Abstract
Background
The coronavirus 2019 (COVID-19) pandemic has had a dramatic impact on health care systems and a variable disease course. Emerging evidence demonstrates that severe acute respiratory syndrome coronavirus 2 is associated with central nervous system disease. We describe central nervous system manifestations in critical patients with COVID-19 at our tertiary center.
Methods
We conducted a single-center retrospective analysis of all actively critical patients with COVID-19 admitted to our tertiary care academic center in New Orleans, Louisiana, on April 22, 2020, with new onset of neurologic disease. Patients were grouped into 1 of 3 categories according to imaging and clinical features; encephalopathy, acute necrotizing encephalopathy, and vasculopathy.
Results
A total of 27 of 76 (35.5%) critical patients with COVID-19 met inclusion criteria. Twenty patients (74%) were designated with COVID-19–associated encephalopathy, 2 (7%) with COVID-19–associated acute necrotizing encephalopathy, and 5 (19%) with COVID-19–associated vasculopathy. Sixty-three percent of neurologic findings were demonstrated on computed tomography, 30% on magnetic resonance imaging, and 44% on electroencephalography. Findings most often included ischemic strokes, diffuse hypoattenuation, subcortical parenchymal hemorrhages, and focal hypodensities within deep structures. Magnetic resonance imaging findings included diffuse involvement of deep white matter, the corpus callosum, and the basal ganglia. For patients with large-territory ischemic stroke, all but one displayed irregular proximal focal stenosis of the supraclinoid internal carotid artery.
Conclusions
Analysis of active critical COVID-19 admissions at our revealed a high percentage of patients with new neurologic disease. Although variable, presentations followed 1 of 3 broad categories. A better understanding of the neurologic sequalae and radiographic findings will help clinicians mitigate the impact of this disease.
Key words: Cerebrovascular disease, Infectious disease, Neurologic surgery, SAR-CoV-2
Abbreviations and Acronyms: ADEM, Acute disseminated encephalomyelitis; ARDS, Acute respiratory distress syndrome; CNS, Central nervous system; COVID-19, Coronavirus disease 2019; CRRT, Continuous renal-replacement therapy; CSF, Cerebrospinal Fluid; CT, Computed tomography; CTA, Computed tomography angiography; DM2, Diabetes mellitus type 2; EEG, Electroencephalography; FLAIR, Fluid-attenuated inversion recovery; IAE, Influenza-associated encephalopathy; ICA, Internal carotid artery; ICH, Intracerebral hemorrhage; ICU, Intensive care unit; LVO, Large-vessel occlusion; MCA, Middle cerebral artery; MERS, Middle Eastern respiratory syndrome; MRA, Magnetic resonance angiography; MRI, Magnetic resonance imaging; NAAT, Nucleic acid amplification technique; PLEX, Plasma exchange; RICU, Respiratory intensive care Unit; RT-PCR, Reverse transcriptase polymerase chain reaction; SARS-CoV-1, Severe Acute Respiratory Distress Syndrome Coronavirus 1; SARS-CoV-2, Severe acute respiratory distress syndrome coronavirus 2; SWI, Susceptibility-weighted imaging; VZV, Varicella zoster virus
Introduction
The coronavirus disease 2019 (COVID-19) pandemic that is due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a dramatic impact on health care systems.1 Although the majority of patients experience mild disease, a subset develops complex multiorgan failure.1 Confirmed disease has afflicted 2.92 million patients globally as of April 26, 2020, with 829,000 recovered and an associated mortality of 204,000 (5.2%).2
SARS-CoV-2 is a member of the coronaviridae family, composed of enveloped positive-sense single-stranded RNA viruses typically responsible for a spectrum of respiratory and gastrointestinal diseases but with occasional neurotropism.1 Notable members include the 2003 severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and 2012 Middle East Respiratory Syndrome (MERS) coronavirus, which have demonstrated the ability to produce neuromuscular and neurologic symptoms.3, 4, 5, 6, 7, 8 Experimental studies suggest early brainstem involvement and secondary damage via exaggerated host immune responses.9 , 10 In addition, known homologues such as the human coronaviruses OC34 and 229E (HcoV-OC34 and HcoV-229E) have been detected in brain tissue of patients with multiple sclerosis, and human T-cell lines have been found to cross-react with myelin basic protein and viral antigens, suggesting molecular mimicry and a potential pathogenesis for central nervous system (CNS) injury.11 , 12
Emerging evidence demonstrates that SARS-CoV-2 is associated with neurologic disease,13, 14, 15 including meningoencephalitis, virus-associated necrotizing encephalopathy similar to the influenza-associated encephalopathy (IAE), and secondary cytokine-induced acute necrotizing encephalopathy syndromes observed in members of the Orthomyxoviridae family such as the hemagglutinin 1 neuraminidase 1 influenza virus.13, 14, 15 These findings highlight the additional challenge facing the neurosciences during this pandemic.16 In this work, we describe and categorize the CNS manifestations observed in critical patients with COVID-19 who were admitted on 1 day to our tertiary academic center.
Methods
This is a single-center retrospective cross-sectional analysis of all patients admitted to our tertiary care academic center in New Orleans, Louisiana, on April 22, 2020, who presented in critical condition and had confirmed SARS-CoV-2. Patients were included if they required intensive care unit (ICU) level of care and had active COVID-19 confirmed by positive qualitative reverse-transcription polymerase chain reaction (RT-PCR) or qualitative RNA nucleic acid amplification technique (NAAT). Demographic data, clinical course, and radiographic findings were reviewed. Patients were excluded if they either did not have clear clinical or radiographic evidence of neurologic disease, if neurologic manifestations were explained completely by a pre-existing condition, or if neurologic involvement preceded active viral disease, as evident by chronology of confirmatory tests.
