Abstract
Background
The use of serological markers to diagnose inflammatory bowel disease (IBD) in humans is well‐established. Because of the frequency of IBD in dogs and resources required for its diagnosis with current methods, new approaches are desired.
Objective
The goal is to evaluate novel serologic markers to differentiate clinical cohorts in dogs with gastrointestinal (GI) disease and assess their potential to develop a serum‐based IBD diagnostic test.
Animals
Seventy dogs diagnosed with biopsy‐confirmed IBD, 23 dogs with non‐IBD predominantly acute GI diseases, and 58 normal dogs.
Methods
Prospective control study. ELISA methods were developed to detect autoantibodies to polymorphonuclear leukocytes (APMNA) and calprotectin (ACNA), antibodies against gliadins (AGA), microbial outer membrane porin C (ACA), and flagellins (AFA) isolated from diseased dogs based on clinical and histopathological scoring.
Results
IBD dogs displayed a 39%‐76% prevalence of seropositivity against selected serologic markers that markedly decreased to 0%‐13% in non‐IBD and normal dogs. ROC analysis showed statistical significance in differentiating the cohorts, with seropositivity against OmpC being the highest single performance marker. The combination of markers such as OmpC and APMNA reached specificities of 93%‐99% and 79%‐98% and sensitivities of 76%‐97% and 66%‐86% when comparing IBD versus normal cohorts and non‐IBD cohorts, respectively.
Conclusion and Clinical Importance
Seropositivity of canine immunoglobulins A against selected serologic markers in dogs appears promising in the detection and differentiation of IBD versus other acute GI conditions. Among them, antibody reactivity to Escherichia coli OmpC and canine autoantibodies against polymorphonuclear leukocytes displayed the highest single marker discriminating performance.
Keywords: calprotectin, flagellin, gliadins, OmpC, polymorphonuclear leukocytes.
Abbreviations
- ACA
anti‐OmpC antibody
- ACNA
anti‐calprotectin antibody
- AFA
anti‐flagellin antibody
- AGA
anti‐gliadin antibody
- APMNA
anti‐polymorphonuclear leukocytes antibody
- ASCA
anti‐Saccharomyces cerevisiae antibody
- AUC
area under the curve
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- Ig
immunoglobulin
- OmpC
outer membrane porin C
- pANCA
antineutrophil cytoplasmic antibodies with perinuclear staining
- ROC
receiver operating characteristics
- UC
ulcerative colitis
- WSAVA
World Small Animal Veterinary Association
1. INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic and relapsing disorder of the gastrointestinal (GI) tract characterized by mucosal inflammation and marked by recurrent diarrhea and vomiting.1, 2 IBD diagnosis is based on a complex approach combining patient history, physical examination and diagnostic tools such as routine hematologic parameters, fecal analysis, abdominal ultrasound, gastroduodenoscopy/colonoscopy, and mucosal biopsy procedures.3, 4 The diagnosis of IBD in dogs remains an expensive, time‐consuming, and invasive process. The identification of serum‐based biomarkers associated with IBD has the potential to improve the diagnostic process while minimizing time and invasiveness. In human medicine, serological markers for IBD have been available for years. 5 Hematologic parameters include leukocyte and thrombocyte counts as well as C‐reactive protein detection. Fecal markers (ie, lactoferrin and calprotectin) are routine but are nonspecific to intestinal inflammation. 6 Human serologic markers that are more specific include antineutrophil cytoplasmic antibodies with perinuclear staining (pANCA), anti‐Saccharomyces cerevisiae antibodies (ASCA), and outer membrane porin C (OmpC), among others. 7
While the pathogenesis of IBD is not completely understood, it is believed to arise from complex interactions involving the immune system, enteric commensal bacteria and genetic factors, both in humans and dogs. Indeed, a unifying hypothesis has emerged that proposes that IBD results from a dysregulated mucosal immune response to the intestinal microbiota in susceptible individuals. 6 Serological markers are important in IBD because their expression represents the host response to translocation of intestinal pathogens into the bloodstream after breakdown of the gut mucosal barrier. 8 In humans, investigations on the cause for intestinal inflammation linked to IBD led to the discovery of antibodies present selectively in patients with ulcerative colitis (UC), Crohn's disease, or both, the 2 main IBD manifestations. 9 Furthermore, human IBD patients also present autoantibodies directed against a component of neutrophil granules.10, 11 Presently, most physicians use a combination of serological markers and autoantibodies to assist in the diagnosis of a complex array of GI diseases, including IBD.12, 13, 14 In veterinary medicine, there is an association between clinical phenotypes in dogs diagnosed with IBD and seroreactivity to microbes and self‐antigens.15, 16
Given the broad usage of serologic markers in human medicine, we set out to explore the development of comparable assays in dogs. Dogs, like humans with IBD, have the potential to present with antibodies directed to various autologous proteins that are associated in the pathogenesis of this chronic disease. These autoantibodies are potentially resulting from extended and sensitized exposure to self‐proteins that can be detected differentially in IBD versus normal cohorts. The objectives of our study were to develop canine‐specific assays that would be formatted as enzyme‐linked immunosorbent assay (ELISA) based on canine‐associated antigens selected among markers known to be associated with IBD in humans. These assays would detect the presence of canine antibodies, specifically of the immunoglobulin A (IgA)‐type, against autoantibodies against canine polymorphonuclear leukocytes (APMNA) and canine calprotectin (ACPA) as well as the presence of antigens derived from microbial presence and dietary contributions. The IgAs are the second most dominant isotype in blood (only after IgG) and known to be largely produced in the mucosal lymphoid tissues and play important roles in mucosal immunity. 17 These assays would be used to assess the potential clinical value of these serological markers to diagnose IBD in dogs.
2. MATERIALS AND METHODS
2.1. Study cohorts
The IBD cohort was composed of 70 dogs of various ages, sex, and breeds that presented to 3 hospitals in Southern California with chronic GI signs and were diagnosed as IBD for our study. Inclusion criteria were vomiting, diarrhea, anorexia, weight loss, or some combination of these signs for at least 3 weeks. No immunosuppressive drugs or antibiotics were administered by the owners for at least 10 days before sample acquisition. Furthermore, a complete clinical evaluation was performed, including hematology, clinical biochemistry, and, as required, fecal flotation, Giardia antigen test, trypsin‐like immunoreactivity (TLI) test and abdominal ultrasound to exclude infectious, endocrine, or neoplastic diseases. Owners gave written consent for their dogs to take part in the study. Gastroduodenoscopy was performed in all dogs of this cohort, and endoscopic biopsy samples from the stomach, duodenum, and in many instances' colon, were taken with flexible endoscopy biopsy forceps. All dogs had intestinal infiltration with inflammatory cells and lesions were graded using the World Small Animal Veterinary Association (WSAVA) guidelines. Based on the chronicity of GI signs, the exclusion of underlying infectious, endocrine, or neoplastic diseases, and the histological inflammatory findings, these dogs were diagnosed as suffering from IBD.
