Abstract
A new strain of human coronaviruses (hCoVs), Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), has been identified to be responsible for the current outbreak of the coronavirus disease 2019 (COVID-19). Though major symptoms are primarily generated from the respiratory system, neurological symptoms are being reported in some of the confirmed cases, raising concerns of its potential for intracranial invasion and neurological manifestations, both in the acute phase and in the long-term. At present, it remains unclear the extent to which SARS-CoV-2 is present in the brain, and if so, its pathogenic role in the central nervous system (CNS). Evidence for neuroinvasion and neurovirulence of hCoVs has been recognised in animal and human studies. Given that SARS-CoV-2 belongs to the same family and shares characteristics in terms of receptor binding properties, it is worthwhile exploring its potential CNS manifestations. This review summarises previous findings from hCoVs in relation to the CNS, and compares these with the new strain, aiming to provide a better understanding of the effects of SARS-CoV-2 on the CNS.
Keywords: SARS-CoV-2, Coronavirus, Human, Brain, Neuroinvasion, Neurological manifestation
1. Coronavirus virology
Human coronaviruses (hCoVs) are a group of enveloped RNA viruses that are composed of a single stranded positive sense RNA genome (~30 kb). The entire genome of hCoVs encodes spike (S) glycoprotein, membrane (M) glycoprotein, envelope (E) glycoprotein, nucleocapsid (N) protein, RNA polymerase and genes for numerous accessory proteins that modulate pathogenesis, among which the N protein is highly conserved in most coronaviruses and abundantly expressed during infection [1], thus acting as a critical antigen for viral detection. The S protein is biologically critical for tropism and modulation [2], and is considered to be integral in the capability of hCoVs to reach the brain [3,4].
The hCoVs include seven strains, three of which, namely SARS-CoV-2, SARS-CoV, and Middle East respiratory syndrome-related coronavirus (MERS-CoV), may cause severe respiratory disease with a relatively high morbidity and mortality. In contrast, the other four strains OC43, 229E, NL63 and HKU1, are often associated with symptoms akin to the ‘common cold’ and hence, are relatively harmless. Based on genome analysis, the OC43 strain shares a 53•1% and a 51•2% identity with SARS-CoV and hCoV-229E, respectively [5]. The new strain, SARS-CoV-2 (previously called 2019-CoV), shares a 79% and 50% identity with SARS-CoV and MERS-CoV, respectively [6].
2. Association between hCoVs and neurological manifestation
Mainly considered as respiratory viruses, a common manifestation from hCoVs related acute respiratory disease is hypoxia, which predisposes the brain to edema, disturbed metabolism, and subsequent neurological manifestation. However, hypoxia is not the only way hCoVs were related to central nervous system. In fact, hCoVs have also been shown to be capable of invading neural cells, and hence manifesting with neurological symptoms and signs (Table 1). The potential for significant neurological deficits recently became a concern following the report of a COVID-19 patient who demonstrated loss of involuntary control of breathing due to presumed involvement of the inspiratory area in the brainstem, as well as reports of SARS-CoV-2 infected patients developing ataxia, loss of smell and convulsions [7].
Table 1.
