Highlights
-
•
Review of conventional and emerging gasification technologies.
-
•
Summary of syngas cleaning and conditioning for FT synthesis.
-
•
Catalyst and reactor technologies for Fischer-Tropsch.
-
•
Overview of waste to aviation fuel projects.
Keywords: Municipal solid waste, Biomass, Gasification, Syngas, Jet fuel, Chemicals
Abstract
This article reviews the production of renewable aviation fuels from biomass and residual wastes using gasification followed by syngas conditioning and Fischer-Tropsch catalytic synthesis. The challenges involved with gasifying wastes are discussed along with a summary of conventional and emerging gasification technologies. The techniques for conditioning syngas including removal of particulate matter, tars, sulphur, carbon dioxide, compounds of nitrogen, chlorine and alkali metals are reported. Recent developments in Fischer-Tropsch synthesis, such as new catalyst formulations are described alongside reactor technologies for producing renewable aviation fuels. The energy efficiency and capital cost of converting biomass and residual wastes to aviation fuels are major barriers to widespread adoption. Therefore, further development of advanced technologies will be critical for the aviation industry to achieve their stated greenhouse gas reduction targets by 2050.
1. Introduction
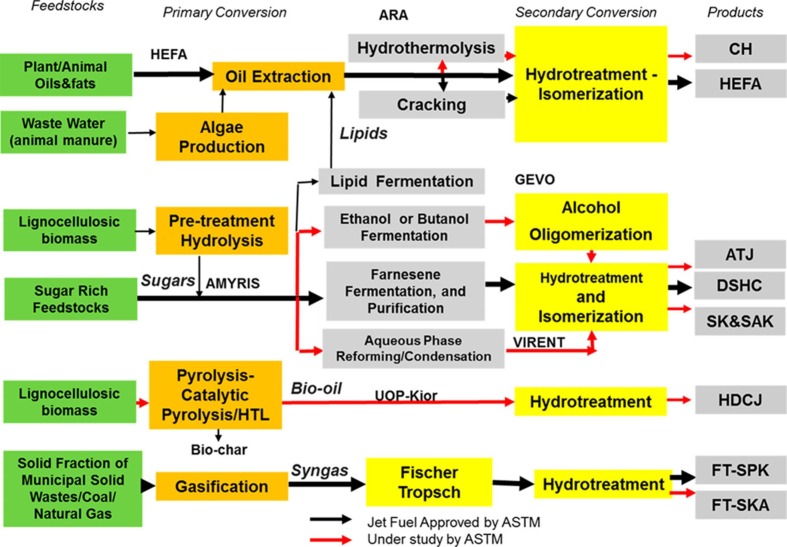
As an alternative source of liquid fuel, biofuel is gaining importance due to its renewability, favourable chemical properties and lower lifecycle emissions. This review is focused on the synthesis of renewable aviation fuels from biomass and residual wastes. Prior to the COVID-19 pandemic, the annual consumption of aviation fuels was around 343 billion litres, of which only 0.015 billion litres were derived from renewable sources. In a business as usual scenario, the aviation sector’s share of global GHG emissions are forecast to grow to 5% by 2050 (Takriti et al., 2017). The international airline industry has committed to ambitious climate change targets including carbon neutral growth from 2020 and the halving of CO2 emissions by 2050 (IATA, 2015). To achieve these targets increased use of sustainable aviation fuels (SAF) is critical. Currently, five production routes are approved by the ASTM D7566 standard (Morgan et al., 2019, Pearlson et al., 2013): Hydrogenated esters and fatty acids (HEFA) fuels derived from used cooking oil, animal fats, algae, and vegetable oils (e.g., camelina) (HEFA-SPK), Fischer-Tropsch (FT) fuels using solid biomass resources (e.g., wood residues) (FT-SPK), FT fuels with aromatics using solid biomass resources (e.g., wood residues) (FT-SKA), Synthetic iso-paraffin (SIP) from fermented hydroprocessed sugar, formerly known as direct-sugar-to-hydrocarbon fuels, which can be blended up to 10% (SIP-SPK) and Alcohol-to-jet (ATJ) fuels produced from isobutanol, which can be blended to a maximum level of 30% (ATJ-SPK) (US Department of Energy, 2020). The routes are based on the five primary conversion techniques as shown in Fig. 1 . This paper reviews the current status of the two routes which involve the synthesis of drop-in aviation fuels from solid biomass and waste feedstocks using gasification and the FT process.