The non‐IBD cohort was composed of 23 dogs that presented with GI clinical signs such as diarrhea or vomiting, and were associated with episodic, short duration, or both clinical signs generally lasting a few days. These patients were confirmed by the attending clinicians to have a wide array of underlying causes for these clinical signs, including, but not limited to, dietary indiscretion, acute viral or bacterial infection, parasitism, and toxin ingestion. Each patient was followed by the attending clinician to ensure that the clinical signs not only resolved with appropriate treatments, but that they did not return for a minimum of 3 months. Although every effort was made to diagnose the non‐IBD cohort appropriately, they were not subjected to endoscopic procedures as it was generally not supported by the owners, considered unethical from a practicing perspective, or both.
The normal cohort consisted of 58 apparently healthy dogs of various ages, sex, and breeds presenting no significant GI clinical signs at the time of visit (ie, there were asymptomatic), no known history of GI distress recurrences, admitted for regular checkups, or all.
2.2. Clinical and histopathological scoring
All IBD dogs were scored according to the canine IBD activity index (CIBDAI). 18 Full thickness biopsies, endoscopy biopsies, or both were immediately placed in ice‐cold phosphate‐buffered saline (PBS) and 4% buffered paraformaldehyde solution until processed. All tissue samples were processed and graded by 1 pathologist (Dr Barbara E. Powers, Colorado State University) according to the WSAVA International Gastrointestinal Standardization Group guidelines. Multiple morphological parameters such as epithelial injury, crypt distension, lacteal dilatation, mucosal fibrosis, and inflammatory histological parameters such as plasma cells, lamina propria lymphocytes, eosinophils, and neutrophils were scored, and the resulting final scores were subdivided into histological severity groups: WSAVA score of 0, normal; 1‐6, mild; 7‐12, moderate; and >13, severe. Endoscopy scoring 19 was performed based on qualitative evaluation of endoscopic mucosal appearance which included friability, granularity, erosions/ulcerations and lymphatic dilatation. The scores range from 1 (normal) to 4 (marked change).
2.3. Antigen cloning and expression
Microorganism cultures were isolated from biopsy samples from 20 dogs with IBD and genotyped using 16S rRNA gene sequencing. Genomic DNA was extracted from frozen microorganism cultures according to the manufacturer's protocol using the ZR fungal/bacterial DNA Isolation Kit (Zymo‐Research) with ultra‐high‐density bashing beads. The DNA preparations were stored at −20°C until analysis. Coding region of the genes of interest (ie, calprotectin and flagellins) were amplified by PCR amplification. PCR reactions were carried out in a 25 μL final volume containing the reaction master mix supplemented with a Taq DNA polymerase (Thermo Fisher scientific), the DNA template, and 0.5 μM of each of the forward and reverse primers, and reactions conducted at 94°C for 4‐5 minutes followed by amplification for 30 cycles (95°C for 30 seconds, 50°C for 30 seconds, 72°C for 60 seconds) and an extension at 72°C for 10 minutes. The PCR products were cloned into the vector pJET1.2 and sequenced to confirm gene identity. Coding regions were then cloned into a bacterial expression vector containing a histidine tag according to the manufacturer's recommendations (Life Technologies). Coding regions of the calprotectin genes were assembled using synthetic oligonucleotides containing a histidine tag. Recombinant products were purified using a nickel‐charged purification resin.
2.4. Determination of antibody levels in dog sera against specific antigens
Canine IgA antibodies and autoantibodies against specific antigens including food derived gliadins were detected by direct ELISA. Sera from healthy and affected dogs were analyzed in duplicate for IgA and IgG reactivity to canine polymorphonuclear leukocytes (APMNA); canine‐isolated bacterial OmpC (ACA), and flagellin (AFA); food derived gliadins (AGA); and canine calprotectin (ACNA) as follows. For APMNA, microtiter plates were coated with 12.5 × 103 to 200 × 103 PMN per well isolated from dog blood sample collected from a single dog. A layer of PMN was recovered after centrifugation of the whole blood at 18‐25°C and treated with a hypotonic solution to lyse red blood cells. PMN were treated with cold 95% methanol and 5% acetic acid for 20 ± 10 minutes to fix the cells. Cells were incubated for 60 ± 30 minutes at 18‐25°C with 1% bovine serum albumin (BSA) in phosphate‐buffered saline to block nonspecific antibody binding. Next, after 3 washes with Tris Buffered Saline‐Tween (TBS‐T, 25.0 mM Tris‐HCl, 2.7 mM potassium chloride, 137 mM sodium chloride, 0.05% Tween‐20, pH 7.4 ± 0.2), control sera and test sample sera were added at a 1:100‐1:200 dilutions to the microtiter plates and incubated for 60 ± 30 minutes at 18‐25°C. After 3 washes with TBS‐T, alkaline phosphatase‐conjugated anti‐canine IgA was added at a 1:2000 dilution to label PMN‐bound antibody and incubated for 60 ± 30 minutes at 18‐25°C. A solution of p‐nitrophenol phosphate substrate was added, and color development was allowed to proceed for 30 ± 10 minutes. The optical density (OD) was measured at 405 nm using an ELISA plate reader.
For all other antigens, microtiter plates were coated overnight at 4°C with 100 μL/well at 0.2‐10 μg/mL antigen in carbonate solution (100.0 mM NaHCO3‐Na2CO3 buffer, pH 9.5 ± 0.5). The plates were washed thrice with TBS‐T (25.0 mM Tris‐HCl, 2.7 mM potassium chloride, 137 mM sodium chloride, 0.05% Tween‐20, pH 7.4 ± 0.2) and blocked with 200 μL/well TBS/BSA (25.0 mM Tris‐HCl, 2.7 mM potassium chloride, 137 mM sodium chloride, pH 7.4 ± 0.2, 1% BSA) for 1 hour at 18‐25°C. After washing the plates thrice with TBS‐T, the standard and sample sera were added to each well and incubated at 18‐25°C for 1 hour. The plates were then washed thrice with TBS‐T and incubated for 1 hour at 18‐25°C with horseradish peroxidase (HRP)‐anti‐dog IgA antibody diluted 1:5000 in TBS/BSA. The plates were washed thrice with TBS‐T and developed using 100 μL/well of 3,3′,5,5′‐tetramethylbenzidine (TMB) substrate. The reaction was stopped with 0.33 M H2SO4 and the OD was measured at 450 nm using an ELISA plate reader.
2.5. Statistical analysis
Statistical analysis was conducted using R (2016, R Foundation for Statistical Computing, Vienna, Austria) or Microsoft Office Excel (2013, Microsoft, Redmond, Washington). Mean, median, minimum, maximum, and percentile were calculated. 20 Data were analyzed by using the Mann‐Whitney U test or Kruskal‐Wallis test, a nonparametric test, depending on the comparison data cohorts. The data were normally distributed after log transformation. A P‐value <.05 was considered significant.
