Abstract
Soybean meal is the main vegetable protein source in animal feed. Soybean meal contains several anti-nutritional factors, which directly affect digestion and absorption of soy protein, thereby reducing growth performance and value in animals. Fermented soybean meal is rich in probiotics and functional metabolites, which facilitates soybean protein digestion, absorption and utilization in piglets. However, the mixed solid-state fermentation (SSF) conditions of soybean meal remain to be optimized. In this study, we investigated the optimal parameters for SSF of soybean meal by Lactobacillus species and Clostridium butyricum. The results showed that two days of fermentation was sufficient to increase the viable count of bacteria, lactic acid levels and degradation of soybean protein in fermented soybean meal at the initial moisture content of 50%. The pH value, lowering sugar content and oligosaccharides in fermented soybean meal, was significantly reduced at the initial moisture content of 50% after two days of fermentation. Furthermore, the exogenous proteases used in combination with probiotics supplementation were further able to enhance the viable count of bacteria, degradation of soybean protein and lactic acid level in the fermented soybean meal. In addition, the pH value and sugar content in fermented soybean meal were considerably reduced in the presence of both proteases and probiotics. Furthermore, the fermented soybean meal also showed antibacterial activity against Staphylococcus aureus and Escherichia coli. These results together suggest that supplementation of both proteases and probiotics in SSF improves the nutritional value of fermented soybean meal and this is suitable as a protein source in animal feed.
Key words: probiotics, proteases, soybean meal, solid-state fermentation
Introduction
The dietary protein source of animal feeds directly affects nutrient utilization and subsequent growth performance in weaning animals, particularly in piglets. Soybean meal is the main source of vegetable protein in animal feed due to its low cost and high nutritional value (Cromwell 2012). However, soybean meal contains several anti-nutritional factors, such as trypsin inhibitor, lectin, α-amylase inhibiting factor and soybean antigens (Grant 1989). These anti-nutritional factors attenuate the nutritional value, utilization and digestibility of soybean protein, leading to digestive and metabolic diseases in animals (Li et al. 1990; Herkelman et al. 1992; Zhao et al. 2008).
Probiotics are live microorganisms, which can confer a health benefit for the host when administered in appropriate and regular quantities (Chaucheyras-Durand and Durand 2010). Previous studies have demonstrated that the soybean meal fermented by probiotics can increase nutrient digestibility, reduce diarrhea and improve growth performance in piglets (Kiers et al. 2003; Hong et al. 2004; Mukherjee et al. 2016). Microbial fermentation of soybean meal efficiently eliminates anti-nutritional factors and enhances nutrient value by producing proteolytic enzymes (Hong et al. 2004). Currently, Aspergillus species are widely used to produce fermented soybean meal due to their capacity to produce multiple hydrolysis enzymes (Pinto et al. 2001; Mathivanan et al. 2006; Feng et al. 2007). In addition to fungi-based fermentation, Lactobacillus species, particularly Lactobacillus plantarum, is another facultative anaerobic bacterial strain, which has also been used to ferment soybean meal (Amadou et al. 2010a, 2010b). L. plantarum produces lactic acid and removes trypsin inhibitor contents during fermentation, thereby increasing protein hydrolysis and liberating free amino acids (Amadou et al. 2010a, 2010b).
Other lactic acid-producing Lactobacillus species, including Lactobacillus acidophilus, Lactobacillus delbrueckii and Lactobacillus salivarius also showed beneficial effects on animals (Dumbrepatil et al. 2008; Deng et al. 2012; De Cesare et al. 2017). In addition, Clostridium butyricum is an anaerobic endospore-forming probiotic and has the potential to improve growth performance and immune function in animals (Yang et al. 2012; Zhang et al. 2014). Since different bacteria strains have different enzyme activities, their capacity to degrade anti-nutritional factors and synthesize different metabolites may vary. It has also been reported that the exogenous enzyme supplementation enhances the degradation of soybean protein and inactive anti-nutritional factors in the SSF of soybean meal (Ma and Wang, 2010; Amadou et al. 2011). However, to our knowledge, there have been no reports about the effects of multistrains, such as L. acidophilus, L. delbrueckii, L. salivarius and C. butyricum on SSF of soybean meal. Further, the available data concerning the interaction between exogenous proteases and probiotics in SSF of soybean meal is still scarce.
Thus, the purpose of this study was to investigate the optimal parameters of mixed SSF of soybean meal by Lactobacillus species and C. butyricum and evaluate the nutritional value in fermented soybean meal. The results provide valuable information about the fermentation of soybean meal by multiple strains and are suitable for use on an industrial scale to produce large amounts of functional fermented products.
