Dear Editor
A number of pneumonia cases of unknown causes have emerged in Wuhan, Hubei, China since December 2019.1 After sequencing analysis of samples from the lower respiratory tract, a coronavirus,2 which was last named as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),3 was newly discovered. On February 11, 2020, the World Health Organization (WHO) announced a new name for the disease caused by 2019-nCoV: coronavirus disease 2019 (COVID-19).4 With the arrival of the Spring Festival, an epidemic SARS-CoV-2 infection has spread rapidly. It has swept across China and all over the world, and became a major global health concern. Chinese scientists found that SARS-CoV-2, like the SARS virus in 2003, enters human cells by recognizing angiotensin-converting enzyme 2 (ACE2) protein, which is the key to the invasion of the “new coronavirus” into the body.5 Decreased ACE2 expression is a cause of hypertension because ACE2 is identified as a major angiotensin 1-7 (Ang1-7)-forming enzyme.6 Based on studies of COVID-19, we found that hypertension initially occurs in many complications in COVID-19 patients.7 However, limited reports on COVID-19 patients with hypertension are available in literature. Whether patients with hypertension who undergo angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) therapy are more likely to suffer SARS-CoV-2 infection and whether ACEI/ARB therapy would have an influence on the clinical outcomes of patients with COVID-19 are controversy.8 , 9 Moreover, the epidemiologic and clinical features of COVID-19 patients with hypertension are also not completely elucidated. Thus, in this study, we describe the demographic, epidemiologic, and clinical characteristics of COVID-19 patients with hypertension. And we also attempted to analyze whether ACEI/ARB treatment would have an influence on the clinical severity and outcomes of COVID-19 patients.
Altogether, 884 COVID-19 patients between January 17, 2020 and February 8, 2020, who confirmed with SARS-CoV-2 infection in Zhejiang Province, diagnosed as having COVID-19 according to WHO interim guidance10 were enrolled in this study. Among various coexisting conditions, the proportion of patients with hypertension (149 patients, 16.86%) was higher than that of others. Compared with COVID-19 patients without hypertension, those patients with hypertension had a higher percentage of male sex (59.06% vs 49.93%, P=0.042), were older (57.00 years vs 43.00 years, P=0.000) and had a higher percentage of age ≥60 years (43.62% vs 13.88%, P=0.000). In this study, 723 patients were diagnosed to have a mild type; 123 patients, severe type; and 37 patients, critical type. Patients with hypertension had a lower rate of mild type (59.06% vs 86.39%, P=0.000), but had a higher rate of severe (26.17% vs 11.43%, P=0.001) and critical types (14.77% vs 2.04%, P=0.000) than patients without hypertension. Compared with patients without hypertension, patients with hypertension had a higher incidence of acute respiratory distress syndrome(ARDS) (24.16% vs 6.67%, P=0.000), were more likely to use glucocorticoids (31.54% vs 12.79%, P=0.000), antibiotic (50.33% vs 39.32%, P=0.013), and intravenous immune globulin therapy (21.48% vs 6.67%, P=0.000) and more likely to need mechanical ventilation (14.77% vs 2.04%, P=0.000) and intensive care unit (ICU) admission (16.11% vs 2.31%, P=0.000), extracorporeal membrane oxygenation (ECMO) (4.03% vs 0.82%, P=0.007) and continuous renal replacement therapy (CRRT) (2.01%vs 0.14%, P=0.016) therapy. The time intervals from illness onset to discharge and from admission to discharge in patients with hypertension (median 25.00 days and 20.00 days, respectively) were longer than those in patients without hypertension (median 22.00 days and 18.00 days, respectively) (P=0.000, P=0.002) (Table 1 ).
Table 1.