Fig. 1.
Approved and under investigation production pathway for the synthesis of biofuel (Morgan et al., 2019).
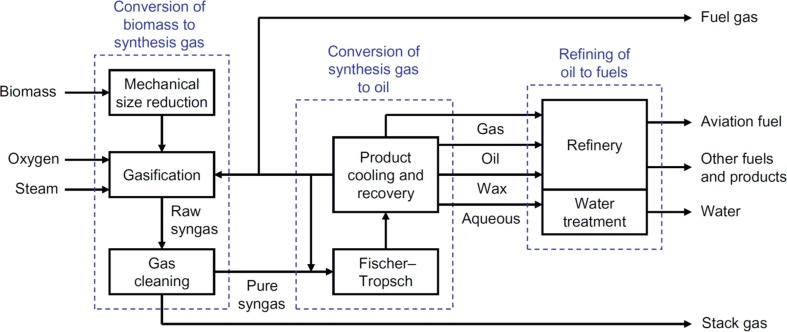
Globally, the most abundant biomass resources are wood and wood wastes, municipal solid wastes and agricultural, forestry and livestock wastes (Ahmad et al., 2016). Lignocellulosic biomass and residual wastes can be transformed into SAF as shown in Fig. 2 . First, the feedstock is pre-treated and often size reduced and then gasified to produce a syngas, which is cleaned to remove contaminants and conditioned to meet the requirements of the FT process. The long chain hydrocarbons from FT are then hydrotreated and hydrocracked to produce aviation fuels meeting the required specifications.
Fig. 2.
Block flow diagram of a generic biomass-to-liquids process based on the Fischer-Tropsch synthesis to produce aviation fuels (from de Klerk, 2016).
The main challenges of producing sustainable aviation fuels from lignocellulosic biomass and residual wastes include: 1) low energy density of the feedstocks, 2) heterogeneity of feedstock in terms of chemical composition, physical properties and moisture content, 3) the complexity and high capital cost of the gasification, gas cleaning and FT process and 4) low carbon efficiency of the overall process. These challenges have resulted in only a very small amount of SAF being produced. In fact, to date, the aviation industry has mostly focused on conducting trials to demonstrate the integration of SAF into existing fuel supply chains and to demonstrate the performance of the fuels in aircraft. However, in recent years a number of commercial projects have been announced and commenced construction (Fulcrum Bioenergy [WWW Document], 2019, Green Car Congress, 2019).
To achieve a greater production of SAF from the FT process, further development of the component technologies are required to improve efficiency and reduce costs. In the following sections, the current status of gasification, syngas cleaning, Fischer-Tropsch catalysis and product purification are reviewed.
2. Gasification
Gasification is a thermochemical process that can be used to convert any carbonaceous material into syngas, being predominately CO and H2. At industrial scale, gasification is most often conducted autothermally, by reacting a sub-stoichometric quantity of oxygen with the carbonaceous feedstock at temperatures in the range 800–1200 °C. A wide variety of gasification technologies are available (Basu, 2013, Higman and van der Burgt, 2008). Many large projects have been constructed to convert fossil fuels such as natural gas, coal and petcoke into syngas and subsequently, hydrogen, ammonia and Fischer-Tropsch liquids (Bell et al., 2011, Higman, 2017). For synthetic aviation fuels, biomass and residual wastes are promising feedstocks due to their renewability and low carbon footprint but are challenged by high variability in composition and low energy density. Recently, the gasification of wastes for electricity and chemicals production has been reviewed (Perkins, 2020). Worldwide 114 biomass gasification projects are in operation while another 15 are idle or on hold (i.e., DP1 + DME pilot and Bio2G projects in Sweden) and 13 plants are currently under construction or in planning (i.e., KSV Koblenz in Germany) (IEA, 2020, Molino et al., 2018). Out of those plants, 106 plants are dedicated to electricity production, 24 plants are used for liquid fuel production, 8 plants are used for gaseous fuel synthesis and the remaining 7 plants are used for chemical production (Molino et al., 2018).
