling, diestrus (days 7‐12 after ovulation) to minimize any effects of reproductive steroid balance. Five OGTTs were performed per pony in 2014, referred to as periods 0 to 4 (Table 2). Four OGTTs were performed per pony in 2015 and referred to as periods 5 to 8 (Table 2). Before an OGTT, the ponies were fasted overnight (between 2400 and 0800). A catheter (Teflon catheter, 12G) was placed in the jugular vein for blood collection, after which 1 g/kg BW glucose monohydrate, dissolved in 2 L of water, was administered by nasogastric tube. Blood samples were collected in 2 4‐mL tubes (a sodium heparin and a sodium fluoride tube) at the start and at t = 30, 60, 90, 120, 180, 240, and 300 minutes after PO glucose administration. Plasma glucose concentrations were measured immediately after blood collection (AU680 Chemistry Analyzer, Beckman Coulter, Woerden, the Netherlands). The heparinized tubes were centrifuged at 3000g for 10 minutes and the plasma was separated and frozen at −20°C until analysis. Plasma insulin concentrations were determined in samples at onset and t = 30, 60, 90, and 120 minutes after glucose administration.
TABLE 2.
Test periods per study year with corresponding number of weeks that Shetland pony mares were fed twice the energy requirements or a control diet. In each period, one oral glucose tolerance test (OGTT) was performed per pony
| Study year | Dietary period | OGTT | Dietary weeks |
|---|---|---|---|
| 2014 | Period 0 | 0 | 0 |
| Period 1 | 1 | 5‐8 | |
| Period 2 | 2 | 10‐12 | |
| Period 3 | 3 | 17‐18 | |
| Period 4 | 4 | 22‐23 | |
| Hay‐only period (100% NE) | ‐ | ‐ | |
| 2015 | Period 5 | 0 | 0 |
| Period 6 | 1 | 9‐13 | |
| Period 7 | 2 | 20‐23 | |
| Period 8 | 3 | 27‐29 |
2.5. Laboratory measurements
In 2014, insulin concentrations were measured using a commercial radioimmunoassay (RIA; Coat‐a‐Count [CAC]: Siemens Diagnostic Products Corp, Los Angeles, California) validated for equine plasma. 16 Because the RIA kit was no longer available in 2015, plasma insulin concentrations were determined using a new commercially available solid‐phase enzyme‐labeled chemiluminescence immunometric assay (CLIA), designed for measuring human insulin (Immulite 2000 Insulin, Siemens Diagnostic Products Corp). The CLIA was validated using samples of equine plasma from year 1 (2014) stored at −20°C, and results were compared to RIA (CAC insulin) results by constructing linearity plots. The relationship between the 2 assays was determined by linear regression, yielding the following formula: CLIA = 0.76 × CAC (R 2 = .98). Although the formula was calculated, it was decided to present data as RIA insulin measurements in year 1 and CLIA insulin measurements in year 2, because the results indicated the absence of a simple linear correlation between CLIA and RIA measurements, as was confirmed in a study that reported fixed and proportional bias in different ranges of insulin concentrations measured by CLIA as compared with RIA. 17
3. STATISTICS
Statistical analysis was performed using the IBM SPSS software (SPSS 22‐24; Quarry Bay, Hong Kong). For all data, normal distribution of the residuals was confirmed using the Shapiro‐Wilk's test or by visual inspection of the Q‐Q plot. To compare the insulin response to glucose and glucose disposal between control and HE ponies, the area under the curve (AUC) was calculated for the glucose and insulin OGTT curves for both groups by the trapezoidal method, using the following formula:
The AUC data for the control and HE groups in each period were analyzed using a generalized linear mixed model with period, group and the interaction between period and group as fixed effects, and individual pony as a random effect. Pairwise comparisons were performed and a post hoc Bonferroni correction was applied by dividing the alpha of P < .05 by the number of pairwise comparisons. The OGTT glucose and insulin curves for both years are shown in Figures 1 and 2 to illustrate the change in curves over time and the difference in AUC between groups. Further description of the curves is presented as basal and peak plasma glucose concentrations and time to reach peak glucose concentration, and basal and peak plasma insulin concentrations and time to reach peak insulin concentration.
FIGURE 1.