Statistical analysis included area under receiver operating characteristics (ROC) curves and calculations of diagnostic sensitivity and specificity as appropriate for each of the markers (univariate analysis) and for a combination of markers (multivariate analysis).20, 21
3. RESULTS
3.1. Canine IBD cohort profile
The IBD cohort was comprised of 57% males and 43% females and the top breeds represented were Labrador Retriever, Golden Retriever, German Shepherd, Poodle, Yorkshire Terrier, Bulldog (English and French), Boxer, Dachshund, Corgi, Great Dane, Beagle, Husky, and Schnauzer, all representatives of the most common breeds according to 2018 AKC list and also present in the other cohorts. Sex compositions were represented by 35% males and 65% females in the non‐IBD cohort and 56% males and 44% females in the normal cohort. Mean (±SD) age was 7.3 ± 3.5 years (range, 0.5‐13.5 years), 7.5 ± 4.5 years (range, 0.3‐14.9 years), and 6.3 ± 3.8 years (range, 0.5‐14.6 years) for IBD, non‐IBD, and normal cohorts, respectively with no significant statistical difference between the 3 cohorts.
Serum samples from 70 dogs diagnosed with IBD were collected and analyzed as described in Materials and Methods. The baseline characteristics of the IBD cohort profile are outlined in Table 1. A large proportion of dogs (>85%) presented with visible endoscopic lesions in either the duodenum or colon. The most frequent diagnosis was lymphoplasmacytic enteritis, followed by mixed or eosinophilic inflammation. This distribution is reflective of the most common and second most common type of IBD described in dogs. 22 Forty‐three of the seventy dogs (61%) were also diagnosed with gastritis based on stomach biopsies. Based on duodenum/colon biopsy results, up to 9% of the cohort was classified as severely affected, 48% were classified as moderate and 43% as mild, according to WSAVA guidelines. Dogs with hypoalbuminemia and clinical signs of protein‐losing enteropathy were not excluded as long as there was histopathologic evidence of intestinal inflammatory cellular infiltrates. In the IBD cohort, there were 6 dogs with concurrent gastric mucosal hyperplasia, 5 with pancreatitis, 2 with adrenal‐dependent hyperadrenocorticism, and 2 with potential liver conditions.
TABLE 1.
Baseline characteristics of 70 dogs with inflammatory bowel disease in the symptomatic IBD population
| Baseline characteristics | IBD group (n = 70) |
|---|---|
| Disease duration | 1‐15 months |
| CIBDAI scores a | 5.6 (2‐14) |
| Endoscopic scores b | |
| Stomach | 1.5 (2‐4) |
| Duodenum | 2.0 (1‐4) |
| Colon | 2.0 (1‐4) |
| Histopathology c | |
| Lymphoplasmacytic | 40% |
| Lymphoplasmacytic + eosinophils | 29% |
| Lymphoplasmacytic + suppurative | 5% |
| Eosinophilic | 10% |
| Severity | |
| Mild | 43% |
| Moderate/severe | 75% |
| Hypoalbumimenic | 25% |
| No additional diagnosis d | 85% |
CIBDAI scoring was done by the attending veterinarian.
All canine patients underwent endoscopy/biopsy.
Pathology was performed by Dr Barbara Powers at CSU.
Additional diagnosis included 5 with pancreatitis, 2 with high ACTH, and 2 with potential liver conditions.
3.2. Canine‐specific or ‐associated antigens and assay development
All assays were developed based on canine‐specific or ‐associated markers and with the purpose to be applied to canine samples. The format selected to perform serum sample analysis was based on ELISA because it allows for a rapid screening and quantification in a specific and highly sensitive manner. Key considerations for the format were the use of canine‐specific or ‐associated antigens in the assay and canine immunoglobulins (Igs) as analytes, which would be quantified by using the appropriate secondary antibodies. The antigens derived from bacterial strains were prepared from microorganisms most representative of the flora isolated and genotyped from biopsy of IBD dogs from multiple centers and obtained before initiation of any treatment. The microbial communities of a total of 36 biopsies from 20 dogs diagnosed with IBD were characterized. Gram negative type bacteria belonging to Pseudomonas (up to 11 species) and Escherichia (with 2 species only that is, E. coli and E. fergusonii) were represented in a higher proportion of the samples with 48 and 10%, respectively. The gram‐positive genus with greater representation were identified as Enterococcus (E. faecium and E. faecalis), detected in 17% of the samples. Some other genus that were detected in significant numbers include Proteus, Enterobacter, and Acinetobacter with 86, and 4% respectively, all of them gram‐negative bacteria.
Bacterial proteins belonging to the porins 23 and flagellins 24 class were cloned from multiple strains isolated from IBD dogs. They were subsequently expressed and purified as described in Section 2 and tested against a subset of the IBD cohort (28 dogs out of the larger canine IBD cohort) versus normal. Four Porin (OmpC) encoding genes from different Escherichia isolates and 5 flagellin‐encoding genes from Pseudomomas isolates were cloned, expressed and assayed to interrogate the differential cohorts. Specific antigens associated with OmpC‐encoding gene from E. coli displayed serum titers ranging between 37.15 ± 7.27 (lowest) and 317.90 ± 48.9 (highest) for the subset IBD cohort. Similar assessments performed with flagellin‐encoding genes showed titers between 181.40 ± 35.0 and 328.90 ± 50.0 in the same cohort. The antigens that presented the highest seropositivity in the largest number of serum samples (specific clone of the OmpC from E. coli, and a flagellin encoding clone from Pseudomonas monteilii) were selected for further characterization.
APMNA and ACNA represent autoantibody detection assays that detect the presence of IgAs against canine polymorphonuclear leukocytes and calprotectin respectively in a quantitative fashion. APMNA would be comparable to pANCA but is ELISA‐ (ie, quantitative) and IgA‐based. ACNA determines the presence of anti‐canine calprotectin IgAs instead of the protein calprotectin itself. In both cases, the assays were optimized using canine‐specific antigens purified as described in Section 2.