Based on imaging, patients were grouped into 1 of 3 informal categories. Patients were categorized as having “COVID-19–associated encephalopathy” if they had either scalp electroencephalography (EEG) showing either generalized encephalopathy or epileptic encephalopathy as well as changes on computed tomography (CT) and/or magnetic resonance imaging (MRI) of the head. Patients were designated “COVID-19–associated acute necrotizing encephalopathy” if they demonstrated significant changes in midline and periventricular structures on MRI and/or CT and had evidence of hemorrhagic conversion on either gradient echo or susceptibility-weighted imaging (SWI) MRI sequences. An informal diagnosis of “COVID-19–associated vasculopathy” was given to patients with evidence of acute ischemic stroke affecting large-vessel territories with or without evidence of large-vessel occlusion (LVO) on computed tomography angiography or magnetic resonance angiography (CTA and MRA, respectively).
Diagnostic criteria for the aforementioned categorizations were derived from existing defined viral-associated disease presentations, such as IAE,13, 14, 15 influenza-associated acute necrotizing encephalopathy,13, 14, 15 human immunodeficiency virus (HIV)-associated vasculitis,17 and human herpes virus (VZV) 3-associated vasculopathy.17
Results
A total of 76 patients were admitted on April 22, 2020, to one of several repurposed respiratory intensive care units (RICUs). The patients were in critical condition and confirmed to have COVID-19. Of these, 27 patients (35.5%) had evidence of new-onset neurologic disease, which ranged from mild headache and dysgeusia to severe focal deficits (Table 1 ). All but 1 patient with neurologic involvement developed altered mental status (AMS) during their hospital course (96.3%). Eight (30%) patients tested positive for SARS-CoV-2 on multiple interval tests. As patients without active disease as well as those who had confirmed negative RT-PCR and/or NAAT testing along with no clinical evidence of COVID-19 at the time of neurologic involvement were excluded, and no recurrent infections were observed, zero patients tested negative. Positive tests were documented on 37 occasions, 8 (22%) of which were confirmed via NAAT and the remainder via RT-PCR. As of April 23, 2020, 3 patients (11%) have died.
Table 1.
Clinical Neurologic Features and Comorbidities of Patients with COVID-19
| Features | n (%) |
|---|---|
| Clinical manifestations | |
| Altered mental status | 26 (96.3) |
| Dysgeusia | 1 (3.7) |
| Generalized weakness | 1 (3.7) |
| Headache | 2 (7.4) |
| Focal deficit | 10 (37.0) |
| Decerebrate posturing | 1 (3.7) |
| Facial droop | 1 (3.7) |
| Fixed pupils | 1 (3.7) |
| Gaze deviation | 3 (11.1) |
| Hemineglect | 2 (7.4) |
| Hemiparesis or hemiplegia | 4 (14.9) |
| Quadriplegia | 1 (3.7) |
| Comorbidities | |
| Acute ischemic stroke | 3 (11.1) |
| Acute myocardial infarction | 1 (3.7) |
| Atrial fibrillation | 2 (7.4) |
| Chronic kidney disease | 6 (22.2) |
| Chronic myeloid leukemia | 1 (3.7) |
| Congestive heart failure | 2 (7.4) |
| Coronary artery disease | 2 (7.4) |
| Diabetes mellitus type 2 | 14 (51.9) |
| Fungal meningitis | 1 (3.7) |
| Hypertension | 17 (63.0) |
| Intravenous drug abuse | 1 (3.7) |
| Neuromyelitis optica | 1 (3.7) |
| Obesity | 7 (25.9) |
| Orthotopic heart transplant | 1 (3.7) |
| Pseudotumor cerebri | 1 (3.7) |
| Systemic lupus erythematous | 1 (3.7) |
N denotes total number of patients with documentation of said finding, and % is relative to all critical patients with COVID-19 with clear neurologic involvement.
COVID-19, coronavirus disease 2019.
Patients had a mean age of 59.8 years (range 35–91 years) and 48% were female. The vast majority of patients had an underlying medical condition, with hypertension (63%), diabetes mellitus type 2 (DM2) (52%), obesity (26%), and chronic kidney disease (22%) being the most common (Table 1). During their clinical course, 51.8% of patients suffered secondary infection, ranging from bacteremia (22%), urinary tract infection (15%), bacterial pneumonia (11%), to fungemia (4%). Patients with neurologic manifestations had a greater proportion of DM2 (52% vs. 40%) but this did not reach statistical significance (P = 0.32).
Seventy-one percent of included patients were imaged with 2 or more modalities. All included patients underwent noncontrast CT of head, 10 patients of whom were scanned for new-onset focal deficit and 13 for persistent AMS in the absence of sedatives. An additional 8 patients underwent MRI for further investigation of suspected large territory infarct, hemorrhagic, or encephalitic process. Three patients underwent CTA without MRI to acutely evaluate suspected LVO, and another 4 underwent MRA in addition to nonvascular magnetic resonance for concern for ongoing vasculopathy. Thirteen patients received continuous EEG for pronounced encephalopathy not explained by previous CT alone (n = 9) or combined CT and MRI (n = 4) findings.
A total of 23 patients (85%) demonstrated radiographic or electrographic evidence of neurologic disease: 63% had positive findings on CT, 30% on MRI, and 15% on noninvasive vascular imaging. Forty-four percent of included patients (100% who received EEG) demonstrated abnormal findings on EEG. CT findings most often included subacute ischemic strokes, diffuse hypoattenuation, subcortical parenchymal hemorrhages, and focal hypodensities in deep structures (Table 2 ). MRI findings often resembled viral encephalitis with diffuse involvement of the deep white matter, corpus callosum, and basal ganglia.