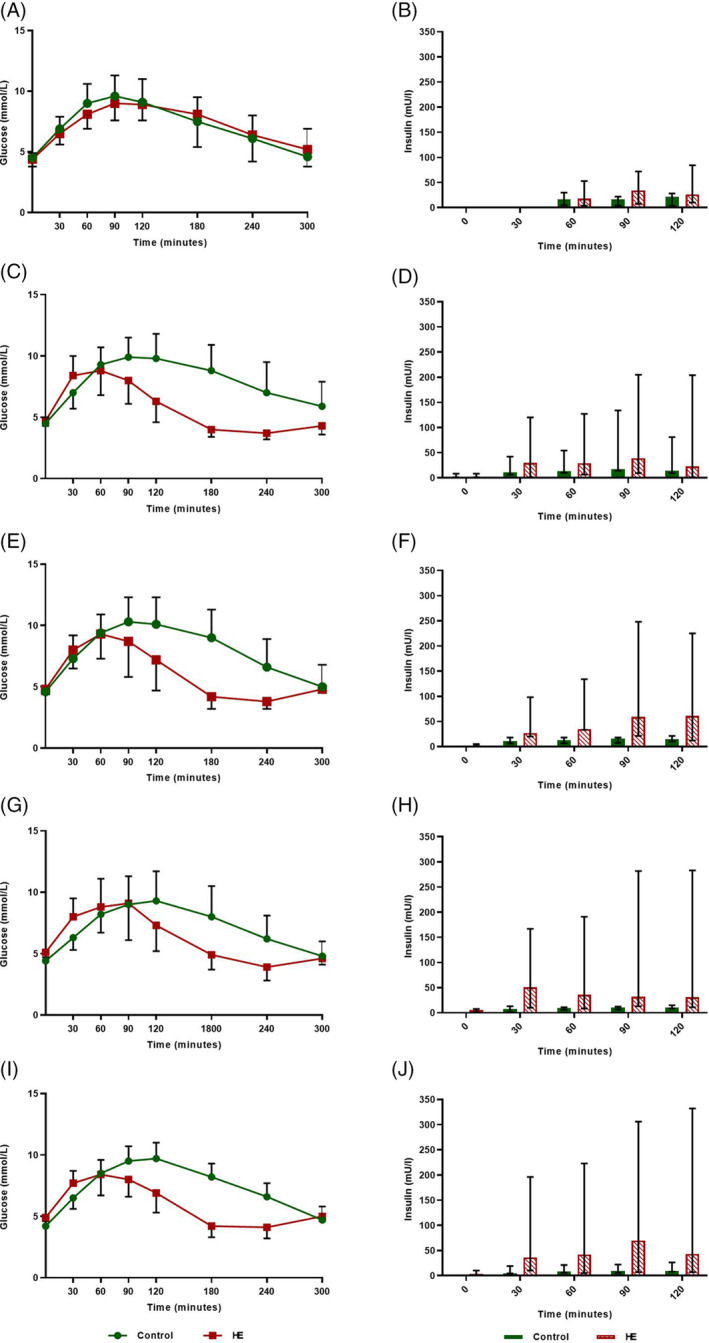
Mean oral glucose tolerance test (OGTT) plasma glucose (SD) and median insulin concentrations (range) for the 4 control (maintenance diet) and 7 high‐energy (HE) Shetland pony mares on a 200% energy requirement diet for 24 weeks (2014). Period 2: n = 5 HE ponies. A,B, period 0; C,D, period 1; E,F, period 2; G,H, period 3; I,J, period 4
FIGURE 2.
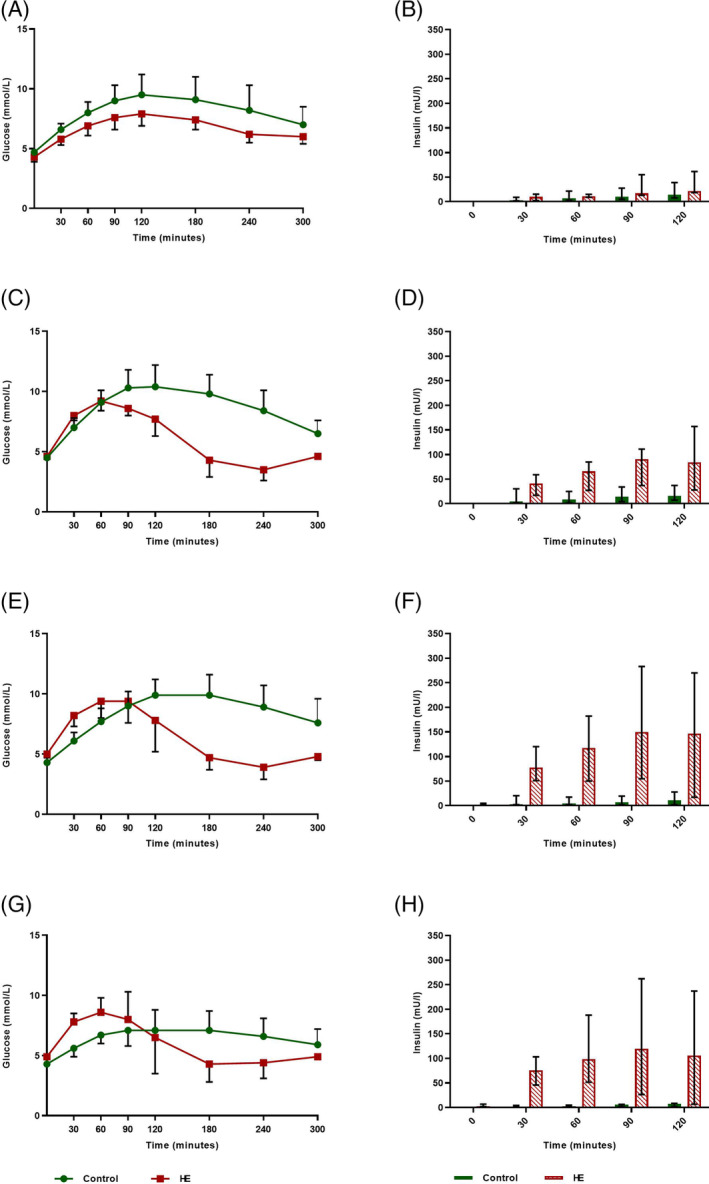
Mean oral glucose tolerance test (OGTT) plasma glucose (SD) and median insulin concentrations (range) for the 6 control (maintenance diet) and 4 high‐energy (HE) Shetland pony mares on a 200% energy requirement diet for 29 weeks (2015). Period 8: n = 3 control ponies. A,B, period 5; C,D, period 6; E,F, period 7; G,H, period 8
An independent samples t test was used to compare basal and peak plasma glucose concentrations and BW between treatment groups. Normally distributed data are presented as mean ± SD (ie, AUC glucose years 1 and 2, basal plasma glucose concentration years 1 and 2, peak plasma glucose concentration years 1 and 2, BW). A log10 transformation was applied to data that were not normally distributed, before analysis, so that data would meet the assumption of normality (AUC insulin years 1 and 2) and data were presented as median (range) values. Data that remained nonnormally distributed after transformation were analyzed using Mann‐Whitney nonparametric tests and presented as median (range) values (basal and peak plasma insulin concentrations). Values of P < .05 were considered significant unless stated otherwise.
