Abstract
Background
Left‐sided congestive heart failure (CHF) is characterized by increased filling pressures and related Doppler echocardiographic (DE) filling patterns.
Hypothesis
Doppler echocardiographic variables of left ventricular filling derived from transmitral flow, pulmonary vein flow, and tissue Doppler can be used to detect CHF in cats with hypertrophic cardiomyopathy (HCM).
Animals
Forty‐seven client‐owned cats.
Methods
Prospective clinical cohort study. Cats underwent physical examination, thoracic radiography, analysis of N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), and transthoracic echocardiography and were divided into 3 age‐matched groups: Group 1 (apparently healthy control), Group 2 (preclinical HCM), and Group 3 (HCM and CHF). Measured and calculated variables included respiratory rate, DE estimates, serum NT‐proBNP concentration, and radiographic CHF score. Groups were compared using ANOVA, and receiver operating characteristic (ROC) curve and multivariate analyses were used to identify diagnostic cutoffs for the detection of CHF.
Results
Fifteen cats were in Group 1, 17 in Group 2, and 15 in Group 3. The ROC analysis indicated that the ratio of peak velocity of early diastolic transmitral flow to peak velocity of late diastolic transmitral flow (area under the curve [AUC], 1.0; diagnostic cutoff, 1.77; P = .001), ratio of left atrial size to aortic annular dimension (AUC, 0.91; diagnostic cutoff, 1.96; P = .003), left atrial diameter (AUC, 0.89; cutoff, 18.5 mm; P = .004), diastolic functional class (AUC, 0.89; cutoff, class 2; P = .005), respiratory (AUC, 0.79; cutoff, 36 breaths per minute [brpm]; P = .02), and the ratio of the peak velocity of fused early and late transmitral flow velocities to the peak velocity of the fused early and late diastolic tissue Doppler waveforms (AUC, 0.74; cutoff, 15.1; P = .05) performed best for detecting CHF.
Conclusions and Clinical Importance
Various DE variables can be used to detect CHF in cats with HCM. Determination of the clinical benefit of such variables in initiating treatments and assessing treatment success needs further study.
Keywords: diastolic function, feline, NT‐ProBNP, respiration rate
Abbreviations
- A
peak velocity of late diastolic transmitral flow
- A′
peak velocity of late diastolic mitral annular motion measured at the lateral mitral annulus
- Adur
duration of late diastolic transmitral flow wave
- Ao
aortic annular dimension
- ARdur
duration of late diastolic pulmonary vein atrial reversal flow wave
- AUC
area under the ROC curve
- CHF
congestive heart failure
- CI
confidence interval
- CV
coefficient of variation
- D
peak velocity of diastolic pulmonary vein flow
- DCM
dilated cardiomyopathy
- DE
Doppler echocardiography
- DMVD
degenerative mitral valve disease
- E
peak velocity of early diastolic transmitral flow
- E′
peak velocity of early diastolic mitral annular motion measured at the lateral mitral annulus
- EAfus
peak velocity of fused early and late transmitral flow velocities
- E′A′fus
peak velocity of the fused early and late diastolic tissue Doppler waveforms measured at the lateral mitral annulus
- HCM
hypertrophic cardiomyopathy
- IVRT
isovolumic relaxation time
- IVSd
thickness of the interventricular septum at end‐diastole
- LA
left atrial
- LAD
left atrial diameter
- LV
left ventricular
- LVFP
left ventricular filling pressure
- LVIDd
left ventricular internal dimension at end‐diastole
- LVIDs
left ventricular internal dimension at end‐systole
- LV‐SF
left ventricular shortening fraction
- NT‐proBNP
N‐terminal pro‐brain natriuretic peptide
- PV
pulmonary vein
- PV AR
peak velocity of the late diastolic pulmonary vein atrial reversal flow wave
- PV ARdur
duration of the late diastolic pulmonary vein atrial reversal flow wave
- ROC curve
receiver operating characteristic curve
- S
peak velocity of systolic pulmonary vein flow
- Sn
sensitivity
- Sp
specificity
- 2D
2‐dimensional
1. INTRODUCTION
Congestive heart failure (CHF) is a life‐threatening clinical syndrome characterized by debilitating clinical signs, cardiac dysfunction with neurohormonal activation, increased filling pressures, and corresponding radiographic abnormalities. The most common cause of CHF in cats is hypertrophic cardiomyopathy (HCM). 1 Diastolic dysfunction is the functional hallmark of this disease leading to increased left ventricular (LV) filling pressure (LVFP).2, 3 Congestive heart failure is recognized by the presence of acute tachypnea, labored breathing, and pulmonary crackles on thoracic auscultation. When available, cage‐side thoracic ultrasound examination may offer supportive evidence by identifying B‐lines and confirming left atrial (LA) enlargement. Ultimately, the final diagnosis is confirmed by thoracic radiography. Differentiating between cardiac and noncardiac causes of respiratory distress in cats can be difficult radiographically (eg, in cats with pleural effusion or concurrent cardiac and pulmonary disease). In addition, the radiographic distribution of pulmonary edema in cats is quite variable, making the rapid and unequivocal diagnosis of CHF difficult in some cats. 4 Accurate detection of increased LVFP and CHF by Doppler echocardiographic (DE) would decrease diagnostic uncertainty.
Many DE variables have been prospectively evaluated for their potential use to estimate increased LVFP in people and dogs.5, 6, 7, 8, 9, 10, 11, 12, 13, 14 In addition to the traditional ratio of peak early (E) diastolic to peak late (A) diastolic transmitral flow velocity (E:A), previous studies identified indices such as the ratio (E:E′) of E to peak early diastolic tissue motion velocity (E′) and the ratio of E to isovolumic relaxation time (E:IVRT) as most useful in the detection of increased filling pressures.15, 16, 17, 18, 19, 20, 21, 22 The value of DE in the echocardiographic diagnosis of CHF has been demonstrated in dogs with degenerative mitral valve disease (DMVD) and dilated cardiomyopathy (DCM).22, 23 Additional variables that aided in the detection of CHF included resting respiratory rate and LV diastolic functional class.22, 23 Diagnostic cutoffs for clinical decision‐making varied considerably between DMVD and DCM in dogs to such a degree that individual variables,22, 23 although very useful in DCM, were without diagnostic value in dogs with DMVD. Because HCM in cats, a disease functionally characterized by diastolic dysfunction with preserved or hyperdynamic systolic function, is very distinct from DMVD and DCM in dogs, data from dogs cannot be extrapolated to cats with HCM. Therefore, the diagnostic value and diagnostic cutoffs used in the detection of CHF in cats with HCM remain unknown. Moreover, as demonstrated in humans with heart failure and preserved ejection fraction (eg, HCM), many DE estimates of LVFP are less reliable in detecting the presence of heart failure.7, 24 Therefore, independent validation of DE variables is necessary in cats with HCM, which was the aim of our study. Our hypothesis was that DE variables can be used to detect the presence of CHF in cats with HCM.
2. MATERIALS AND METHODS
The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee (Protocol #2016A00000096) and the Clinical Research Committee (Protocol #2016V10) of the Department of Veterinary Clinical Sciences, College of Veterinary Medicine, The Ohio State University, Columbus, Ohio.
2.1. Cats, clinical examinations, and group assignment
Forty‐seven client‐owned cats were prospectively studied. Cats were recruited over a period of 2 years based on an echocardiographic diagnosis of either an apparently normal heart 25 or diagnosis of HCM characterized by idiopathic LV wall thickening of ≥6 mm. 25 All cats underwent a thorough physical examination and a 2‐dimensional (2D), M‐mode, and DE study (Vivid E9 with EchoPac software package BT13 version 113.1.3, GE Medical Systems, Waukesha, WI, USA). In addition, thoracic radiography (Digital radiography system EDR‐6, Sound‐Eklin, Carlsbad, CA, USA), systolic blood pressure measurement (ultrasonic Doppler flow detector, Parks Medical Electronics Inc, Aloha, OR, USA), and measurement of serum N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) concentration were performed in all cats with HCM. Serum thyroid hormone concentrations were measured in HCM cats >6 years of age. Heart rate and respiratory rate were determined during the initial physical examination and recorded as the number of beats and respirations per minute, respectively. Cats with increased blood pressure (repeated measurements >170 mm Hg), hyperthyroidism, arterial thromboembolism, atrial fibrillation, and ventricular tachycardia and with evidence of concurrent diseases or conditions such as pulmonary disease, renal failure, or neoplasia were excluded. Using clinical, radiographic, and echocardiographic data, cats were divided into 3 groups for statistical analysis: apparently healthy (Group 1), occult HCM (Group 2), and HCM with CHF (Group 3). Congestive heart failure was diagnosed based on history, physical examination findings, and the presence of pulmonary edema, pleural effusion, pericardial effusion, or some combination of these, detected during imaging. Plasma NT‐pro‐BNP concentration provided supportive data for group assignment. In Group 2, cardiac medications were not permitted (eg, atenolol, pimobendan, angiotensin‐converting enzyme inhibitors) except for clopidogrel. In cats with CHF at presentation (Group 3), no treatments other than clopidogrel and furosemide (given within <24 hours of thoracic radiographs and echocardiography) were permitted. All cats were sedated using butorphanol (Butorphanoltartrate injection, Butorphic; 0.2‐0.25 mg/kg IM). Acepromazine (Acepromazine maleate injection, Vetone; 0.03 mg/kg IM) was administered in 1 cat that required additional sedation. Six cats received gabapentin (Gabapentin capsules, Ascend) before the hospital visit (100 mg/cat, PO).