Table 2.
Neuroimaging and Electroencephalography Results in Patients with COVID-19
| Type of Testing and Results | n (%) |
|---|---|
| Computed tomography | |
| Deep lobar hypoattenuation | 6 (22.2) |
| Deep supratentorial hypodensity | 14 (51.9) |
| Caudate head | 1 (3.7) |
| Corona radiata | 6 (22.2) |
| Globus pallidus | 2 (7.4) |
| Internal capsule | 5 (18.5) |
| Subacute ischemic stroke | 4 (14.8) |
| Subcortical parenchymal hematoma | 3 (11.1) |
| Magnetic resonance imaging | |
| DWI | 5 (18.5) |
| Ep2d_tra_hemo | 1 (3.7) |
| FLAIR | 6 (22.2) |
| MP-RAGE | 1 (3.7) |
| SWI | 3 (11.1) |
| Electroencephalography | |
| Generalized encephalopathy | 11 (40.1) |
| NCSE | 1 (3.7) |
| Vascular imaging | |
| Focal stenosis—ICA terminus | 3 (11.1) |
| Large vessel occlusion—PCA P2B | 1 (3.7) |
N denotes total number of patients with documentation of said finding, and % is relative to all critical patients with COVID-19 with clear neurologic involvement.
COVID-19, coronavirus disease 2019; DWI, diffusion-weighted imaging, ep2d_tra_hemo, gradient echo imaging; FLAIR, fluid-attenuated inversion recovery; MP-RAGE, magnetization prepared–rapid gradient echo; SWI, susceptibility-weighed imaging; NCSE, nonconvulsive status epilepticus; ICA, internal carotid artery; PCA, posterior cerebral artery.
Vascular imaging was obtained most often to rule out acute ischemic stroke. Four large-territory infarcts were discovered on MRI—3 involving an extensive middle cerebral artery (MCA) territory and one involving the posterior cerebral artery territory. Of the posterior circulation strokes, one study demonstrated LVO of the posterior cerebral artery P2B segment that corresponded with evolving infarct. The other 3 studies showed irregular proximal focal stenosis of the supraclinoidal internal carotid artery (ICA), particularly at the area of the terminus, with patent anterior circulation vasculature (Table 2).
EEG on affected patients yielded a high proportion of generalized encephalopathy, with 1 patient showing activity suggestive of nonconvulsive status epilepticus (Table 2). Encephalopathy typically consisted of irregular slowing with delta and theta frequency oscillations.
Twenty patients (74%) were diagnosed with COVID-19–associated encephalopathy, 2 (7%) with COVID-19–associated acute necrotizing encephalopathy, and 5 (19%) with COVID-19–associated vasculopathy. Of the last group, 1 patient had evidence of LVO, whereas the other 4 had focal stenosis at the ICA terminus and distal supraclinoid segments with patent distal vasculature.
Representative Cases
COVID-19–Associated Encephalopathy
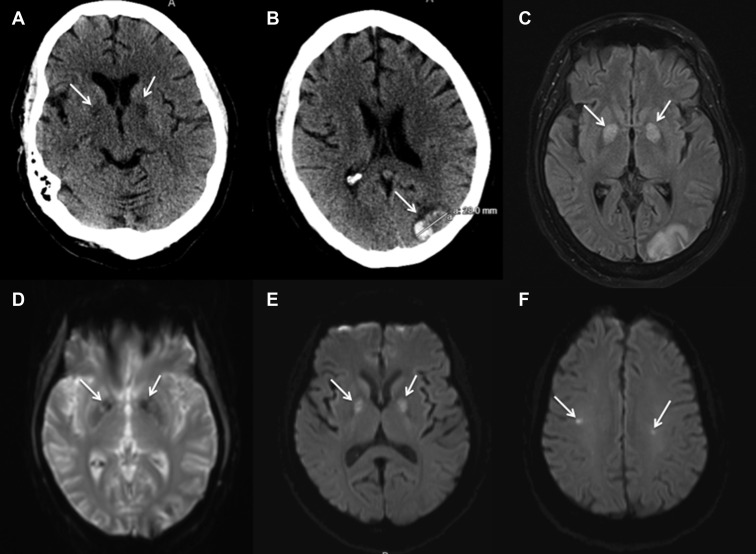
A 63-year-old female patient with hypertension and obesity presented with 4 days of fever, cough, shortness of breath, and chest pain. Due to respiratory failure and hypoxemia, the patient was intubated and transferred to the RICU. RT-PCR confirmed SARS-CoV-2 infection. She developed multisystem disease, including renal failure requiring continuous renal-replacement therapy (CRRT) and fungal pneumonia. She was treated with glucocorticoids and a 5-day course of hydroxychloroquine. Quantitative D-dimer assay was elevated at 12.02 mg/L and systemic anticoagulation was initiated with heparin titrated to anti-factor Xa (range 0.3–0.7 IU/mL) on day 5 of admission. On ventilator day 10, the patient was weaned from sedation and found to have significant AMS without focal deficit. Lumbar puncture showed normal glucose and protein levels with a cell differential suggestive of a traumatic tap. Noncontrast CT of the head demonstrated hypodensities within bilateral globus pallidi as well as a focal parenchymal hemorrhage in the left occipital pole (Figure 1 ). MRI showed fluid-attenuated inversion recovery (FLAIR) changes at the same locations and diffusion restriction in bilateral globus pallidi and bilateral centrum semiovale (Figure 1). Gradient echo showed subtle changes in bilateral globus pallidi. Supportive critical care was continued with plans for viral testing of cerebrospinal fluid (CSF).