Experimental
Materials and Methods
Microorganisms and culture conditions. L. acidophilus (BCRC 10695), L. delbrueckii (BCRC 10696), and L. salivarius (BCRC12574) were purchased from the Food Industry Research and Development Institute (Hsinchu, Taiwan). C. butyricum MIYAIRI 588 was purchased from Miyarisan Pharmaceutical (Tokyo, Japan). Staphylococcus aureus (BCRC 10780) was provided by the Department of Food Science, National Ilan University (Ilan, Taiwan). Escherichia coli DH5α was provided by the Department of Animal Science, National Taiwan University (Taipei, Taiwan). After thawing, L. acidophilus, L. delbrueckii and L. salivarius were inoculated into an Erlenmeyer flask containing De Man, Rogosa and Sharpe broth (MRS; Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 24 hours with shaking. C. butyricum was inoculated into an Erlenmeyer flask containing brain heart infusion broth (BHI; Sigma-Aldrich, St. Louis, MO, USA) and incubated at 37°C for 48 hours with shaking. S. aureus and E. coli were grown in Luria-Bertani broth (LB; Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 18 hours with shaking.
Solid-state fermentation. The procured substrates including soybean meal and molasses were ground to fine powder. Each substrate was mixed with water to give the required relative moisture content in a space bag and autoclaved at 121°C for 30 min. The cooled substrates were inoculated with 3% (v/w) inoculums containing L. acidophilus, L. delbrueckii, L. salivarius and C. butyricum, mixed carefully under sterile conditions and incubated in a chamber at 37°C. To study the effects of initial moisture content and fermentation duration, fermentations were performed with different initial moisture contents (40, 45 and 50%, w/w) and different fermentation durations (2–6 days). To study the effects of exogenous protease on SSF, acid protease (0.37%, w/w) in powder form with the activity of 50 000 IU/g (Life Rainbow Biotech, Taiwan) was added at the initial moisture content of 50%. Different fermentation durations (12–48 hours) were employed to study their effects on fermented soybean meal. All experiments were performed 3 times. After fermentation, samples of fermented soybean meal were dried at 50°C for 12 hours and homogenized by mechanical agitation. The fermented powder was then stored at 4°C prior to analysis.
Determination of CFU and spore production. Fermented soybean meal was diluted serially in 0.85% NaCl and then plated on selective media for microbial enumeration. MRS agar was used for determination of Lactobacillus species and BHI agar was used for determination of C. butyricum. Bacterial colonies counts were statistically analyzed and expressed as colony forming units per gram (CFU/g). For determination of the number of spores, fermented soybean meal was diluted in 0.85% NaCl and then heated at 80°C for 10 min before plating on agar plates. After incubation at 37°C for 18 h, the colonies were counted and expressed as colony forming units per gram (CFU/g).
Determination of lactic acid, pH and residual reducing sugars. The pH level of the fermentation products was measured with the aid of a portable digital pH meter (Mettler Toledo, Greifensee, Switzerland). The content of residual reducing sugars in the supernatants of fermentation products was analyzed by a dinitrosalicylic acid method (Miller 1959), and lactic acid levels were determined by HPLC.
Determination of antimicrobial activity. Antimicrobial activity of fermented soybean meal was analyzed using an agar-well diffusion assay. The S. aureus BCRC10780 and E. coli DH5α were used as indicator organisms for the determination of antimicrobial activity. Fermented soybean meal was diluted in 0.85% NaCl and transferred into a well in the lysogeny broth agar (LB agar; Sigma-Aldrich, St. Louis, MO, USA) containing S. aureus BCRC10780 or E. coli DH5α. The plates were incubated at 37°C for 24 h and then examined for zones of inhibition. The control discs were impregnated with ampicillin.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The crude protein extracts from fermented soybean meal were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis, the gel was stained with Coomassie Brilliant Blue R-250 (CBB R-250; Sigma-Aldrich, St. Louis, MO, USA) to visualize total protein contents.
Thin layer chromatography. Sucrose, raffinose and stachyose standards were purchased from Sigma (St. Louis, MO, USA) and dissolved in 80% aqueous ethanol. The fermentation products were dissolved in 80% ethanol and then heated at 70°C for 1 hour. After cooling, the supernatant of the fermentation products was centrifuged at 10 000 rpm for 10 minutes. Standards and samples were applied on a silica gel plate (Merck Silica Gel 60F 254, Darmstadt, Germany) and developed in a propanol, acetic acid, and water mixture (1:1:0.1). The plates were sprayed with α-naphthol, phosphoric acid, and ethanol mixture (1:100:900, g/v/v), followed by heating in an oven to detect saccharides.
Statistical analysis. All experimental data were analyzed by ANOVA using the general linear model (GLM) procedure of SAS (SAS Institute, Cary, NC, USA). Duncan’s new multiple range test was used to evaluate the differences between means. P values of less than 0.05 were considered statistically significant.