2.2. Thoracic radiography
Thoracic radiographs were taken in 3 to 4 different imaging planes (right lateral, left lateral, and either ventral dorsal or dorsal ventral projections). They were assessed by the attending clinician for initial group allocation (Group 2 versus Group 3). The radiographic images also were assessed collectively by a board‐certified radiologist (A. H.) at the end of the recruitment period. All radiographs were ordered randomly and coded. The observer was aware of the principal diagnosis (HCM), but was blinded to other patient and diagnostic data. Initially, CHF status (yes/no) was determined, and cardiac mensuration performed (vertebral heart scale, VHS) using standard methods. 26 In addition, a radiographic CHF score (see Appendix) was applied using visual criteria and variable weights: presence of cardiomegaly, pulmonary venous congestion, pulmonary infiltrates compatible with cardiogenic edema, pleural effusion, and caudal vena cava distention.4, 26, 27 This process led to a final quantitative radiograph CHF score for each cat with HCM (minimum score, 0; maximum score, 12). A score of >2.0 was considered diagnostic of CHF.
2.3. Collection of blood and analysis of N‐terminal‐proBNP
In cats with HCM with or without CHF, 3 to 5 mL of blood was collected from the medial saphenous vein and placed into serum tubes. After approximately 20 minutes of storage at room temperature (22°C‐24°C) to allow for stable clot formation, samples were centrifuged at 3000g for 10 minutes and further processed within 15 minutes. The serum was separated and placed in plastic cryotubes and stored at −80°C for a maximum of 16 weeks until batch analysis. Samples were shipped on dry ice to the reference laboratory (IDEXX Laboratories, Westbrook, MA, USA) where analysis was performed.
Serum NT‐proBNP concentration was determined using the second‐generation enzyme‐linked immunosorbent assay for cats using antibodies raised against the N‐terminal portion of proBNP. The maximum measurable concentration was 1500 pmol/L. Coefficients of variation for intra‐assay accuracy are reported as 1.6% to 6.3%. 28
2.4. Echocardiography
Transthoracic 2D, M‐mode, and DE examinations were performed primarily by a single operator (M. N. R) under the supervision of a board‐certified cardiologist. The cats were imaged in right and left lateral recumbency with a digital ultrasound system (Vivid E9 with EchoPac software package BT13 version 113.1.3, GE Medical Systems, Waukesha, WI, USA) and a sector transducer with a nominal frequency of 6 or 12 MHz. Right parasternal standard imaging views optimized for the left atrium (LA), left ventricle (LV; long and short axes), and LV outflow tract (long axis), and left apical parasternal standard imaging views optimized for the LV inflow tract and longitudinal motion of the lateral mitral annulus or the LV outflow tract were used for data acquisition.11, 25 Two‐dimensional cine loops and Doppler tracings were recorded and stored. A simultaneous 1‐lead ECG was recorded. Heart rate was determined from R‐R intervals on the ECG at the time IVRT was measured. Measurements were obtained from digital still images as an average of 3 to 5 cardiac cycles, unrelated to the phase of respiration. Only high‐quality images were used for data analysis. All measurements were performed collectively at the end of the recruitment period by 1 investigator (M. N. R). All studies were labeled by medical record number only and ordered randomly before analysis.
2.5. Echocardiographic data analysis
Nineteen variables were measured and 7 variables were calculated as described previously in cats.11, 29, 30 A 2D right parasternal 4‐chamber image was used to obtain the maximum (systolic) septal‐to‐caudal dimension of the LA (LAD)29, 31 using the distance from blood‐tissue interface to blood‐tissue interface and digital calipers provided by the ultrasound system. Maximum area of the LA (LAA) 32 was quantified by planimetry in the same imaging view. The thickest dimensions of the interventricular septum (IVSd) and left ventricular free wall (LVFWd) at end‐diastole also were obtained from 2D right parasternal long axis images, using the leading edge‐to‐trailing edge method. From an LV short‐axis image and at the level of the papillary muscles, the end‐diastolic thickness of the IVSd and LVFWd were measured (leading edge‐to‐trailing edge). Using LV short‐axis M‐mode at the same level, LV dimensions in systole (LVIDs) and diastole (LVIDd) were measured using the leading edge‐to‐leading edge method. From the right parasternal short‐axis heart base view, the LA and aortic dimensions (Ao) were obtained at early diastole (inner edge‐to‐inner edge method) and the LA:Ao ratio calculated. 33 From a left apical 5‐chamber recording, IVRT was measured as the time period from the aortic valve closure click to the beginning of transmitral flow using a pulsed wave sample volume of 3 to 6 mm length placed in an intermediate position between the LV inflow and outflow tract.11, 29 Transmitral flow was recorded as recommended.29, 34 Peak velocity of E and A were measured. When the E and A waves were fused, the peaks of the fused waveforms were measured (EAfus). When the E wave was clearly distinguishable from the A wave, but partially fused, only the E velocity was recorded and the A velocity was considered unreliable. Partially fused E and A waves were measured when the velocity intercept between the deceleration phase of the E wave and the acceleration phase of the A wave was <0.4 m/s, although it is recommended in people to measure if the intercept is <0.2 m/s.29, 35 When possible, duration of the A wave (Adur) was measured from the beginning to the end of the A wave. Pulmonary venous flow was recorded from either the right parasternal short‐axis view at the heart base, the left apical 4‐chamber view, or the left cranial parasternal view optimized for the LA appendage, with minimized baseline filter, optimized velocity scale, and a pulsed wave sample volume of 3 to 4 mm placed at approximately 5 mm within the pulmonary vein (PV).29, 34 Peak velocity of systolic (S wave) and diastolic (D wave) flow were quantified, and the flow pattern was determined to be either systolic dominant (S > D) or diastolic‐dominant (D > S). The velocity and duration of the late diastolic reversal wave (PV AR and PV ARdur) were measured. Pulsed wave Doppler‐derived velocities of myocardial wall motion were recorded from an apical left parasternal 4‐chamber view optimized for the lateral mitral annulus, with a sample volume of 3 to 4 mm placed at the lateral aspect of the mitral annulus. Peak E′ was measured. When E′ and late (A′) diastolic mitral annulus motion waves were summated, peak velocity of the summated wave was recorded (E′A′fus). Variables calculated included LV shortening fraction (LV‐SF = [LVIDd‐LVIDs]/LVIDd × 100), the ratio between E to A (E:A), the ratio between the duration of A to the duration of AR (Adur:ARdur), the ratio of E to E′ (E:E′), and the ratio of E to IVRT (E:IVRT). When the waveforms were fused, the following ratios were calculated: EAfus:E′A′fus and EAfus:IVRT. Left ventricular diastolic function was classified primarily based on the E:A ratio (E:A class) 29 : Class 1: normal pattern (1.0 ≤ E:A ≤ 2.0), Class 2: relaxation delay pattern (E:A < 1.0), Class 3: pseudonormal pattern (1.0 ≤ E:A ≤ 2.0), and Class 4: restrictive pattern (E:A > 2.0). Classes 1 and 3 were discriminated by evaluating for the presence of LA dilatation, hypertrophy of the left ventricle, and a low E′ (<6 cm/s) 29 all indicating pseudonormal rather than normal filling if E:A was between 1.0 and 2.0. In alignment with anecdotal evidence, for cats >12 years of age, any observed E:A between 1.0 and 2.0 was considered pseudonormal transmitral flow because a physiologic age‐related relaxation delay pattern would be expected in such cats. 29 Tachycardia led to a high number of cats with fused transmitral waveforms, making classification of LV diastolic function using solely the E:A ratio impossible. Therefore, an alternative method was applied and led to the variable, diastolic class (or D‐class). This method considered 7 variables based on LA size, IVRT, E:A, pulmonary venous flow, Adur:ARdur, E′, and E:E′ for classification. 29 If nonsummated, with clearly separated E and A waves, preference was given to the E:A ratio for diastolic class assignment. If E and A waves were summated, at least 2 of the remaining 6 variables had to be interpretable for final class assignment.