Figure 1.
A 63-year-old female patient with coronavirus disease 2019–associated encephalopathy is shown. Noncontrast computed tomography scan of the head showed (A) hypodensities in bilateral globus pallidus pars interna (arrows) and (B) focal intraparenchymal hematoma at occipital pole (arrow). Magnetic resonance fluid-attenuated inversion recovery (C) and gradient echo (D) showed changes at bilateral globus pallidus (arrows) and occipital pole. Diffusion-weighted imaging showing restriction at bilateral globus pallidus (arrows) (E) and at focal points in the centrum semiovale (arrows) (F).
COVID-19–Associated Acute Necrotizing Encephalopathy
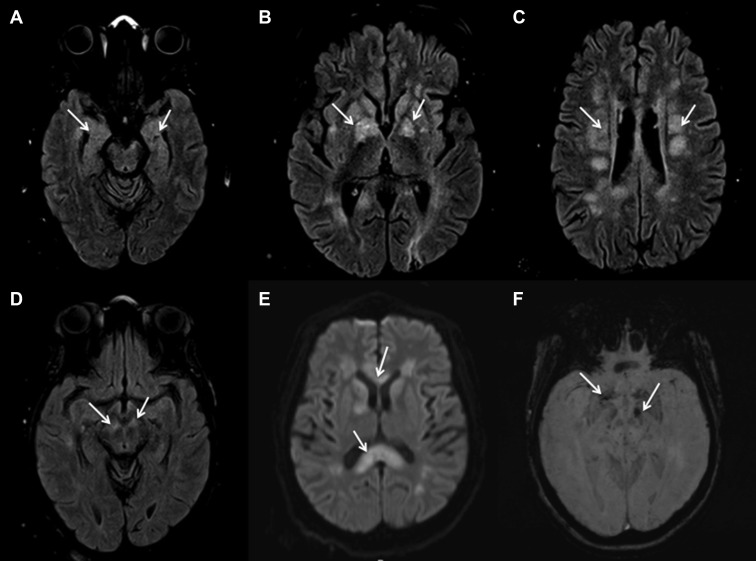
A 43-year-old female patient with hypertension and DM2 presented with cough and dyspnea. She developed hypoxia requiring mechanical ventilation and was transferred to the RICU. Two interval RT-PCR tests confirmed active SARS-CoV-2 infection. Similarly, the patient developed multisystem organ failure, including renal failure requiring CRRT and bacteremia requiring broad-spectrum antibiotics. The patient was treated with glucocorticoids and started on therapeutic heparin anticoagulation titrated to anti-factor Xa for an elevated D-dimer of 3.44 mg/L. On ventilator day 15, the patient was weaned from sedation and found to be significantly altered; she demonstrated extensor posturing of the right extremities and left hemiplegia. MRI of the brain showed FLAIR changes in bilateral mesial temporal structures, lenticular nuclei, crus cerebri, and centrum semiovale (Figure 2 ). Diffusion-weighted imaging showed restriction of those areas together with the splenium, body, and genu of the corpus callosum (Figure 2). SWI sequences showed hemorrhagic conversion in the left cerebral peduncle and bilateral basal ganglia (Figure 2). The patient was presumptively diagnosed with acute disseminated encephalomyelitis (ADEM) and started on a 5-day course of plasma exchange (PLEX). The patient remains in critical condition with poor neurologic function.
Figure 2.
A 43-year-old female patient with coronavirus disease 2019–associated acute necrotizing encephalopathy is shown. Changes (arrows) noted on magnetic resonance fluid-attenuated inversion recovery in (A) the bilateral medial temporal structures, (B) the bilateral lentricular nuclei, (C) the bilateral centrum semiovale, and (D) the bilateral crus cerebri. Diffusion-weighted imaging showing restriction (arrows) at (E) the genu and splenium of the corpus callosum as well as several deep white structures. Susceptibility-weighted imaging showing changes (arrows) in the crus cerebri and basal ganglia (F).