3.3. IgA titers against specific antigens
Sera from the different cohorts were examined by ELISA and single‐marker‐based results are illustrated in Figure 1. The overall analysis involved titer measurements for a broad set of Igs including the main types and subtypes (IgA, IgG1, IgG2, IgM) 25 done on a subset of the dog IBD cohort and compared with the normal group (data not shown). Of the different markers included in the analysis, Figure 2 displays those that are most discriminating among the different cohorts with statistical significance. Marker positivity (seropositivity) was defined as serum level of IgA antibodies against the specific marker exceeding 2 standard deviations above the mean for the normal control serum level. In this respect, the results indicated that IBD patients had seropositivity against canine polymorphonuclear leukocytes (APMNA, 77%), OmpC (ACA, 76%), gliadins (AGA, 54%), canine calprotectin (ACNA, 43%), and flagellins (AFA, 39%). For non‐IBD dogs, 52% of them displayed positive titers against gliadins, 13% of the cohort members tested positive for APMNA, ACA, and ACNA while none displayed seropositivity for AFA. Regarding the normal cohort, the percentage that showed reactivity against ACA were just 3%, while 8‐9% tested positive for all other markers. Furthermore, the overall mean levels (mean ± SD) of these selected markers in the IBD cohort (ACA, 251.5 ± 29.4; APMNA, 121.8 ± 12.42; AFA, 189.7 ± 31.82; ACNA, 47.22 ± 11.04; AGA, 208.3 ± 28.57) were higher than the normal cohort mean levels (ACA, 10.15 ± 1.96; APMNA, 20.96 ± 1.42; AFA, 26.66 ± 5.14; ACNA, 6.85 ± 0.68; AGA, 29.05 ± 4.6) and higher than the non‐IBD cohort mean levels (ACA, 31.51 ± 18.48; APMNA, 26.04 ± 5.15; AFA, 13.5 ± 3.11; ACNA, 9.07 ± 1.5; AGA, 109.3 ± 28.23) as graphically displayed in Figure 1 with these differences being statistically significant (P‐values results for the 4 markers range between .0001 and .009 by the Mann‐Whitney U test) when comparing IBD with normal cohorts. In the case of IBD versus non‐IBD, difference between titers were statistically significant for all markers (P‐value <.001 for ACA, APMNA and AFA; and <.05 for ACNA) except gliadins (P > .05). By contrast, seropositivity to gliadins (P < .05) was the only marker with statistically significant difference between the normal and non‐IBD cohorts.
FIGURE 1.
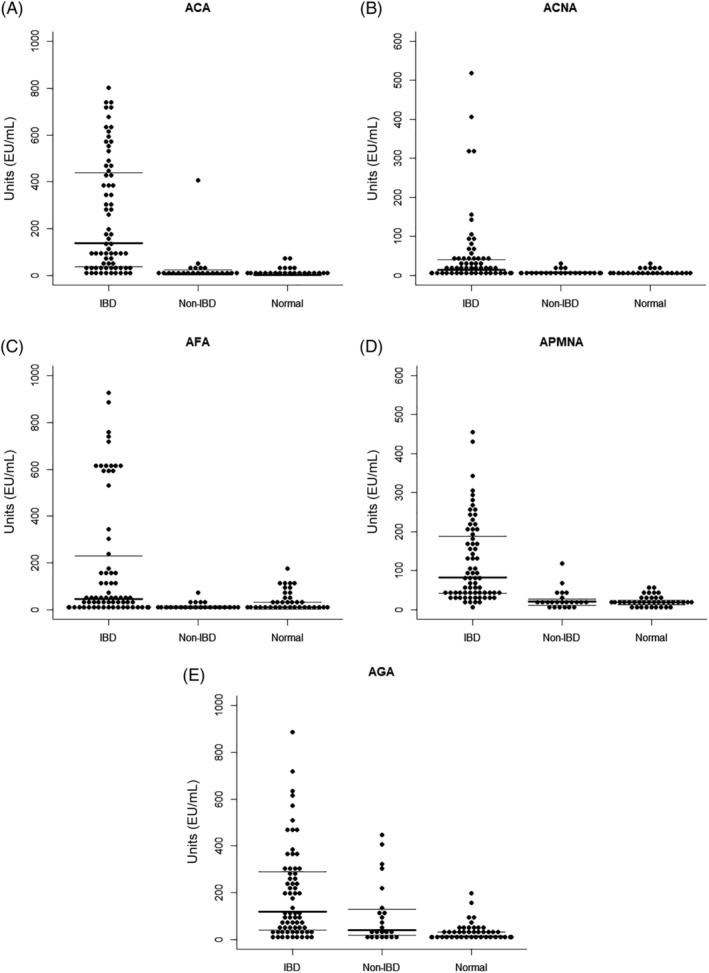
Dog sero‐reactivity of the IgA class specific to selected antigens. Dog sera from the IBD, non‐IBD, and normal cohorts were tested by ELISA as described in Material and Methods to detect anti‐OmpC (ACA, panel A), anti‐calprotectin (ACNA, panel B) IgA antibodies, anti‐flagellin (AFA, panel C), anti‐polymorphonuclear leukocytes (APMNA, panel D), and anti‐gliadins (AGA, panel E) IgA antibodies. Mean of ELISA values (EU/mL) are indicated by thick horizontal bars, and 25th and 75th percentile by light horizontal bars respectively
FIGURE 2.
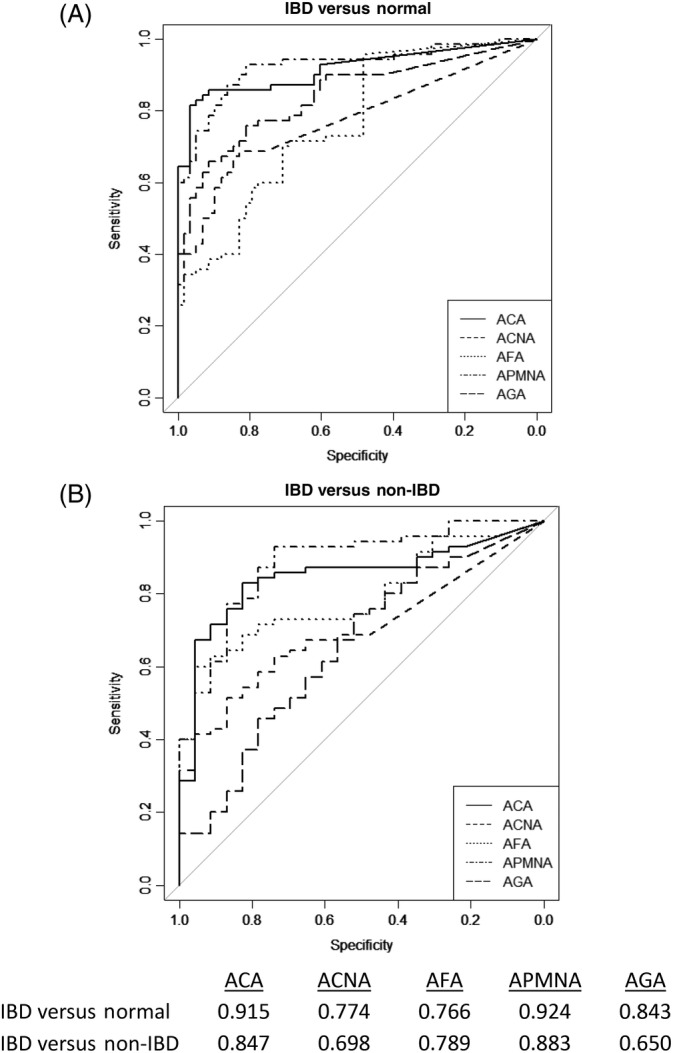
Receiver operator characteristics curves for discriminating the IBD, Normal and non‐IBD cohorts for continuous serological markers and autoantibodies. Area under the curve (AUC) represents the discriminating performance of each marker. All AUC values for each of the markers when tested versus identity were statistically significant (Mann‐Whitney U statistic, P < .001)
3.4. Receiver operator characteristics of selected markers and multiple markers
A receiver operating characteristic curve was generated for each marker and plotted against different cohorts. The area under the curve (AUC) was derived using ROC analysis to plot true positivity (ie, sensitivity) versus false positive rates (ie, specificity) thereby representing an effective way to determine which individual markers are best at differentiating cohorts. AUCs are used to examine relative differences between chance (0.5) and markers. 21 Figure 2 shows that the markers were differentially reactive with IBD dog sera when compared to normal dog sera (Figure 2A) and non‐IBD dog sera (Figure 2B) and that the differences were statistically significant.