Results
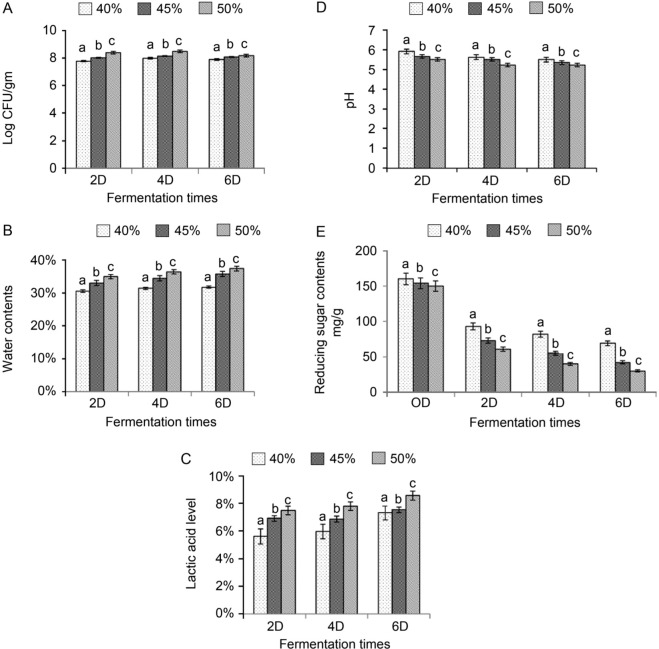
Optimization of mixed SSF on soybean meal-based medium. Since initial moisture content of fermentation is critical for microorganism growth in SSF, we first investigated the relation between initial moisture content and fermentation duration on soybean fermentation by Lactobacillus species (L. acidophilus, L. delbrueckii and L. salivarius) and C. butyricum. The results showed that 50% initial moisture had the highest bacterial growth compared with other initial moisture contents (Fig. 1A). Similarly, the effect of different initial moisture content on bacterial growth was observed for different fermentation durations (Fig. 1A). The highest water content of fermented soybean meal was also found at the initial moisture of 50% in SSF (Fig. 1B). The lactic acid levels of fermented soybean meal increased linearly with the increased initial percentage of moisture (Fig. 1C). Consistently, the pH of the fermented soybean meal was negatively correlated with the initial percentage of moisture (Fig. 1D). Furthermore, the reducing sugar content in fermented soybean meal was significantly reduced at the initial moisture of 50% in SSF (Fig. 1E). Similar results were also found for different fermentation durations (Fig. 1E).
Fig. 1.
Effect of different initial moisture and fermentation duration on the soybean meal-based medium in SSF.
(A) Effect of different initial moisture (40%, 45% and 50%) and fermentation duration (2 days; 2D, 4 days; 4D; 6 days; 6D) on colony forming units (CFU) of probiotics in the fermented soybean meal. (B) Effect of different initial moisture (40%, 45% and 50%) and fermentation duration (2 days; 2D, 4 days; 4D; 6 days; 6D) on water contents of the fermented soybean meal. (C) Effect of different initial moisture (40%, 45% and 50%) and fermentation duration (2 days; 2D, 4 days; 4D; 6 days; 6D) on lactic acid level of the fermented soybean meal. (D) Effect of different initial moisture (40%, 45% and 50%) and fermentation duration (2 days; 2D, 4 days; 4D; 6 days; 6D) on pH level of the fermented soybean meal. (E) Effect of different initial moisture (40%, 45% and 50%) and fermentation duration (0 day; 0D, 2 days; 2D, 4 days; 4D; 6 days; 6D) on reducing sugar contents of the fermented soybean meal. Values are expressed as mean ± SD (n = 3). a-cMeans in the same superscript followed by different letters are significantly different (p < 0.05).
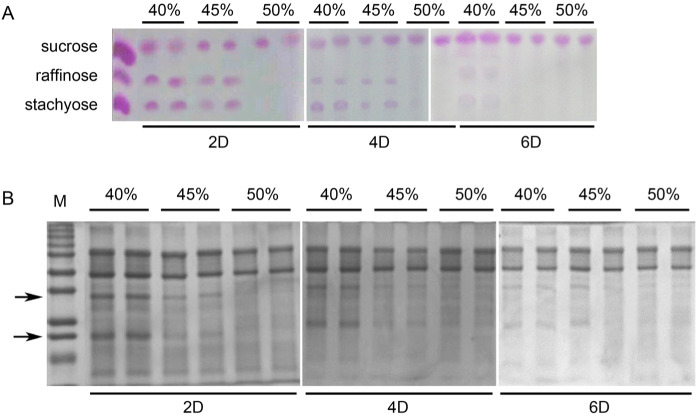
We then examined whether the different initial percentage of moisture and fermentation duration have an impact on the degradation of soybean oligosaccharides in SSF. The results showed that soybean oligosaccharides, including raffinose and stachyose were more efficiently degraded at the initial moisture of 50% compared with other initial moisture contents (Fig. 2A). In addition, two days of SSF was sufficient to degrade soybean oligosaccharides compared with four and six days of SSF (Fig. 2A). Furthermore, we investigated whether the different initial percentage of moisture and fermentation duration could affect the degradation of soybean crude protein in SSF. Consistently, two days of fermentation duration in combination with 50% of initial moisture content in SSF was sufficient to degrade soybean crude proteins compared with other conditions (Fig. 2B, indicated by an arrow). After six days of fermentation, degradation of soybean proteins was achieved at almost all initial moisture conditions (Fig. 2B, indicated by an arrow). Taken together, these results demonstrate that the ideal initial percentage of moisture and fermentation duration in mixed SSF of soybean meal by Lactobacillus species and C. butyricum are initial moisture of 50% and two days of fermentation. Therefore, 50% moisture level was selected as the optimum parameter for the proliferation of multiple strains of probiotics and used for the next optimization process.