Clinical characteristics of COVID-19 patients with and without hypertension
| With Hypertension (n=149) |
Without Hypertension (n=735) | P-Value# | ||||
|---|---|---|---|---|---|---|
| Total (n=149) | ACEI/ARB (n=65) | Non-ACEI/ARB (n=84) | P-Value* | |||
| Sex (male) | 88 (59.06%) | 40 (61.54%) | 48 (57.14%) | 0.588 | 367 (49.93%) | 0.042 |
| Age (years) | 57.00 (49.50-66.00) | 56.00 (48.00-64.00) | 58.00 (52.00-67.00) | 0.043 | 43.00 (34.00-54.00) | 0.000 |
| ≥60 yr | 65 (43.62%) | 25 (38.46%) | 40 (47.62%) | 0.264 | 102 (13.88%) | 0.000 |
| Coexisting Condition | ||||||
| Diabetes | 30 (20.13%) | 16 (24.62%) | 14 (16.67%) | 0.230 | 35 (4.76%) | 0.000 |
| Heart disease | 7 (4.70%) | 2 (3.08%) | 5 (5.95%) | 0.469 | 8 (1.09%) | 0.006 |
| COPD | 2 (1.34%) | 1 (1.54%) | 1 (1.19%) | 1.000 | 3 (0.41%) | 0.200 |
| Chronic liver disease | 9 (6.04%) | 5 (7.69%) | 4 (4.76%) | 0.691 | 26 (3.54%) | 0.153 |
| Chronic renal disease | 6 (4.03%) | 4 (6.15%) | 2 (2.38%) | 0.404 | 2 (0.27%) | 0.000 |
| Cancer | 3 (2.01%) | 0 (0.00%) | 3 (3.57%) | 0.257 | 6 (0.82%) | 0.379 |
| Clinical Type | ||||||
| Mild Type | 88 (59.06%) | 37 (56.92%) | 51 (60.71%) | 0.641 | 635 (86.39%) | 0.000 |
| Severe Type | 39 (26.17%) | 20 (30.77%) | 19 (22.62%) | 0.262 | 84 (11.43%) | 0.000 |
| Critical Type | 22 (14.77%) | 8 (12.31%) | 14 (16.67%) | 0.457 | 15 (2.04%) | 0.000 |
| General symptoms | ||||||
| Fever | 127 (85.23%) | 58 (89.23%) | 69 (82.14%) | 0.226 | 587 (79.86%) | 0.129 |
| Fatigue | 32 (21.48%) | 17 (26.15%) | 15 (17.86%) | 0.221 | 126 (17.14%) | 0.208 |
| headache | 7 (4.70%) | 4 (6.15%) | 3 (3.57%) | 0.699 | 74 (10.07%) | 0.038 |
| Muscle ache | 22 (14.77%) | 11 (16.92%) | 11 (13.10%) | 0.514 | 77 (10.48%) | 0.130 |
| Respiratory symptoms | ||||||
| Nasal obstruction | 3 (2.01%) | 1 (1.54%) | 2 (2.38%) | 1.000 | 48 (6.53%) | 0.031 |
| Sore throat | 20 (13.42%) | 11 (16.92%) | 9 (10.71%) | 0.270 | 103 (14.01%) | 0.849 |
| Cough | 100 (67.11%) | 47 (72.31%) | 53 (63.10%) | 0.235 | 471 (64.08%) | 0.480 |
| Sputum production | 56 (37.58%) | 26 (40.00%) | 30 (35.71%) | 0.592 | 245 (33.33%) | 0.318 |
| Hemoptysis | 7 (4.70%) | 5 (7.69%) | 2 (2.38%) | 0.240 | 7 (0.95%) | 0.003 |
| Shortness of breath | 21 (14.09%) | 10 (15.38%) | 11 (13.10%) | 0.690 | 20 (2.72%) | 0.000 |
| Gastrointestinal symptoms | ||||||
| Nausea and vomiting | 7 (4.70%) | 4 (6.15%) | 3 (3.57%) | 0.699 | 24 (3.27%) | 0.386 |
| Diarrhea | 12 (8.05%) | 7 (10.77%) | 5 (5.95%) | 0.284 | 59 (8.03%) | 0.991 |
| Complications | ||||||
| Acute respiratory distress syndrome | 36 (24.16%) | 16 (24.62%) | 20 (23.81%) | 0.909 | 49 (6.67%) | 0.000 |
| Shock | 2 (1.34%) | 1 (1.54%) | 1 (1.19%) | 1.000 | 2 (0.27%) | 0.134 |
| Treatment | ||||||
| Glucocorticoids | 47 (31.54%) | 19 (29.23%) | 28 (33.33%) | 0.559 | 94 (12.79%) | 0.000 |
| Antibiotic treatment | 75 (50.33%) | 31 (47.69%) | 44 (52.38%) | 0.570 | 289 (39.32%) | 0.013 |
| Intravenous immune globulin therapy | 32 (21.48%) | 14 (21.54%) | 18 (21.43%) | 0.987 | 49 (6.67%) | 0.000 |
| Admission to intensive care unit | 24 (16.11%) | 9 (13.85%) | 15 (17.86%) | 0.509 | 17 (2.31%) | 0.000 |
| Mechanical ventilation | 22 (14.77%) | 8 (12.31%) | 14 (16.67%) | 0.457 | 15 (2.04%) | 0.000 |