The potential use of multiple markers in a combined fashion to further differentiate the disease phenotype is shown in Figure 3. Serum levels associated with multiple markers can be plotted against each other and their combination, association, or both can be used to strengthen the separation of cohorts. Figure 3A,B visualizes boundary data when plotting bivariable markers that separate cohorts with the IBD diagnosis versus normal patients and defines potential cutoff levels for the selected markers. The markers selected, APMNA and ACA, have been shown to be present in a significant proportion of the canine IBD cohort (ie, 77 and 76%, respectively). With these dominant markers, the graphs display scatterplots comparing data obtained from 2 cohorts with different underlying pathological conditions associated with bowel disease (IBD versus normal in Figure 3A; and IBD versus non‐IBD in Figure 3B). Given the data set, specific cutoffs can be selected to provide read outs with sensitivity ranging from 79 to 97% and specificity ranging from 93 to 99% for IBD versus normal, and with sensitivity ranging from 66 to 86% and specificity from 79 to 98% for IBD versus non‐IBD depending on the specific cut off selected and the purpose for which the multivariate test is developed for. The multivariate analysis can be extended to incorporate additional markers described here associated with infection and/or inflammation, both of which proven to be relevant in IBD pathophysiology.
FIGURE 3.
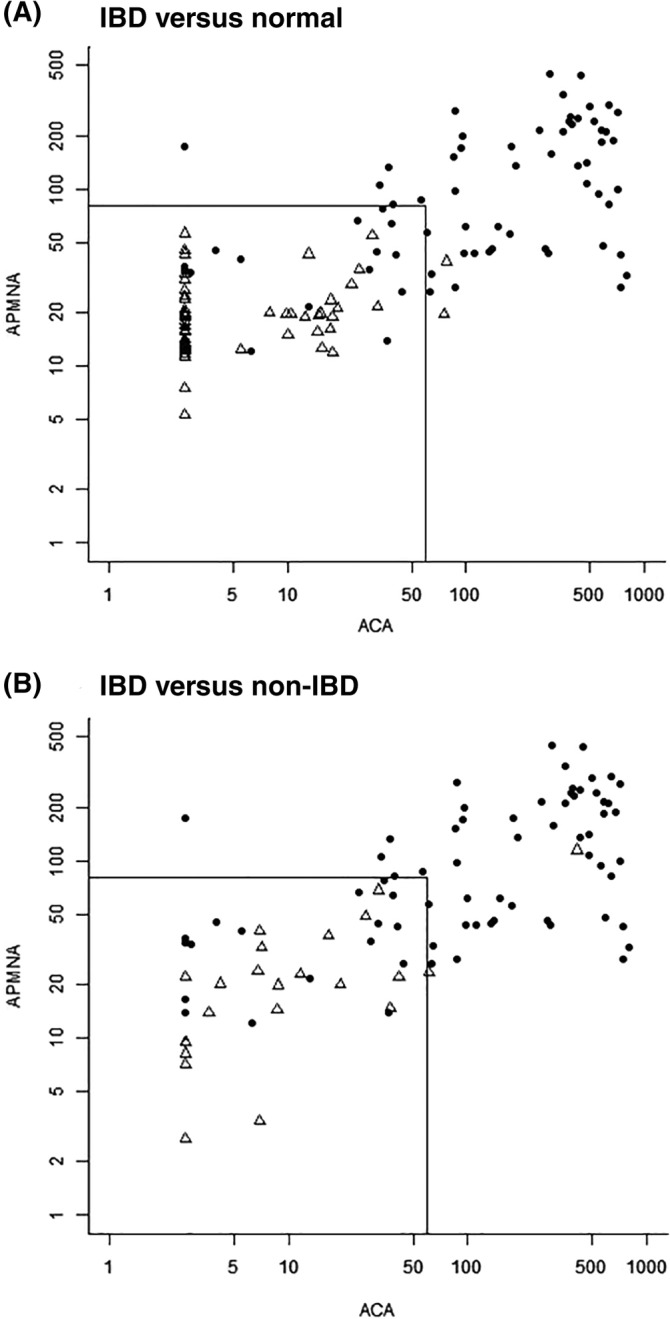
Scatterplots of sero‐reactivity against 2 markers determined in canine populations diagnosed with GI conditions. A, IBD versus normal cohorts; B, IBD versus non‐IBD populations. Filled circles represent individual data sets from patients of the IBD cohort in A and B. Unfilled triangles represent individual data sets from patients belonging to the normal cohort in A, and non‐IBD cohort in B. Sensitivity ranging from 76 to 90% and specificity ranging from 86 to 97% for IBD versus normal, and 74 to 96% for IBD versus non‐IBD were obtained per individual markers depending on marker titers selected. Smaller squares represent how the algorithm works in practical terms when applying specific cutoff values
4. DISCUSSION
Canine IBD is often a disease characterized by relapsing and remitting clinical signs including recurrent diarrhea, abdominal pain, weight loss, and anorexia. Its diagnosis remains a challenge. Our study focused on the characterization and quantification of canine‐specific serum markers that measure the presence of antibodies against selected targets (ie, selected seropositivity) differentially expressed in IBD, non‐IBD, and normal dog cohorts by using ELISA methodology based on its quantitative properties, scalability, and broad adoption across human and veterinary medicines. A key objective of the study was to ascertain how the different conditions would impact the seropositivity of selected markers. It is worthwhile to note that the non‐IBD cohort was primarily populated with dogs with more acute conditions, and that 9 members of the IBD cohort presented with concurrent conditions as described in Table 1, in both cases representing study limitations.