Measurement reliability was determined for selected echocardiographic variables. Nine echocardiograms (all groups represented) were randomly selected from the study pool for repeated analyses by 1 observer (M. N. R) to determine intraobserver measurement variability. The same 9 echocardiograms underwent repeated analyses by a second independent observer (K. E. S.) to determine interobserver measurement variability. Both investigators were blinded to the results of prior studies.
2.6. Statistical analysis
Statistical analyses were performed using commercially available software (SAS version 9.4 [2016], Cary, NC, USA; GraphPad Prism, version 8, GraphPad Software, Inc, San Diego, CA, USA). All data were visually inspected and tested for normality using the Shapiro‐Wilk test. An extreme value was found for respiratory rate and was replaced by the group mean value of that variable. Descriptive statistics were calculated, and frequencies or median and 5th and 95th percentiles were reported for each variable. Continuous variables were compared among groups using 1‐way ANOVA or the Kruskal‐Wallis test, whichever was appropriate. When significance was detected among the 3 groups, a Dunn's or Tukey's post hoc test was performed. Depending on the expected cell counts of the corresponding contingency tables, chi‐square or Fisher's exact test was used to explore the association between Groups 2 and 3 and other categorical variables. Receiver operating characteristic (ROC) curve analysis was performed to assess the diagnostic accuracy of each variable to detect the presence of CHF. The area under the ROC curve was used as a summary measure for diagnostic accuracy and is reported with 95% confidence intervals (CIs). Diagnostic cutoffs for each variable were chosen on the basis of the highest of various combinations of sensitivity (Sn) and specificity (Sp) using Youden's index (Y = Sn + Sp − 1). Multivariable ROC analysis was performed using the variables that were significant (P ≤ .05) in the univariate ROC analysis to identify the combination of variables that best detected the presence of CHF. For this purpose, different submodels were tested against the full model by taking a submodel at a time as a reference model. Significance level was set as P ≤ .05.
Observer variability of echocardiographic measurements was calculated by (SD/average of measurements) × 100 and expressed as the coefficient of variation (CV) in percent and also as an absolute value. 36
3. RESULTS
Demographic data and results of physical examination are summarized in Table 1. In Group 3, 7 cats (47%) had received furosemide within 12 hours before the study, but they were determined to be in active CHF at study time based on radiographic or echocardiographic findings or both. Results of the radiographic composite score, NT‐proBNP and selected echocardiographic variables are presented in Tables 2 and 3. All cats in Group 2 had a radiographic CHF score ≤2.0, whereas 12 of the 15 cats in Group 3 had a radiographic CHF score >2. The score of ≤2 found in 3 cats of Group 3 is attributed to their imaging diagnosis of CHF being based primarily on pericardial effusion (in addition to clinical signs, LV wall thickening, and LA enlargement), which cannot easily be identified on radiographs. Because the NT‐proBNP measurements were performed using a second‐generation ELISA with an maximum measurable concentration of 1500 pmol/L and data points were not normally distributed, 1500 pmol/L was identified as both the median and the upper value of the 95th percentile for Group 3. Repeating analyses (summarized in Tables 2 and 3) without the cats with pericardial effusion neither changed the significance level reported nor caused clinically relevant changes of the AUCs as presented in Table 3. A high frequency of summated waveforms for transmitral flow and tissue Doppler (EAfus and E′A′fus) was observed in the cats of our study owing to sinus tachycardia or poor atrial function (38% of all cats, 73% of cats in Group 3). The majority of cats in Groups 1 and 2 had separated waveforms, but only 4 of the cats in Group 3 had separated E and A waves. Therefore, E:A class only could be determined in 33% of the cats in Group 3. In contrast, the method for determining D‐class based on a composite of multiple variables 29 allowed for determination of diastolic class in all cats. Of note, 3 cats with CHF had prolonged IVRT (>60 ms) 29 including a 14‐year‐old cat with restrictive LV filling (E:A > 2.0, diastolic‐dominant PV flow, and E:E′ > 15) with an IVRT of 66 ms and a 3‐year old cat with pseudonormal LV filling and an of IVRT 64 ms.
TABLE 1.
Demographic data and results of physical examination in 15 control cats and 32 cats with hypertrophic cardiomyopathy (HCM)
| Group | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| n | 15 | 17 | 15 |
| Age (years) | 6.43 (2.87‐12.25) | 6.68 (1.67‐12.65) | 7.37 (2.0‐15.5) |
| Body weight (kg) | 4.21 (2.8‐7.41) | 5.05 (30.1‐7.25) | 5.86 (4.31‐8.30) a |
| Sex (female:male) | 13:2 | 6:11 | 3:12 |
| Respiratory rate (min−1) | 36 (20‐52) | 34 (20‐60) | 48 (36‐120) a , b |
| Heart rate (min−1) | 200 (150‐245) | 200 (130‐250) | 200 (150‐270) |
| Systolic heart murmur | 1 (7%) | 16 (94%) | 9 (60%) b |
| Gallop | 0 | 3 (18%) | 4 (27%) |
| Arrhythmia | 0 | 4 (24%) | 3 (20%) |
Notes: Group 1, apparently healthy; Group 2, occult HCM; Group 3, HCM with CHF. Median (5th and 95th percentiles) for continuous data and number (n) or percent (%) for frequency data. Frequency data (systolic heart murmur, gallop, and arrhythmia) only statistically compared between Groups 2 and 3.
Within a row, values between Groups 1 and 3 differ (P ≤ .05).
Within a row, values between Groups 2 and 3 differ (P ≤ .05).
TABLE 2.
Radiographic composite congestive heart failure (CHF) score, serum concentrations of NT‐proBNP, and selected echocardiographic variables in 15 control cats and 32 cats with hypertrophic cardiomyopathy
| Group 1 | n | Group 2 | n | Group 3 | n | |
|---|---|---|---|---|---|---|
| Radiographic CHF score | N/A | 0 | 1 (0‐2) | 17 | 5 (0.25‐10.75) a | 15 |
| NT‐proBNP (pmol/L) | N/A | 0 | 496 (24‐1500) | 16 | 1500 (496‐1500) a | 13 |
| HR (min−1) | 162 (120‐236) | 15 | 185 (113‐240) | 17 | 198 (155‐275) | 15 |
| LAD (mm) | 13.20 (11.07‐15.43) | 15 | 14.78 (12.1‐24) b | 17 | 20.57 (18.53‐25.17) a , c | 15 |
| LA:Ao | 1.40 (1.25‐1.67) | 15 | 1.51 (1.29‐2.35) b | 17 | 2.24 (1.62‐2.82) a , c | 15 |
| IVRT (ms) | 49 (38‐58) | 15 | 60 (29‐78) | 17 | 37 (26‐89) | 15 |
| E (m/s) | 0.66 (0.50‐0.93) | 13 | 0.64 (0.54‐0.93) | 12 | 0.82 (0.49‐0.92) | 4 |
| A (m/s) | 0.53 (0.35‐1.03) | 13 | 0.78 (0.55‐1.07) b | 9 | 0.25 (0.21‐0.29) a , c | 4 |
| EAfus (m/s) | 0.91 (0.73‐1.03) | 6 | 0.94 (0.64‐1.22) | 13 | 0.85 (0.70‐1.46) | 13 |
| E′ (cm/s) | 7.46 (5.55‐9.58) | 12 | 4.23 (3.3‐10.10) | 10 | 4.09 (2.72‐4.80) | 4 |
| E′A′fus (cm/s) | 9.84 (7.97‐13.00) | 5 | 8.66 (4.87 10.87) | 10 | 4.90 (3.40‐11.43) | 12 |
| E:A ratio | 1.19 (0.80‐1.90) | 13 | 0.81 (0.57‐1.70) | 9 | 3.30 (1.77‐4.45) a , c | 4 |
| E:IVRT | 1.44 (0.89‐2.05) | 13 | 1.27 (0.76‐3.21) | 12 | 2.22 (1.14‐3.55) | 4 |
| EAfus:IVRT | 1.69 (1.49‐2.45) | 6 | 2.28 (0.82‐4.04) | 11 | 2.34 (0.81‐4.16) | 13 |
| E:E′ | 9.49 (6.29‐12.48) | 12 | 14.34 (7.54‐19.52) b | 8 | 14.64 (10.26‐19.01) | 2 |
| EAfus:E′A′fus | 9.36 (7.38‐9.73) | 5 | 11.43 (8.5‐13.46)a, | 10 | 17.59 (7.39‐24.62)b.c | 12 |
| PV AR (m/s) | 0.22 (0.17‐0.30) | 13 | 0.26 (0.16‐0.53) b | 15 | 0.29 (0.18‐0.61) c | 13 |
| PV ARdur (ms) | 56 (49‐84) | 13 | 59 (44‐102) | 15 | 70 (53‐95) a , c | 12 |
| Adur:ARdur | 0.91 (0.6‐1.31) | 11 | 0.98 (0.38‐1.18) | 6 | 0.69 (0.66‐0.71) | 2 |
| PV S dominant/D dominant | 8/6 | 14 | 16/0 | 16 | 7/8 a | 15 |
Notes: Group 1, apparently healthy; Group 2, preclinical HCM; Group 3, HCM with CHF. Median (5th and 95th percentiles) for continuous data and number (n) or percent (%) for frequency data. Frequency data (PV S dominant/D dominant) only statistically compared between Groups 2 and 3.