4. RESULTS
The diet was tolerated well by all overfed ponies, which consumed their full ration of concentrates. Some hay was left on occasion. After 2 years of consuming the HE diet, all overfed ponies moved slightly stiffly and, on a firm floor, walked with short strides increasing suspicion of subclinical laminitis, which was evaluated further as described previously. 18
4.1. Measurements of adiposity
Mean BCS (±SD) of the HE group increased by 3 units from BCS 5 ± 1 to 8 ± 1 in 2014, and by 2 units from BCS 7 ± 1 to 9 ± 0 in 2015. Mean BCS of the control group decreased by 1 unit from BCS 4 ± 1 to 3 ± 1 in 2014, and did not change in 2015 (mean BCS, 4 ± 1). Mean (±SD) BW of each group is presented per year and per period in Figure 3. The mean percentage increase in BW for the control and HE groups is shown in Table 3, and presented per year for each OGTT. The HE group showed a mean total increase in BW of >27% for each of the overfeeding periods. Mean BW of the 4 HE ponies that received the diet in 2 periods increased by 86 kg (range, 73‐100 kg) equating to 51% (range, 44%‐59%) total BW increase. Mean (±SD) BW of the 4 HE ponies before and after the hay‐only period did not decrease significantly (215 ± 42 versus 203 ± 43 kg, respectively; P = .07). Similarly, the 3 control ponies did not show a change in BW (P = .41) over the hay‐only period (162 ± 27 kg before versus 161 ± 23 kg after).
FIGURE 3.
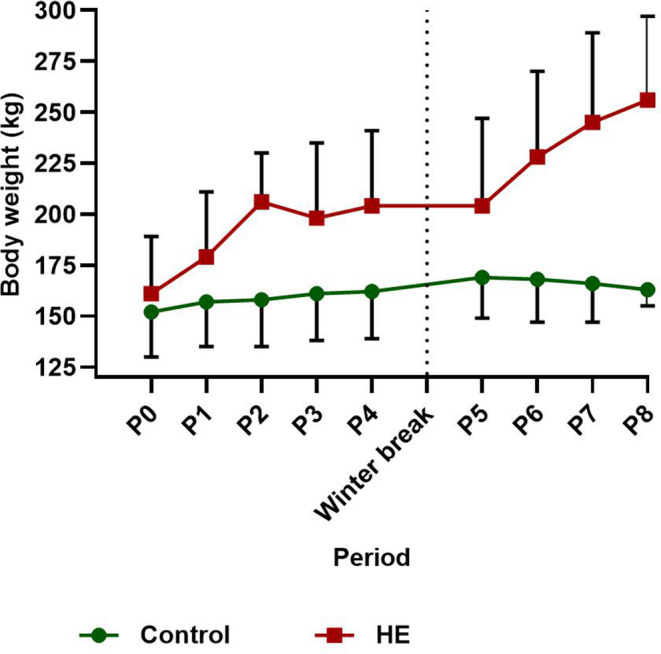
Mean ± SD body weight for the control and high‐energy (HE) group per dietary period for year 1 (control n = 4, HE n = 7; periods 0‐4) and year 2 (control n = 6, HE n = 4; periods 5‐8) with a hay‐only winter break in between
TABLE 3.
Mean percentage increase in body weight (BW) per oral glucose tolerance test (OGTT) of Shetland pony mares on a control or high‐energy (HE; 200% of energy requirements) diet for 24 weeks (2014), 17 weeks hay‐only (winter break 2014‐2015), and another 29 weeks control or HE diet (2015)
| Mean ± SD % change in BW | ||||
|---|---|---|---|---|
| Study year | Dietary period (weeks) | OGTT | Control group | HE group |
| 2014 | Period 0 (0) | 0 | 0 | 0 |
| Period 1 (5‐8) | 1 | 3.5 ± 4 | 12.4 ± 3.9 | |
| Period 2 (10‐12) | 2 | 5 ± .8 | 17.7 ± 2.6 | |
| Period 3 (17‐18) | 3 | 5.5 ± 1.3 | 23 ± 3.3 | |
| Period 4 (22‐23) | 4 | 6.2 ± 2.1 | 27.8 ± 3.6 | |
| 2015 | Period 5 (0) | 0 | 0 | 0 |
| Period 6 (9‐13) | 1 | −1.2 ± 3.4 | 12.8 ± 4.9 | |
| Period 7 (20‐23) | 2 | −2.5 ± 1 | 22.2 ± 7.4 | |
| Period 8 (27‐29) | 3 | −1.4 ± 4.6 | 27.2 ± 8.8 | |
Note: Percentage change in BW is presented per year and per period, relative to initial BW at the start of each year.
4.2. Oral glucose tolerance test
4.2.1. Year 1—2014
Results for t = 30 minutes were not collected for period 0, and OGTT data for 2 HE ponies were missing for period 2. Significant group (P = .03) and group × period effects (P = .001) were found for AUC glucose. After Bonferroni correction, P < .01 was used to indicate statistical significance. Pairwise comparison indicated a significantly smaller AUC glucose levels for the HE group in periods 1 (P = .002) and 2 (P = .006) compared with the control group (Figure 1C,E,I; Table 4). Mean results for basal and peak plasma glucose concentrations and time to reach peak glucose concentrations are presented in Table 4. All basal plasma glucose concentrations fell into the normoglycemic range (ie, 3.9‐5.6 mmol/L). The HE group experienced a significant increase in mean basal plasma glucose concentration compared to the control group in periods 3 (P = .02) and 4 (P = .001). No significant difference in peak plasma glucose concentration was found between the groups.
TABLE 4.