All different classes of Igs against the selected targets were measured. However, the study focused on IgA because of its key role in mucosal immunity. 17 Specifically, IgA seropositivity against polymononuclear leukocytes, bacterial OmpC, calprotectin, gliadins, and bacterial flagellins was found in a significant percentage of the IBD cohort with prevalence ranging from 39 to 77% depending on the marker. Thus, these results highlight the potential to develop a tool to aid the current diagnostic process involving from more invasive diagnostic procedures when attempting to diagnose IBD in dogs.
On the basis of the experience in humans, the use of serological markers with relevance to various physiological aspects known to be compromised or impaired in IBD patients have resulted in a significant contribution to the diagnostic effort.5, 6, 7, 9, 10, 11, 12, 13, 14 Initially, 2 serological markers such as anti‐S. cerevisiae antibodies (ASCA) directed to yeast cell wall phosphopeptidomannan and perinuclear antineutrophil cytoplasmic antibodies (pANCA) showed utility in defining IBD cohorts and differentiating the 2 predominant types of IBD in humans, that is, UC and Crohn's (CD). 11 While the prevalence of ASCA positivity is highest in CD patients (35%‐76%), pANCA staining pattern has been found in 30%‐83% of patients with UC. There are a few publications in veterinary medicine that report on the detection of ASCA and pANCA antibodies in dogs with IBD as an attempt to assess their potential clinical value as serologic markers.15, 16 While the prevalence of yeast population is high in Crohn's patients, 6 efforts in our study to isolate yeast microorganisms in gut samples obtained from IBD dogs yielded extremely low populations. Furthermore, ELISA‐based quantification of antibodies against such yeast microorganisms inserum samples of IBD, non‐IBD and normal dog cohorts detected no differential titers between them irrespective of the antibody types (data not shown). Allenspach et al 15 reported on the potential usefulness of markers like pANCA and ASCA in the detection of IBD in dogs by using staining techniques. The specificities of these tests range between 88 and 95% for pANCA, and 56 and 79% for ASCA. These tests which titrate primarily IgG type antibodies against perinuclear antineutrophilic cytoplasmic and S. cerevisiae antigens and are qualitative, generally have low sensitivities (51 and 44% for pANCA and ASCA, respectively). These results associate abnormal markers with clinical manifestations of the disease. In human medicine, the application of immunofluorescence techniques based on these markers has revealed a perinuclear pattern of staining in neutrophils that enabled a differential classification among the different IBD subtypes when compared to healthy controls. 26 Fluorescence patterns revealed by anti‐pANCA antibodies in canine samples were consistent with such perinuclear fluorescent staining patterns observed in humans. Therefore, Mancho et al 16 suggested the use of pANCA in the IBD diagnostic protocol as additional test to differentiate between IBD and other GI conditions with similar clinical signs. Although pANCA proved to have significant specificity, the reported determinations have all been based on immunostaining techniques with the practical limitations associated with such approaches such as lack of quantitation and complexity in the mixture of antigens. Furthermore, the sensitivity both in humans and canine seem to be limited (in the 40% range). 22
One of the serology makers identified in our study with a significant prevalence (77%) in the IBD cohort is IgA to polymorphonuclear leukocytes (APMNA). These types of leukocytes are likely to play a crucial role in the development and maintenance of inflammation in chronic enteropathies as shown in animal models and human IBD patients. 27 These are circulating autoantibodies directed against certain leukocyte components which appear elevated in canine IBD cohorts. Since its introduction in 1998, pANCA use in clinical practice in humans has increased significantly because of its high specificity (high 90s) for UC even though the sensitivity is in the 50s. 28 In fact, pANCA in combination with ASCA and OmpC represents a reliable tool to classify human IBD into UC or Crohn's patients depending on the profile. 28
Serologic markers are important in IBD because their expression represents the host response to translocation, otherwise exposure of intestinal bacteria to the host immune system, or both as a result of the breakdown of the gut mucosal barrier. The canine intestinal tract is colonized by a vast assortment of commensal microbial species, while also being occasionally exposed to bacteria that are potentially pathogenic. 29 These bacteria may express surface antigens with the power to stimulate strong immune response in the host by activating T‐cells and leading to the expression of higher titers of specific antibodies. Our study showed that 2 of the most significant serum markers differentially associated with the IBD cohort correspond to surface antigens linked to bacteria commonly found in the dog guts. Several studies have demonstrated that IBD is associated with alterations of the small intestinal and fecal microbial communities characterized by increases in members of the Proteobacteria, particularly Escherichia and Pseudomonas with a concurrent decrease in members of Firmicutes. 30 Consistent with these findings, our study showed that significant number of samples isolated from IBD dogs had Pseudomonas and Escherichia. As the prevalence of Enterobacteriaceae (particularly E. coli) have been shown to be overrepresented in dogs with IBD, together with the well‐documented increased of intestinal permeability also associated with IBD dogs, that would explain why surface antigens were some of the most significant serum markers differentially associated with the IBD cohort. Indeed, seropositive response to OmpC, an OmpC protein from E. coli, is the best single differential serologic marker associated with canine IBD among the ones tested in our study. While ACA response was detected in 76% of the IBD cohort, they were present in less than 13% in the other 2 cohorts including the non‐IBD one. The analysis of our OmpC assay displayed performance parameters such as AUC 0.915, 95% specificity and 85% sensitivity that proves it to be highly associated with the diagnosis of with canine IBD. In the context of humans, the OmpC antigen in the form of IgG and IgA antibodies response represents a marker with limited sensitivity on its own but of significant impact when considered in a 4‐marker diagnostic panel identifying Crohn's disease in 65% of children and UC in 74% of children with a 94% specificity. 31 To our knowledge, this is the first study demonstrating the presence of selected seropositive response against OmpC in IBD dogs.
In addition to the porin proteins, subsets of commensal and pathogenic bacteria are motile as result of the expression of flagella, whose primary structural component is the 35 kDa‐50 kDa flagellin. Flagellin has been found to be a major target of both innate and adaptive immune responses that are associated with IBD in humans. 32 We have shown that a significant number of canine IBD cohort members (39%) displayed IgA‐type antibody responses against flagellins. Furthermore, although there have been 1 report detecting the presence of antibodies against flagellins in IBD dogs, 33 the findings were preliminary, nonquantitative and limited to commercially available flagellins. Our study focused on ELISA‐based assays in which, both OmpC and flagellin antigens, were purified and cloned specifically from bacteria that colonized the gut of diseased dogs diagnosed with IBD. As we continue to expand our understanding of the microbiome associated with chronic GI conditions, further evaluation of novel bacterial antigens would be warranted.
