Abstract
The aim of this study was to characterize the echocardiographic phenotype of patients with COVID-19 pneumonia and its relation to biomarkers. Seventy-four patients (59 ± 13 years old, 78% male) admitted with COVID-19 were included after referral for transthoracic echocardiography as part of routine care. A level 1 British Society of Echocardiography transthoracic echocardiography was used to assess chamber size and function, valvular disease, and likelihood of pulmonary hypertension. The chief abnormalities were right ventricle (RV) dilatation (41%) and RV dysfunction (27%). RV impairment was associated with increased D-dimer and C-reactive protein levels. In contrast, left ventricular function was hyperdynamic or normal in most (89%) patients.
Résumé
Cette étude visait à caractériser le phénotype échocardiographique des patients atteints de pneumonie causée par la COVID-19 et sa relation avec des biomarqueurs. Soixante-quatorze patients (âgés de 59 ± 13 ans, 78 % de sexe masculin) hospitalisés en raison de la COVID-19 ont participé à l’étude après leur orientation vers le service de cardiologie pour subir une échocardiographie transthoracique dans le cadre des soins courants. Une échocardiographie transthoracique de niveau 1 de la British Society of Echocardiography a été effectuée en vue d’évaluer la taille et la fonction des cavités cardiaques, la valvulopathie et le risque d’hypertension pulmonaire. Les principales anomalies étaient la dilatation du ventricule droit (41 %) et la dysfonction du ventricule droit (27 %). L’atteinte du ventricule droit était associée à une augmentation des taux de D-dimères et de protéines C-réactives. En revanche, la fonction ventriculaire gauche était hyperdynamique ou normale chez la plupart (89 %) des patients.
In the face of the COVID-19 pandemic, Birmingham has emerged as an epicentre within the United Kingdom. This has led to increasing demand for transthoracic echocardiography (TTE) in critically ill patients being considered for mechanical ventilation and/or circulatory support. Although cardiac biomarkers such as high-sensitivity troponin are emerging as strong predictors of outcome in COVID-19 patients, there are only limited data on echocardiographic findings in these patients. A description of the echocardiographic phenotype and its relation to biomarkers and outcomes is therefore warranted. The aim of this study was to characterize the TTE findings in consecutive patients admitted with proven COVID-19 pneumonia.
Methods
This retrospective observational cohort study included adults 18 years of age or older with COVID-19 pneumonia who underwent TTE between March 22 and April 17, 2020, at a 1215-bed quaternary referral centre. All cases were confirmed through reverse transcriptase polymerase chain reaction assays performed on nasopharyngeal swabs and all patients had pulmonary infiltrates on chest radiograph. Patients were referred for TTE at the discretion of the clinician responsible for the patient’s care, with one of the following indications: chest pain, arrhythmia, abnormal electrocardiogram changes, or hemodynamic instability. To minimize the risk of unnecessary exposure to COVID-19 on our echocardiographers, each referral for inpatient TTE was confirmed as appropriate by one of 3 imaging consultant physicians (W.E.M., W.M.B., R.P.S.). Suspected or proven COVID-19 patients proceeded to TTE only after documentation of an elevated high-sensitivity Troponin I (hsTnI) > 14 ng/L, or if unavailable at the time of triage, when urgent assessment was needed to guide escalation or withdrawal of care. During the period of study, departmental echocardiographers were available 24/7 and to our knowledge, performed all scanning in COVID-19 patients to the exclusion of other point of care ultrasound examinations. Patients with previously abnormal echocardiography (n = 3) were excluded from the analysis, with the aim of isolating as far as possible abnormalities due to COVID-19.
Echocardiography was performed (Sparq 795090CC ultrasound system, Philips Healthcare, Amsterdam, Netherlands) using a phased array S5 probe by experienced, accredited echocardiographers (level 2 proficiency accreditation British Society of Echocardiography [BSE]) following a modified level 1 focused protocol with assessment of chamber size and function, valvular disease, and likelihood of pulmonary hypertension.1 The BSE level 1 protocol does not require measurements to be performed and does not include tissue Doppler.