Glucose parameters of oral glucose tolerance tests (OGTTs) performed in Shetland pony mares on a control or high‐energy (HE; 200% of energy requirements) diet for 24 weeks (2014), 17 weeks hay‐only (winter break 2014‐2015), and another 29 weeks control or HE diet (2015)
| Mean basal plasma glucose concentration (mmol/L) (SD) | Mean peak plasma glucose concentration (mmol/L) (SD) | Median time of peak glucose concentration (range) | Mean (SD) area under the curve for glucose (mmol/L × min) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2014 | Dietary period | Control | HE | Control | HE | Control | HE | Control | HE |
| OGTT 0 | 0 | 4.5 (.42) | 4.4 (.59) | 9.6 (1.6) | 9.2 (1.2) | 90 (60‐90) | 90 (90‐180) | 2197 (424) | 2194 (344) |
| OGTT 1 | 1 | 4.5 (.48) | 4.7 (.40) | 10.3 (1.7) | 9.0 (1.8) | 105 (60‐120) | 60 (30‐90) | 2419 (488)g | 1705 (247)h |
| OGTT 2 | 2 | 4.6 (.34) | 4.8 (.40) | 10.4 (2.1) | 9.5 (2.1) | 105 (60‐120) | 60 (60‐90) | 2456 (594)i | 1799 (300)j |
| OGTT 3 | 3 | 4.4 (.33)a | 5.1 (.60)b | 9.5 (2.0) | 9.9 (2.6) | 120 (60‐120) | 60 (30‐90) | 2181 (528) | 1823 (317) |
| OGTT 4 | 4 | 4.2 (.17)c | 4.9 (.30)d | 9.8 (1.3) | 8.7 (1.4) | 120 (90‐120) | 60 (30‐90) | 2261 (245) | 1754 (182) |
| 2015 | |||||||||
| OGTT 0 | 5 | 4.7 (.23) | 4.3 (.41) | 9.6 (1.34) | 7.9 (.98) | 120 (90‐180) | 120 (60‐120) | 2445 (417) | 2026 (195) |
| OGTT 1 | 6 | 4.5 (.20) | 4.6 (.09) | 10.5 (1.3) | 9.3 (.62) | 120 (90‐180) | 75 (60‐90) | 2613 (386) | 1793 (92) |
| OGTT 2 | 7 | 4.3 (.24)e | 5.0 (.30)f | 10.3 (1.3) | 9.7 (1.54) | 120 (90‐180) | 75 (60‐90) | 2552 (390) | 1889 (196) |
| OGTT 3 | 8 | 4.3 (0) | 4.9 (0) | 7.3 (1.8) | 8.8 (1.52) | 120 (90‐120) | 75 (60‐90) | 1994 (422) | 1760 (245) |
Note: 2014: 4 control, 7 HE ponies (period 2, n = 5 HE); 2015: 6 control, 4 HE ponies (period 8, n = 3 control). Different superscripts within the same row indicate significance at P < .05; ab P = .02; cd P = .001; ef P = .02; gh P = .002; ij P = .006.
Significant group × period effects (P = .001) were found for AUC insulin measured by RIA. After Bonferroni correction, P < .01 was used to indicate statistical significance. Pairwise comparison indicated a significantly larger AUC insulin for the HE group in periods 3 (P = .008), and 4 (P = .007) compared to the control group (Figure 1F,H,J; Table 5). Median basal and peak plasma insulin concentrations and time to reach peak insulin concentrations are presented in Table 5. The HE group experienced a significant increase in peak plasma insulin concentrations in periods 2 (P = .02), 3 (P = .01), and 4 (P = .04), compared with controls.
TABLE 5.
Insulin parameters of oral glucose tolerance tests (OGTTs) performed in Shetland pony mares on a control or high‐energy (HE; 200% of energy requirements) diet for 24 weeks (2014), 17 weeks hay‐only (winter break 2014‐2015), and another 29 weeks control or HE diet (2015)
| Median basal plasma insulin concentration (mU/L) (range) | Median peak plasma insulin concentration (mU/L) (range) | Median time of peak insulin concentration (min) (range) | Median area under the curve for insulin (mU/L × min) (range) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Year 1 (RIA) | Dietary period | Control | HE | Control | HE | Control | HE | Control | HE |
| OGTT 0 | 0 | 1 (0) | 1 (0) | 25.5 (4.9‐30) | 34 (10‐84) | 90 (60‐120) | 120 (90‐120) | 1822 (405‐2013) | 2310 (577‐5835) |
| OGTT 1 | 1 | 1 (1‐8.3) | 1 (1‐8.4) | 17.5 (14‐134) | 39 (12‐205) | 90 (90) | 90 (60‐120) | 1448 (1122‐8239) | 3300 (699‐16 746) |
| OGTT 2 | 2 | 1 (0) | 1 (1‐4.8) | 16 (10‐21)a | 64 (21‐248)b | 120 (90‐120) | 90 (60‐120) | 1405 (756‐1950) | 4590 (2040‐17 847) |
| OGTT 3 | 3 | 1 (0) | 2.5 (1‐7.4) | 11.5 (8.7‐15)c | 51 (15‐283)d | 120 (90‐120) | 90 (30‐120) | 857 (772‐1320)o | 3690 (1860‐23 556)p |
| OGTT 4 | 4 | 1 (0) | 3.7 (1‐10) | 10.2 (8.8‐26)e | 70 (10‐332)f | 120 (90‐120) | 90 (30‐120) | 816 (606‐2265)q | 5595 (778‐26 854)r |
| Year 2 (CLIA) | |||||||||
| OGTT 0 | 5 | 2 (0) | 2 (0) | 13.8 (7.7‐39) | 21.7 (18.6‐61.3) | 120 (120) | 120 (120) | 820 (439‐2916) | 1372 (960‐3405) |
| OGTT 1 | 6 | 2 (0) | 2 (0) | 16.2 (7.1‐37.2)g | 94.7 (36.9‐157)h | 120 (120) | 105 (90‐120) | 1103 (376‐3264)u | 7177 (2805‐9975)v |
| OGTT 2 | 7 | 2 (0) | 2.1 (2‐4.7) | 11.0 (2‐27.8)i | 153.4 (55.5‐283)j | 120 (120) | 105 (90‐120) | 858 (240‐2160)u | 12 247 (5505‐21 630)v |
| OGTT 3 | 8 | 2 (0) | 2.5 (2‐6.9) | 7.7 (2‐8.4)k | 140.4 (51.6‐262)l | 120 (120) | 75 (60‐120) | 495 (240‐586)u | 10 147 (4320‐20 175)v |
Note: 2014:4 control, 7 HE ponies (period 2, n = 5 HE); 2015:6 control, 4 HE ponies (period 8, n = 3 control). Different superscripts within the same row indicate significance ab P = .02; cd P = .01; ef P = .04; gh P = .02; ij P = .01; kl P = .03; op P = .008; qr P = .007; uv P = <.001.