Fig. 2.
Effect of different initial moisture content and fermentation duration on soy carbohydrate contents and soy protein contents of the fermented soybean meal.
(A) Effect of different initial moisture (40%, 45% and 50%) and fermentation duration (2 days; 2D, 4 days; 4D; 6 days; 6D) on disaccharide (sucrose) and soy oligosaccharide (raffinose and stachyose) contents of the fermented soybean meal. (B) Effect of different initial moisture (40%, 45% and 50%) and fermentation duration (2 days; 2D, 4 days; 4D; 6 days; 6D) on soy protein of the fermented soybean meal. M represents the standard protein marker. Three experiments were carried out, and one representative result is shown.
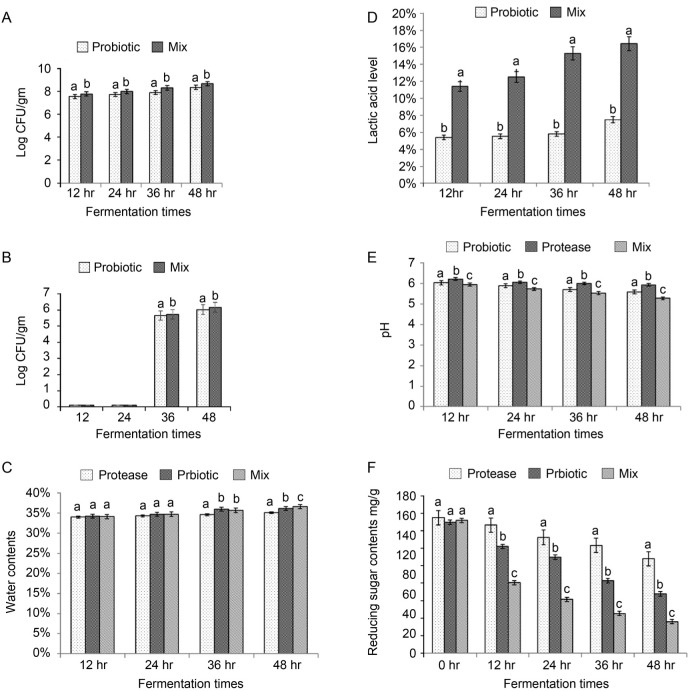
Effect of mixed probiotics in combination with protease supplementation on SSF of soybean meal. We further tested whether proteases and probiotics could further improve the nutritional value of soybean meal at the initial moisture of 50% and two days of SSF. The results showed that bacterial growth was further increased in the presence of both proteases and probiotics in SSF of soybean meal from 12 hours to 48 hours of fermentation compared with probiotics supplementation alone (Fig. 3A). The spore production was further elevated in the presence of both proteases and probiotics from 36 to 48 hours of fermentation compared with probiotics alone (Fig. 3B). The additive effect of proteases and probiotics on the water content of fermented soybean was only observed at 48 hours of SSF (Fig. 3C). Probiotics and protease supplementation further increased the lactic acid levels of fermented soybean meal compared with probiotics supplementation alone (Fig. 3D). The pH of fermented soybean meal was significantly elevated by protease supplementation compared with probiotics supplementation alone, while the pH of fermented soybean meal was further decreased in the presence of both probiotics and protease supplementation (Fig. 3E). Similar to previous results, probiotics supplementation significantly reduced the reducing sugar content in fermented soybean in SSF (Fig. 3F). However, the reducing sugar content was further remarkably decreased in the presence of both probiotics and protease supplementation (Fig. 3F).
Fig. 3.
Effect of different treatments and fermentation duration on the soybean meal-based medium in SSF.