2.1. Types of gasification reactors
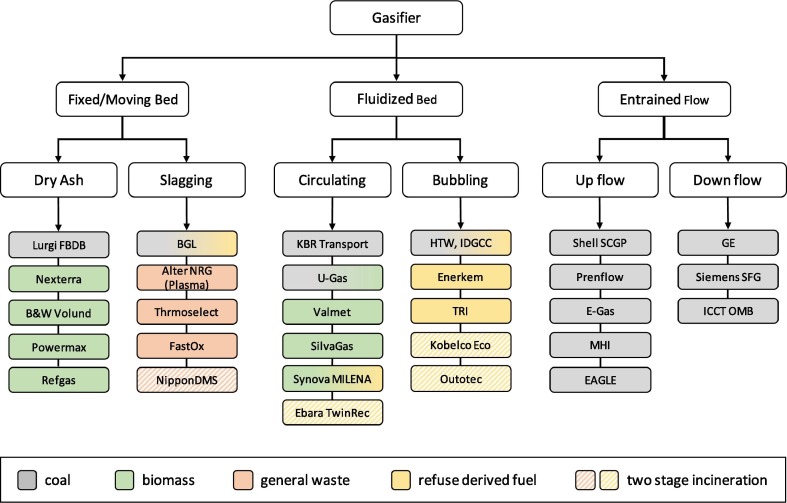
Depending on the configuration, gasifiers are classified into three main types as fixed bed, fluidized bed and entrained flow. These generic gasifiers can be further divided into categories which are shown in Fig. 3 . Fixed bed dry ash gasifiers with either updraft or downdraft configurations are ideal for small scale biomass feedstocks, with a capacity < 50 MWth (Basu, 2013, Sikarwar et al., 2016). For unprocessed general waste feedstocks, high temperature slagging conditions are used to melt the inorganic materials. Fluidized bed gasifiers can be used to process biomass and refuse derived fuel (RDF) from pre-treated waste feedstocks, which need to meet specifications on size, composition and moisture content (Molino et al., 2016). The fluidized bed gasifiers are further classified as bubbling and circulating types (Sikarwar et al., 2017, Sikarwar et al., 2016). Circulating fluidized beds are mostly used for biomass, whereas bubbling beds can be used to process pre-treated wastes such as RDF. Entrained flow gasifiers, which can be classified based on whether they are up flow or down flow, require the feedstock to be sized in the micrometre range and typically operate at pressures of 20 – 80 bar. Entrained flow gasifiers are designed to process coal, though some of the technologies have been tested for co-processing of coal and biomass (Basu, 2013). It is not feasible to pre-treat general wastes to meet the requirements of entrained flow gasifiers. Table 1 shows the summary of the global biomass based liquid fuel production plants.
Fig. 3.
Classification of gasifiers and commercially available technologies by feedstock type.
Table 1.
Summary of commercial fuel liquid production plants using biomass and waste (Molino et al., 2018).