Representative cases of CNS infection with hCoVs
| hCoV | Age/Sex | Onset Symptoms | Neurological Symptoms/Signs | Chest Radiography | Brain CT/MRI | CSF | Other Special Tests | Outcome | Ref |
|---|---|---|---|---|---|---|---|---|---|
| SARS-CoV | 39/M | fever, chills, malaise, headache, dizziness, myalgia | obscured monocular vision (26) →dysphoria, vomiting, deliria (28) →coma (33) →brain herniation (35) | left lower lobe infiltrates (11) →obvious resolution (28) →progressive bibasilar infiltrations (35) | CT: broad encephalic pathological changes of probably ischemia and necrosis and brain edema (33) | none | Eye-ground exam: an exudation around the visual yellow zone (26) | Death (35) | [25] |
| SARS-CoV | 32/F | myalgia, fever, chills, rigor, unproductive cough (w26 of pregnancy) | generalized tonic-clonic convulsion with loss of consciousness and up-rolling eyeballs for 1 min (29) | Patchy consolidations over the right upper lobe and both lower lobes (7) | MRI: no abnormalities (53) | opening pressure of 15cm of water, clear, cultures (-), SARS-CoV by RT-PCR (+) (29) | Electroencephalogram: no abnormalities (46) | Recovery (34), pregnancy terminated by CS (15) | [28] |
| SARS-CoV | 59/F | swinging fever, chills, productive cough, diarrhea | vomiting, episodes of four-limb twitching, confused, disorientated →recurred and prolonged (>30min) seizures | CT: progressive bilateral consolidation | CT: no abnormalities | Normal opening pressure, clear, cultures (-), SARS-CoV by RT-PCR (+) | none | Recovery | [29] |
| MERS-CoV | 74/M | fever | ataxia, vomiting, confusion (0) / dysmetria, decreased motor power on the left side (3) →coma, GCS 3-4 (27) | infiltrate in the mid right lung zone (3) →progression in air space disease (10) | CT: multiple chronic lacunar strokes but no acute changes (3) →an interval development of numerous patchy and one large hypodensities (27) MRI: moderate chronic small vessel ischemic changes (8) →multiple bilateral patchy areas of signal abnormality (31) |
MERS-CoV by RT-PCR (-) | none | Death (37) | [30] |
| MERS-CoV | 57/M | flu-like illness, fever, diabetic foot | unresponsive, hypotensive with left-sided facial paralysis (7) | CT: two subtle hypodensities at right semiovale and left basal ganglia, likely small lacunar infarctions, near total occlusion at origin of internal carotid arteries with M1 narrowing of left MCA (7) CT: interval multiple patchy hypodensities. MRI: bilateral signal abnormalities consistent with acute infarction (11) |
none | none | Death (12) | [30] | |
| MERS-CoV | 45/M | productive cough, dyspnea, rigors, fever, diarrhea | Low GCS (34) | infiltrate in the lower and mid right zones (10) | CT: no acute abnormality (34) MRI: confluent T2W1/FLAIR hyperintensity within the white matter of both cerebral hemispheres and along the corticospinal tract (35) |
MERS-CoV by RT-PCR (-) | Recovery (117) | [30] | |
| MERS-CoV | 34/F | high-grade fever, generalized bone pain and fatigue | Severe headache, nausea, vomiting, GCS 3/15 (15) | right lung homogenous opacity | CT: right frontal lobe intracerebral hemorrhage with massive brain edema and midline shift (15) | Death (∼60) | [31] | ||
| MERS-CoV | 28/M | fever, generalized myalgia, dizziness, productive cough | Weakness in both legs and inability to walk with numbness and tingling in stocking distribution | MRI: normal | normal | NCV: length dependent axonal polyneuropathy | Recovery | [31] | |
| OC43 | 15/M | upper respiratory tract illness | numbness in the lower extremities, difficulty walking, clumsiness in right hand, increased irritability / mild distal weakness in the right hand and foot, patchy loss of vibration and temperature sensation below T10, mild dysmetria of the left hand, poor heel-to-toe walking, antalgic gait (7) | MRI: lesions on T2-weighted imaging at C4-C5 and at T7-T8. The spinal cord lesions were non-enhancing. Patchy areas of hyperintensity (7) →MRI: improvement of the lesions in the brain and cerebellum (∼42) →MRI: a possible new lesion in the left hemisphere of the cerebellum. The periventricular lesion in the right cerebral hemisphere appeared brighter and larger. The spinal cord lesions had resolved (90) | OC43 by RT-PCR (+) | Recovery | [13] | ||
| OC43 + 229E | 3/F | fever, rhinorrhea, cough, weakness | inability to walk / damaged swallowing, chewing and speech functions, muscle strength 0/5, absent deep tendon reflexes, flexor plantar response (1) | normal | MRI: no abnormalities | normal pressure, glucose and protein, culture (-) | EMG: no pathological findings. | Recovery | [15] |
All cases had fever and/or flu-like symptoms at the onset. Half of the patients (5/10) had headache, dizziness or vomiting as an early sign of neurological manifestation. 2/3 SARS patients had seizures, whereas 3/5 MERS patients and both OC43-infected cases had facial/limb paralysis. Unfortunately, MERS-CoV has not been detected in CSF samples of any of the reported cases. Ischaemic changes have been detected in images of half of the patients, while haemorrhagic pathology has been identified on one MERS patient. The OC43-related CNS-infected patients were younger compared to SARS and MERS patients. GCS: Glasgow Coma Scale; NCV: Nerve Conduct Velocity; EMG: Electroneuromyography.
