Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and carries a fatality rate of 1.0% to 13.5% in confirmed cases.1,2 Infection is mainly through droplets reaching the eyes, mouth, or nose of an individual, and transmission can be mitigated by measures including hand washing, wearing masks, and social distancing.3 A further measure to protect those extremely vulnerable patients is shielding; a means of guarding individuals from coming into contact with others, achieved by minimizing social interaction for a defined period.
A large number of dialysis patients are of older age with coexisting health conditions, including cardiovascular disease, hypertension, diabetes, and lung disease and are immune-compromised, which affects their ability to produce specific responses to infectious pathogens and to develop seroconversion.4 They are therefore at increased risk of COVID-19 infection and its complications and meet the UK government criteria for shielding. Logistically this cannot be rigorously adhered to by hemodialysis (HD) patients, as they are required to attend dialysis units, to be in close proximity to other patients during HD, and often use shared transport to their dialysis unit.
Various preventive strategies that can be used in outpatient HD facilities have been proposed, which aim to minimize the spread of COVID-19, and dialysis providers have adopted these measures to varying degrees depending on resources.5,6 Guy’s and St. Thomas’ NHS Foundation Trust provide in-center HD (ICHD) in 2 hospital-based dialysis units and 6 satellite units: 4 in inner London boroughs, 1 in outer London, and 1 in a rural location. We serve a diverse population of patients, including a large proportion (52%) from black, Asian, and minority ethnic backgrounds. We report our experience and reflect on the effectiveness of measures taken to reduce viral transmission (Supplementary Methods) while delivering optimal HD in our patient cohort during the initial phase of the COVID-19 pandemic in the United Kingdom.
Results
Between March 14, 2020, and April 20, 2020, of the 670 regular HD patients who dialyse at Guy’s and St. Thomas’ NHS Foundation Trust, 76 (11.3%) tested positive for COVID-19 infection. Of these patients, all received ICHD, and 31 (40.1%) attend ICHD using hospital-organized patient transport. The first positive swab for COVID-19 from our HD cohort was on March 14, 2020. Among those patients who tested positive for COVID-19, the median age was 61.5 years (range, 23–85 years), 47 of 76 (61.8%) were male and 29 of 76 (38.2%) were female (Table 1). Seventy-two (94.7%) infections were identified in patients attending the inner London units, with only 4 (5.3%) in the outer London unit and none in the rural unit, reflecting the early community circulation of SARS-Cov-2 in these inner London boroughs. Ethnicities and cause of end-stage renal disease were representative of our HD population and are shown in Table 2. Thirteen of 76 (17.1%) patients had previously received a renal transplant, 11 (14.5%) of these remain on immunosuppression. The overall median duration on dialysis was 28 months (range, 4–384 months). Table 2 compares the demographics of the cohort of COVID-19–positive patients with our HD population as a whole.
Table 1.
Summary demographics of coronavirus disease 2019–positive hemodialysis patients
| Demographics | All patients | Deceased patients | P |
|---|---|---|---|
| Total number, N | 76 | 7 | — |
| Male:female | 47:29 | 6:1 | 0.151 |
| Median age, yr (range) | 61.5 (23–85) | 79 (56–85) | 0.025 |
| Time on dialysis, mo | 28 (4–384) | 60 (11–84) | 0.545 |
| Cause of end-stage renal failure, n (%) | |||
| Diabetes | 20 (26.3) | 6 (85.7) | 0.007 |
| Hypertension | 8 (10.5) | 0 | |
| Glomerulonephritis | 8 (10.5) | 0 | |
| Other | 10 (13.2) | 0 | |
| Unknown | 30 (39.5) | 1 (14.3) | |
| Ethnicity, n (%) | |||
| Black | 39 (51.3) | 3 (42.9) | 0.721 |
| White | 21 (27.6) | 2 (28.6) | |
| Asian | 8 (10.5) | 1 (14.3) | |
| Other | 8 (10.5) | 1 (14.3) | |
| Previous treatment | 13 | 0 | 0.34 |
| Hospital transportation | 31 | 4 | 0.438 |
Table 2.