| EMCO | 6 (4.03%) | 3 (4.62%) | 3 (3.57%) | 1.000 | 6 (0.82%) | 0.007 |
| CRRT | 3 (2.01%) | 1(1.54%) | 2 (2.38%) | 1.000 | 1 (0.14%) | 0.016 |
| Interval between illness onset to hospital outpatient (Days) | 2.00(0.00-5.00) | 2.00(1.00-4.00) | 1.00(0.00-5.00) | 0.688 | 2.00 (1.00-4.00) | 0.931 |
| Interval between illness onset to admission (Days) | 4.00(1.00-7.00) | 4.00(2.50-6.00) | 4.00(1.00-7.00) | 0.548 | 3.00 (1.00-6.00) | 0.206 |
| Interval between illness onset to confirmation (Days) | 4.00(2.00-8.00) | 4.00(2.50-7.00) | 4.50(2.00-8.00) | 0.782 | 4.00 (2.00-7.00) | 0.576 |
| Interval between illness onset to discharge (Days) | 25.00(19.00-32.00) | 26.00(18.25-32.00) | 25.00(19.00-31.50) | 0.955 | 22.00(17.00-28.00) | 0.000 |
| Interval between admission to discharge (Day) | 20.00(14.00-27.00) | 20.50(14.00-26.75) | 20.00(14.50-27.00) | 0.915 | 18.00(13.00-23.00) | 0.002 |
| Death | 1 (0.67%) | 1 (1.54%) | 0 (0.00%) | 0.436 | 0 (0.00%) | 0.169 |
| Laboratory detection | ||||||
| Leucocytes (× 109 /L; normal range 4-10) | 5.40 (4.29-6.48) | 5.30 (4.26-6.32) | 5.45 (4.30-6.64) | 0.749 | 4.70 (3.80-5.90) | 0.000 |
| Neutrophils (× 109 /L; normal range 2-7) | 3.60 (2.80-4.80) | 3.43 (2.92-4.95) | 3.65 (2.71-4.79) | 0.928 | 2.90 (2.20-3.83) | 0.000 |
| Lymphocyte (× 109 /L; normal range 0.8-4.0) | 1.00 (0.71-1.37) | 0.96 (0.70-1.40) | 1.04 (0.77-1.33) | 0.896 | 1.20 (0.90-1.60) | 0.000 |
| Platelets (× 109 per L; normal range 83-303(Male), 101-320 (female) | 171.00 (138.00-220.50) | 166.00 (140.00-196.50) | 179.00 (137.00-225.50) | 0.336 | 183.00 (149.00-223.00) | 0.095 |
| INR(normal range 0.85-1.15) | 1.02 (0.97-1.10) | 1.02 (0.97-1.11) | 1.02 (0.98-1.08) | 0.871 | 1.01 (0.97-1.08) | 0.190 |
| Alb (g/L; normal range 40-55) | 40.34 (36.73-42.60) | 40.70 (37.2-42.89) | 39.20 (36.20-42.40) | 0.339 | 41.30 (38.50-43.80) | 0.001 |
| ALT (U/L; normal range 9-50 (Male), 7-40 (Female)) | 25.00 (18.20-39.00) | 26.00 (19.00-41.00) | 25.00 (17.00-32.75) | 0.375 | 21.00 (15.00-33.00) | 0.001 |
| AST (U/L; normal range 15-40 (Male),13-35 (Female)) | 28.00 (21.50-40.00) | 27.00 (19.95-40.10) | 28.00 (22.00-39.75) | 0.857 | 25.00 (19.00-32.00) | 0.001 |
| TB (umol/L; normal range 0-26 (Male), 0-21 (Female)) | 10.60 (7.70-14.95) | 11.40 (7.50-16.95) | 10.25 (7.73-14.40) | 0.716 | 9.50 (6.90-13.10) | 0.010 |
| Scr (µmol/L; normal range: 57-97(Male), 41-73 (Female)) | 72.00 (58.00-85.00) | 71.00 (57.00-85.25) | 73.00 (58.30-85.00) | 0.829 | 66.00 (55.00-76.43) | 0.000 |
| BUN (mmol/L; normal range 3.1-8.0 (Male), 2.6-7.5 (Female) | 4.30(3.50-5.93) | 4.20 (3.31-5.71) | 4.33 (3.56-6.15) | 0.277 | 3.77 (3.00-4.50) | 0.000 |
| CK (U/L; normal range 50-310 (Male), 40-200 (Female) | 83.00 (53.5-130.50) | 72.00 (49.50-131.00) | 90.00 (55.00-129.75) | 0.521 | 69.00 (47.00-105.00) | 0.003 |
| LDH (U/L; normal range 120-250) | 250.00 (194.50-315.00) | 244.00 (181.00-318.00) | 253.00 (204.00-311.50) | 0.332 | 211.00 (169.00-256.00) | 0.000 |
| Serum potassium (mmol/L; normal range 3.5-5.3) | 3.80 (3.50-4.05) | 3.84 (3.49-4.03) | 3.79 (3.50-4.06) | 0.576 | 3.85 (3.60-4.14) | 0.031 |