Measurements were performed retrospectively, offline using the archived images by 2 independent observers (H.M.M.-E. and J.S.) blinded to the clinical data, in accordance with the 2015 joint guidelines from the American Society of Echocardiography and the European Association of Cardiovascular Imaging.2 The right ventricle (RV) was assessed in an appropriately focused view. The RV was defined as dilated if the RV basal diameter measured > 41 mm; RV systolic dysfunction was defined as a fractional area change < 35% or a tricuspid annular plane systolic excursion < 17 mm. The echocardiographic probability of pulmonary hypertension was assessed in accordance with European Association of Cardiovascular Imaging guidelines.3 Left ventricular (LV) systolic function was assessed visually, as per the BSE level 1 guidance.1
All patients underwent routine chest radiography. Computed tomography pulmonary angiography was performed in selected cases at the discretion of the clinical team.
Data were manually extracted from electronic health records. For patients with COVID-19 disease, the need for individual consent was waived by national United Kingdom guidance covering research during the COVID-19 pandemic because the data were collected by members of usual clinical care teams for the primary purposes of clinical need and/or locally approved service evaluation. This study was reviewed and approved by the University Hospitals Birmingham NHS Foundation Trust COVID-19 Related Research and Audit Board.
Results
Baseline demographic characteristics, risk factors, and clinical, laboratory, and echocardiographic characteristics are presented in Table 1 . The mean age was 59 ± 13 years, 78% were male, and 72% white. Most patients had severe type 1 respiratory failure (partial pressure of oxygen < 8 kPa or < 60 mm Hg) requiring mechanical ventilation (82%), with more than half (58%) requiring vasopressor support (norepinephrine) during admission.
Table 1.
Baseline demographic characteristics, risk factors, and laboratory, clinical, and echocardiographic characteristics (N = 74)
| Variable | All patients (N = 74) | Normal right ventricular systolic function (n = 54) | Impaired right ventricular systolic function (n = 20) | P |
|---|---|---|---|---|
| Baseline demographic characteristics and risk factors | ||||
| Age, years | 59 ± 13 | 59 ± 13 | 58 ± 13 | 0.73 |
| Sex | 0.27 | |||
| Male | 58 (78) | 40 (74) | 18 (90) | |
| Female | 16 (22) | 14 (26) | 2 (10) | |
| Ethnicity | 0.68 | |||
| White | 46 (62) | 33 (61) | 13 (65) | |
| Asian | 26 (35) | 19 (35) | 7 (35) | |
| Afro-Caribbean | 2 (3) | 2 (4) | 0 (0) | |
| Hypertension | 31 (42) | 24 (44) | 7 (35) | 0.47 |
| Diabetes mellitus | 27 (36) | 18 (33) | 9 (45) | 0.36 |
| Chronic kidney disease | 8 (11) | 8 (15) | 0 (0) | 0.10 |
| Stroke | 5 (7) | 5 (9) | 0 (0) | 0.31 |
| Current smoker | 5 (7) | 5 (9) | 0 (0) | 0.32 |
| Lung disease | 10 (14) | 7 (13) | 3 (15) | 1.00 |
| Coronary artery disease | 7 (9) | 7 (13) | 0 (0) | 0.18 |
| History of malignancy | 5 (7) | 4 (7) | 1 (5) | 1.00 |
| Laboratory findings | ||||
| Full blood count | ||||
| Hemoglobin, g/L | 128 ± 24 | 127 ± 23 | 133± 26 | 0.35 |
| Platelets, n/mm3 | 226 (172-287) | 218 (170-280) | 243 (184-315) | 0.34 |