Abbreviations: A, peak velocity of late diastolic transmitral flow; Adur, duration of the late diastolic transmitral flow wave; E, peak velocity of early diastolic transmitral flow; E′, peak velocity of early diastolic lateral mitral annular motion; N/A, not applicable; EAfus, peak velocity of fused early and late diastolic transmitral flow waves; E′A′fus, peak velocity of fused early and late diastolic lateral mitral annular motion waves; HR, heart rate during echocardiography; IVRT, isovolumic relaxation time; LA:Ao, ratio of left atrial diameter to the aortic annular dimension measured from a right parasternal short‐axis view; LAD, maximum left atrial cranial‐caudal dimension measured from a right parasternal long axis view; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PV AR, peak velocity of the late diastolic pulmonary vein atrial reversal flow wave; PV ARdur, duration of the late diastolic pulmonary vein atrial reversal flow wave; PV D dominant, diastolic‐dominant pulmonary vein flow (systolic flow wave < diastolic flow wave); PV S dominant, systolic dominant pulmonary vein flow (systolic flow wave > diastolic flow wave).
Within a row, values between Groups 2 and 3 differ (P ≤ .05).
Within a row, values between Groups 1 and 2 differ (P ≤ .05).
Within a row, values between Groups 1 and 3 differ (P ≤ .05).
TABLE 3.
Distribution of categories (number of observations and %) of left ventricular (LV) diastolic dysfunction and frequency of LV systolic dysfunction as determined by E:A class and D‐class and shortening fraction, respectively, in control cats and cats with hypertrophic cardiomyopathy
| Group 1 (n = 15) | Group 2 (n = 17) | Group 3 (n = 15) | |
|---|---|---|---|
| E:A class | |||
| Normal flow | 10 (67) | 0 | 0 |
| Delayed relaxation flow pattern | 3 (20) | 10 (59) | 1 (6.5) |
| Pseudonormal flow | 0 | 2 (12) | 1 (6.5) |
| Restrictive flow | 0 | 0 | 3 (20) |
| n.d. | 2 (13) | 5 (29) | 10 (67) |
| Diastolic class (D‐class) | |||
| Normal flow | 11 (73) | 1 (6) | 0 |
| Delayed relaxation flow pattern | 4 (27) | 11 (65) | 0 |
| Pseudonormal flow | 0 | 4 (24) | 8 (53) |
| Restrictive flow | 0 | 1 (5) | 7 (47) |
| Systolic dysfunction (SF < 30%) | 0 | 0 | 27 |
Notes: Group 1, apparently healthy; Group 2, preclinical HCM; Group 3, HCM with CHF. Frequency data (systolic dysfunction) only statistically compared between Groups 2 and 3. The distribution of the categories of LV diastolic dysfunction (diastolic flow patterns) between Groups 2 and 3 were significantly different (P ≤ .05) for both E:A class and D‐class.
Abbreviations: D‐class, designation of diastolic functional class based on a composite of 7 Doppler echocardiographic variables including LAD, IVRT, transmitral flow, tissue Doppler and pulmonary venous flow variables 37 ; E:A class, diastolic functional class determined primarily from the E:A ratio 10 and using the presence of left atrial dilation, hypertrophy of the left ventricle, and a low E′ to distinguish “normal” from “pseudonormal”; n.d., not determined because of fused flow waves; SF, shortening fraction.
Figure 1 illustrates the median and scatter plots of respiratory rate, LAD, E:A ratio, D‐class IVRT, EAfus:E′A′fus, E:IVRT, and Adur:ARdur between groups of cats with HCM.
FIGURE 1.
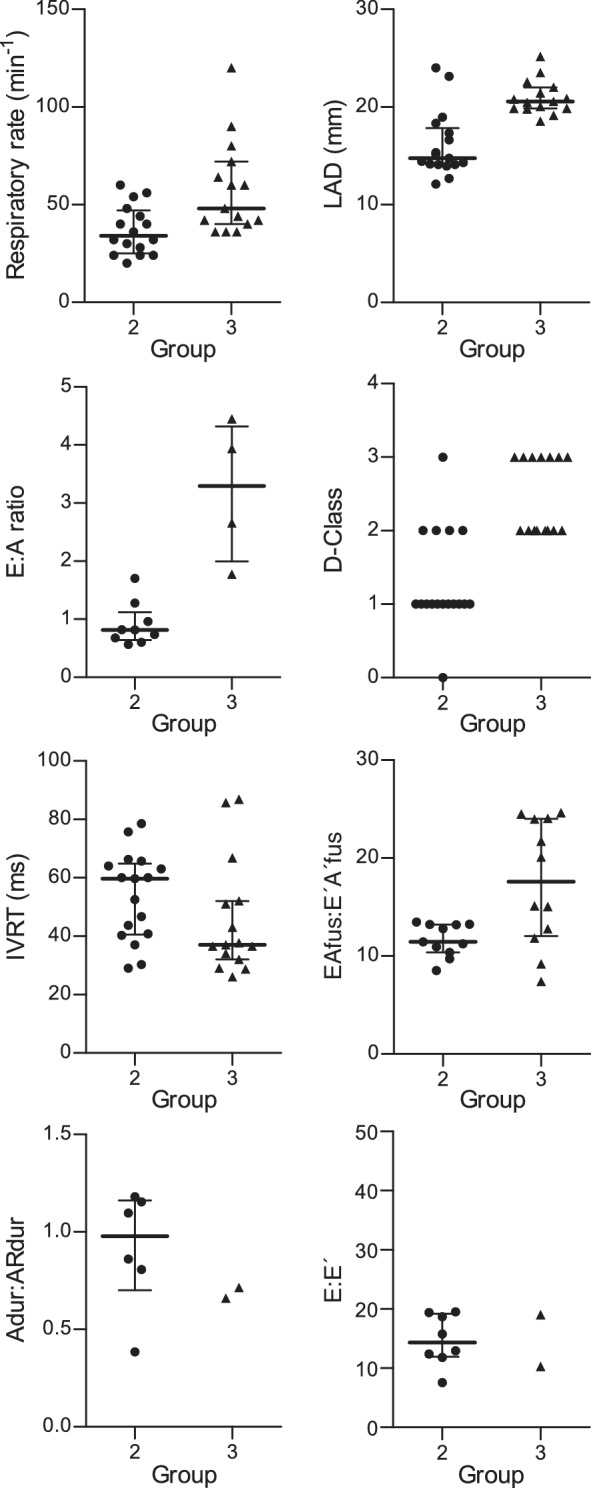
Scatter plots of respiratory rate, 2‐dimensional, and Doppler echocardiographic variables of left ventricular filling in 17 cats with occult HCM (Group 2) and 15 cats with congestive heart failure secondary to HCM (Group 3). Respiratory rate (median, 34 versus 48 brpm, P = .001); LAD (median, 14.78 versus 20.57, P < .001); E:A ratio (median, 0.81 versus 3.3, P < .001); class of LV diastolic function (D‐class, median, 1 versus 2, P < .05); IVRT (median, 59 versus 37 ms, P = .28), EAfus:E′A′fus (median 11.4 versus 17.6, P = .03), Adur:ARdur (median, 0.98 versus 0.69, P = .22), and E:E′ (median 14.34 versus 14.64, P = .90). The bars represent median and interquartile range. Bars are not present for the categorical variable (D‐class) or when there were less than 3 observations per group
Table 4 summarizes the results of the univariate ROC analysis and presents variables that best detected the presence of CHF (P < .05). In contrast, IVRT (P = .41), E (P = .85), EAfus (P = .77), Adur:ARdur (P = .21), Adur − AR dur (P = .18), E:IVRT (P = .21), EAfus:IVRT (P = .41), and E:E′ (P = .33) did not perform well in detecting CHF. Results of multivariate ROC analysis are presented in Table 5. Data on intra‐ and interobserver measurement variability of echocardiographic indices are summarized in Table 6. Coefficients of variation were <10% for all variables except EAfus:IVRT.