4.2.2. Year 2—2015
The OGTT data for 3 control ponies are missing for period 8. Significant group (P = .002) and period (P = .04) effects were found for AUC glucose in year 2, but no significant interaction was found (Figure 2; Table 4). The HE group experienced a significant increase in basal plasma glucose concentration in period 7 (P = .02) compared with the control group (Table 4).
Significant group (P < .001), period (P < .001), and group × period (P < .001) effects were found for AUC insulin measured by CLIA. After Bonferroni correction, P < .01 was used to indicate statistical significance. Pairwise comparison identified a significantly larger AUC for the HE group for periods 6, 7, and 8 (P < .001) compared with the control group (Figure 2B,D,F,H; Table 5). The HE group experienced a significant increase in peak plasma insulin concentration, compared with the control group, during periods 6 (P = .02), 7 (P = .01), and 8 (P = .03; Table 5). Time of peak insulin concentration for the HE group was earlier than in controls during periods 6, 7, and 8.
5. DISCUSSION
Our aim was to determine the effect of long‐term provision of an HE diet on the development of ID and obesity in Shetland pony mares. It was hypothesized that ID would appear soon after mares were started on the HE concentrate feed and become progressively more severe over time. Body weight was predicted to increase gradually over time, and it was assumed that, once an animal was obese, a change to a roughage‐only diet fed to meet maintenance energy intake, would not affect the ID status. In the HE group, consuming an HE concentrate diet led to more efficient glucose metabolism (smaller AUC for glucose), followed by an increase in the insulin response to PO glucose (larger AUC for insulin, higher peak insulin) only after weight gain had increased to the point of obesity (BCS ≥7/9). Prolongation of the diet further increased the hyperinsulinemic response to glucose, whereas the OGTT response remained the same. A 17‐week hay‐only period did not result in significant weight loss but did normalize the OGTT‐related insulin response curve (ie, it became comparable to that of the control group), indicating that adaptation to diet played a more substantial role in development of ID than body condition itself.
At the start of the study, all ponies had a normal OGTT response, as defined by a peak plasma glucose concentration 2 hours after administration of PO glucose that returned to resting concentrations 4 hours later, 19 without a marked hyperinsulinemic response. 20 After 5 to 8 weeks of overfeeding (period 1—2014), the OGTT curve for the HE group changed to a smaller AUC for glucose and an earlier plasma glucose concentration peak. Over time, a significantly higher peak plasma insulin concentration (period 2—2014) occurred and eventually an increased insulin AUC developed after 17 to 18 weeks of overfeeding (period 3—2014). This diet‐induced change in the OGTT curve might indicate improved glucose uptake, which is similar to findings of previous studies in ponies 21 and probably relates to upregulated expression of intestinal sodium‐glucose cotransporters (SGLT1) and increased capacity of the gut to absorb glucose in response to increased dietary carbohydrates, as well as increased activity of the enteroinsular axis.22, 23 To our knowledge, no studies in horses are available on the reversibility of upregulated SGLT1‐expression. In our study, a switch to a hay‐only diet for 17 weeks seemed to slow glucose uptake in the HE group, as shown by a delay in reaching peak plasma glucose concentration (period 5—2015; median time to reach peak glucose concentration, 120 min) compared to the end of the first dietary period (period 4—2014; median time to reach peak glucose concentration, 60 minutes). This finding might indicate adaptation, but measurement of SGLT1 expression was not performed and other factors also might have played a role.
After restarting the HE diet in year 2, diet‐induced adaptation toward an earlier OGTT plasma glucose concentration peak recurred after 9 to 13 weeks of overfeeding (period 6—2015), as evidenced by a mean peak glucose concentration time of 75 minutes in the HE group, as compared to 120 minutes in the control group (Table 4). However, this adaptation was not reflected by a smaller AUC for glucose in the HE group compared to the control group (no significant period × group interaction), as had been seen in year 1. The absence of changes in AUC for glucose in year 2 might be a consequence of missing data for 3 control ponies during period 8—2015, or might be influenced by the fact that the AUC for glucose in the HE group did not return to preoverfeeding values during the hay‐only period between year 1 and 2. This latter possibility is supported by the fact that the main factor “group” had a significant effect in year 2, indicating an overall difference between groups, independent of period.