| White cell count, n/mm3 | 9 (7-13) | 8.5 (6-12) | 11.2 (8-15) | 0.04 |
| Neutrophils, n/mm3 | 7.4 (5.2-11.6) | 7 (4.8-11.2) | 9.5 (6.4-12.4) | 0.07 |
| Lymphocytes, n/mm3 | 0.97 ± 0.53 | 0.94 ± 0.55 | 1.1 ± 0.49 | 0.37 |
| Neutrophil to lymphocyte ratio | 9.0 (5.2-16.6) | 8.2 (4.5-16.6) | 11.2 (6.3-16.5) | 0.46 |
| hs-Troponin I, ng/L | 14 (6-67) | 15 (6-46) | 12 (6-252) | 0.19 |
| D-dimer, ng/L | 657 (365-2066) | 635 (365-1396) | 724 (410-6362) | 0.33 |
| C-reactive protein, mg/dL | 307 ± 114 | 298 ± 112 | 328 ± 119 | 0.19 |
| Chest radiograph findings | ||||
| Bilateral pulmonary infiltrates | 74 (100) | 54 (100) | 20 (100) | 1.0 |
| Ventilatory parameters∗ | ||||
| Mechanical ventilation at time of echocardiogram | 58 (78) | 42 (78) | 16 (80) | 0.84 |
| Fraction of inspired oxygen | 0.57 ± 0.17 | 0.55 ± 0.17 | 0.62 ± 0.19 | 0.17 |
| PO2/FiO2 | 142 ± 44 | 142 ± 41 | 139 ± 52 | 0.80 |
| Positive end-expiratory pressure, cm H2O | 9.3 ± 2.9 | 9.1 ± 3.2 | 9.8 ± 2.2 | 0.46 |
| Echocardiographic parameters | ||||
| Left ventricular size | ||||
| End-diastolic dimension, mm | 42 ± 8 | 42 ± 7 | 40 ± 10 | 0.40 |
| Small | 15 (20) | 8 (15) | 7 (35) | 0.14 |
| Normal | 56 (76) | 44 (82) | 12 (60) | |
| Dilated | 3 (4) | 2 (4) | 1 (4) | |
| Left ventricular systolic function, visual assessment | ||||
| Hyperdynamic | 36 (48) | 23 (43) | 13 (65) | 0.35 |
| Normal | 30 (41) | 25 (46) | 5 (25) | |
| Mildly impaired | 4 (5) | 3 (6) | 1 (5) | |
| Moderately impaired | 2 (3) | 1 (2) | 1 (5) | |
| Severely impaired | 2 (3) | 2 (4) | 0 (0) | |
| Right ventricular size | ||||
| Normal | 44 (59) | 40 (74) | 4 (20) | < 0.0001 |
| Dilated | 30 (41) | 14 (26) | 16 (80) | |
| RV basal diameter, mm | 40 (35-44) | 39 (34-43) | 44 (40-49) | 0.004 |
| Right ventricular systolic function | ||||
| Normal | 54 (73) | - | - | - |
| Impaired | 20 (27) | - | - | - |
| TAPSE, mm | 23 ± 5 | 23 ± 4 | 21 ± 6 | 0.17 |
| Fractional area change, % | 40 (34-45) | 40 (37-50) | 30 (30-34) | < 0.001 |
| Peak TR velocity, m/s† | 2.9 ± 0.6 | 2.9 ± 0.7 | 2.8 ± 0.4 | 0.75 |
| Pulmonary hypertension | ||||
| Low probability | 12 (16) | 12 (22) | 0 (0) | 0.006 |
| Intermediate probability | 13 (18) | 5 (9) | 8 (40) | |
| High probability | 12 (16) | 9 (17) | 3 (15) | |
| Unable to estimate‡ | 37 (50) | 28 (52) | 9 (45) | |
| Pericardial effusion | 3 (4) | 3 (6) | 0 (0) | 0.56 |
| During hospital stay | ||||
| Vasopressor support | 43 (58) | 31 (57) | 12 (60) | 0.84 |
| Invasive mechanical ventilation | 61 (82) | 45 (83) | 16 (80) | 0.74 |
| Pulmonary embolism§ | 5 (7) | 1 (2) | 4 (20) | 0.02 |
| Death | 28 (38) | 21 (39) | 7 (35) | - |
Data are presented as n (%), mean ± SD, or median (interquartile range). The normality of distribution for continuous variables was determined using the Kolmogorov–Smirnov test. Variables not normally distributed were log-transformed. Baseline data were analyzed using independent samples Student t, χ2, or where appropriate, Fisher exact tests.
CT, computed tomography; hs, high-sensitivity; PO2/FiO2 (also known as the Horowitz or P/F ratio), the ratio of arterial oxygen concentration in mm Hg to the fraction of inspired oxygen; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
In the 58 patients with mechanical ventilation at the time of the echocardiogram.