TABLE 4.
Results of univariate receiver operating characteristic (ROC) curve analysis and optimal diagnostic cutoffs of clinical and echocardiographic variables in the prediction of congestive heart failure (CHF) in 32 cats without heart failure (Groups 1 and 2 combined) and 15 cats with hypertrophic cardiomyopathy (HCM) and CHF (Group 3) or 17 cats with HCM without CHF (Group 2) and 15 cats with HCM and CHF (Group 3)
| Variable | AUC | 95% CI | Cutoff | Sn | Sp | P |
|---|---|---|---|---|---|---|
| Groups 1 and 2 combined versus Group 3 | ||||||
| E:A ratio | 0.97 | 0.93‐0.99 | 1.77 | 1.00 | 0.90 | <.001 |
| LAD (mm) | 0.94 | 0.86‐0.99 | 18.5 | 1.00 | 0.90 | <.001 |
| D‐class | 0.94 | 0.88‐0.99 | 2 | 1.00 | 0.84 | <.001 |
| LA:Ao | 0.94 | 0.88‐0.99 | 1.61 | 1.00 | 0.78 | <.001 |
| E:A class | 0.85 | 0.74‐0.95 | 2 | 1.00 | 0.71 | .001 |
| Respiratory rate (brpm) | 0.79 | 0.64‐0.92 | 36 | 1.00 | 0.51 | .02 |
| EAfus:E′A′fus | 0.78 | 0.57‐0.98 | 15.0 | 0.63 | 1.00 | .02 |
| Group 2 versus Group 3 | ||||||
| E:A ratio | 1.00 | 1.00‐1.00 | 1.77 | 1.00 | 1.00 | .001 |
| LA:Ao | 0.91 | 0.79‐0.99 | 1.96 | 0.84 | 0.82 | .003 |
| LAD (mm) | 0.89 | 0.75‐0.99 | 18.5 | 1.00 | 0.85 | .004 |
| D‐class | 0.89 | 0.78‐0.99 | 2 | 1.00 | 0.71 | .005 |
| E:A class | 0.79 | 0.64‐0.95 | 3 | 0.91 | 0.71 | .006 |
| Respiratory rate (brpm) | 0.79 | 0.61‐0.94 | 36 | 1.00 | 0.51 | .02 |
| EAfus:E′A′fus (cm/s) | 0.74 | 0.51‐0.98 | 15.1 | 0.63 | 1.00 | .05 |
TABLE 5.
Results of multivariate receiver operating characteristic (ROC) curve analysis of clinical and echocardiographic variables in the prediction of congestive heart failure (CHF) in 32 cats without heart failure (Groups 1 and 2 combined) and 15 cats with hypertrophic cardiomyopathy (HCM) and CHF (Group 3) or 17 cats with HCM without CHF (Group 2) and 15 cats with HCM and CHF (Group 3)
| Model | AUC | 95% CI | P |
|---|---|---|---|
| Groups 1 and 2 combined versus Group 3 | |||
| D‐class + LAD | 0.96 | 0.90‐1.00 | <.05 |
| LAD + EAfus:E′A′fus | 0.94 | 0.86‐1.00 | <.05 |
| Group 2 versus Group 3 | |||
| D‐class + LAD | 0.92 | 0.82‐1.00 | <.05 |
| LAD + EAfus:E′A′fus | 0.92 | 0.80‐1.00 | <.05 |
TABLE 6.
Intra‐ and interobserver measurement variability of 13 echocardiographic variables assessed in 9 randomly selected cats
| Intraobserver | Interobserver | |||
|---|---|---|---|---|
| CV% | Absolute | CV% | Absolute | |
| LAD (mm) | 1.50 | 0.24 | 5.63 | 0.92 |
| LA:Ao | 3.00 | 0.05 | 5.55 | 0.09 |
| IVRT (ms) | 4.47 | 2 | 6.69 | 3 |
| E (m/s) | 2.35 | 0.02 | 1.11 | 0.01 |
| EAfus (m/s) | 1.80 | 0.02 | 5.08 | 0.05 |
| E′ (cm/s) | 5.69 | 0.31 | 6.07 | 0.34 |
| E′A′fus (cm/s) | 4.31 | 0.35 | 4.90 | 0.37 |
| E:A ratio | 3.12 | 0.05 | 2.66 | 0.06 |
| E:IVRT | 4.66 | 0.08 | 7.51 | 0.15 |
| Adur:ARdur | 4.01 | 0.04 | 5.62 | 0.04 |
| E:E′ | 4.26 | 0.48 | 6.82 | 0.71 |
| EAfus:E′A′fus | 2.88 | 0.33 | 4.70 | 0.65 |
| EAfus:IVRT | 6.10 | 0.14 | 12.95 | 0.27 |
Note: See Table 2 for remainder of the key.
Abbreviation: CV, coefficient of variation.
4. DISCUSSION
Most cats present with tachypnea and labored breathing as the first manifestation of CHF. Clinical assessment, along with thoracic radiographs or NT‐proBNP or both, is the first step in the evaluation of such patients. However, there are limitations to these methods and thus a noninvasive assessment of LVFP is necessary. Increased filling pressure is a pathophysiological hallmark of CHF.6, 7, 8, 9, 19, 22, 38 The results of our prospective study confirm earlier findings in dogs 22 that presence of CHF (and thus, increased LVFP) also can be detected by DE in cats with HCM. The transmitral E:A ratio, measures of LA size, and diastolic functional class best allowed for the detection of CHF in our study. In addition, the echocardiographic variables and diagnostic thresholds found to be most useful in the detection of CHF in cats with HCM differed relevantly from those reported in dogs with DCM and DMVD. 22 Therefore, disease‐specific echocardiographic variables and diagnostic cutoffs must be considered in the echocardiographic diagnosis of CHF.
4.1. Detection of CHF using single echocardiographic variables of LA size and LV diastole
In our study of cats with HCM, LA enlargement measured by LAD and LA:Ao performed equally well in supporting the diagnosis of CHF in cats with respiratory distress. In the absence of hemodynamically relevant mitral regurgitation, LA enlargement is largely caused by LV diastolic dysfunction with a resulting increase in LVFP. The importance of other causes such as atrial myocarditis, neurohormonal activation, or primary genetic abnormalities is unknown. Therefore, LA enlargement currently is considered a morphophysiologic expression 39 of LV diastolic dysfunction, with increasing LA size corresponding to progressively worse LV diastolic function and atrial hypertension.39, 40 Our results confirm previous findings on the association between LA size and CHF status in cats with HCM.41, 42 Progressive LA dilatation (and dysfunction) reflects the severity and chronicity of the disease37, 38, 43 and consistently has been reported as a negative prognostic indicator in people40, 44 and cats41, 42, 45 with HCM. Left atrial size is 1 of the 4 major diagnostic criteria in the echocardiographic evaluation of LV diastolic function and the noninvasive assessment of LVFP in people based on the 2016 American Society of Echocardiography/European Association of Cardiovascular Imaging guidelines. 38 Left atrial enlargement, found in cats with respiratory distress and thus increased pretest probability for heart failure, is a simple and easy‐to‐acquire echocardiographic finding to not only identify heart disease in cats but also to determine the severity of LA pressure overload and thus support the clinical diagnosis of left‐sided CHF.