Hyperinsulinemia has been widely considered to represent compensation for systemic insulin resistance (IR), but evidence suggests that hyperinsulinemia precedes IR or occurs independently of IR.3, 4 In our study, it was not clear whether the hyperinsulinemic response to glucose in the HE group occurred because of tissue resistance to insulin, decreased insulin clearance, or increased secretion based on gastro‐enteral etiology (ie, enteroinsular axis), 4 because no IV glucose tolerance tests were performed. However, the improved glucose response seems to indicate that the onset of hyperinsulinemia was not related to tissue resistance to insulin. Diet‐induced improvement in insulin sensitivity has been documented previously in horses and ponies fed starch‐rich, sugar‐rich, or both diets.9, 24, 25 A previous study showed that horses and ponies consuming a ration containing a once daily glycemic load for 12 weeks had improved insulin sensitivity based on a frequently sampled intravenous glucose tolerance test (FSIGTT), despite induction of obesity. 9 However, no changes were found in postprandial insulin and glucose responses. 9 Recently, another study showed that 7 weeks of adaptation to a starch‐ or sugar‐rich diet improved insulin sensitivity at the peripheral tissue level during an FSIGTT, but enhanced postprandial hyperinsulinemia after a dietary meal challenge in healthy nonobese adult and aged Thoroughbred and Standardbred horses. 26 However, OGTT (in the form of Karo light syrup) results were not altered by dietary adaptation and it was questioned whether the Karo light syrup test was sensitive enough to detect small changes in healthy, nonobese horses. Moreover, the amount of sugar in the diet was much higher (2 g/kg BW) than in the syrup (Karo light, 0.25 mL/kg BW) and also higher than in our study (1 g/kg). In our study, a significant hyperinsulinemic response to glucose (peak insulin concentration) was seen after 10 to 12 weeks of overfeeding (period 2—2014), which is later than the 7‐week study period reported previously, 26 possibly confirming that it takes time to develop a hyperinsulinemic response that can be detected by an OGTT and that the amount of sugar administered is relevant for detecting small changes. The insulin response during the OGTT corresponded with the glucose response curves, in particular the ranking of peak times between the glucose and insulin curves was consistent within groups and over time. Shifts in the curves occurred in the same direction for both glucose and insulin, whereby peak insulin concentrations consistently occurred slightly later than peak glucose concentrations. However, whereas mean peak plasma glucose concentration and AUC for glucose for the HE group remained the same, mean peak insulin concentration increased over time, possibly indicating downregulation of the insulin receptor and downstream signaling. 4
Impaired glucose tolerance, evidenced by a delay to peak glucose concentrations and failure to return to pretest concentrations, 27 along with increased insulin concentrations, did not occur during our study. However, basal glucose concentrations in the HE group were higher at the end of year 1 (periods 3 and 4—2014) than in control animals. According to the definition of ID (insulin concentrations >80 mU/L during an OGTT), 3 of 4 HE ponies exhibited ID in year 2. 3 This definition is only applicable to insulin concentrations measured by CLIA, and therefore no conclusions can be drawn for year 1.
In our study, consuming a ration consisting of an energy‐dense concentrate (36% sugar and starch, 13% fat) and hay resulted in a gradual but continuous increase in BW, with total weight gain reaching 27% of starting weight, during both overfeeding periods (years 1 and 2) in the HE Shetland pony mares (Table 3). This result is similar to the reported 20% increase in BW of Arabian horses that were fed an energy‐dense concentrate diet (35% sugar and starch, 5.5% fat) over a 16‐week period at a feeding level set at twice the energy requirement. 6 Offering a diet that contained twice the amount of energy required did not lead to self‐regulation of energy intake in the initially nonobese HE ponies at the onset of our study, as has been described previously in mice. 28 At the beginning of our study, the HE ponies were not obese but nevertheless showed no self‐regulation of energy intake, before developing obesity (BCS, 8‐9/9). Failure to regulate caloric intake could be explained by the high amount of sugar present in the diet and the preference of horses for hydrolyzable carbohydrate and protein‐rich diets over lipid‐rich diets, 29 which may have stimulated consumption of the concentrate. The decrease in mean BCS of the control group by 1 unit during year 1, despite a mean ± SD increase in BW of 6.2% ± 2.1%, could be explained by growth in the young group of ponies and possibly loss of muscle mass because of decreased exercise during the experimental period. A previous study reported measuring fat depths SC and retroperitoneally in some of the ponies used in our study, and found no significant changes in ultrasound detectable fat reserves in year 1, suggesting that the decrease in BCS in control ponies might not be related to fat loss alone. 15 However, the first measurements in that study were performed 12 weeks after starting the diet, and it possible that some fat loss preceded this first measurement.
Altering the diet of ponies fed twice the energy requirement, provided by an energy‐dense concentrate feed, to ad libitum roughage for 17 weeks did not result in significant weight loss (P = .07). Mean ± SD BCS of the HE group did, however, decrease by 1 unit from BCS 8 ± 1 to 7 ± 1 over the winter period. A previous study reported a decrease in withers and axillary fat thickness in the HE group over the winter period. 15 However, retroperitoneal fat depth did not decrease appreciably. The decrease in BCS, regardless of constant BW, also could be explained by growth of the young ponies. Another explanation for the decrease in BCS could be an increase in energy expenditure, because of environmental changes from individual to group housing. Group dynamics, such as playing, could have led to an increase in exercise. A decrease in consumption of roughage is a third possibility. Obesity, especially intra‐abdominal fat, can constrain gut capacity thereby limiting the DM intake of HE ponies on an ad libitum hay diet. 30
After the hay‐only period between years 1 and 2, the OGTT response curve in the HE group had changed to become more comparable in shape to that of the control group (ie, basal plasma glucose concentrations had decreased and the OGTT glucose peak occurred later than in the last OGTT performed before the hay‐only period). The HE ponies were able to normalize ID status on the reduced diet despite remaining obese, which suggests that dietary changes impact ID status more than body condition. This finding contrasts with the observations of another study that reported that 5 weeks of a hay‐only diet did not result in normalization of ID status in obese horses. 6 In that study, obese Arabian horses were fed a hay‐only diet at 133% of energy requirements for 5 weeks while maintaining their obese state, after a 16‐week period in which twice the energy requirement was provided. Our study found that 17 weeks was sufficient for normalization of the OGTT response in Shetland pony mares after a long period on a HE diet. However, resuming the HE diet in year 2 resulted in a rapid return to a hyperinsulinemic response with a significantly larger AUC for insulin, which was evident after 9 to 13 weeks instead of the 17 to 18 weeks observed in year 1, suggesting that chronic changes or metabolic adaptations still were present in the ponies and might take longer to regress.