| Institution | Start-up year | Technology | Feedstock | Output (Stream Flow) | Country |
|---|---|---|---|---|---|
| Cutec | 1990 | Atmospheric gasifier | straw, wood, dried silage, organic residues | FT liquids (0.02 t/year) | Germany |
| Lahti Energia Oy | 1998 | Circulating fluidized bed gasifier | wood waste | renewable diesel (HVO) (70 MWth) | Finland |
| CHP Agnion Biomasse Heizkraftwerk Pfaffenhofen | 2001 | Agnion Heatpipe-Reformer | wood waste (80,000 t/year) | SNG (32.5 MWth) | Germany |
| West Biofuels | 2007 | Dual fluidized bed thermal reforming | clean wood, waste wood (5 t/day) | FT liquids | USA |
| H2Herten GmbH | 2009 | Multi-stage reforming Process | roadside greenery/syngas (13 MW) | H2 (150 m3/h | Germany |
| TUBITAK MRC-ENERGY INSTITUTE | 2009 | Down draft fixed bed gasifier | biomass | SNG (0.2 MW) | TURKEY |
| Greasoline GmbH | 2011 | Catalytic cracking of bio-based oils + fats primarily produces diesel fuel-range hydrocarbons | bio-based oils and fats, residues of plant bio-based oils and fats (3 t/year oil processing, free fatty acids, used | diesel-type hydrocarbons (2 t/year) | Germany |
| Karlsruhe Institute of Technology (KIT) | 2012 | Fast pyrolysis, high pressure entrained flow gasification, hot gas cleaning, DME- and gasoline synthesis | straw (0.5 t/h) | gasoline-type fuels (608 t/year) | Germany |
| TUBITAK | 2013 | Pressurised fluidized bed gasifier | combination of hazelnut shell, olive cake, wood chip and lignite blends (0.2 t/h) | FT liquids (250 t/year) | TURKEY |
| Goteborg Energi AB | 2014 | Repotec indirect gasification technology and Haldor Topsoe fixed bed methanation | forest residues, wood pellets, branches and tree tops | SNG (11,200 t/year) | Sweden |
| Karlsruhe Institute of Technology (KIT | 2014 | Fast pyrolysis, high pressure entrained flow gasification, hot gas cleaning, DME- and gasoline synthesis | straw (0.5 t/h) | DME (608 t/year), gasoline-type fuels (360 t/year) | Germany |
| Enerkem | 2016 | Bubbling fluidized bed | 100,000 dry tonnes of MSW per year | Methanol and ethanol, 38 million lt/yr | Canada |
| BioMCN | 2017 | Not reported | wood chip | Methanol (413,000 t/year) | Netherlands |
| Total | 2017 | Not reported | straw, forest waste, dedicated energy crops | FT liquids (200,000 t/year) | France |
| Go Green Fuels Ltd. | 2018 | Not reported | refuse derived fuel and waste wood (7500 t/year) | SNG (1500 t/year) | United Kingdom |
| Fulcrum BioEnergy Sierra Biofuels Plant | 2019 | Not reported | waste (20,000 t/year) | FT liquids (314,913 t/year) | United States |
2.2. Feedstock pre-treatment
Biomass and wastes can be the promising fuel sources for gasification. However, they consist of a variety of combustible and non-combustible materials. For example, municipal solid waste (MSW) consists of paper, plastic, cardboard, wood, textiles as well as metals, glass and many other materials. Therefore, for many gasification technologies the waste must be pre-treated to form a refuse derived fuel. The pre-treatment generally involves removing non-combustible products such as steel, concrete and glass, reducing the moisture content and homogenising the waste to minimise operational problems. The treatment of biomass is classified as mechanical and biological treatment (Stapf et al., 2019). Biological treatment includes bio-stabilisation and composting. Several studies have been conducted using these pre-treatment techniques and found favourable results by reducing the formation of coke and tar while increasing syngas yield (Fang, 2008, Tanksale et al., 2007). Biomass and waste can also be pre-treated using chemical techniques to change the organic and inorganic properties of the feedstock. Further details can be found in Shahabuddin et al. (2020).
2.3. Biomass and waste gasification technologies
As shown in Fig. 3, gasification technologies are available to process a wide range of coal, biomass and waste feedstocks. This section describes several of the gasification technologies that are suitable for producing aviation fuels from the gasification of biomass and solid wastes.