In the 37 patients with a measurable tricuspid regurgitation continuous wave Doppler signal.
Due to an incomplete tricuspid regurgitation continuous wave Doppler signal.
Diagnosis made using CT pulmonary angiography. CT pulmonary angiography was performed in 20 of the 74 patients (27%).
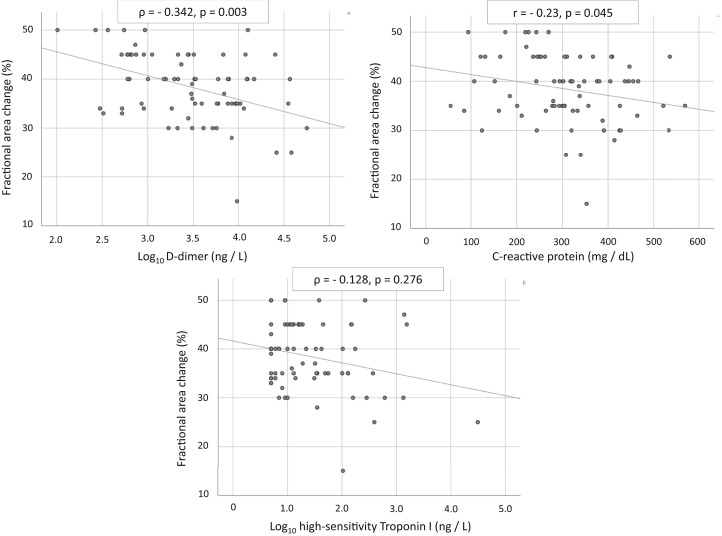
The median time between admission and echocardiography was 5 days (interquartile range [IQR], 3-10 days). LV systolic function was hyperdynamic or normal in most cases (89%), the RV was dilated in 41%, and impaired in 27%. RV systolic dysfunction was associated with pulmonary embolism (20%). The median time between echocardiography and the peak levels of D-dimer, C-reactive protein (CRP), and hsTnI was 3 days (IQR, 1-5 days). RV systolic dysfunction (defined by fractional area change) was significantly associated with elevated D-dimer (ρ = −0.34; P = 0.003) and CRP (r = −0.23; P = 0.045) but was not related to hsTnI (Fig. 1 ). RV size and function was not related to use of vasopressors or mechanical ventilation. Patients with RV dysfunction during mechanical ventilation had lower mean ratio of arterial oxygen concentration in mm Hg to the fraction of inspired oxygen (a marker of acute respiratory distress syndrome severity), and higher mean fraction of inspired oxygen and positive end-expiratory pressure, although results did not meet statistical significance. No patient had more than mild regurgitation and none had any degree of valvular stenosis.
Figure 1.
Relationship between biomarkers and right ventricular systolic function. Pearson correlation was used for normally distributed data, and Spearman rank correlation for data not normally distributed; a 2-tailed P < 0.05 was considered significant.
Twenty-three patients (31%) underwent repeat echocardiography after a median interval of 8 days (IQR, 3-20 days). Across the cohort, there were no significant differences in LV or RV size and function compared with the baseline study.
At the time of submission, 28 patients (38%) had died, of whom 14 (41%) had a RV abnormality (13 dilated, 7 impaired), whereas only 2 (7%) had LV impairment. To date, 15 (20%) have been discharged.
Discussion
In patients with COVID-19 pneumonia, RV dilatation and dysfunction is common and its presence is associated with a prothrombotic, inflammatory state reflected in elevated D-dimer and CRP levels. In contrast, LV size is normal and LV function is hyperdynamic in most, whereas significant valvular abnormalities, either primary or secondary, are absent.