The presence of active gut inflammation in patients with IBD is associated with migration of leukocytes to the gut, and this is transferred into the production of several proteins which may be detected in serum or feces. Calprotectin is an abundant protein contained in infiltrated leukocytes, predominantly granulocytes, but also lymphocytes at sites of inflammation where it is released into the extracellular spaces as result of cell disintegration. 34 In humans, the increase of serum concentration of calprotectin has been associated with various infections and inflammatory conditions. In practice, fecal calprotectin testing has been used to distinguish between inflammatory and non‐IBD. 35 The test for calprotectin might be suitable for selecting patients with IBD clinical signs for endoscopy. In dogs, a radioimmunoassay for the quantification of canine calprotectin in serum and feces has been validated. 36 When it was applied to samples from idiopathic IBD dogs, calprotectin levels are elevated compared to normal cohorts (median 227.3 μg/L; range 91.2 and 528.4 μg/L for normal versus median 408.4 μg/L; range 188.5‐1093 μg/L for mild‐to‐moderate IBD cohort). More recently, dogs with severe signs of idiopathic IBD have displayed more often abnormal levels of serological and fecal markers than dogs having less severe disease 37 and such levels changed after treatment.
In our study, we focused on developing an ELISA‐based method to assess any significant serum changes associated with the distinctive process of leukocyte migration underlying mucosal inflammation. Although the clinical target is the same as in humans (ie, calprotectin), our approach is distinctive by measuring seropositivity against canine calprotectin, not the protein itself, using ELISA methods. Our results showed that canine patients with IBD displayed a significantly higher titer of IgA antibodies against calprotectin (47.22 EU ± 11.04) than their non‐IBD (9.07 ± 1.5) and normal (6.85 ± 0.68) counterparts. Our observations support further that underlying gut inflammation is a key component of IBD condition in dogs. In addition, they reveal that the assessment of anti‐calprotectin IgA titer is potentially a high‐quality marker for such disease.
The presence of IgA against gliadins in a significant percentage of the symptomatic cohorts (54% for IBD and 52% for non‐IBD cohorts) while substantially absent in normal cohorts (less than 9%) is also reported. In humans, the presence of anti‐gliadin titers has been generally associated with food sensitivity to ingredients containing these highly immunogenic proteins causing different degrees of pathophysiology ranging from GI discomfort to celiac disease. 38 Previous studies have shown the presence of anti‐gliadin antibodies in the sera of dogs with chronic enteritis 39 using Western Blot analysis and intestinal T‐cell lymphoma 40 both by Western and ELISA, and also in dogs diagnosed with paroxysmal‐gluten sensitivity dyskinesia. 41 In our study we report that a significant proportion of dogs suffering GI conditions present with high titers of anti‐gliadin IgA antibodies in a statistical differential manner to asymptomatic (normal) dogs highlighting the potential value of gliadin seropositivity as indicator for canine GI conditions when use in combination with other relevant markers.
The use of a panel of serologic markers such as the combination of 2 or more markers, has been developed in human medicine to address the complexities of IBD populations.9, 13, 14, 29 In this regard, the addition of OmpC to the basic ASCA and pANCA markers resulted in an increased sensitivity for Crohn's diagnosis reaching 94%. The concept of combining multiple relevant markers resulting from the differential seroconversion for those antigens in patients suffering from IBD should be regarded as a promising approach to improve diagnosing of IBD in the dog as well. In human medicine, the clinical practice of using serologic markers has provided mounting evidence that increased seroconversion toward certain microbial antigens and inflammation related autoantibodies can be used as a valuable adjunctive tool for IBD diagnosis.42, 43 The findings in our study demonstrate that there are serologic markers of microbial origin and associated with the underlying physiological processes of canine patients suffering from IBD that can also be harnessed to aid in the diagnosis of canine IBD. This report sets the foundation for additional studies in which the markers described here can be used as single, combination, or both assays to differentiate IBD from non‐IBD dogs and to better define subsets of IBD dogs with distinctive prognosis and different treatment requirements. Although the data sets presented here are substantial, additional studies are ongoing with multiple cohorts and expanded follow up procedures on how the markers can be used in clinical settings.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for our study.
ACKNOWLEDGMENTS
The authors thank the technical staff members of the California Veterinary Specialists Hospital network for their technical support; and Dr Jacqueline Johnston, BVMS, MS, DABVP for reviewing earlier versions of the manuscript.
Estruch JJ, Barken D, Bennett N, et al. Evaluation of novel serological markers and autoantibodies in dogs with inflammatory bowel disease. J Vet Intern Med. 2020;34:1177–1186. 10.1111/jvim.15761
REFERENCES
- 1. Hall EJ, German EJ. Diseases of the small intestine In: Ettinger SJ, Feldman ED, eds. Textbook of Veterinary Internal Medicine. 6th ed. Philadelphia, PA: WB Saunders Co; 2005:1332‐1378. [Google Scholar]
- 2. German AJ, Hall EJ, Day MJ. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med. 2003;17(1):8‐20. [DOI] [PubMed] [Google Scholar]
- 3. Craven M, Simpson JW, Ridyard AE, Chandler ML. Canine inflammatory bowel disease: retrospective analysis of diagnosis and outcome in 80 cases (1995‐2002). J Small Anim Pract. 2004;45(7):336‐342. [DOI] [PubMed] [Google Scholar]
- 4. Jergens AE, Simpson KW. Inflammatory bowel disease in veterinary medicine. Front Biosci. 2012;4:1404‐1419. [DOI] [PubMed] [Google Scholar]
- 5. Nakamura RM, Matsutani M, Barry M. Advances in clinical laboratory tests for inflammatory bowel disease. Clin Chim Acta. 2003;335:9‐20. [DOI] [PubMed] [Google Scholar]
- 6. Sandborn WJ. Serological markers in inflammatory bowel disease: state of the art. Rev Gastroenterol Disord. 2004;4:167‐174. [PubMed] [Google Scholar]
- 7. Peyrin‐Biroulet L, Standaert‐Vitse A, Branche J, Chamaillard M. IBD serological panels: facts and perspectives. Inflamm Bowel Dis. 2007;13:1561‐1566. [DOI] [PubMed] [Google Scholar]
- 8. Xavier RY, Podolsky DK. Unravelling the pathogenensis of inflammatory bowel disease. Nature. 2007;448:427‐434. [DOI] [PubMed] [Google Scholar]