(A) Effect of probiotics (Lactobacillus species and C. butyricum) and combination (probiotics in combination with proteases), and fermentation duration (12 hours; 12, 24 hours; 24, 36 hours; 36; 48 hours; 48) on colony forming units (CFU) of probiotics of the fermented soybean meal. (B) Effect of probiotics and combination, and fermentation duration (12 hours; 12, 24 hours; 24, 36 hours; 36; 48 hours; 48) on spore production by probiotics of the fermented soybean meal. (C) Effect of probiotics, proteases and combination, and fermentation duration (12 hours; 12, 24 hours; 24, 36 hours; 36; 48 hours; 48) on water contents of the fermented soybean meal. (D) Effect of probiotics, combination and fermentation duration (12 hours; 12, 24 hours; 24, 36 hours; 36; 48 hours; 48) on lactic acid level of the fermented soybean meal. (E) Effect of probiotics, proteases, combination, and fermentation duration (12 hours; 12, 24 hours; 24, 36 hours; 36; 48 hours; 48) on pH level of the fermented soybean meal. (F) Effect of probiotics, proteases and combination, and fermentation duration (12 hours; 12, 24 hours; 24, 36 hours; 36; 48 hours; 48) on reducing sugar contents of the fermented soybean meal. Values are expressed as mean ± SD (n = 3). a-cMeans in the same superscript followed by different letters are significantly different (p < 0.05).
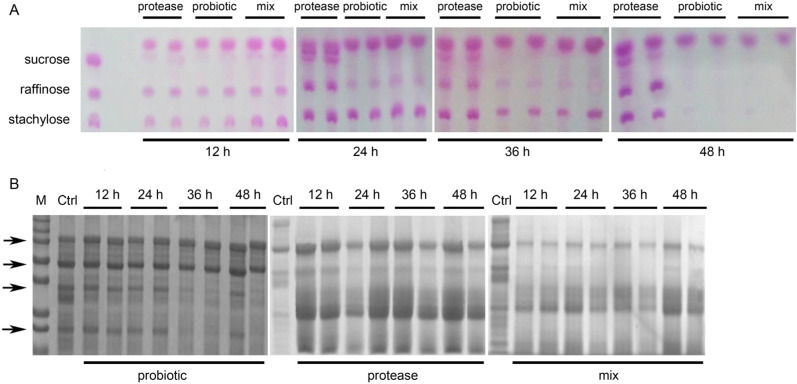
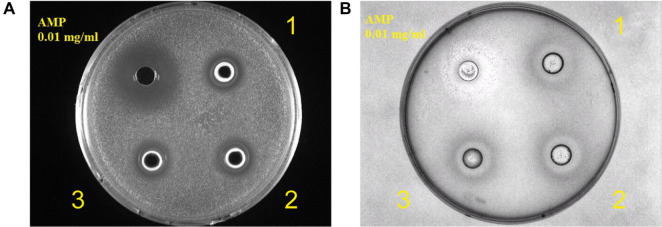
We then examined whether proteases and probiotics could affect the degradation of soybean oligosaccharides in SSF. As expected, probiotics supplementation significantly promoted oligosaccharide degradation in fermented soybean meal from 12 to 48 hours of SSF (Fig. 4A). However, protease supplementation did not affect the degradation of soybean oligosaccharide in fermented soybean meal in SSF (Fig. 4A). In addition, supplementation of both proteases and probiotics in SSF did not further increase the degradation of soybean oligosaccharide in fermented soybean meal in SSF (Fig. 4A). Furthermore, we investigated whether supplementation of both proteases and probiotics in SSF could affect the degradation of soybean crude protein in SSF. Similar to previous findings, probiotics supplementation gradually degraded soybean proteins during the fermentation (Fig. 4B, indicated by an arrow), whereas protease supplementation alone did not have a significant effect on the degradation of soybean crude proteins. Furthermore, the degradation of soybean protein was rapidly achieved in the presence of both proteases and probiotics at the early stage of SSF (Fig. 4B, indicated by an arrow). In addition, we also demonstrated that fermented soybean meal harvested from two days of SSF with supplementation of proteases and probiotics showed antimicrobial activity against S. aureus (Fig. 5A) and E. coli (Fig. 5B) compared with ampicillin. These results suggest that the supplementation of both proteases and probiotics in SSF is able to improve the nutritional value of fermented soybean meal.
Fig. 4.
Effect of different treatments and fermentation duration on soy carbohydrate contents and soy protein contents of the fermented soybean meal.
(A) Effect of different treatments (probiotics, protease and combination) and fermentation duration (12 hours; 12 h, 24 hours; 24 h, 36 hours; 36 h; 48 hours; 48 h) on disaccharide (sucrose) and soy oligosaccharide (raffinose and stachyose) contents of the fermented soybean meal. (B) Effect of different treatments (probiotics, protease and combination) and fermentation duration (12 hours; 12 h, 24 hours; 24 h, 36 hours; 36 h; 48 hours; 48 h) on soy protein of the fermented soybean meal. M represents the standard protein marker. Three experiments were carried out, and one representative result is shown.
Fig. 5.
Assessment of antimicrobial activity of the fermentation product.
(A) Antimicrobial activity of the fermented soybean meal against S. aureus compared with ampicillin (AMP). Three experiments were carried out, and one representative result is shown. (B) Antimicrobial activity of the fermented soybean meal against E. coli compared with ampicillin (AMP). 1, 2 and 3 represent the antimicrobial results from three independent fermentation experiments.