COVID-19–Associated Vasculopathy
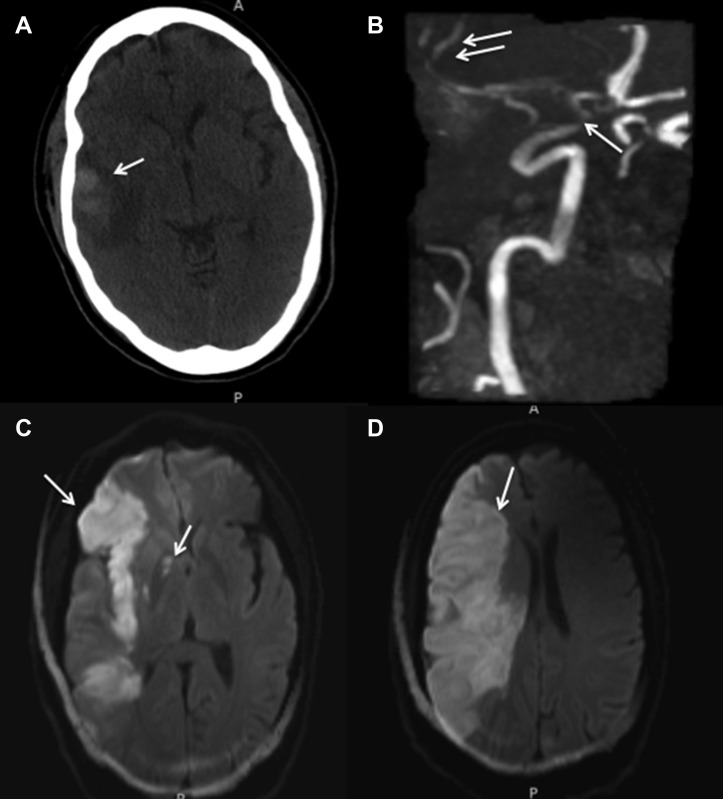
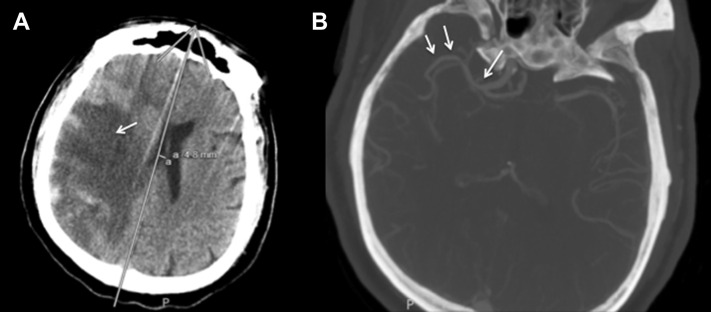
A 53-year-old male patient with hypertension presented with dyspnea and ultimately required mechanical ventilation and RICU care. CRRT was started for acute renal failure and broad-spectrum antibiotics were administered for bacterial pneumonia. The patient was started on therapeutic heparin anticoagulation for an elevated D-dimer of 9.52 mg/L. SARS-CoV-2 infection was confirmed with RT-PCR. On ventilator day 8, the patient was extubated but shortly thereafter developed left hemiparesis and right gaze deviation. Noncontrast CT of the head showed a subcortical parenchymal hemorrhage at the right temporal pole with associated vasogenic edema (Figure 3 ). The patient was reintubated and followed with interval imaging, which showed suspicion for cytotoxic edema secondary to acute ischemic stroke. Diffusion-weighted imaging sequences confirmed restriction of the entire right MCA territory suspicious of LVO, as well as within the lateral lenticulostriate territory (Figure 3). MRA was negative for LVO, demonstrating focal stenosis of the ICA supraclinoidal segments and patency in the anterior and posterior MCA M2 trunks (Figure 3). The patient remains in critical condition. The findings of ICA terminus stenosis with patent distal vessels were demonstrated on MRA in several other cases of vasculopathy, as well as on CTA in a 65-year-old male patient who developed a large territory infarct of the MCA territory (Figure 4 ).
Figure 3.
A 54-year-old male patient with coronavirus disease 2019–associated vasculopathy is shown. Noncontrast computed tomography scan of the head (A) showed initial temporal pole parenchymal hematoma (arrow) with surrounding vasogenic edema. Later magnetic resonance angiography (B) showed focal stenosis of the supraclinoid internal carotid artery (arrow) with preserved flow into the posterior and anterior middle cerebral artery trunks distally (double arrows). Diffusion-weighted imaging showed restriction (arrow) of the total right middle cerebral artery territory (C and D) and within the right posterior limb of the internal capsule at its junction with the genu (C).
Figure 4.
A 65-year-old male patient with coronavirus disease 2019–associated vasculopathy is shown. Noncontrast computed tomography (CT) scan of the head (A) showed large territory middle cerebral artery infarct with petechial hemorrhagic conversion. CT (B) angiography showing no large vessel occlusion and focal stenosis at internal carotid artery terminus (arrow) with patent middle cerebral artery trunks (double arrows).
Discussion
Evolving evidence concerning critically hospitalized patients with confirmed COVID-19 links comorbidities of DM2, hypertension, chronic obstructive pulmonary disease, chronic kidney disease, obesity, and cardiovascular disease with an increased risk of more severe disease course and multisystem effects.13, 14, 15, 16 , 18, 19, 20, 21, 22, 23 Given that the aforementioned comorbidities are seen in patients undergoing treatment for neurologic conditions, particularly in the context of ischemic occlusive and hemorrhagic cerebrovascular disease, neurologic and neurosurgical patients are at heightened risk.13, 14, 15, 16 , 18, 19, 20, 21, 22, 23 In contrast to the typical bimodal patterns of age distribution of moderate and severe viral disease, data have demonstrated that increased age is significantly associated with greater odds of mortality for every additional year of life.21, 22, 23 Patients in our cohort carried a high proportion of comorbidities and were relatively young. It is possible that the age phenomenon is confounded by the presence of complex multisystemic disease states such DM2 and CKD, as every year of life increases the probability of developing a comorbid disease. We hypothesize that our patient population's high rate of comorbid disease is responsible for the more serious course of illness being experienced.
It is intuitive that severe strain on pulmonary function via acute respiratory distress syndrome (ARDS) would exacerbate pre-existing systemic disease via various pathophysiologic pulmonary mechanisms and accelerate such conditions.13, 14, 15, 16 , 18, 19, 20, 21, 22, 23 Furthermore, a pre-existing history of ischemic or hemorrhagic stroke has been demonstrated to be a significant risk factor for the development of ARDS in the neurointensive setting.13, 14, 15, 16 , 18, 19, 20, 21, 22, 23 Severe COVID-19 carries an association of vascular thrombosis.24 It thus becomes critical to accurately characterize disease pathophysiology to rule out direct injury secondary to viral infection from consequent systemic pathophysiology.