2.3.1. Plasma gasification
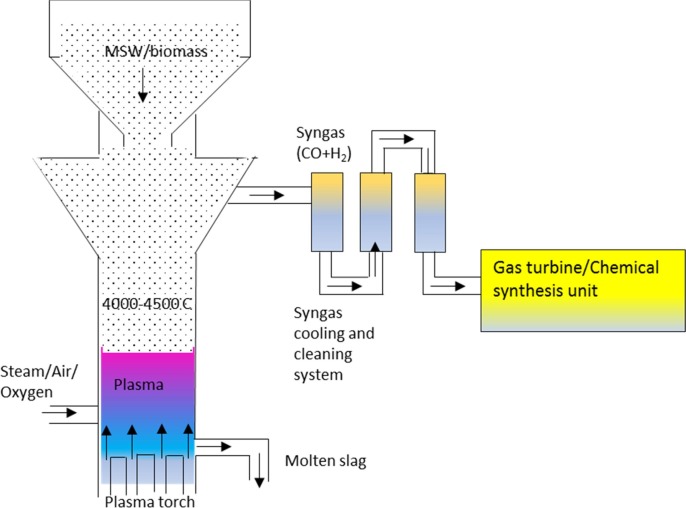
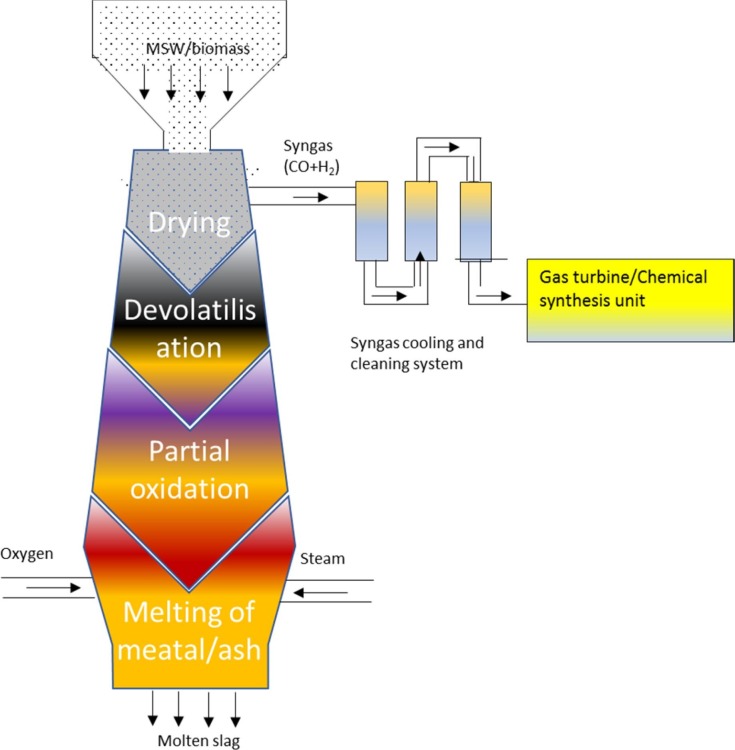
A schematic diagram of a plasma gasifier is shown in Fig. 4 . The gasifier operates at over 2500 °C and can handle unprocessed general wastes, medical wastes and hazardous wastes and achieve full carbon conversion regardless of feedstock type. The waste feedstock is fed from the side or top of a large refractory lined vessel and is reacted with air and/or oxygen injected via tuyeres. The plasma torches are directed to heat and melt the waste forming a molten liquid at ~1600 °C. The high-temperature syngas is collected from the upper part of the gasifier at ~950 °C which means that tars are converted and reformed into smaller molecules such as CO, H2, CH4 and CO2 (Alter NRG, 2018). The hot syngas is cooled before being passed through gas cleaning processes and used for power, chemicals or biofuel production. Due to very high temperature, this gasifier is able to melt any inorganic material in the waste, which is tapped as a molten slag from the bottom of the gasifier (Favas et al., 2017, Pourali, 2010). However, due to the very high-temperature application and complicated design, the capital cost is very high. Besides, the plasma torches can require up to 40–50% of the electrical power generated to sustain the process (Sierra Energy, 2019). The technology also has high maintenance and operational costs (Matveev et al., 2013, Minutillo et al., 2009). While high cold gasification efficiency has been reported for plasma gasification (Mazzoni et al., 2017), net electric efficiency is generally low due to the power consumption of the plasma torches and auxiliary utility loads. AlterNRG has four operating project references, two in China, one in India and one in Japan - all designed to produce electric power (Alter NRG, 2018).