- 9. Plevy S, Silverberg MS, Lockton S, et al. Combined serological, genetic, and inflammatory markers differentiate non‐IBD, Crohn's disease and ulcerative colitis patients. Inflamm Bowel Dis. 2013;19(6):1139‐1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saxon A, Shanahan F, Landers C, et al. A distinct subset of antineutrophil cytoplasmic antibodies is associated with inflammatory bowel disease. J Allergy Clin Immunol. 1990;86:202‐210. [DOI] [PubMed] [Google Scholar]
- 11. Targan SR. The utility of ANCA and ASCA in inflammatory bowel disease. Inflamm Bowel Dis. 1998;5:61‐63. [DOI] [PubMed] [Google Scholar]
- 12. The Synergistic Role of Serology, Genetics and Inflammation in the Diagnosis of Inflammatory Bowel Disease. IBD Monograph. San Diego, CA: Prometheus Laboratories; 2011:1‐20. [Google Scholar]
- 13. Smids C, Horjus‐Talabur Horje CS, Groenen MJM, et al. The value of serum antibodies in differentiating inflammatory bowel disease, predicting disease activity and disease course in the newly diagnosed patient. Scan J Gastroenterol. 2017;52:1104‐1112. [DOI] [PubMed] [Google Scholar]
- 14. Bourgonje AR, von Martels JZH, Gabriels RY, et al. A combined set of four serum inflammatory biomarkers reliably predict endoscopic disease activity in inflammatory bowel disease. Front Med. 2019;6:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allenspach K, Luckschander N, Styner M, et al. Evaluation of assays for perinuclear antineutrophilic cytoplasmic antibodies and antibodies to Saccharomyces cerevisiae in dogs with inflammatory bowel disease. Am J Vet Res. 2004;65:1279‐1283. [DOI] [PubMed] [Google Scholar]
- 16. Mancho C, Sainz A, Garcia‐Sancho M, et al. Detection of perinuclear antineutrophil cytoplasmic antibodies and antinucelar antibodies in the diagnosis of canine inflammatory bowel disease. J Vet Diagn Invest. 2010;22:553‐558. [DOI] [PubMed] [Google Scholar]
- 17. Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17:291‐297. [DOI] [PubMed] [Google Scholar]
- 19. Slovak JE, Wang C, Morrison KL, et al. Endoscopic assessment of the duodenum in dogs with inflammatory bowel disease. J Vet Intern Med. 2014;28:1442‐1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou XH, Obuchowski NA, McClish DK. Statistical Methods in Diagnostic Medicine. New York, NY: Wiley and Sons; 2002. [Google Scholar]
- 21. Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver‐operating characteristics analysis for diagnostic tests. Prev Vet Med. 2000;45:23‐41. [DOI] [PubMed] [Google Scholar]
- 22. Cerquetella M, Spaterna A, Laus F, et al. Inflammatory bowel disease in the dog: differences and similarities with humans. World J Gastroenterol. 2010;16:1050‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nguyen TX, Alegre ER, Kelley ST. Phylogenetic analysis of general bacterial porins: a phylogenomic case study. J Mol Microbiol Biotechnol. 2006;11:291‐301. [DOI] [PubMed] [Google Scholar]
- 24. Beatson SA, Minamino T, Pallen MJ. Variation in bacterial flagellins: from sequence to structure. Trends Microbiol. 2006;14:151‐155. [DOI] [PubMed] [Google Scholar]
- 25. Pastoret PP. Immunology of the dog In: Pastoret PP, Griebel P, Bazin H, et al., eds. Handbook of Vertebrate Immunology. USA: Elsevier Science Publishing Co. Inc., 1998:261‐288. [Google Scholar]
- 26. Vasiliauskas EA, Plevy SE, Landers CJ, et al. Perinuclear antineutrophil cytoplasmic antibodies in patients with Crohn's disease define a clinical subgroup. Gastroenterology. 1996;110:1810‐1819. [DOI] [PubMed] [Google Scholar]
- 27. Rivera‐Nieves J, Gorfu G, Ley K. Leukocyte adhesion molecules in animal models of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1715‐1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tesija KA. Serological markers of inflammatory bowel disease. Biochem Med. 2013;23:28‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suchodolski JS. Microbes and gastrointestinal health in dogs and cats. J Anim Sci. 2011;89:1520‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Honneffer JB, Minamoto Y, Suchodolski JS. Microbiota alterations in acute and chronic gastrointestinal inflammation in cats and dogs. World J Gastroenterol. 2014;20:16489‐16497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zholudev A, Zurakowski D, Young W, Leichtner A, Bousvaros A. Serologic testing with ANCA, ASCA, and anti‐OmpC in children and young adults with Crohn's disease and ulcerative colitis: diagnostic value and correlation with disease phenotype. Am J Gastroenterol. 2004;99:2235‐2241. [DOI] [PubMed] [Google Scholar]
- 32. Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn's disease. J Clin Invest. 2004;113:1296‐1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Procoli F, Elson‐Riggins KG, de Ambrogi M, et al. Seroreactivity against bacterial flagellin in dogs with inflammatory bowel disease: preliminary findings. J Vet Intern Med. 2011;25:1487. [Google Scholar]
- 34. Lehmann FS, Burri E, Beglinger C. The role and utility of faecal markers in inflammatory bowel disease. Therap Adv Gastroenterol. 2015;8:23‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waugh N, Cummins E, Royle P, et al. Faecal calprotectin testing for differentiating amongst inflammatory and non‐inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess. 2013;17:1‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heilmann RM, Jergens AE, Ackermann MR, Barr JW, Suchodolski JS, Steiner JM. Serum calprotectin concentrations in dogs with idiopathic inflammatory bowel disease. Am J Vet Res. 2012;73:1900‐1907. [DOI] [PubMed] [Google Scholar]
- 37. Otoni CC, Heilmann RM, Garcia‐Sancho M, et al. Serological and fecal markers to predict response to induction therapy in dogs with idiopathic inflammatory bowel disease. J Vet Intern Med. 2018;32:999‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fasano A, Sapone A, Zevalos V, et al. Non‐celiac gluten sensitivity. Gastroenterol. 2015;148:1195‐1204. [DOI] [PubMed] [Google Scholar]
- 39. Vincenzetti S, Rossi G, Mariani P, Pengo G, Cammertoni N, Vita A. Evidence of anti‐gliadin and transglutaminase antibodies in sera of dogs affected by lymphoplasmacytic enteritis. Vet Res Commun. 2006;30:219‐221. [Google Scholar]
- 40. Matsumoto I, Uchida K, Nakashima K, et al. IgA antibodies against gliadin and tissue transglutaminase in dogs with chronic enteritis and intestinal T‐cell lymphoma. Vet Pathol. 2018;55:98‐107. [DOI] [PubMed] [Google Scholar]
- 41. Lowrie M, Garden OA, Hadjivassiliou M, Sanders DS, Powell R, Garosi L. Characterization of paroxysmal gluten‐sensitive dyskinesia in border terriers using serological markers. J Vet Intern Med. 2018;32:775‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barken DM, McGinniss MJ, Nakamura RM, et al. Prediction of inflammatory bowel disease (IBD) using serological testing: a retrospective analysis. Paper presented at: North American Society for Pediatric Gastroenterology, Hepatology and Nutrition; September, 2008; San Diego, CA.
- 43. Hanauer SB, Sandborn W, Practice parameters committee of the American College of Gastroenterology . Management of Crohn's disease in adults. Am J Gastroenterol. 2001;96:635‐643. [DOI] [PubMed] [Google Scholar]