Much of SARS-CoV-2 pathophysiology has been derived from literature concerning SARS-CoV-1.25, 26, 27, 28, 29, 30, 31 Both viruses have been shown to enter lower respiratory epithelium via attachment to the angiotensin-converting enzyme 2 receptor.25, 26, 27, 28, 29, 30, 31 Although angiotensin-converting enzyme 2 receptor is found primarily in lung alveolar epithelium, it is present on the surface of CNS neurons, suggesting potential neurotropism.25, 26, 27, 28, 29, 30, 31 An axodendritic trans-synaptic route has been reported as a potential mechanism for CNS dissemination.9 Rodent models infected with intranasal SARS-CoV-1 have demonstrated particular tropism for the cerebrum, thalamus, and rhombencephalon derivatives typical of viral encephalitis.9
Although coronaviridae CNS entry is well documented, its role in neurologic disease remains under investigation. Reports implicate potential central involvement of respiratory failure compounding primary pulmonary injury via direct infection of the pontine and medullary respiratory groups in COVID-19.9 With a median onset of 8 days for the development of ARDS, SARS-CoV-2 CNS involvement is possible by the time of ICU admission, reinforcing the hypothesis of a mixed central and peripheral etiology of pulmonary compromise.25, 26, 27, 28, 29, 30, 31
In addition, SARS-CoV-2 may incite noninflammatory encephalopathy, which has been previously implicated in SARS-CoV-1 infection.25, 26, 27, 28, 29, 30, 31 In MERS, viral-associated encephalopathy has been reported with resulting confusion, coma, ataxia, and focal motor deficits.8 A significant proportion of our patients showed evidence of direct neurologic injury. Diffuse hypoattenuation of deep white matter tracts and focal hypodensities of both white and gray deep structures on noncontrast CT observed in our patients are similar to the focal encephalitides and vasculolitides associated with members of the Herpesviridae family.17 MRI was seldom ordered due to containment precautions and instability of the patients; however, when completed, findings were most often consistent with viral encephalitis: restriction and FLAIR changes in bilateral deep structures as well as the corpus callosum. Basal ganglia involvement with focal restriction, diffuse FLAIR changes on MRI, or discrete hypodensities on CT were common, and several patients had petechial changes noted on SWI similar to necrotizing encephalopathy.13, 14, 15 One patient had FLAIR changes in the cortical region of the bilateral pre-central frontal gyri and deep structure changes characteristic of anoxic injury.
Reported cases of severe neurologic disease in COVID-19 are rare but have heterogenous presentation and progress rapidly.13, 14, 15 Our patients progressed variably along 1 of 3 presentation spectrums. The majority of patients developed severe electrographic nonspecific encephalopathy during their disease course, with clinical AMS ranging from mild to obtunded. These patients often had nonspecific imaging findings such a hypodensity or hypoattenuation of deep structures. It would be reasonable to classify these patients as having COVID-19–associated encephalopathy. One of our patients developed nonconvulsive status epilepticus, which is similar to literature descriptions of COVID-19–associated epileptic encephalopathy.12
In severe cases, CT and MRI findings were consistent with other viral encephalatides, particularly IAE and acute necrotizing encephalopathy. It appears that acute necrotizing encephalopathy is not a diagnosis restricted to hemagglutinin 1 neuraminidase 1 influenza virus Orthomyxoviridae infection and may be a sequela of SARS-CoV-2 pathogenesis. As previously mentioned, coronaviridae can alter neuroinflammatory cascades, and given the suspected pathogenesis of COVID-19–associated ARDS, it is plausible that necrotizing encephalopathy progresses similarly. These diagnoses remain subjective and clinical. None of our patients had their CSF tested for viral positive-sense single-stranded RNA, although there may be utility in doing so, given reports of CSF infection despite a negative nasopharyngeal swab.13 This is particularly relevant when performing intrathecal procedures, such as lumbar puncture, as CSF to mucous membrane transmission has been demonstrated to be a viable mechanism in HIV 1 and 2.32 Furthermore, there is evidence that a negative oropharyngeal swab does not rule out active COVID-19 disease.1 , 32
Available literature on the treatment of CNS involvement in COVID-19 is variable and sparse, with reports including intravenous immunoglobulin and corticosteroids in the treatment of encephalitis.12 Intravenous immunoglobulin, which has been used in treating other necrotizing forms of IAE, is theorized to treat severe cytokine storming and may ameliorate secondary vasogenic edema from blood–brain barrier compromise.14 Similarly, PLEX may help relieve secondary disease patterns radiographically similar to ADEM.14 One patient in our cohort, who was presumed to have ADEM, and likely had early sequelae of COVID-19–associated acute necrotizing encephalopathy, underwent a 5-day course of PLEX. Seizure activity should be managed per protocol with antiepileptics,13 and anecdotal reports of treatment success with anti-inflammatories/antiparasitics such as hydroxychloroquine and viral protease inhibitors such lopinavir and ritonavir may be considered,13 although either option has yet to yield promising results. Patients with elevated levels of D-dimer have been reported to have a worse prognosis and greater risk of thromboembolic complications in COVID-19, presumably secondary to consumptive coagulopathy.33, 34, 35, 36 In accordance with recent literature,37 all critical patients with COVID-19 at our center are therapeutically anticoagulated with heparin titrated against anti-factor Xa unless otherwise contraindicated.
The increased prevalence of acute cerebrovascular disease in patients with severe infection is thought to be related to elevated D-dimer levels, which have been previously shown to correlate with unfavorable outcomes in stroke patients.33 Furthermore, cerebrovascular disease is a known predisposing factor for the development of ARDS in a neurocritical care setting.33 Although there are no specific guidelines concerning pre-existing stroke or elevated D-dimer in the setting of COVID-19, it is easy to speculate that these patients likely have an increased likelihood of progressing to ARDS and warrant increased vigilance in monitoring disease course.