| Serum sodium (mmol/L; normal range 137-147) | 137.00 (135.00-139.90) | 137.00 (135.14-139.85) | 137.15 (135.00-139.90) | 0.942 | 138.60 (136.39-140.08) | 0.000 |
| CRP (mg/L; normal range 0-8) | 16.88 (7.20-44.55) | 21.00 (7.29-40.90) | 15.53 (6.39-51.00) | 0.556 | 7.80 (2.30-19.00) | 0.000 |
Note 1: *, P value of comparison between ACEI/ARB and non- ACEI/ARB; #, P value of comparison between with and withour hypertention.
Note 2: Alb, Albumin; ALT, Alanine aminotransferase; AST: Aspartate aminotransferase; BUN, Blood urea nitrogen; CK, Creatine kinase; COPD, Chronic obstructive pulmonary disease; CRP, CRRT: Continuous renal replacement therapy;C-reactive protein; ECMO, Extracorporeal membrane oxygenation; INR: International normalized ratio; LDH: Lactate dehydrogenase; Scr, Serum creatinine; TB,Total bilirubin.
We found that the level of leukocyte count (median 5.40 × 109/L vs 4.70 × 109/L, P=0.000) and neutrophil count (median 3.60 × 109/L vs 2.90 × 109/L, P=0.000) was higher, but the level of lymphocyte count (median 1.00 × 109/L vs 1.20 × 109/L, P=0.000) was lower in patients with hypertension than in patients without hypertension. In terms of liver function, patients with hypertension had a lower level of albumin (median 40.34 vs 41.30, P=0.001), but a higher level of alanine aminotransferase (ALT)(median 25.00 vs 21.00, P=0.001), aspartate aminotransferase (AST) (median 28.00 vs 25.00, P=0.001), and total bilirubin (TB)(median 10.60 vs 9.50, P=0.010). As for renal function, patients with hypertension had a higher level of serum creatinine (Scr)(median 72.00 µmol/L vs 66.00µmol/L, P=0.000) and blood urea nitrogen (BUN) (median 4.30 mmol/L vs 3.77 mmol/L, P=0.000) . In addition, patients with hypertension had a higher level of creatine kinase (CK) (median 83.00 U/L vs 69 U/L, P=0.003), lactate dehydrogenase (LDH) (median 250.00 U/L vs 211 U/L, P=0.000), and C-reactive protein(CRP) (median 16.88 mg/L vs 7.80 mg/L, P=0.000), but lower level of serum potassium (median 3.80 mmol/L vs 3.85 mmol/L, P=0.031) and serum sodium (median 137.00 mmol/L vs 138.60 mmol/L, P=0.000). (Table 1)
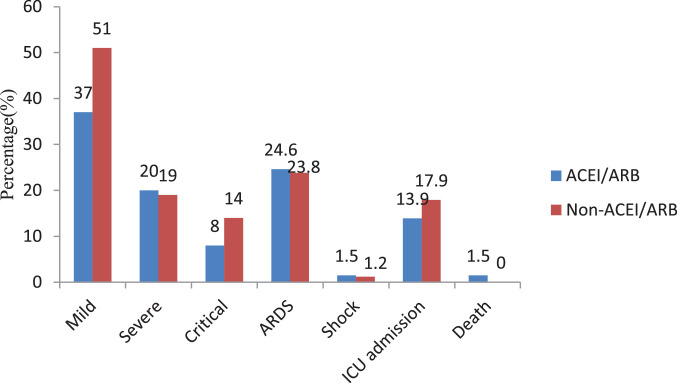
Among the 149 patients with hypertension, most patients (102 patients) treated with CCB including 62 patients used CCB alone. 149 patients were divided into two groups according to whether or not ACEI or ARB included in the antihypertensive drug regimen, 65 patients in ACEI/ARB group and 84 patients in non-ACEI/ARB group. Compared with patients treated without ACEI/ARB, the clinical presentations and laboratory results between the two groups did not reach significant difference (all P>0.05). No difference was found in severe/critical cases between patients with and those without ACEI/ARB treatment (all P>0.05). There were no significant differences of the complication (Shock, ARDS), the treatments, including anti-coronavirus treatment, glucocorticoids treatment, antibiotic treatment, mechanical ventilation, ECMO, CRRT and so on (all P>0.05). In addition, no difference was found in the rate of death, admission to ICU, interval between illness onset to discharge and the time of hospitalization (all P>0.05). (Table 1, Fig. 1 ).
Fig. 1.
Clinical type, complications and outcome of COVID-19 patients with hypertension of different anti-hypertensive drugs.