2.1. hCoV-OC43 and hCoV-229E
In vitro studies have shown that 229E and OC43 hCoV strains can infect a wide range of human neural cell cultures, including neuroblastoma, neuroglioma, astrocytoma, microglial and oligodendrocytic cell lines [8], [9], [10]. In addition, animal studies have also revealed the neuroinvasion and neurovirulence of hCoV-OC43 [4,5,[11], [12], [13]]. Importantly, a significantly higher prevalence of the OC43 strain in terms of viral RNA detection has been shown in human brain autopsy samples from multiple sclerosis (MS) patients when compared to other neurological diseases and normal controls, which is consistent with the capability for neuroinvasion of this hCoV [3]. Furthermore, OC43 has also been identified in a teenager patient with demyelinating disease, in whom the virus was detected in both CSF and nasopharyngeal secretions by PCR technology [14]. The hCoV-OC43 has also been associated with a fatal encephalitis in an infant although the underlying circumstances are still unclear [15]. Additionally, co-infection with the 229E and OC43 strains has been reported in a young girl who developed an acute flaccid paralysis [16].
2.2. SARS-CoV and MERS-CoV
SARS-CoV and MERS-CoV have also been linked with neurological manifestations. SARS-CoV has been shown to be capable of infecting human neural cells [17], and neuroinvasion and neurovirulence have been found in studies involving both SARS-CoV [18], [19], [20], [21], [22], [23] and MERS-CoV [24,25]. An association of these two more highly pathogenic viruses with neurological manifestations have also been reported. For instance, SARS-CoV particles and genomic sequences have been detected from post-mortem brain tissues of SARS patients [26], [27], [28]. They have also been detected using RT-PCR in CSF samples from a 32-year-old pregnant female patient who presented with a brief duration generalized convulsion and accompanying loss of consciousness [29] and within 24 h of a first seizure in a 59-year-old female patient [30]. Although there is less of direct evidence of viral presence in the CNS, MERS patients have also presented with neurological findings, such as altered consciousness, as well as manifested with a wide range of abnormalities on brain MRI [31,32].
Regarding the regional distribution of the virus in the CNS, data from the post-mortem studies have shown that infection from SARS-CoV was confined to neurons within selected areas of the brain, including thalamus, cerebrum, brainstem, hypothalamus and cortex [22,27]. Intriguingly, SARS-CoV has been detected in cerebrum, but not in cerebellum, in both animal [22] and human [28] studies. In animals infected in the CNS with MERS-CoV, the thalamus and brain stem were found to be the highest infected sites [25].
3. Dissemination pathways for coronavirus to gain access to the CNS
Data from multiple hACE2 transgenic mouse models has revealed that SARS-CoV detection in the brain is significantly delayed compared to that within the lung, consistent with the initial establishment of infection within the respiratory system before dissemination to the CNS [21], [22], [23]. Several dissemination routes have been proposed for coronaviruses to gain access to the CNS (Fig. 1).
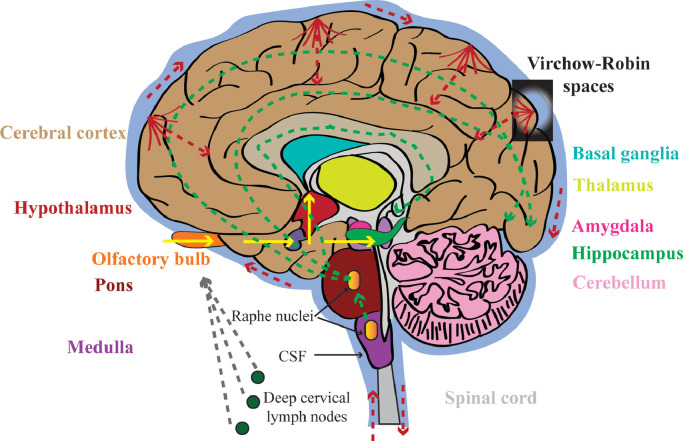
Fig. 1.