Demographics of whole population versus COVID-19–positive HD population
| Demographics | Whole HD population (%) | COVID-19–positive HD Patients (%) | P |
|---|---|---|---|
| Total number, N | 670 | 76 | — |
| Male:female | 399:271 | 47:29 | 0.711 |
| Median age, yr (range) | 63.2 (21–92) | 61.6 (23–85) | 0.380 |
| Median time on dialysis, mo (range) | 32.4 (1–384) | 28 (5–384) | 0.303 |
| Cause of end-stage renal failure, n (%) | |||
| Diabetes | 144 (21.5) | 20 (26.3) | 0.789 |
| Hypertension | 70 (10.4) | 8 (10.5) | |
| Glomerulonephritis | 94 (14) | 8 (10.5) | |
| Other | 90 (272) | 10 (13.2) | |
| Unknown | 272 (40.6) | 30 (39.5) | |
| Ethnicity, n (%) | |||
| Black | 279 (41.6) | 39 (51.3) | 0.151 |
| White | 251 (37.5) | 21 (27.6) | |
| Asian | 53 (7.9) | 8 (10.5) | |
| Other | 87 (13) | 8 (10.5) | |
| Previous treatment, n (%) | 124 (18.5) | 13 (17.3) | 0.738 |
COVID-19, coronavirus disease 2019; HD, hemodialysis.
Thirty-one of 76 (40.8%) patients required admission to hospital, the median length of hospital stay was 28.5 days (range, 9–40 days). Fifteen of 37 (40.5%) of those admitted remain as current inpatients with a length of stay ranging from 3 to 98 days. Two of these patients, however, have been in hospital for 98 and 75 days, related to intra-abdominal collections and post endovascular treatment of an abdominal aortic aneurysm, respectively. Both of these patients acquired COVID-19 infections during their current inpatient stay. Excluding these 2 patients from analysis gives a median length of hospital stay for current inpatients of 12 days (range, 3–29 days). Of the remaining 13 patients, only 3 have been in hospital for 20 or more days, but remain medically fit for discharge while awaiting social planning.
Seven of 76 (9.2%) HD patients have died, all from respiratory failure secondary to COVID-19 pneumonitis. Of these, 6 (85%) (P = 0.15) were male and 5 (71%) (P = 0.72) were from a black, Asian, and minority ethnic background. The median age of those who died was 79 (range, 56–85 years old), and the median length of survival from time of first positive swab for COVID-19 was 28 days (range, 6–38 days) (Table 1). Only 1 (14.3%) died from COVID-19 following a stay on the intensive care unit. All of the patients who died had a number of additional comorbidities and a high level of clinical frailty. None of the patients who died had previously received a renal transplant. The median dialysis duration of patients who died was 60 months (range, 11–84 months).
We compared our data with that of other dialysis unit cohorts in London, from UK renal registry weekly national reports of COVID-19 positivity, and associated mortality. Our unit appears to have had both fewer cases of COVID-19, as well as fewer deaths following infection.7 Table 3 compares our data with that of other dialysis units across London.
Table 3.
UK Renal Registry data comparing number of coronavirus disease 2019–positive cases and deaths among ICHD at our unit (Guy’s) and different London renal units
| Renal center | Total no. dialysis patients | No. cumulative positive tests |
No. cumulative deaths |
||
|---|---|---|---|---|---|
| ICHD | % Total | ICHD | % | ||
| London Guy’s | 755 | 76 | 10 | 7 | 0.93 |
| Other London units | 340–1583 | 31–268 | 5.65–17.37 | 8–44 | 1.34–3.24 |
ICHD, in-center hemodialysis.
Adapted from UK renal registry. UK Renal Registry (2020) Weekly COVID-19 Surveillance Report For Renal Centres in the UK; London – up to 22 April 2020. Bristol, UK: The Renal Association; 2020.7
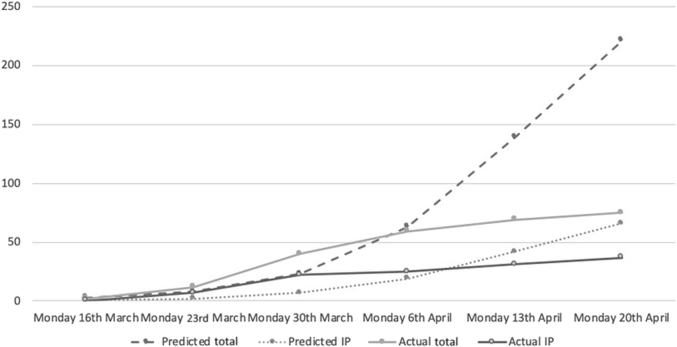
We used a dialysis-specific SIR (susceptible-infectious-recovered) prediction model designed by Imperial College London to help predict and plan for COVID-19 positivity in our HD patient population. The “best guess” values calculated by this model, based on an R0 of 2.0, predicted that there would be a total of 221 (33%) confirmed HD patients testing positive for COVID-19 by April 20, 2020. This same model predicted that of these, 66 (9.9%) would be admitted to hospital. Figure 1 shows how the best guess predicted total number of COVID-19–positive HD patients and COVID-19–positive inpatients, compares with actual numbers recorded at our Trust between March 30, 2020 and April 20, 2020.