Five patients in our cohort experienced largescale cerebrovascular accident, 4 of which occurred after presentation. Two of these were purely parenchymal hematomas located in the subcortical occipital lobe. One of these patients presented with a focal intracerebral hematoma (ICH) in the left inferior occipital gyrus toward the occipital pole and the other with a similar location, though below the lateral occipital sulcus in the second occipital gyrus. Both of these patients had negative vascular imaging on MRA and CTA but had deep structure involvement including corpus callosal and focal hypodensities in the basal ganglia. These findings suggest a COVID-19–associated small vessel vasculopathy, such as that observed in VZV infection.17 One patient with a history of an occipital pole infarct was found to have restriction of bilateral frontotemporoparietal and insular cortices. Although MRA did not show any LVO, it revealed focal stenosis of the ICA terminus with preserved distal flow. A fourth patient with a subtotal infarct of the right MCA M2 superior division experienced an interval expansion of his stroke several days into his stay to include the entire MCA territory with hemorrhagic conversion, hemorrhagic infarction type 2. On MRA, the patient was also found to have bilateral focal non-occlusive stenosis of the ICA terminus with patent distal anterior vasculature. Another patient with a subcortical superior temporal gyrus ICH near the temporal pole progressed to total infarct of the MCA territory, including the region supplied by the lateral lenticulostriates in the basal ganglia. This patient was found to have focal supraclinoidal ICA stenosis with patent distal vasculature on MRA. Only 1 patient in our cohort developed an ischemic stroke secondary to LVO, and this occurred at the distal end of the P2B segment of the posterior cerebral artery, an atypical location too distal for neuroendovascular intervention.
In our cohort of patients with COVID-19, stroke involved either focal subcortical lobar ICH, large-territory anterior circulation infarcts in the presence of focal ICA terminus stenosis without LVO, or both. Although COVID-19 is known to induce thrombosis-associated complications, including stroke, reports did not confirm LVO to be the actual etiology.24 Our patient population suggests large territory infarct may occur via transient or hemodynamic ischemic condition in the absence of LVO. Observation in other viral infections with similar presentation are often deemed to derive from associated vasculopathies.17
All of our patients with vasculopathy had elevated D-dimer, 4 of whom were on therapeutic anticoagulation at the time of insult. The sole patient who was not receiving anticoagulation suffered LVO. There may be separate mechanisms accounting for acute ischemic stroke in COVID-19. The first may be LVO as a direct consequence of hypercoagulability, similar to reports of CTA-confirmed pulmonary embolism.37 This was likely the sequela suffered by the patient with a posterior territory infarct. The remaining 4 with evidence of focal ICA terminus stenosis were therapeutically anticoagulated at the time of infarct, suggesting a separate mechanism. Large-vessel vasculopathy has been documented in both HIV-1 and 2 as well as VZV infection.38 , 39 VZV vasculopathy, in particular, presents with focal segmental stenosis of both large and small vessels with secondary ischemic infarcts,39 whereas HIV vasculopathy has typical dilatation of the proximal large vessels of the anterior circulation and small vessel thickening.38 Both HIV and VZV vasculopathy may present with both lacunar and large hemispheric strokes in the absence of LVO on noninvasive imaging.38 , 39 Likewise, both MERS and SARS have been associated with varying degrees of direct cardiovascular and cerebrovascular involvment.40 , 41
Limitations
We acknowledge our report is limited by the retrospective nature of analysis and our limiting inclusion criteria to patients actively admitted to our center in critical condition with confirmed COVID-19. Future investigations will seek to expand our data in a prospective manner for all patients with confirmed SARS-CoV-2–positive assay. Likewise, at the time of data collection, we were not actively evaluating patients' CSF for the presence of viral genetic material. Prospective CSF analysis also will be included in ongoing studies, as delineation of associated clinical factors and chronology of positive viral tests in CSF as compared with other mediums is critical in understanding disease pathobiology and transmissibility. Furthermore, as neurologic disease in COVID-19 is actively being characterized, we did not have a specific imaging protocol for such patients, and all patients were imaged secondary to clinical findings. As such, not all patients received combined CT, EEG, vascular studies, and MRI, potentially masking relevant radiographic findings.
Conclusions
As the SARS-CoV-2 pandemic continues, we increasingly appreciate the neurologic manifestations of COVID-19. We present the neurologic and ischemic sequelae observed in 27 of 76 critically ill patients with COVID-19 who were admitted on 1 day to our tertiary academic center in New Orleans, Louisiana. Presentations were heterogeneous but fit into one of several categories. These included COVID-19–associated encephalopathy, COVID-19–associated acute necrotizing encephalopathy, and COVID-19–associated vasculopathy. No disease pattern is likely mutually exclusive. Further investigations exploring pathogenesis, management, and outcomes are warranted.
CRediT authorship contribution statement
Tyler Scullen: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing. Joseph Keen: Conceptualization, Validation, Investigation, Data curation, Resources, Writing - review & editing, Supervision. Mansour Mathkour: Validation, Writing - review & editing. Aaron S. Dumont: Conceptualization, Resources, Writing - review & editing, Supervision. Lora Kahn: Conceptualization, Validation, Investigation, Data curation, Resources, Writing - review & editing, Supervision, Project administration.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Coronavirus disease 2019 (COVID-19): situation report – 83. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200412-sitrep-83-covid-19.pdf?sfvrsn=697ce98d_4 Available at: Accessed April 22, 2020.