Possible dissemination routes of CNS infection with hCoVs. Route 1 (yellow solid arrows): olfactory nerve to olfactory cortex of temporal lobe to hippocampus to amygdala, or to hypothalamus; Route 2 (green dot arrows): via serotoninergic dorsal raphe system; Route 3 (red dot arrows): via hematogenous route and Virchow-Robin spaces; Route 4 (gray dot arrows): via lymphatic system. Dissemination routes with empiric data are indicated by solid arrows, and speculative ones are indicated by dot arrows. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.1. Transneuronal route
The mouse hepatitis virus (MHV), a neurotropic coronavirus, has been shown to enter the CNS via a transneuronal route and disseminate within the brain via neuroanatomic pathways [33]. In mice transgenic for hACE2, SARS-CoV seems to enter the brain via the olfactory bulb post intranasal inoculation before disseminating transneuronally to distal connected neurons and causing the dysfunction/death of these infected neurons [18]. A similar dissemination pattern has also been detected in OC43-inoculated mice [5,13]. However, different observations were found in another hACE2 transgenic mouse model, in which no SARS-CoV was detected in the olfactory bulb, or preferentially in sites transneuronally connected to the olfactory bulb [22]. In addition, a recent study has revealed that ACE2 (Angiotensin-converting Enzyme 2) and TMPRSS2 (transmembrane protease, serine 2), two key genes involved in SARS-CoV-2 entry, are not expressed in olfactory sensory or bulb neurons, but rather in olfactory epithelial cells [63], suggesting the involvement of other dissemination mechanisms independent of axonal transport.
3.2. Neurotransmitter pathway
It has also been proposed that hCoVs can gain access to the CNS via the certain neurotransmitter pathways such as serotoninergic dorsal raphe system [18], which primarily projects to cerebral cortex, neostriatum, amygdala, substantia nigra, pons, hippocampus, entorhinal cortex and locus coeruleus. However, this hypothesis is speculative and requires to be further tested in vivo models.
3.3. Hematogenous route
Viremia has been observed following intranasal inoculation with MHV [34] and SARS-CoV [21] in transgenic mice. In addition, viral RNA has been measured in the blood of patients infected with SARS-CoV and MERS-CoV [35] and thus, the dissemination of these two viruses to the CNS may be mediated by the hematogenous route through a damaged blood-brain barrier (BBB).
Once crossing the BBB, hCoVs-infected cells could potentially invade the brain through perivascular spaces, also known as Virchow-Robin spaces. These fluid-filled spaces are implicated as sites in which macrophages and lymphocytes interact to initiate immune response in patients with viral encephalitis [36]. For instance, the cryptococcal organisms in AIDS patients have been shown to disseminate through the Virchow-Robin spaces before further propagation into other brain regions [37]. Whether hCoVs could disseminate via the same route remains unclear.
3.4. Lymphatic systems
The intranasal inoculation of rodents with MHV has also revealed a lymphatic dissemination of coronavirus via cervical and mesenteric lymph nodes in addition to viremia [34]. Additional studies are required to determine the role of this potential dissemination route.
4. Long-term effects post acute infection
The infection of hCoVs may also result in long-term neurological deficits. The pathologic examination of brain tissue collected from a SARS patient revealed necrosis of neurons and broad hyperplasia of gliocytes, indicative of the presence of a chronic progressive encephalitis during the course of viral infection [26]. In addition, scattered red degeneration of neurons, a sign of neuronal hypoxia or ischaemia [27], as well as oedema around small veins with demyelination of nerve fibres [38], have been detected in brains of SARS patients, which may provide an explanation for an incidence of long-term neurological [39] and/or psychological [40] abnormalities observed in SARS survivors.
In surviving mice post hCoV-OC43 infection, spongiform-like degeneration was a residual finding post acute infection, indicating the possibility of neurodegenerative neuropathological sequelae [4]. Further, a decline of locomotor activity and an abnormality in the limb clasping reflex were observed starting several months after viral infection, and neuronal loss especially in the hippocampal region was still detectable one year post infection [41]. Although the majority of infectious virus (80%) was cleared in surviving animals, viral RNA could persist for months [41]. Even for cells that have survived the initial viral infection, little is known about their long-term functional status. To better understand this, CoV-infected models that allow the identification and isolation of survival cells, such as the Cre reporter mouse model of MHV infection, may be helpful [42]. Recovery from acute infection does not necessarily promise complete clearance of the virus, and if an infection were to becomes chronic, it may result in long-term sequelae including chronic neurological diseases. However, given the limited clinical data, the long-term effects of hCoVs on the CNS post acute infection are unknown and thus, more studies involving the long-term follow-up of survivors are needed.