Fig. 4.
Plasma gasification technology for the gasification of general waste.
More recently, plasma torches have been integrated with conventional gasifier technologies to treat the syngas in a process called advanced plasma gasifier technology (Nair et al., 2005, Pemen et al., 2003). In this technology, processed and dried waste and biomass are firstly gasified in a conventional fluidized bed gasifier, which produces crude syngas and tar. This crude syngas and tar along with unconverted solids are fed to the plasma reactor and are further reacted at very high temperature by plasma torches to produce relatively clean high-temperature syngas. The high-temperature syngas (~1200 °C) generated from the plasma reactor is then transferred to the heat recovery unit where the temperature is dropped to around 200 °C. The heat recovered from the heat recovery unit is utilized for the generation of steam, which is used as a fluidizing medium (reactant) in the first reactor. The cooled syngas is then used for either power generation or chemical/biofuel synthesis. While this plasma technology enables very high temperatures and breaks the feedstocks down into almost entirely CO and H2, leading to a clean raw syngas, like the direct plasma gasifiers, the technology is expensive and only a few plants have been built and put into operation, mostly for treating medical and hazardous wastes.
Zhang et al. studied the conversion of municipal solid waste under plasma gasification conditions in a updraft moving bed gasifier at a temperature of up to 6000 °C (Zhang et al., 2012). The results showed that increasing plasma power from 240 kW to 260 kW lead to increase in the ratio of H2/CO from 1.5 to 2.0. The increased H2/CO ratio using higher plasma power, led to higher temperature and increased cracking of tar. The heating value of the product gases were between 6 and 7 MJ/Nm3 under different operating conditions and the maximum energy efficiency was determined to be 58%. The H2/CO ratio from the plasma gasification was determined to be much higher than that of gasification under conventional gasification using air, oxygen and steam (Seo et al., 2018).
While gasifying hazardous industrial waste under plasma gasification condition, it is observed that the ratio of H2/CO is about one. A study conducted by Mountouris et al. showed similar gas composition using sewage sludge under plasma gasification conditions (Mountouris et al., 2006, Moustakas et al., 2005). In contrast, Lemmens et al. conducted a study using RDF and reported a H2/CO ratio of about 0.5 (2007). The quality of syngas using plasma gasification depends on fuel type and quality, moisture content, oxidant used, plasma power and other conditions. The important advantage of plasma gasification is its capability of handling fuel with low quality for example high moisture content fuel. Mountouris et al. showed that plasma gasification can handle the moisture content as high as 40 wt% without compromising the syngas quality (Mountouris et al., 2006). However, this flexibility comes at a high cost, which may be prohibitive if the aim is to produce aviation fuels from general wastes.
2.3.2. Melting gasification
Fixed/moving bed melting gasifiers have been widely used in the gasification of solid waste and several commercial technologies are available. Nippon Steel & Sumikin Engineering Co. Ltd. developed the direct melting system (DMS) waste gasification technology using a moving bed shaft-furnace type gasifier at atmospheric pressure (Tanigaki and Ishida, 2014). In this technology, untreated MSW or RDF is reacted with enriched air and coke. Although>38 DMS plants have been built, they all feed the syngas to a boiler to generate steam, and therefore this technology would need to be adapted to produce syngas for synthesizing aviation fuels (Tanigaki et al., 2013, Tanigaki and Ishida, 2014).