Our study had a number of limitations. In year 2, the number of ponies was small and the control group included different animals than did year 1. In addition, only mares were included because of the wider scope of the study. The insulin assay had to be changed because of the cessation of supply by the manufacturer in year 2, which interfered with comparison of results over the 2 project years. Moreover, the recalculation of insulin concentrations from RIA to CLIA does not appear to be justifiable based on recent literature showing nonlinearity in correlation. 17 Insulin measurements also could have been extended over a longer period during the OGTT, but the additional financial costs were not considered to be justified by expected additional information. Another interesting addition to our study would have been measurement of postprandial glucose and insulin dynamics in response to the supplied diet.
Although more efficient glucose metabolism was shown in overfed ponies in our study, sustained hyperinsulinemia is highly associated or considered to be the causal factor in the development of endocrinopathic laminitis.31, 32 As mentioned, there were indications that subclinical laminitis may have developed in the overfed ponies by the end of the study. 18 Therefore, feeding a diet rich in sugar and starch over a long period of time is not recommended.
Feeding an HE diet at twice the energy requirement to healthy nonobese Shetland pony mares led to more efficient glucose metabolism within 5 weeks, followed by development of significant postprandial hyperinsulinemia and increased BW. Insulin dysregulation status was reversed during a 17‐week hay‐only feeding period regardless of body condition, but the rapid return of ID after resuming the HE diet indicates the existence of more deeply integrated changes that take longer to resolve.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Ethical approval for the study was granted by the Committee on Animal Welfare of Utrecht University, the Netherlands (ethical committee approval No. 2014.III.01.004).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors thank Pavo for the semipurified concentrate diet. Funding for the project was provided by the FP7 Marie Curie International Training Network “EpiHealthNet” (project number 317146). Part of the study was presented as an oral presentation at the European College of Equine Internal Medicine (ECEIM) 2015 and International Equine Endocrinology Summit (IEES) in Florida 2017.
d' Fonseca NMM, Gibson CME, van Doorn DA, de Ruijter‐Villani M, Stout TAE, Roelfsema E. Effect of long‐term overfeeding of a high‐energy diet on glucose tolerance in Shetland pony mares. J Vet Intern Med. 2020;34:1339–1349. 10.1111/jvim.15788
Funding information FP7 People: Marie‐Curie Actions, Grant/Award Number: 317146; PAVO
REFERENCES
- 1. Durham AE, Frank N, McGowan CM, et al. ECEIM consensus statement on equine metabolic syndrome. J Vet Intern Med. 2019;33(2):335‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Timpson AJ, de Mestre AM, Elliott J, et al. Seasonal and dietary influences on adipose tissue and systemic gene expression in control and previously laminitic ponies. J Equine Vet. 2018;69:84‐95. [Google Scholar]
- 3. de Laat MA. Equine hyperinsulinemia: investigation of the enteroinsular axis during insulin dysregulation. Am J Physiol Endocrinol Metab. 2015;310(1):E61‐E72. [DOI] [PubMed] [Google Scholar]
- 4. Frank N, Tadros EM. Insulin dysregulation. Equine Vet J. 2014;46(1):103‐112. [DOI] [PubMed] [Google Scholar]
- 5. Pratt SE, Geor RJ, McCutcheon LJ. Effects of dietary energy source and physical conditioning on insulin sensitivity and glucose tolerance in standardbred horses. Equine Vet J Suppl. 2006;36:579‐584. [DOI] [PubMed] [Google Scholar]
- 6. Carter RA, McCutcheon LJ, George LA, Smith TL, Frank N, Geor RJ. Effects of diet‐induced weight gain on insulin sensitivity and plasma hormone and lipid concentrations in horses. Am J Vet Res. 2009;70(10):1250‐1258. [DOI] [PubMed] [Google Scholar]
- 7. Lindåse SS, Nostell KE, Müller CE, Jensen‐Waern M, Bröjer JT. Effects of diet‐induced weight gain and turnout to pasture on insulin sensitivity in moderately insulinresistant horses. Am J Vet Res. 2016;77(3):300‐309. [DOI] [PubMed] [Google Scholar]
- 8. Quinn RW, Burk AO, Hartsock TG, et al. Insulin sensitivity in thoroughbred geldings: effect of weight gain, diet, and exercise on insulin sensitivity in thoroughbred geldings. J Equine Vet. 2008;28(12):728‐738. [Google Scholar]
- 9. Bamford NJ. Effect of increased adiposity on insulin sensitivity and adipokine concentrations in horses and ponies fed a high fat diet, with or without a once daily high glycaemic meal. Equine Vet J. 2016;48(3):368‐373. [DOI] [PubMed] [Google Scholar]
- 10. Hoffman RM, Boston RC, Stefanovski D, Kronfeld DS, Harris PA. Obesity and diet affect glucose dynamics and insulin sensitivity in Thoroughbred geldings. J Anim Sci. 2003;81(9):2333‐2342. [DOI] [PubMed] [Google Scholar]
- 11. Bamford NJ. Effect of increased adiposity on insulin sensitivity and adipokine concentrations in different equine breeds adapted to cereal‐rich or fat‐rich meals. Vet J. 2016;214:14‐20. [DOI] [PubMed] [Google Scholar]
- 12. Bamford NJ, Baskerville CL, Harris PA, Bailey SR. Postprandial glucose, insulin, and glucagon‐like peptide‐1 responses of different equine breeds adapted to meals containing micronized maize. J Anim Sci. 2015;93(7):3377‐3383. [DOI] [PubMed] [Google Scholar]
- 13. Henneke DR, Potter GD, Kreider JL, Yeates BF. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet J. 1983;15(4):371‐372. [DOI] [PubMed] [Google Scholar]
- 14. Centraal Veevoederbureau (Het EWpa en VREp systeem. CVB documentatierapport No. 31, Centraal Veevoederbureau, Lelystad, the Netherlands 497 (in Dutch). 2004.