Discussion
In this study, we demonstrated that the optimal initial percentage of moisture and fermentation duration for L. acidophilus, L. delbrueckii, L. salivarius, and C. butyricum was 50% and two days in SSF of soybean meal. Supplementation of exogenous protease in combination with probiotics further improved the SSF of soybean meal. Fermented soybean meal was able to inhibit the growth of S. aureus and E. coli in vitro.
The initial moisture content of dry substrates is a critical factor in the proliferation of bacteria (Zhao et al. 2008). It has been reported that initial moisture content of 50% in SFF increases the growth of L. plantarum (Patel et al. 2004). The maximum viable counts of L. reuteri was observed at an initial moisture content of 50% in SSF (Zhang et al. 2014). Similarly, we herein found that the maximum viable counts of probiotics were achieved at the initial moisture content of 50% in SSF of soybean meal by Lactobacillus species and C. butyricum. Moreover, prolonged fermentation duration did not significantly improve bacterial growth in SSF of soybean meal. This result was in accord with the previous studies (Patel et al. 2004; Ying et al. 2009; Zhang et al. 2014). The correlation between initial moisture content, water content and viable counts of bacteria in fermented soybean meal in SSF are relatively less studied. Here, we found that initial moisture content was strictly associated with water content in fermented soybean meal. However, the water content in fermented soybean meal was not positively correlated with viable counts of probiotics, particularly after six days of fermentation. It seems that an optimal range of water content for bacteria growth may occur in SSF of soybean meal. It has been reported that the pH of the fermented product shows a gradual drop in the initial stage of fermentation and then remains steady (Ying et al. 2009; Zhang et al. 2014). In the present study, we used Lactobacillus species and C. butyricum to ferment the soybean meal and demonstrated that the lactic acid production was linearly increased at the same initial moisture content during the fermentation. The pH value of fermented soybean meal was also consistently decreased at the same initial moisture content after six days of fermentation. These results are similar to the previous findings that lactic acid content increased with increasing initial moisture content during SSF, while the pH value decreased (Dumbrepatil et al. 2008; Ying et al. 2009). Since C. butyricum also could reduce pH value in the fermented products by producing butyric acid during fermentation (Zhang et al. 2009), the concentration of butyric acid during SSF of soybean meal in the present study remains to be further examined. It has been shown that residual reducing sugar content is negatively associated with viable counts of bacteria in SSF (Zhang et al. 2014). Our findings were generally in line with an earlier report that lower reducing sugar content indicates that the bacteria proliferated more rapidly (Zhang et al. 2014).
Several reports have demonstrated that microbes could produce a variety of enzymes during fermentation and these enzymes could degrade the soybean oligosaccharides and crude proteins (Hong et al. 2004; Adeyemo and Onilude 2014). We also found that soybean oligosaccharides and crude protein content decreased with increasing initial moisture content in SSF, implying that some hydrolytic enzymes were produced by Lactobacillus species or C. butyricum. It has been demonstrated that protease supplementation significantly improved the nutritional value of soybean meal in SSF by degradation of the large molecular weight of soybean protein (Wang et al. 2014). The proteases are classified into acidic, neutral and alkaline proteinase according to its optimum pH value. Since Lactobacillus species are lactic acid-producing probiotics, we then used acidic protease in the present study. Similar to a previous study (Wang et al. 2014), we found that the viable counts of bacteria were significantly increased in the presence of both protease and probiotics. It is possible that amino acids hydrolyzed from soybean proteins by exogenous protease were efficiently utilized by bacteria for growth. Our results including lactic acid production, pH value, residual reducing sugar content and degradation of soybean crude protein supported this hypothesis. Herein, we also found that the spore production of C. butyricum was further increased in the presence of both proteases and probiotics in SSF. These results may contribute to the Lactobacillus species becoming the dominant strains at the final stage of fermentation and then create a more acidic environment by lactic acid production. Thus, the increased spore production of C. butyricum was achieved to resist the harsh environment at the later stages of fermentation. In addition, the more acidic environment caused by Lactobacillus species during fermentation also further enhanced the enzyme activity of exogenous acid proteinase. Therefore, the degradation of soybean protein was greatly increased in the presence of both protease and probiotics in SSF compared with supplementation of protease or probiotics alone.
A previous study reported that the pH value was elevated linearly with increased levels of protease supplementation in SSF (Wang et al. 2014). In the present study, we also observed that protease supplementation alone in SSF also consistently increased the pH value.