In general, DE estimation of LVFP is more challenging when LV systolic function is preserved.38, 43, 44 Under such circumstances, individual variables of transmitral flow have rather weak correlations with filling pressures, as demonstrated in people with HCM.7, 24, 44, 46 Although LV filling variables correlate closely with heart failure status in DCM, a condition characterized by a systolic dysfunction‐dominant influence on LV filling,7, 8, 22, 38 they are much less accurate for DMVD22, 38 and HCM,6, 7, 24, 38 conditions characterized by volume overload dominance or delayed relaxation‐dominant influence on LV filling, respectively. Although early validation studies 7 rejected the usefulness of DE in the detection of increased LVFP in humans with HCM, it was found later that filling pressures can be estimated with reasonable accuracy in HCM using the E:E′ ratio (r = 0.82, P < .001) whereas both IVRT and E:A were not suitable. 6 The modest correlation between DE variables and LVFP in HCM likely is related to the phenotypic and functional heterogeneity of the disease with variable degrees of wall thickness, muscle mass, amount of fiber disarray and fibrosis, and obstructive versus nonobstructive physiology.38, 43 These differences lead to different combinations of abnormal relaxation, chamber compliance, and myocardial stiffness with resultant variations of transmitral flow patterns. 38 Recent guidelines 38 for the evaluation of LV diastolic function in humans with HCM recommend a more comprehensive approach in the determination of LVFP not based on single DE variables but rather considering a variety of indices including transmitral flow (E:A and E), tissue Doppler (E′ and E:E′), 2D (LA volume), PV flow (AR dur and Adur:ARdur), and peak velocity of tricuspid regurgitation. Our results confirm, at least in part, prior findings on LV filling flow patterns in HCM in cats41, 47 and people.6, 8, 48, 49 The E:A ratio, if available, was significantly different among groups and most useful in the detection of CHF, with a ratio ≥1.77 suggesting presence of CHF with high accuracy. In contrast, E, IVRT, E:IVRT, E:E′, and variables of PV flow were less useful in heart failure diagnosis. In humans with HCM, an E:A ratio ≤0.80 and a mitral E velocity ≤0.50 m/s reliably rule out increased filling pressures whereas an E:A ≥ 2.0 confirms increased LVFP with high accuracy.38, 43, 50, 51, 52, 53 For results that do not fall within these 2 groups, E′ and E:E′, LA volume index and tricuspid regurgitation velocity also are considered. 43 Similar guidelines do not exist for cats, but a comprehensive, multivariable approach should be given precedence over the evaluation of individual variables in the detection of increased filling pressures in cats with HCM.
The high incidence of summated transmitral filling flow waveforms observed in our study (approximately 75% in cats with CHF) was challenging in the evaluation of LV diastolic function and led to the investigation of the novel variable EAfus:E′A′fus to detect increased filling pressures and CHF. This variable was significantly different among groups, and a diagnostic threshold of 15.0 (Group 1 and Group 2 versus Group 3) and 15.1 (Group 2 versus Group 3) had high Sp (100%) for the diagnosis of CHF but modest Sn (63%) with AUC of 0.78 and 0.74, respectively. Although diagnostic accuracy was relatively low with only 3 of 4 cats correctly identified (considering the AUC), the use of this variable may be appealing in cats with fused EA and E′A′ waves when other, more useful variables are not readily available.
In addition to the E:A ratio, other ratio indices frequently have been used in the detection of increased filling pressures and CHF. The rationale for the use of E:E′ and E:IVRT is to correct for the influence of impaired relaxation on a variable that is largely dependent on relaxation and filling pressure (E velocity).10, 29, 44 Controversy exists regarding the use of E:E′ in people with HCM, with some studies reporting reasonably good correlation with LVFP,6, 16, 17 and others rejecting such associations.7, 24, 46 Our results support the lack of correlation between the presence of CHF and E:E′ because no significant difference between E:E′ in cats with occult HCM and those with CHF was found. However, because of the small number of observations, our study is likely underpowered to reliably detect differences between groups. More data are needed to fully assess the diagnostic value of E:E′ in cats with HCM.
The E:IVRT ratio is another index used to correct for the effects of relaxation on E, and thus relates to LVFP. This variable recently was found to best detect the presence of CHF in dogs with pacing‐induced heart failure 21 and CHF secondary to DCM and DMVD. 22 However, E:IVRT performed poorly in detecting heart failure in our study, which is in agreement with results of a previous retrospective study in cats with HCM, 41 although both studies were limited by a small number of data points, minimizing their validity. Absence of a true relationship between E:IVRT and LVFP in HCM because of the diverse morphological and functional features of HCM as well as a disproportionate effect of delayed relaxation on DE variables in HCM are potential reasons for the poor performance of E:IVRT.
The time between completion of systole and onset of transmitral flow determines IVRT. The latter is a surrogate marker of relaxation11, 54 but is also largely influenced by LVFP. 55 Because impaired relaxation is an integral part of the pathophysiology of HCM and high filling pressure is a key hemodynamic characteristic of CHF, IVRT should be prolonged in preclinical disease and shortened in HCM with CHF. Low IVRT has been found very useful diagnostically in the DE detection of CHF in dogs.21, 22, 23 Similar observations were made in our study, and most cats with CHF had low IVRT. However, prolonged IVRT did not always rule out CHF as would be expected. Three of 15 cats (20%) with CHF had prolonged IVRT. In 1 cat with E:A > 2.0 and diastolic‐dominant pulmonary venous flow (both suggestive of a restrictive filling pattern with high LVFP), IVRT was increased at 66 ms (normal, 37‐60 ms). 29 Another cat with pseudonormal LV filling (E:A between 1.0 and 2.0 suggestive of increased LVFP) also had increased IVRT at 64 ms. In these cases, the interpretation of IVRT was misleading because prolonged IVRT suggested relatively normal filling pressure, thus masking the presence of CHF.
4.2. Detection of CHF using echocardiographic composite variables (class of diastolic function)
For a variety of reasons outlined above, echocardiographic detection of increased LVFP and CHF cannot be accomplished reliably using single echocardiographic variables.38, 43, 44 Instead, grading systems that consider a composite of multiple key echocardiographic indices and establishment of diastolic functional classes have been used. Echocardiographic staging of LV diastolic dysfunction in humans with HCM in accordance with disease severity and prognosis has been reported as early as 1987 49 and refined later.5, 8, 38, 44, 48, 56 Four basic patterns have been described primarily based on the E:A ratio: normal, delayed relaxation flow (mild diastolic dysfunction with normal LVFP), pseudonormal flow (moderate diastolic dysfunction with increased LVFP), and restrictive flow (severe diastolic dysfunction with severely increased filling pressure). 29 Classification into stages implies that progression of disease occurs in a relatively predictable manner with concurrent worsening of function as filling pressure increases.5, 7, 48 In our study, LV diastolic function was classified based on the E:A ratio alone (“E:A class”) and also based on a composite of 7 echocardiographic variables 29 of which only 2 had to be positive for class assignment (diastolic class or D‐class). Although E:A class detected CHF with reasonable accuracy, it was affected by missing values as a consequence of E‐A fusion (13% in control cats, 29% in preclinical HCM cats, and 67% in CHF cats). In addition, it was inferior to D‐class, with a 10% higher diagnostic accuracy of D‐class versus E:A class. Diastolic class could be evaluated in all cats, making it the preferred variable for staging diastolic dysfunction. Our findings confirmed previous observations45, 47, 57, 58 that most cats (8/12, 67%) with HCM and pseudonormal LV filling (moderate diastolic dysfunction) and almost all cats (7/8, 88%) with restrictive LV filling (severe LV diastolic dysfunction) were in CHF, whereas a delayed relaxation pattern and normal filling flow were less likely to be associated with CHF. Classification of diastolic function using D‐class seems clinically feasible and provides flexibility because it can be applied despite missing individual variables. However, further validation studies, involving larger groups, are needed to assess its use in clinical practice.
4.3. Detection of CHF using respiratory rate
Tachypnea is a common finding in cats with CHF and thus appears to be a simple variable that can be monitored by the owner to identify possible decompensation of cardiac disease. In our study, in‐hospital respiratory rate to detect the presence of CHF did not perform as well as in dogs with CHF as documented in an earlier investigation.22, 23 A diagnostic cutoff of 36 breaths per minute was highly sensitive (100%) but not specific (51%) for identifying CHF despite a highly significant difference between groups. Moreover, clinically relevant overlap was found between the in‐hospital respiratory rates for symptomatic and asymptomatic cats with HCM. This overlap possibly is associated with increased hospital stress commonly observed in cats that could increase respiratory rate regardless of CHF status. Thus, in‐hospital respiratory rate is not a reliable indicator of CHF in cats with HCM and is inferior to DE variables.