Figure 1.
Predicted versus actual total and inpatient coronavirus disease 2019 (COVID-19)–positive hemodialysis (HD) patients.
Discussion
It is widely acknowledged that managing patients receiving ICHD with respect to control and prevention of infectious diseases differs from that in the general population because of their close proximity to each other while on treatment, in the waiting area, and on shared transport to and from dialysis facilities.8 SARS-Cov-2 is a highly transmissible virus, and is associated with cluster outbreaks.9 Most dialysis patients have other comorbidities, which in combination with the logistical considerations relating to dialysis facilities, have resulted in these patients being identified as a group that is at high risk for acquiring COVID-19 and its complications. Emerging data from Wuhan and Italy reported death rates of 13.1% in dialysis patients with COVID-19 compared with 4% in the general population.1 The demographics, prevention measures, treatments, and outcomes of our ICHD patients infected with COVID-19 have been described. Facing the challenges of this worldwide pandemic we wish to share the strategies used to minimize transmission and to reflect on measures taken in managing patients, as well as consider those factors that may improve patient survival (summarized in Table 4).
Table 4.
Summary of strategies to optimize care and limit spread of severe acute respiratory syndrome coronavirus 2 among HD patients
| Reducing the spread of infection |
|
|
|
|
|
|
| Optimizing care for patients |
|
|
|
COVID-19, coronavirus disease 2019; HD, hemodialysis.
With respect to planning, in anticipation of the potential impact of COVID-19 on the health care system as a whole, we used dialysis-specific SIR modeling data to obtain best guess predictions of how many of our HD population may be affected. We hoped that this information would allow us to prepare for a potentially large influx of HD patients into hospital, adapt our outpatient HD service to optimize renal support for patients, and limit cross-contamination between COVID-19–positive and –negative patients. Real-time modeling data have been shown to be of benefit in previous pandemics, particularly when it comes to planning of service provision in the early days of a pandemic.S1,S2 The SIR model we used assumed an R0 (i.e., the expected number of cases generated by 1 case) of 2.0. The data presented herein highlight the low rate of COVID-19–positive HD patients identified at our hospital, compared with those predicted by the SIR model until April 20, 2020; 221 (32.7%) predicted versus 76 (11.3%) confirmed cases. Similarly, the model predicted a large influx of patients into hospital compared with our actual data: 66 (9.9%) versus 37 (5.5%) being admitted to hospital. Our data would suggest that, with the measures we have put in place, we have reduced the R0 to a value between 1.7 and 1.8. Further to this, we found that the death rate among COVID-19–positive patients is markedly lower than anticipated, with a total of only 7 (1.03%) deaths in this cohort. When compared with UK Renal Registry data from other units in London (Table 3), the rate of COVID-19 positivity and death from COVID-19 in our Trust, appears to be lower at this early stage.7
The overestimation of affected patients predicted by the model is possibly related to the fact that so much remains unknown about SARS-CoV-2 and how it affects different populations. Much of the modeling data is based on analysis of how the virus has spread in other populations; however, this does not necessarily mean this information is directly applicable to the population of patients we serve. Differences in not only population demographics, but also population behavior, government interventions, and local hospital responses will likely affect the spread of disease, and therefore the accuracy of modeling data. Conversely, it could be that the modeling data predicting the total number of patients with COVID-19 were accurate, but our testing of only symptomatic, and not asymptomatic, patients resulted in lower numbers being recorded.
We implemented a strict protocol for retesting of confirmed COVID-19 cases at our COVID-19–positive HD unit. By using this approach, we have previously shown that by day 15 following the initial positive swab, 41% of patients have not cleared the virus and could not be repatriated back to their original HD unit.S3 Although it is unclear if detection of viral RNA represents ability to transmit virus, this may have prevented the cross-contamination in this population. In addition, 35% of our patients could be repatriated in their base unit at day 12, which is crucial for the capacity of the dedicated COVID-19–positive dialysis unit.