It has been reported that increased antimicrobial activity of fermented product is mainly due to bacterial metabolites, including acidic substances and bacteriocins (Fuller 1989). Lactic acid from Lactobacillus species is able to inhibit the synthesis of bacterial proteins (Wang et al. 2015). C. butyricum also can reduce pH value to inhibit the growth of pathogens by producing butyric acid (Zhang et al. 2009). In the current study, the lactic acid production was linearly increased during fermentation and the fermented soybean meal also possessed antimicrobial activity. Thus, it is possible that the antimicrobial activity in the fermented soybean meal might contribute to lactic acid or butyric acid, although the butyric acid production was not measured in our study.
The multistrain probiotics for fermentation have been reported to be more effective than monostrain probiotics, but related research or products are limited (Timmerman et al. 2004; Choi et al. 2011). The previous research reported that the viable counts of Lactobacillus species were significantly increased when Bacillus subtilis was added to the solid substrate (Zhang et al. 2014). B. subtilis followed by Enterococcus faecium supplementation effectively improved the quality of corn-soybean meal mixed feed (Shi et al. 2017). Further, fermented soybean meal using multistrain probiotics in combination with protease supplementation improved the nutritional value of soybean meal (Wang et al. 2014). Here, we also demonstrated that SSF by multistrain probiotics also has beneficial effects. Whether the anti-nutritional factors, such as trypsin inhibitor are reduced in SSF and the effects of fermented soybean meal on animals remain to be further investigated.
In conclusion, our results showed that the optimal initial moisture content and fermentation duration for mixed SSF of soybean meal is 50% of initial moisture and two days fermentation. The exogenous protease supplementation in mixed SSF of soybean meal was able to improve the nutritional value of fermented soybean meal.
Acknowledgements
This work was supported by the Ministry of Science and Technology (MOST 105-2622-B-197-001-CC2) in Taiwan.
Literature
- Adeyemo SM, Onilude AA.. 2014. Reduction of oligosaccharide content of soybeans by the action of Lactobacillus plantarum isolated from fermented cereals. Afr J Biotechnol. 13:3790–3796. [Google Scholar]
- Amadou I, Tidjani A, Foh MBK, Kamara MT, Le GW.. 2010a. Influence of Lactobacillus plantarum Lp6 fermentation on the functional properties of soybean protein meal. Emir J Food Agric. 22: 456–465. [Google Scholar]
- Amadou I, Kamara MT, Tidjani A, Foh MBK, Le GW.. 2010b. Physicochemical and nutritional analysis of fermented soybean protein meal by Lactobacillus plantarum Lp6. World J Dairy Food Sci. 5:114–118. [Google Scholar]
- Amadou I, Le GW, Shi YH, Gbadamosi OS, Kamara MT, Jin S.. 2011. Optimized Lactobacillus Plantarum Lp6 solid-state fermentation and proteolytic hydrolysis improve some nutritional attributes of soybean protein meal. J Food Biochem. 35:1686–1694. [Google Scholar]
- Cromwell GL. 2012. Soybean meal: an exceptional protein source. Soybean Meal INFOcenter. http://www.soymeal.org. accessed 05.09.2012.
- Chaucheyras-Durand F, Durand H.. 2010. Probiotics in animal nutrition and health. Benef Microbes 1:3–9. [DOI] [PubMed] [Google Scholar]
- Choi J, Shinde P, Ingale S, Kim J, Kim Y, Kim K, Kwon I, Chae B.. 2011. Evaluation of multi-microbe probiotics prepared by submerged liquid or solid substrate fermentation and antibiotics in weaning pigs. Livest Sci. 138:144–151. [Google Scholar]
- Deng J, Li Y, Zhang J, Yang Q.. 2012. Co-administration of Bacillus subtilis RJGP16 and Lactobacillus salivarius B1 strongly enhances the intestinal mucosal immunity of piglets. Res Vet Sci. 94:62–68. [DOI] [PubMed] [Google Scholar]
- Dumbrepatil A, Adsul M, Chaudhari S, Khire J, Gokhale D.. 2008. Utilization of molasses sugar for lactic acid production by Lactobacillus delbrueckii subsp. delbrueckii mutant Uc-3 in batch fermentation. Appl Environ Microb. 74:333–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cesare A, Sirri F, Manfreda G, Moniaci P, Giardini A, Zampiga M, Meluzzi A.. 2017. Effect of dietary supplementation with Lactobacillus acidophilus D2/CSL (CECT 4529) on caecum microbioma and productive performance in broiler chickens. PLoS One. 12(5):e0176309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Liu X, Xu ZR, Liu YY, Lu YP.. 2007. Effects of Aspergillus oryzae 3.042 fermented soybean meal on growth performance and plasma biochemical parameters in broilers. Anim Feed Sci Technol. 134:235–242. [Google Scholar]