5. Receptors or antibodies for virus entry in the CNS
5.1. Angiotensin-converting enzyme 2 (ACE2)
ACE2 was reported as a functional cellular receptor for SARS-CoV due to the ability of the S1 domain of the spike (S) protein to efficiently bind to ACE2 for subsequent viral replication in the cytoplasm of sensitive cells [43]. In addition, previous autopsy studies have inferred that only ACE2-postive cells were susceptible to SARS-CoV infection because the S protein and its RNA could not be detected in ACE2-negative cells [44]. It has been reported that SARS-CoV-2 might also use ACE2 as a cellular receptor, despite a variation at some key residues of the receptor-binding domain compared to that of SARS-CoV [6].
The lack of ACE2 expression in neuronal cells at both transcription and protein level contrasts with the viral susceptibility of these cells and cytopathogenicity during the acute phase of infection in neural cell lines of human and rat origins [17] as well as in humans brains [45]. Even in hACE2 transgenic mice, when specific brain regions were heavily infected by SARS-CoV, the expression levels of hACE2 in the brain were no more than 0•1 to 1% of those found in the lungs [18]. Furthermore, even in cells highly expressing hACE2 in the transgenic animal model, SARS-CoV infection could be absent, indicating that the expression of hACE2 alone might not be sufficient for maintaining viral infection [46]. These findings suggest that other receptor(s) or co-receptor(s) are involved in the viral entry into neural cells.
5.2. Dipeptidyl peptidase-4 (DPP-4)
DPP-4 (also known as CD26) was identified as a cellular receptor for MERS-CoV [47]. Two lines of human DPP-4 transgenic mice were found to have high affinity to infection by MERS-CoV [24,25]. DPP-4 is expressed in human brains [48], therefore, when the virus gains access to the brain, cells expressing DPP-4 are potentially available for viral replication within the CNS.
5.3. Other potential receptors
Aminopeptidase N (APN, also known as CD13), a cell-surface metalloprotease, was proposed as a receptor for hCoV 229E [49], as well as SARS-CoV [50]. Human aminopeptidase N was shown, in vitro, to be a cellular receptor for hCoV 229E infection of human neuronal, astrocytic and oligodendrocytic cell lines [51]. Another glycoprotein, namely CD209L (also called L-SIGN) has also been identified as an alternative cellular receptor for SARS-CoV [52]. In addition, the infection of SARS-CoV on ACE2-expressing cells seemed to be dependent on a proteolytic enzyme Cathepsin L (CTSL1) in a pH sensitive manner [53,54]. The expression levels of these receptors in human brain, however, require further elucidation to determine the extent to which they may be responsible for the hCoVs infection in the CNS.
5.4. Antibodies
In addition to known and putative receptors, antibody-dependent enhancement (ADE) of viral entry using the fusogenic spike protein has also been reported as a pathway to transfer coronavirus (MHV4) from infected cells to non-infected cells [55]. In vitro studies have shown that coronavirus entry could be mediated by special antibodies that bind the virus surface spike protein and facilitate subsequence viral entry of MERS-CoV [56] and SARS-CoV [57] in a receptor-like manner. The ADE pathway is particularly important for vaccine design and the development of antibody-based drug therapies.