4.4. Limitations
Our study had several limitations. Group size was relatively small, limiting power to detect statistically significant differences between groups. Moreover, small group size limited the ability to further stratify groups based on the presence or absence of left ventricular outflow tract obstruction or the presence of pericardial effusion as a manifestation of left‐sided heart failure. Many variable sets were incomplete largely owing to the summation of filling waves and absence of late diastolic waves, possibly because of poor atrial function. In addition, we applied an arbitrary cutoff of 0.4 m/s as the point at which partial fusion would be tolerated when assessing transmitral E and A waves. This decision may have influenced our results. The fact that all cats were sedated and some cats in the CHF group were treated with furosemide may have affected DE variables. A true gold standard for assessing LVFP was not applied. Instead, clinical signs and thoracic radiographic findings were used as surrogate methods to diagnose CHF and thus increased filling pressures. Left atrial dimension and IVRT were not corrected for body weight and heart rate, respectively, which may have affected their diagnostic performance. Lastly, population bias is likely present because all cases were recruited from a single tertiary referral clinic.
In conclusion, echocardiography can be used to detect the presence of CHF in cats with HCM with good accuracy. The transmitral E:A ratio, variables of LA size, and diastolic functional class performed best whereas IVRT, E:IVRT, E:E′, and in‐hospital respiratory rate were diagnostically inferior. Variables and diagnostic cutoffs applicable to cats with HCM and CHF differed relevantly from dogs with DCM and DMVD and CHF,21, 22, 23 making use of disease‐specific variables and discrimination limits in the echocardiographic diagnosis of LVFP and CHF in cats with HCM necessary. Use of the indices suggested here may improve patient care in cats with HCM. Larger prospective studies are needed to further validate our findings.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approval of this study by IACUC and the local Clinical Research Committee was granted.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
The authors thank Alicia Byrd and Olivia Stepp (cardiology technicians), Emily Chapel and Samantha Kochie (cardiology residents), and Marc Hardman (education assistant). An abstract of this study was presented at the 2019 ACVIM Forum, Phoenix, AZ.
Rohrbaugh MN, Schober KE, Rhinehart JD, Bonagura JD, Habing A, Yildiz V. Detection of congestive heart failure by Doppler echocardiography in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2020;34:1091–1101. 10.1111/jvim.15777
This work was completed at The Ohio State University, Columbus, OH.
Funding information American College of Veterinary Internal Medicine, Grant/Award Number: Cardiology Resident Research Grant; College of Veterinary Medicine, Ohio State University, Grant/Award Number: Intramural Feline Grant
REFERENCES
- 1. Payne JR, Brodbelt DC, Luis Fuentes V. Cardiomyopathy prevalence in 78 apparently healthy cats in rehoming centers (the CatScan study). J Vet Cardiol. 2015;17:S244‐S257. [DOI] [PubMed] [Google Scholar]
- 2. Soufer R, Wohlgelernter D, Vita NA, et al. Intact systolic left ventricular function in clinical congestive heart failure. Am J Cardiol. 1984;53:567‐571. [DOI] [PubMed] [Google Scholar]
- 3. Dougherty AH, Naccarelli GV, Gray EL, Hicks CH, Goldstein RA. Congestive heart failure with normal systolic function. Am J Cardiol. 1984;54:778‐782. [DOI] [PubMed] [Google Scholar]
- 4. Benigni L, Morgan N, Lamb CR. Radiographic appearance of cardiogenic pulmonary edema in 23 cats. J Small Anim Pract. 2009;50:9‐14. [DOI] [PubMed] [Google Scholar]
- 5. Appleton CP, Hatle LK. The natural history of left ventricular filling abnormalities: assessment by two‐dimensional and Doppler echocardiography. Echocardiogr. 1992;9:437‐457. [Google Scholar]
- 6. Nagueh SF, Lakkis NM, Middleton KJ, Spencer WH III, Zoghbi WA, Quiñones MA. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation. 1999;99:254‐261. [DOI] [PubMed] [Google Scholar]
- 7. Nishimura RA, Appleton C, Redfied MM, et al. Noninvasive Doppler echocardiographic evaluation of left ventricular filling pressure in patients with cardiomyopathies: a simultaneous Doppler echocardiographic and cardiac catheterization study. J Am Coll Cardiol. 1996;28:1226‐1233. [DOI] [PubMed] [Google Scholar]
- 8. Hurrell DG, Nishimura RA, Ilstrup DM, Appleton CP. Utility of preload alteration in assessment of left ventricular filling pressure by Doppler echocardiography: a simultaneous catheterization and Doppler echocardiographic study. J Am Coll Cardiol. 1997;30:459‐467. [DOI] [PubMed] [Google Scholar]
- 9. Appleton CP, Galloway JM, Gonzales MS, et al. Estimation of left ventricular filling pressures using two‐ dimensional and Doppler echocardiography in adult patients with cardiac disease. J Am Coll Cardiol. 1993;22:1972‐1982. [DOI] [PubMed] [Google Scholar]
- 10. Choong CY, Abascal VM, Thomas JD, Guerrero JL, McGlew S, Weyman AE. Combined influence of ventricular loading and relaxation on transmitral flow velocity profile in dogs measured by Doppler echocardiography. Circulation. 1988;78:672‐683. [DOI] [PubMed] [Google Scholar]
- 11. Schober KE, Luis Fuentes V, Bonagura JD. Comparison between invasive hemodynamic measurements and noninvasive assessment of left ventricular diastolic function by use of Doppler echocardiography in healthy anesthetized cats. Am J Vet Res. 2003;64:93‐103. [DOI] [PubMed] [Google Scholar]
- 12. Schober KE, Luis Fuentes V. Quantitative Doppler‐echocardiographic assessment of left ventricular diastolic function in dogs. Tierärztl Prax. 1998;26:13‐20. [PubMed] [Google Scholar]
- 13. Schober KE, Luis Fuentes V. Doppler echocardiographic assessment of left ventricular diastolic function in 74 boxer dogs with aortic stenosis. J Vet Cardiol. 2002;4:7‐16. [DOI] [PubMed] [Google Scholar]
- 14. Sohn DW, Chai IH, Lee DJ, et al. Assessment of mitral annulus velocity by tissue Doppler imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474‐480. [DOI] [PubMed] [Google Scholar]
- 15. Naqvi TZ. Diastolic function assessment incorporating new techniques in Doppler echocardiography. Rev Cardiovasc Med. 2003;4:81‐99. [PubMed] [Google Scholar]
- 16. Nagueh SF, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527‐1533. [DOI] [PubMed] [Google Scholar]
- 17. Kim YK, Sohn DW. Mitral annulus velocity in the estimation of left ventricular filling pressure: prospective study in 200 patients. J Am Soc Echocardiogr. 2000;13:980‐985. [DOI] [PubMed] [Google Scholar]
- 18. Gonzales‐Vilchez F, Ayuela J, Ares M, et al. Comparison of Doppler echocardiography, color M‐mode Doppler, and Doppler tissue imaging for the estimation of pulmonary capillary wedge pressure. J Am Soc Echocardiogr. 2002;15:1245‐1250. [DOI] [PubMed] [Google Scholar]
- 19. Oyama MA, Sisson DD, Bulmer B, Constable PD. Echocardiographic estimation of mean left atrial pressure in a canine model of acute mitral valve insufficiency. J Vet Intern Med. 2004;18:679‐692. [DOI] [PubMed] [Google Scholar]
- 20. Schober KE, Bonagura JD, Scansen BA, Stern JA, Ponzio NM. Estimation of left ventricular filling pressure by use of Doppler echocardiography in healthy anesthetized dogs subjected to acute volume loading. Am J Vet Res. 2008;69:1034‐1049. [DOI] [PubMed] [Google Scholar]
- 21. Schober KE, Stern J, DaCunha D, et al. Estimation of left ventricular filling pressure by Doppler echocardiography in dogs with pacing‐induced heart failure. J Vet Intern Med. 2008;22:578‐585. [DOI] [PubMed] [Google Scholar]