Thermoselect developed a technology which gasifies unprocessed waste into synthesis gas at ~1200 °C and generates molten slag. In the Thermoselect process the waste is compacted and pyrolyzed in an externally heated horizontal channel at 800 °C ( Schilli, 2004, Perkins, 2020). The partially decomposed waste then enters a vertical retort and is gasified with oxygen at 1600 °C, while mineral matter is heated to over 2000 °C using oxygen and natural gas to form a liquid melt which is tapped from the bottom of the reactor. The gas cleaning and conditioning system of the Thermoselect technology is involved and consists of acid scrubber, alkaline scrubber, de-dusting stage, de-sulfurization and gas drying. The cold gas efficiency for the gasification module is reported as 59% (Campbell, 2008). Nine plants varying in capacity from 38 to 289 ktpa of waste feedstock have been developed using the Thermoselect technology with seven constructed in Japan (Frank Campbell, 2008, Gersham et al., 2013). None have been designed to upgrade the syngas into synthetic fuels.
Recently, Sierra Energy has developed the FastOx gasification technology which is based on the blast furnace as illustrated in Fig. 5 . This gasifier is a fixed bed type gasifier in which steam and oxygen are injected from the bottom of the gasifier through tuyeres (Sierra Energy, 2019). The steam/oxygen reactants generate a high temperature of 2200 °C. The cold gas efficiency is between 66 and 79% and the parasitic load in this gasification system is estimated at 16–20% compared to 40–50% in plasma gasifiers (Sierra Energy, 2019). The produced syngas is drawn from the upper part of the gasifier for further downstream treatment. Mineral matter in the waste is melted forming slag, which is tapped from the bottom of the gasifier. Sierra Energy has built a 20 tonnes per day (tpd) pilot plant at a U.S. Army site in California and is currently constructing a 50 tpd demonstration unit. Therefore, the technology will need further scale up before being suitable for use in producing aviation fuels. Although some may consider FastOx as a new concept, there are several commercial gasifiers which are very similar in design and operation. The BGL gasifier, originally developed for processing coal and adapted for co-processing wastes, has the same general layout, though is generally operated at high pressure (Hirschfelder and Olschar, 2010). The Nippon direct melting system (DMS) mentioned above is also similar in concept (Perkins, 2020, Tanigaki and Kashiwabara, 2017).
Fig. 5.
Schematic of the working principle of the FastOx melting gasification technology from Sierra Energy.
Like plasma gasification, melting gasifiers are designed to process untreated general wastes and operate at very high temperatures, which requires complex and costly reactor designs. The main cost items include feeding systems, air separation unit, refractory lined reactors, syngas reforming and need for high alloy metals in gas cooling systems.
2.3.3. Fluidized bed gasification
For biomass feedstocks there are a number of circulating fluidized bed (CFB) gasification technologies which have been commercialized. The U-Gas technology was originally designed for coal but can co-process biomass (Higman and van der Burgt, 2008). Valmet has developed a CFB system for biomass feedstocks and built a number of plants with up to 300 MWth scale in Finland (Valmet, 2017). A number of fluidized bed technologies such as the Ebara TwinRec circulating fluidized bed system, and the Kobelco Eco and Outotec bubbling bed gasifiers have been designed to combust syngas to generate electric power, and would need significant adaption to produce aviation fuels (Tanigaki et al., 2013, Tanigaki et al., 2012, Yoshikawa, 2013). While dual fluidized bed gasifiers have been implemented at semi-commercial scale, like the biomass gasification plant in Güssing, Austria, they have not been adopted widely or scaled up significantly (Corella et al., 2007).