In summary, we reported the largest cases of COVID-19 patients with hypertension. This study showed that patients with hypertension might have more severe respiratory symptoms, more abnormality laboratory indication, and more proportion of severe/critical type of COVID-19. Moreover, they may need more antibiotic, hormone, and intravenous immune globulin therapy and intensive care unit admission and have a longer hospital stay. Treatment with ACEI/ARB have no influence on the severity and the clinical outcome of COVID-19 patients with hypertention.
Funding
This study was supported by the Medical Science and Technology Project of Zhejiang Province (2020KY550), the national S&T major project (2017ZX10202202, 2017ZX10204401-001-002), the Zhejiang Provincial Natural Science Foundation (LQ19H190001).
Potential conflicts of interest
None.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
References
- 1.Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. Journal of medical virology. 2020 doi: 10.1002/jmv.25689. 2020-Jan-29. PubMed PMID: MEDLINE:31994742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J. A Novel Coronavirus from Patients with Pneumonia in China, 2019. The New England journal of medicine. 2020 Feb 20;382(8):727–733. doi: 10.1056/NEJMoa2001017. PubMed PMID: 31978945. Pubmed Central PMCID: 7092803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander E. Gorbalenya SCB, Ralph S. Baric, Raoul J. de Groot, Christian Drosten, Anastasia A. Gulyaeva, Bart L. Haagmans, Chris Lauber, Andrey M. Leontovich, Benjamin W. Neuman, Dmitry Penzar, Stanley Perlman, Leo L.M. Poon, Dmitry Samborskiy, Igor A. Sidorov, Isabel Sola, John Ziebuhr. Severe acute respiratory syndrome-related coronavirus: The species and its viruses - a statement of the Coronavirus Study Group. 2020.
- 4.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020 Feb 17 doi: 10.1016/j.ijantimicag.2020.105924. 105924. PubMed PMID: 32081636. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Feb 3 doi: 10.1038/s41586-020-2012-7. PubMed PMID: 32015507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuba K, Imai Y, Penninger JM. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ J. 2013;77(2):301–308. doi: 10.1253/circj.cj-12-1544. PubMed PMID: 23328447. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. PubMed PMID: 31986264. Pubmed Central PMCID: 7159299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J. Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circulation research. 2020 Apr 17 doi: 10.1161/CIRCRESAHA.120.317134. PubMed PMID: 32302265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunden-Cullberg J. Chronic Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers Is High Among Intensive Care Unit Patients With Non-COVID-19 Sepsis but Carry a Moderately Increased Risk of Death. Hypertension (Dallas, Tex: 1979) 2020 Apr 10 doi: 10.1161/HYPERTENSIONAHA.120.15178. HYPERTENSIONAHA12015178. PubMed PMID: 32275190. Epub 2020/04/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. Jan 11, 2020. https://wwwwhoint/internal-publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected(accessed Jan 30,2020) 2020.