6. Is SARS-CoV-2 likely to be present in the central nervous system?
At present, it remains unknown whether SARS-CoV-2 is present in the CNS, possibly due to limited access to brain tissue and CSF from patients infected with the virus. Autopsies are being increasingly carried out, however, initial biopsies have been taken only from lung, liver and heart for histopathology [58]. Given that several strains of hCoVs have been shown to be present in the brain, as well as CSF [[14], [15], [16],[26], [27], [28], [29], [30]], with neuroinvasion and neurovirulence shown in cell culture and animal models [4,5,[11], [12], [13],[17], [18], [19], [20], [21], [22], [23], [24], [25]], it is reasonable to consider that the SARS-CoV-2 may also be present in the CNS. This is further supported by the fact that some COVID-19 patients present with headache, nausea and vomiting, symptoms that potentially indicates neurological involvement [59]. The frequency of neurological manifestations including changes in consciousness and acute cerebrovascular disease increase in parallel with the severity of the disease [60]. One study on nasal epithelium samples taken from a subset of COVID-19 patients exhibiting olfactory dysfunction has suggested that the disturbance of smell in these patients is more possibly due to SARS-CoV-2 infection of non-neuronal cells rather than olfactory neurons [63]. More recently, the report of a cohort of 58 COVID-19 patients showed a correlation between acute respiratory distress syndrome (ARDS) and encephalopathy, mainly agitation, confusion and corticospinal tract signs [61]. However, these findings need to be confirmed by studies on brain samples or CSF. The most efficient way to identify involvement of neurological systems remains to be determined. The presence of SARS-CoV-2 in CSF was confirmed by gene sequencing in a 56-year-old patient with COVID-19, which was the first direct evidence for the neuroinvasion of this novel coronavirus [62]. Brain samples remain the gold standard of confirmation, however, access to such samples will almost certainly remain very limited and it is not a practical or ethical consideration in larger cohorts, especially in mild to moderate cases. Several pathways of invasion for hCoVs have been proposed, including via the olfactory nerve, neurotransmitter pathways, hematogenous route, Virchow-Robin space surrounding arterioles and venules, lymphatic systems and receptors, from which alternative parameters and types of samples may offer opportunity for proof of CNS involvement. More studies are needed to evaluate and compare these alternative pathways and parameters. In addition, the timing of sampling needs further investigation, as the change of viral load in the human body at different stages of COVID-19-related infection and recovery remains unclear.
7. Outlook and perspective
Though considered mainly as respiratory viruses, several strains of hCoVs have been detected in brains from patients with encephalitis and multiple sclerosis. In addition, the detection of SARS-CoV in CSF of patients with neurological manifestation has also provided direct evidence for the neuroinvasion and neurovirulence of hCoVs. However, the role of the virus in the process of the disease in acute phase as well as in the long term still remains elusive. The current strain SARS-CoV-2 can also potentially infect neuronal cells in the brain. Therefore, a better understanding of dissemination characteristics and neuropathogenesis of these hCoVs in the CNS is urgently needed.
7.1. Outstanding questions
Catching a viral infection with ensuing cytokine release and tissue lesions may lead to a variety of CNS-related manifestations (delirium, headache, vomiting, etc.), which are recognizable for many viruses including hCoVs, however, these non-specific clinical symptoms and signs may be constitutional and represent systemic involvement rather than direct CNS infection. Therefore, we should be cautious when reporting cases with neurological symptoms and/or signs that lack solid evidence for CNS infection (e.g. absence of viral detection in CSF samples or abnormalities supportive of infection on brain imaging). It is particularly challenging when evaluate the neurological deficits during the advanced stage of COVID-19-related disease. The clinical diagnosis and treatment strategy has to be made on the basis of a differential diagnosis which includes hypoxia, respiratory, metabolic acidosis, ARDS-related encephalopathy, the effect or withdrawal of medications, and viral infection of the CNS.
8. Search strategy and selection criteria
PubMed search of articles: “human coronavirus”, “SARS”, “MERS”, “OC43”, “229E”, “neurological symptom”, “neurological sign”, “neurological manifestation”, “neuroinvasion”, and “neurovirulence”. Additional articles were selected based on articles in these searches and as suggested by reviewers.
Declaration of Competing Interest
We declare no conflicts of interest.
Acknowledgments/Funding
We thank A/Prof Dennis Cordato, Senior Specialist/Neurologist, Liverpool Hospital, Sydney, Australia, for critical review and grammar check of the manuscript. We received no funding for this review.