- 15. Siegers EW, De Ruijter‐Villani M, Van Doorn DA, Stout TAE, Roelfsema E. Ultrasonographic measurements of localized fat accumulation in Shetland pony mares fed a normal v. a high energy diet for 2 years. Animal. 2018;12(8):1602‐1610. [DOI] [PubMed] [Google Scholar]
- 16. van der Kolk JH, Wensing T, Kalsbeek HC, Breukink HJ. Laboratory diagnosis of equine pituitary pars intermedia adenoma. Domest Anim Endocrinol. 1995;12(1):35‐39. [DOI] [PubMed] [Google Scholar]
- 17. Carslake HB, Pinchbeck GL, McGowan CM. Evaluation of a chemiluminescent immunoassay for measurement of equine insulin. J Vet Intern Med. 2017;31(2):568‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sleutjens J, Serra Bragança FM, van Empelen MW, et al. Mouldable, thermoplastic, glue‐on frog‐supportive shoes change hoof kinetics in normal and obese Shetland ponies. Equine Vet J. 2018;50(5):684‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roberts MC, Hill FWG. The oral glucose tolerance test in the horse. Equine Vet J. 1973;5(4):171‐173. [DOI] [PubMed] [Google Scholar]
- 20. Freestone JF, Shoemaker K, Bessin R, Wolfsheimer JK. Insulin and glucose response following oral glucose administration in well‐conditioned ponies. Equine Vet J Suppl. 1992;11:13‐17. [DOI] [PubMed] [Google Scholar]
- 21. Murphy D, Reid SWJ, Love S. The effect of age and diet on the oral glucose tolerance test in ponies. Equine Vet J. 1997;29(6):467‐470. [DOI] [PubMed] [Google Scholar]
- 22. Dyer J, Al‐Rammahi M, Waterfall L, et al. Adaptive response of equine intestinal Na+/glucose co‐transporter (SGLT1) to an increase in dietary soluble carbohydrate. Pflug Arch Eur J Physiol. 2009;458(2):419‐430. [DOI] [PubMed] [Google Scholar]
- 23. Shirazi‐Beechey SP, Moran AW, Batchelor DJ, Daly K, Al‐Rammahi M. Glucose sensing and signalling; regulation of intestinal glucose transport. Proc Nutr Soc. 2011;70(2):185‐193. [DOI] [PubMed] [Google Scholar]
- 24. Rapson JL, Schott HC, Nielsen BD, McCutcheon LJ, Harris PA, Geor RJ. Effects of age and diet on glucose and insulin dynamics in the horse. Equine Vet J. 2018;50(5):690‐696. [DOI] [PubMed] [Google Scholar]
- 25. Suagee JK, Corl BA, Swyers KL, Smith TL, Flinn CD, Geor RJ. A 90‐day adaptation to a high glycaemic diet alters postprandial lipid metabolism in non‐obese horses without affecting peripheral insulin sensitivity. J Anim Physiol Anim Nutr. 2013;97(2):245‐254. [DOI] [PubMed] [Google Scholar]
- 26. Jacob SI, Geor RJ, Weber PSD, Harris PA, McCue ME. Effect of age and dietary carbohydrate profiles on glucose and insulin dynamics in horses. Equine Vet J. 2018;50(2):249‐254. [DOI] [PubMed] [Google Scholar]
- 27. Jeffcott LB, Field JR, McLean JG, O'dea K. Glucose tolerance and insulin sensitivity in ponies and Standardbred horses. Equine Vet J. 1986;18(2):97‐101. [DOI] [PubMed] [Google Scholar]
- 28. Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet‐induced obesity and leptin resistance in C57B1/6J mice. Int J Obes (Lond). 2000;24(5):639‐646. [DOI] [PubMed] [Google Scholar]
- 29. Redgate SE, Cooper JJ, Hall S, Eady P, Harris PA. Dietary experience modifies horses’ feeding behavior and selection patterns of three macronutrient rich diets. J Anim Sci. 2014;92(4):1524‐1530. [DOI] [PubMed] [Google Scholar]
- 30. Dugdale AHA, Curtis GC, Cripps PJ, Harris PA, Argo CM. Effects of season and body condition on appetite, body mass and body composition in ad libitum fed pony mares. Vet J. 2011;190(3):329‐337. [DOI] [PubMed] [Google Scholar]
- 31. de Laat MA, McGowan CM, Sillence MN, Pollitt CC. Equine laminitis: induced by 48 h hyperinsulinaemia in Standardbred horses. Equine Vet J. 2010;42(2):129‐135. [DOI] [PubMed] [Google Scholar]
- 32. Asplin KE, Sillence MN, Pollitt CC, McGowan CM. Induction of laminitis by prolonged hyperinsulinaemia in clinically normal ponies. Vet J. 2007;174(3):530‐535. [DOI] [PubMed] [Google Scholar]