Following data from Wuhan province reporting the major cause of death in HD patients with COVID-19 infection to be related to cardiovascular events and hyperkalemia, we protected our patients by maintaining their regular 3-times-a-week HD schedules.S4 We did, however, counsel patients starting dialysis for the first time during the COVID-19 pandemic that we should adopt incremental twice-weekly dialysis to limit their time in the dialysis units and away from home.S5
In addition to the measures taken to limit patient exposure to the virus, we took steps to ensure that our cohort of COVID-19–positive HD patients were monitored closely by renal physicians to limit the impact of the pandemic on their regular care. By maintaining a cohort of COVID-19–positive patients in a single unit and actively monitoring them for both deterioration or clearance of infection, we ensured timely referral to inpatient care or repatriation to their base HD unit, respectively. This further allowed us to ensure that there were an adequate number of HD slots available to allow all COVID-19–positive patients to continue on 3-times-a-week HD treatment.S3
The data presented should be interpreted with a degree of caution in view of some inherent limitations. First, the number of COVID-19–positive cases we report is likely to be an underestimate, as only symptomatic patients were screened for the virus. A number of patients are likely to have remained asymptomatic in spite of infection, and therefore not included in our testing. The true accuracy of the SIR model is therefore difficult to determine. Second, when comparing data with other units, it is important to bear in mind that they may have had different protocols for testing (e.g., testing of asymptomatic patients), and that they may have had different starting points for testing of ICHD patients. This makes the validity of comparison between different units less reliable. Last, the total number of reported patients on dialysis at different hospitals is based on UK Renal Registry data from the end of 2018 (Table 3). We have assumed that current populations have not changed meaningfully and so actual percentages of patients with COVID-19 are not vastly different. Therefore, the comparison of our infection rates with those in other units can only be viewed as an approximation to how our measures are affecting infection rates compared with other units.
We have shown that the measures we have taken have had a positive impact, slowing the spread of COVID-19 infection, and protecting our HD patients while maintaining optimal dialysis treatment. This package of interventions (Table 4) required rigorous implementation by staff, and the commitment from our HD patient cohort, to make a difference and ultimately help protect this vulnerable group of patients. Although these interventions are shown to have been effective in the initial phase of this pandemic, it will be important to maintain them in subsequent phases of increased SARS-Cov-2 transmission, such as may occur when social distancing strategies for the general population are relaxed.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors acknowledge the following individuals: Glenda Baillie, Head of Nursing in Transplant, Renal and Urology; Lisa Silas, Matron for living donor; Kevin Evans, Matron for satellite units; and Philomina Kwofie, Manager for New Cross Gate Satellite dialysis unit, for their contributions in developing and implementing our strategic plan during the COVID-19 pandemic; Kieron Clark, data manager in Renal for collecting the data; and Dr Damian Ashby, Renal Consultant at Imperial College London, for providing the prediction model.
Author Contributions
TR, D-AM, and TL-M analyzed data. TR wrote the main body of text. D-AM reviewed and amended the main text. NK wrote the introduction. NK, VM, TK, DG, and CB reviewed and amended the drafts. D-AM, NK, VM, TK, DG, and CB approved of the final draft.
Footnotes
Supplementary Material
References
- 1.Verity R., Okell L.C., Ddorigatti I., et al Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University Center for Systems Science and Engineering (JHU CSSE) COVID-19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://github.com/CSSEGISandData/COVID-19 Available at:
- 3.World Health Organization Coronavirus disease (COVID-19) technical guidance: infection prevention and control. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/infection-prevention-and-control Available at: [PubMed]
- 4.Sarnak M.J., Jaber B.L. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;58:1758–1764. doi: 10.1111/j.1523-1755.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Interim additional guidance for infection prevention and control recommendations for patients with suspected or confirmed COVID-19 in outpatient hemodialysis facilities. https://www.cdc.gov/coronavirus/2019-ncov/hcp/dialysis.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhealthcare-facilities%2Fdialysis.html Available at:
- 6.American Society of Nephrology . 2020. Nephrologists Transforming Dialysis Safety (NTDS), coronavirus disease 2019.https://www.asn-online.org/ntds/ Available at: [Google Scholar]
- 7.UK renal registry . The Renal Association; Bristol, UK: 2020. UK Renal Registry (2020) Weekly COVID-19 Surveillance Report For Renal Centres in the UK; London – up to 22 April 2020. [Google Scholar]
- 8.Karkar A. Infection control guidelines in hemodialysis facilities. Kidney Res Clin Pract. 2018;37:1–3. doi: 10.23876/j.krcp.2018.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pung R., Chiew C.J., Young B.E. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020;395:1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.