Visual RV assessment has highlighted many subjects with marked reduction in radial RV systolic function but relative preservation of longitudinal shortening. This likely explains the discrepancy between the objective tricuspid annular plane systolic excursion and FAC measurements among patients with reduced RV function. Indeed, many patients exhibited the McConnell sign (exemplar case, see Video 1
 view video online), which involves severe free wall hypokinesia or akinesia with apical sparing. This sign is not specific for acute pulmonary embolus (especially in patients receiving mechanical ventilation) and we believe in most COVID-19 patients this could instead represent acute cor pulmonale secondary to acute respiratory distress syndrome. Although this study has not shown a link between mechanical ventilation and the presence of RV dysfunction and pulmonary hypertension, identification of RV impairment might prompt physicians to limit positive end-expiratory pressure and avoid hypercapnic acidosis, which could otherwise adversely affect RV performance by inducing pulmonary arteriolar vasoconstriction and increased RV afterload.
view video online), which involves severe free wall hypokinesia or akinesia with apical sparing. This sign is not specific for acute pulmonary embolus (especially in patients receiving mechanical ventilation) and we believe in most COVID-19 patients this could instead represent acute cor pulmonale secondary to acute respiratory distress syndrome. Although this study has not shown a link between mechanical ventilation and the presence of RV dysfunction and pulmonary hypertension, identification of RV impairment might prompt physicians to limit positive end-expiratory pressure and avoid hypercapnic acidosis, which could otherwise adversely affect RV performance by inducing pulmonary arteriolar vasoconstriction and increased RV afterload.
Echocardiography might also be used to help identify which patients with COVID-19 could benefit from therapeutic anticoagulation; although there is no current consensus, it was used empirically to good effect in Wuhan patients with very high D-dimer levels.4 Prospective randomized trials might be warranted to determine whether TTE-guided systemic anticoagulation confers survival benefit in hospitalized patients with COVID-19.5 Acute myocarditis causing severe LV dysfunction has also been reported in COVID-19 patients,6 but on the basis of our cohort, there is a low prevalence of LV impairment despite the ubiquitous presence of bilateral lung infiltrates on chest radiograph.
There are limitations to this study. As a retrospective observational cohort study, it is inevitably subject to selection bias. TTE was restricted to patients with COVID-19 pneumonia with elevated HS Tn for safety reasons; these findings are not, therefore, generalizable to the many asymptomatic or paucisymptomatic individuals with COVID-19. Furthermore, only patients with positive reverse transcriptase polymerase chain reaction assays for COVID-19 were included and we acknowledge that the limited sensitivity of this assay (approximately 70%) means that patients with false negative nasopharyngeal swab results who underwent echocardiography were excluded. We acknowledge that echocardiographic estimation of pulmonary hypertension is challenging in critically ill patients and the studies that validated this approach did not include patients receiving mechanical ventilation; however, TTE was performed according to current appropriate guidelines. Finally, not all of the patients underwent computed tomography pulmonary angiography and it is conceivable, therefore, that a proportion of patients with RV dysfunction had undiagnosed thromboembolic disease.
In conclusion, RV dilatation and dysfunction is common in patients with COVID-19 pneumonia and elevated HS Tn. In contrast, the left ventricle is seldom impaired and more often hyperdynamic.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 1207 for disclosure information.
To access the supplementary material accompanying this article, visit the online version of the Canadian Journal of Cardiology at www.onlinecjc.ca and at https://doi.org/10.1016/j.cjca.2020.05.030.
Supplementary Material
Transthoracic echocardiography, apical 4-chamber view. These echocardiographic appearances typify those of the current COVID-19 cohort. This cine shows a dilated right ventricle and severe impairment of radial right ventricle systolic function with relative preservation of long axis function. The left ventricle is small and hyperdynamic. There is also abnormal paradoxical septal wall motion and a thin rim of pericardial effusion lying adjacent to the right atrium.
References
- 1.Hindocha R., Garry D., Short N. Minimum dataset for a level 1 echocardiogram: a guideline protocol from the British Society of Echocardiography. Echo Res Prac. 2020;7 doi: 10.1530/ERP-19-0060. G51-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Galiè N., Humbert M., Vachiery J.L. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 4.Tang N., Bai H., Chen X. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paranjpe I., Fuster V., Lala A. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) [e-pub ahead of print]. JAMA Cardiol 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiography, apical 4-chamber view. These echocardiographic appearances typify those of the current COVID-19 cohort. This cine shows a dilated right ventricle and severe impairment of radial right ventricle systolic function with relative preservation of long axis function. The left ventricle is small and hyperdynamic. There is also abnormal paradoxical septal wall motion and a thin rim of pericardial effusion lying adjacent to the right atrium.