References
- 1.Lin Y., Shen X., Yang R.F. Identification of an epitope of SARS-coronavirus nucleocapsid protein. Cell Res. 2003;13:141–145. doi: 10.1038/sj.cr.7290158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almazan F., Gonzalez J.M., Penzes Z. Engineering the largest RNA virus genome as an infectious bacterial artificial chromosome. Proc Natl Acad Sci USA. 2000;97:5516–5521. doi: 10.1073/pnas.97.10.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbour N., Day R., Newcombe J., Talbot P.J. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74:8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacomy H., Talbot P.J. Vacuolating encephalitis in mice infected by human coronavirus OC43. Virology. 2003;315:20–33. doi: 10.1016/S0042-6822(03)00323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.St-Jean J.R., Jacomy H., Desforges M., Vabret A., Freymuth F., Talbot P.J. Human respiratory coronavirus OC43: genetic stability and neuroinvasion. J Virol. 2004;78:8824–8834. doi: 10.1128/JVI.78.16.8824-8834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baig A.M. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Ther. 2020;26(5):499–501. doi: 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbour N., Ekande S., Cote G. Persistent infection of human oligodendrocytic and neuroglial cell lines by human coronavirus 229E. J Virol. 1999;73:3326–3337. doi: 10.1128/jvi.73.4.3326-3337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arbour N., Cote G., Lachance C., Tardieu M., Cashman N.R., Talbot P.J. Acute and persistent infection of human neural cell lines by human coronavirus OC43. J Virol. 1999;73:3338–3350. doi: 10.1128/jvi.73.4.3338-3350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonavia A., Arbour N., Yong V.W., Talbot P.J. Infection of primary cultures of human neural cells by human coronaviruses 229E and OC43. J Virol. 1997;71:800–806. doi: 10.1128/jvi.71.1.800-806.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler N., Pewe L., Trandem K., Perlman S. Murine encephalitis caused by HCoV-OC43, a human coronavirus with broad species specificity, is partly immune-mediated. Virology. 2006;347:410–421. doi: 10.1016/j.virol.2005.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacomy H., Talbot P.J. Susceptibility of murine CNS to OC43 infection. Adv Exp Med Biol. 2001;494:101–107. doi: 10.1007/978-1-4615-1325-4_16. [DOI] [PubMed] [Google Scholar]
- 13.Dube M., Le Coupanec A., Wong A.H.M., Rini J.M., Desforges M., Talbot P.J. Axonal Transport Enables Neuron-to-Neuron Propagation of Human Coronavirus OC43. J Virol. 2018;92 doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeh E.A., Collins A., Cohen M.E., Duffner P.K., Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113:e73–e76. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
- 15.Morfopoulou S., Brown J.R., Davies E.G. Human Coronavirus OC43 Associated with Fatal Encephalitis. N Engl J Med. 2016;375:497–498. doi: 10.1056/NEJMc1509458. [DOI] [PubMed] [Google Scholar]
- 16.Turgay C., Emine T., Ozlem K., Muhammet S.P., Haydar A.T. A rare cause of acute flaccid paralysis: human coronaviruses. J Pediatr Neurosci. 2015;10:280–281. doi: 10.4103/1817-1745.165716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita M., Yamate M., Li G.M., Ikuta K. Susceptibility of human and rat neural cell lines to infection by SARS-coronavirus. Biochem Biophys Res Commun. 2005;334:79–85. doi: 10.1016/j.bbrc.2005.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass W.G., Subbarao K., Murphy B., Murphy P.M. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J Immunol. 2004;173:4030–4039. doi: 10.4049/jimmunol.173.6.4030. [DOI] [PubMed] [Google Scholar]
- 20.Roberts A., Deming D., Paddock C.D. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3:e5. doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tseng C.T., Huang C., Newman P. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human Angiotensin-converting enzyme 2 virus receptor. J Virol. 2007;81:1162–1173. doi: 10.1128/JVI.01702-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCray P.B., Jr., Pewe L., Wohlford-Lenane C. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81:813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshikawa N., Yoshikawa T., Hill T. Differential virological and immunological outcome of severe acute respiratory syndrome coronavirus infection in susceptible and resistant transgenic mice expressing human angiotensin-converting enzyme 2. J Virol. 2009;83:5451–5465. doi: 10.1128/JVI.02272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agrawal A.S., Garron T., Tao X. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol. 2015;89:3659–3670. doi: 10.1128/JVI.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li K., Wohlford-Lenane C., Perlman S. middle east respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human Dipeptidyl peptidase 4. J Infect Dis. 2016;213:712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J., Zhong S., Liu J. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu J., Gong E., Zhang B. Multiple organ infection and the pathogenesis of SARS. JExp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Y., He L., Zhang Q. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau K.K., Yu W.C., Chu C.M., Lau S.T., Sheng B., Yuen K.Y. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10:342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung E.C., Chim S.S., Chan P.K. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. 2003;49:2108–2109. doi: 10.1373/clinchem.2003.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arabi Y.M., Harthi A., Hussein J. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Algahtani H., Subahi A., Shirah B. Neurological Complications of Middle East Respiratory Syndrome Coronavirus: a Report of Two Cases and Review of the Literature. Case Rep Neurol Med. 2016;2016 doi: 10.1155/2016/3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perlman S., Evans G., Afifi A. Effect of olfactory bulb ablation on spread of a neurotropic coronavirus into the mouse brain. J Exp Med. 1990;172:1127–1132. doi: 10.1084/jem.172.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barthold S.W., Smith A.L. Viremic dissemination of mouse hepatitis virus-JHM following intranasal inoculation of mice. Arch Virol. 1992;122:35–44. doi: 10.1007/BF01321116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan J.F., Lau S.K., To K.K., Cheng V.C., Woo P.C., Yuen K.Y. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esiri M.M., Gay D. Immunological and neuropathological significance of the Virchow-Robin space. J Neurol Sci. 1990;100:3–8. doi: 10.1016/0022-510x(90)90004-7. [DOI] [PubMed] [Google Scholar]
- 37.Wehn S.M., Heinz E.R., Burger P.C., Boyko O.B. Dilated Virchow-Robin spaces in cryptococcal meningitis associated with AIDS: CT and MR findings. J Comput Assist Tomogr. 1989;13:756–762. doi: 10.1097/00004728-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Ding Y., Wang H., Shen H. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tso E.Y., Tsang O.T., Choi K.W. Persistence of physical symptoms in and abnormal laboratory findings for survivors of severe acute respiratory syndrome. Clin Infect Dis. 2004;38:1338. doi: 10.1086/383580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu K.K., Chan S.K., Ma T.M. Posttraumatic stress, anxiety, and depression in survivors of severe acute respiratory syndrome (SARS) J Trauma Stress. 2005;18:39–42. doi: 10.1002/jts.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacomy H., Fragoso G., Almazan G., Mushynski W.E., Talbot P.J. Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice. Virology. 2006;349:335–346. doi: 10.1016/j.virol.2006.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheeler D.L., Athmer J., Meyerholz D.K., Perlman S. Murine Olfactory Bulb Interneurons Survive Infection with a Neurotropic Coronavirus. J Virol. 2017;91 doi: 10.1128/JVI.01099-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li W., Moore M.J., Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He L., Ding Y., Zhang Q. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.To K.F., Tong J.H., Chan P.K. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases. J Pathol. 2004;202:157–163. doi: 10.1002/path.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raj V.S., Mou H., Smits S.L. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lambeir A.M., Durinx C., Scharpe S., De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 49.Yeager C.L., Ashmun R.A., Williams R.K. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kontoyiannis D.P., Pasqualini R., Arap W. Aminopeptidase N inhibitors and SARS. Lancet. 2003;361:1558. doi: 10.1016/S0140-6736(03)13186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lachance C., Arbour N., Cashman N.R., Talbot P.J. Involvement of aminopeptidase N (CD13) in infection of human neural cells by human coronavirus 229E. J Virol. 1998;72:6511–6519. doi: 10.1128/jvi.72.8.6511-6519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeffers S.A., Tusell S.M., Gillim-Ross L. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci USA. 2004;101:15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci USA. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang I.C., Bosch B.J., Li F. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J Biol Chem. 2006;281:3198–3203. doi: 10.1074/jbc.M508381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallagher T.M., Buchmeier M.J., Perlman S. Dissemination of MHV4 (strain JHM) infection does not require specific coronavirus receptors. Adv Exp Med Biol. 1993;342:279–284. doi: 10.1007/978-1-4615-2996-5_43. [DOI] [PubMed] [Google Scholar]
- 56.Wan Y., Shang J., Sun S. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virol. 2020;94 doi: 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kam Y.W., Kien F., Roberts A. Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcgammaRII-dependent entry into B cells in vitro. Vaccine. 2007;25:729–740. doi: 10.1016/j.vaccine.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Z., Yi Y., Luo X. Development and Clinical Application of A Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis. J Med Virol. 2020 doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Helms J., Kremer S., Merdji H. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou L., Zhang M., Wang J., Gao J. Sars-Cov-2: underestimated damage to nervous system. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brann, D.H., Tsukahara, T., Weinreb, C., Lipovsek, M., Van den Berge, K., Gong, B., et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. bioRxiv preprint doi: 10.1101/2020.03.25.009084. [DOI] [PMC free article] [PubMed]