- Fuller R. 1989. Probiotics in man and animals. J Appl Bacteriol. 66:365–378. [PubMed] [Google Scholar]
- Grant G. 1989. Anti-nutritional effects of soyabean: a review. Prog Food Nutr Sci. 13:317–348. [PubMed] [Google Scholar]
- Herkelman KL, Cromwell GL, Stahly TS, Pfeiffer TW, Knabe DA.. 1992. Apparent digestibility of amino acids in raw and heated conventional and low-trypsin-inhibitor soybeans for pigs. J Anim Sci. 70:818–826. [DOI] [PubMed] [Google Scholar]
- Hong KJ, Lee CH, Kim SW.. 2004. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J Med Food. 7:430–435. [DOI] [PubMed] [Google Scholar]
- Kiers JL, Meijer JC, Nout MJ, Rombouts FM, Nabuurs MJ, van der Meulen J.. 2003. Effect of fermented soya beans on diarrhoea and feed efficiency in weaned piglets. J Appl Microbiol. 95:545–552. [DOI] [PubMed] [Google Scholar]
- Li DF, Nelssen JL, Reddy PG, Blecha F, Hancock JD, Allee GL, Goodband RD, Klemm RD.. 1990. Transient hypersensitivity to soybean meal in the early weaned pig. J Anim Sci. 68:1790–1799. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wang T.. 2010. Deactivation of soybean agglutinin by enzymatic and other physical treatments. J Agric Food Chem. 58: 11413–11419. [DOI] [PubMed] [Google Scholar]
- Miller GL. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 31:426–428. [Google Scholar]
- Mukherjee R, Chakraborty R, Dutta A.. 2016. Role of fermentation in improving nutritional quality of soybean meal – a review. Asian-Australas J Anim Sci. 29:1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan R, Selvaraj P, Nanjappan K.. 2006. Feeding of fermented soybean meal on broiler performance. Int J Poult Sci. 5:868–872. [Google Scholar]
- Patel HM, Wang R, Chandrashekar O, Pandiella SS, Webb C.. 2004. Proliferation of Lactobacillus plantarum in solid-state fermentation of oats. Biotechnol Prog. 20:110–116. [DOI] [PubMed] [Google Scholar]
- Pinto GAS, Leite SGF, Terzi SC, Couri C.. 2001. Selection of tannase-producing Aspergillus niger strains. Braz J Microbiol. 32:24–26. [Google Scholar]
- Shi C, Zhang Y, Lu Z, Wang Y.. 2017. Solid-state fermentation of corn-soybean meal mixed feed with Bacillus subtilis and Enterococcus faecium for degrading anti-nutritional factors and enhancing nutritional value. J Anim Sci Biotechnol. 8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman H, Koning C, Mulder L, Rombouts F, Beynen A.. 2004. Monostrain, multistrain and multispecies probiotics – a comparison of functionality and efficacy. Int J Food Microbiol. 96:219–233. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu XT, Wang HL, Li DF, Piao XS, Lu WQ.. 2014. Optimization of processing conditions for solid-state fermented soybean meal and its effects on growth performance and nutrient digestibility of weanling pigs. Livest Sci. 170:91–99. [Google Scholar]
- Wang L, Liu C, Chen M, Ya T, Huang W, Gao P, Zhang H.. 2015. A novel Lactobacillus plantarum strain P-8 activates beneficial immune response of broiler chickens. Int Immunopharmacol. 29: 901–907. [DOI] [PubMed] [Google Scholar]
- Yang CM, Cao GT, Ferket PR, Liu TT, Zhou L, Zhang L, Xiao YP, Chen AG.. 2012. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult Sci. 91:2121–2129. [DOI] [PubMed] [Google Scholar]
- Ying W, Zhu R, Lu W, Gong L.. 2009. A new strategy to apply Bacillus subtilis MA139 for the production of solid-state fermentation feed. Lett Appl Microbiol. 49:229–234. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Qin G, Sun Z, Zhang X, Bao N, Wang T, Zhang B, Zhang B, Zhu D, Sun L.. 2008. Disappearance of immunoreactive glycinin and β-conglycinin in the digestive tract of piglets. Arch Anim Nutr. 62:322–330. [DOI] [PubMed] [Google Scholar]
- Zhao S, Hu N, Huang J, Liang Y, Zhao B.. 2008. High-yield spore production from Bacillus licheniformis by solid state fermentation. Biotechnol Lett. 30:295–297. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yang H, Yang F, Ma Y.. 2009. Current progress on butyric acid production by fermentation. Curr Microbiol. 59:656–663. [DOI] [PubMed] [Google Scholar]
- Zhang YR, Xiong HR, Guo XH.. 2014. Enhanced viability of Lactobacillus reuteri for probiotics production in mixed solid-state fermentation in the presence of Bacillus subtilis. Folia Microbiol. 59:31–36. [DOI] [PubMed] [Google Scholar]
- Zhang L, Cao GT, Zeng XF, Zhou L, Ferket PR, Xiao YP, Chen AG, Yang CM.. 2014. Effects of Clostridium butyricum on growth performance, immune function, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult Sci. 93:46–53. [DOI] [PubMed] [Google Scholar]