- 3.Lu R., Zhao X., Li J., Niu P., Yang B., Song H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H. Neurological complications during treatment of Middle East respiratory syndrome. J Clin Neurol. 2017;13:227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai L.K., Hsieh S.T., Chao C.C., Chen Y.C., Lin Y.H., Chang S.C. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004;61:1669–1673. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- 6.Chao C.C., Tsai L.K., Chiou Y.H., Tseng M.T., Hsieh S.T., Chang S.T. Peripheral nerve disease in SARS: report of a case. Neurology. 2003;61:1820–1821. doi: 10.1212/01.wnl.0000099171.26943.d0. [DOI] [PubMed] [Google Scholar]
- 7.Algahtani H., Subahi A., Shirah B. Neurological complications of Middle East respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep Neurol Med. 2016;2016:3502683. doi: 10.1155/2016/3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arabi Y.M., Harthi A., Hussein J., Bouchama A., Johani S., Hajeer A.H. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [e-pub ahead of print] https://doi.org/10.1002/jmv.25728 J Med Virol. accessed April 22, 2020. [DOI] [PMC free article] [PubMed]
- 10.Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dube M. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12 doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray R.S., Brown B., Brian D., Cabirac G.F. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann Neurol. 1992;31:525–533. doi: 10.1002/ana.410310511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boucher A., Desforges M., Duquette P., Talbott P.J. Long-term human coronavirus-myelin cross-reactive T-cell clones derived from multiple sclerosis patients. Clin Immunol. 2007;123:258–267. doi: 10.1016/j.clim.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filatov A., Sharma P., Hindi F., Espinosa P. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12:e7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poyiadji N. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [e-pub ahead of print] https://doi.org/10.1148/radiol.2020201187 Radiology. accessed April 22, 2020. [DOI] [PMC free article] [PubMed]
- 15.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J. A first Case of Meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waldman G., Mayeux R., Claassen J., Agarwal S., Willey J., Anderson E. Preparing a neurology department for SARS-CoV-2 (COVID-19): early experiences at Columbia University Irving Medical Center and the New York Presbyterian Hospital in New York City. Neurology. 2020;94:886–891. doi: 10.1212/WNL.0000000000009519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egdell R., Egdell D., Solomon T. Herpes simplex encephalitis. BMJ. 2012;344:e3630. doi: 10.1136/bmj.e3630. [DOI] [PubMed] [Google Scholar]
- 18.Simonnet A., Chetboun M., Poissy J. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation [e-pub ahead of print] https://doi.org/10.1002/oby.22831 Obesity (Silver Spring) accessed April 22, 2020. [DOI] [PMC free article] [PubMed]
- 19.Lighter J., Phillips M., Hochman S. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission [e-pub ahead of print] https://doi.org/10.1093/cid/ciaa415 Clin Infect Dis. acessed April 22, 2020. [DOI] [PMC free article] [PubMed]
- 20.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou F., Yu T., Du R., Fan G., Liu Y., Xiang J. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porcheddu R., Serra C., Kelvin D., Kelvin N., Rubino S. Similarity in case fatality rates (CFR) of COVID-19/SARS-COV-2 in Italy and China. J Infect Dev Ctries. 2020;14:125–128. doi: 10.3855/jidc.12600. [DOI] [PubMed] [Google Scholar]
- 24.Klok F.A., Kruip M., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19 [e-pub ahead of print] https://doi.org/10.1016/j.thromres.2020.04.013 Thromb Res. accessed April 22, 2020. [DOI] [PMC free article] [PubMed]
- 25.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia H.P., Look D.C., Shi L. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi G.P., Sanga V., Barton M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. Elife. 2020;9:e57278. doi: 10.7554/eLife.57278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng J., Xiao G., Zhang J. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danser A.H.J., Epstein M., Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75:1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broussard I.M., Kahwaji C.I. Treasure Island; FL: StatPearls: 2020. Universal Precautions. [PubMed] [Google Scholar]
- 33.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China [e-pub ahead of print] https://doi.org/10.1001/jamaneurol.2020.1127 JAMA Neurol. accessed April 22, 2020. [DOI] [PMC free article] [PubMed]
- 34.Panigada M., Bottino N., Tagliabue P. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis [e-pub ahead of print] https://doi.org/10.1111/jth.14850 J Thromb Haemost. accessed April 22, 2020. [DOI] [PMC free article] [PubMed]
- 35.Zhang L., Yan X., Fan Q. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19 [e-pub ahead of print] https://doi.org/10.1111/jth.14859 J Thromb Haemost. accessed April 22, 2020. [DOI] [PMC free article] [PubMed]
- 36.Leonard-Lorant I., Delabranche X., Severac F. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels [e-pub ahead of print] https://doi.org/10.1148/radiol.2020201561 Radiology. accessed April 22, 2020. [DOI] [PMC free article] [PubMed]
- 37.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action [e-pub ahead of print] https://doi.org/10.1111/bjh.16727 Br J Haematol. accessed April 22, 2020. [DOI] [PMC free article] [PubMed]
- 38.Chen B., Morris S.R., Panigrahi S. Cytomegalovirus coinfection is associated with increased vascular-homing CD57(+) CD4 T cells in HIV infection. J Immunol. 2020;204:2722–2733. doi: 10.4049/jimmunol.1900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau A., Essien E.O., Tan I.J. Zoster sine herpete masquerading as central nervous system vasculitis. Cureus. 2020;12:e7231. doi: 10.7759/cureus.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato T., Ushiroda Y., Oyama T., Nakatomi A., Motomura H., Moriuchi H. Kawasaki disease-associated MERS: pathological insights from SPECT findings. Brain Dev. 2012;34:605–608. doi: 10.1016/j.braindev.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Hu S.S., Yang Y.J., Zhu M.L. Effects of underlying cerebrocardiovascular diseases on the incidence of critical conditions and multiple organs dysfunction syndrome in severe acute respiratory syndrome cases. Zhonghua Yi Xue Za Zhi. 2004;84:1257–1259. [in Chinese] [PubMed] [Google Scholar]