- 22. Schober KE, Hart TM, Stern JA, et al. Detection of congestive heart failure in dogs by Doppler echocardiography. J Vet Intern Med. 2010;24:1358‐1368. [DOI] [PubMed] [Google Scholar]
- 23. Schober KE, Hart TM, Stern JA, et al. Assessment of the effects of treatment on respiratory rate, serum natriuretic peptides concentrations, and Doppler variables of filling pressure in dogs with congestive heart failure secondary to degenerative mitral valve disease and dilated cardiomyopathy. J Am Vet Med Assoc. 2011;239:468‐479. [DOI] [PubMed] [Google Scholar]
- 24. Bhella PS, Pacini EL, Prasad A. Echocardiographic indices do not reliably track changes in left‐sided filling pressure in healthy subjects or patients with heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2011;4:289‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Häggström J, Luis Fuentes V, Wess G. Screening for hypertrophic cardiomyopathy in cats. J Vet Cardiol. 2015;17(Suppl 1):S134‐S149. [DOI] [PubMed] [Google Scholar]
- 26. Litster A, Buchanan JW. Vertebral scale system to measure heart size in radiographs of cats. J Am Vet Med Assoc. 2000;216:210‐214. [DOI] [PubMed] [Google Scholar]
- 27. Guglielmini C, Diana A. Thoracic radiography in the cat: identification of cardiomegaly and congestive heart failure. J Vet Cardiol. 2015;17:S87‐S101. [DOI] [PubMed] [Google Scholar]
- 28. Mainville CA, Clark GH, Esty KJ. Analytical validation of an immunoassay for the quantification of N‐terminal pro‐B‐type natriuretic peptide in feline blood. J Vet Diagn Invest. 2015;27(4):414‐421. [DOI] [PubMed] [Google Scholar]
- 29. Schober KE, Chetboul V. Echocardiographic evaluation of left ventricular diastolic function in cats: hemodynamic determinants and pattern recognition. J Vet Cardiol. 2015;17:S102‐S133. [DOI] [PubMed] [Google Scholar]
- 30. Boon JA. Evaluation of size, function, and hemodynamics In: Boon JA, ed. Manual of Veterinary Echocardiography. 2nd ed. Chichester, UK: Wiley‐Blackwell; 2011:153‐156. [Google Scholar]
- 31. Schober KE, März I, Ludewig E, et al. Diagnostic accuracy of electrocardiography and thoracic radiography in the assessment of left atrial size in cats: comparison with transthoracic 2‐dimensional echocardiography. J Vet Intern Med. 2007;21:709‐718. [DOI] [PubMed] [Google Scholar]
- 32. O'Grady MR, Bonagura JD, Powers JD, et al. Quantitative cross‐sectional echocardiography in the normal dog. Vet Radiol. 1986;27:34‐49. [Google Scholar]
- 33. Abbott JA, MacLean HN. Two‐dimensional echocardiographic assessment of the feline left atrium. J Vet Intern Med. 2006;20:111‐119. [DOI] [PubMed] [Google Scholar]
- 34. Santilli RA, Bussadori C. Doppler echocardiographic study of left ventricular diastole in non‐anesthetized healthy cats. Vet J. 1998;156:203‐215. [DOI] [PubMed] [Google Scholar]
- 35. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107‐133. [DOI] [PubMed] [Google Scholar]
- 36. Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217‐238. [DOI] [PubMed] [Google Scholar]
- 37. Yang WI, Shim CY, Kim YJ, et al. Left atrial volume index: a predictor of adverse outcome in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2009;22(12):1338‐1343. [DOI] [PubMed] [Google Scholar]
- 38. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277‐314. [DOI] [PubMed] [Google Scholar]
- 39. Tsang TSM, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284‐1289. [DOI] [PubMed] [Google Scholar]
- 40. Bauer F, Shiota T, White RD, et al. Determinants of left atrial dilatation in patients with hypertrophic cardiomyopathy: a real‐time 3‐dimensional echocardiographic study. J Am Soc Echocardiogr. 2004;17:968‐975. [DOI] [PubMed] [Google Scholar]
- 41. Linney CJ, Dukes‐McEwan J, Stephenson HM, López‐Alvarez J, Fonfara S. Left atrial size, atrial function and ventricular diastolic function in cats with hypertrophic cardiomyopathy. J Small Anim Pract. 2014;55:198‐206. [DOI] [PubMed] [Google Scholar]
- 42. Payne J, Luis Fuentes V, Boswood A, Connolly D, Koffas H, Brodbelt D. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997‐2005). J Small Anim Pract. 2010;51:540‐547. [DOI] [PubMed] [Google Scholar]
- 43. Nagueh SF. Non‐invasive assessment of left ventricular filling pressure. Eur J Heart Fail. 2018;20:38‐48. [DOI] [PubMed] [Google Scholar]
- 44. Andersen OS, Smiseth OA, Dokainish H, et al. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol. 2017;69(15):1937‐1948. [DOI] [PubMed] [Google Scholar]
- 45. Payne JR, Borgeat K, Connolly DJ, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2013;27(6):1427‐1436. [DOI] [PubMed] [Google Scholar]
- 46. Geske JB, Sorajja P, Nishimura RA. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation. 2007;116(23):2702‐2708. [DOI] [PubMed] [Google Scholar]
- 47. Payne JR, Luis Fuentes V, Connolly D, et al. Transmitral flow velocity patterns and survival in feline hypertrophic cardiomyopathy [abstract]. Proceedings of the 20th ECVIM‐CA Congress, Toulouse, France; 2010.
- 48. Ohno M, Cheng CP, Little WC. Mechanism of altered patterns of left ventricular filling during the development of congestive heart failure. Circulation. 1994;89:2241‐2250. [DOI] [PubMed] [Google Scholar]
- 49. Maron BJ, Spirito P, Green KJ, Wesley YE, Bonow RO, Arce J. Noninvasive assessment of left ventricular diastolic function by pulsed Doppler echocardiography in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1987;10:733‐742. [DOI] [PubMed] [Google Scholar]
- 50. Pinamonti B, Di Lenarda A, Nucifora G, et al. Incremental prognostic value of restrictive filling pattern in hypertrophic cardiomyopathy: a Doppler echocardiographic study. Eur J Echocardiogr. 2008;9(4):466‐471. [DOI] [PubMed] [Google Scholar]
- 51. Biagini E, Spirito P, Rocchi G, et al. Prognostic implications of the Doppler restrictive filling pattern in hypertrophic cardiomyopathy. Am J Cardiol. 2009;104:1727‐1731. [DOI] [PubMed] [Google Scholar]
- 52. Maskatia SA, Decker JA, Spinner JA, et al. Restrictive physiology is associated with poor outcomes in children with hypertrophic cardiomyopathy. Pediatr Cardiol. 2012;33:141‐149. [DOI] [PubMed] [Google Scholar]
- 53. Kubo T, Gimeno JR, Bahl A, et al. Prevalence, significance, and genetic basis of hypertrophic cardiomyopathy with restrictive phenotype. J Am Coll Cardiol. 2007;49:2419‐2426. [DOI] [PubMed] [Google Scholar]
- 54. Riesen SC, Schober KE, Smith DN, Otoni CC, Li X, Bonagura JD. Effects of ivabradine on heart rate and left ventricular function in healthy cats and cats with hypertrophic cardiomyopathy. Am J Vet Res. 2012;73:202‐212. [DOI] [PubMed] [Google Scholar]
- 55. Myreng Y, Smiseth OA. Assessment of left ventricular relaxation by Doppler echocardiography. Comparison of isovolumic relaxation time and transmitral flow velocities with time constant of isovolumic relaxation. Circulation. 1990;81:260‐266. [DOI] [PubMed] [Google Scholar]
- 56. Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol. 1988;12:426‐440. [DOI] [PubMed] [Google Scholar]
- 57. Payne JR, Borgeat K, Brodbelt DC, Connolly DJ, Luis Fuentes V. Risk factors associated with sudden death vs. congestive heart failure or arterial thromboembolism in cats with hypertrophic cardiomyopathy. J Vet Cardiol. 2015;17:S318‐S328. [DOI] [PubMed] [Google Scholar]
- 58. Schober KE, Hart T. Left ventricular diastolic dysfunctionand diastolic heart failure in cats with hypertrophic cardiomyopathy: value of Doppler echocardiography in disease staging [abstract]. J Vet Intern Med. 2008;22:1469. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