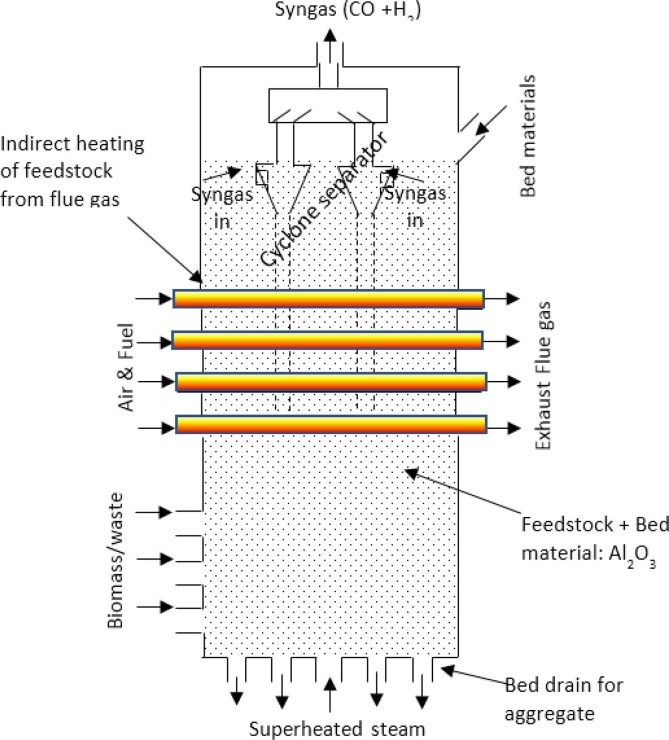
For waste feedstocks, bubbling fluidized bed technologies have been developed by Enerkem and by ThermoChem Recovery International (TRI) to process RDF. In the Enerkem design, the bed is heated by direct injection of oxygen and steam (Enerkem, 2019), while in the TRI design, the heat is supplied by high temperature flue gas passing through tubes inserted into the gasifier (ThermoChem Recovery International Inc., 2020). Fig. 6 shows a schematic of the TRI gasifier technology. Biomass and/or RDF are fed from the bottom sidewall, which reacts with the superheated steam supplied from the bottom of the gasifier. A cyclone separator is installed at the upper part of the gasifier, which separates the particles from the syngas. The key advantages of this gasifier include high quality of syngas due to indirect heating and easy controlling of H2/CO ratio by tuning operating parameters. Also, the particulate removal from the syngas is integrated within the gasifier, which makes the system compact and lower cost. The technology has been tested in a four tonne per day (capacity: 1 MWth) pilot plant within a fully integrated biorefinery process and has demonstrated over 10,000 h of smooth operation (ThermoChem Recovery International Inc., 2020). The technology has been selected for several projects, including the Sierra biofuels plant in Nevada, USA which is under construction and will annually process up to 175,000 of MSW to produce 42 million litres of transport fuels (Fulcrum Bioenergy, 2019).
Fig. 6.
Schematic diagram of the Thermo-Chem Recovery International (TRI) Gasifier technology.
2.3.4. Supercritical water gasification
Supercritical water gasification is a form of hydrothermal gasification usually carried out in the presence of a high volume of water for the production of H2 and CH4 (Rodriguez Correa and Kruse, 2018). Generally, the yield of this process is very high. However, the factors affecting its product output depend on the selection of temperature, biomass to water ratio and catalyst. The most advantageous aspect of hydrothermal gasification is its ability to handle wet biomass feedstocks with up to 70 wt% moisture, substantially saving the costs of drying, which is a prerequisite for conventional thermal gasification (Dahmen et al., 2010, Kruse et al., 2013).
Supercritical gasification is typically performed either at low temperature of 374–550 °C or high temperature of 550–700 °C with or without a catalyst (Azadi and Farnood, 2011). Kruse and Dahmen (2015) studied the hydrothermal gasification of high moisture content biomass under non-catalytic condition. Results showed that at lower temperature, the production of CH4 is favoured compared to that of H2. However, increasing temperature increases the yield (mol%) of H2 but decreases CH4. The yield of H2 and CH4 reach equilibrium at a temperature around 600 °C. In low temperature supercritical gasification, the reaction rate is so low that transition metal based catalysts need to be employed (Elliott et al., 2006, Osada et al., 2006). In contrast, the reaction rate in high temperature supercritical gasification is very high and complete gasification can be achieved at 700 °C without the presence of any catalyst (Osada et al., 2006, Schmieder et al., 2000).
Supercritical water gasification is at an early stage of maturity and the CH4 in the syngas will require further reforming for use in the FT process to produce SAF.
2.3.5. Microwave gasification
Microwave assisted pyrolysis and gasification is an effective method for the conversion of biomass. A significant number of studies have been reported in the scientific literature predominantly for the pyrolysis of biomass (Chen et al., 2015). The advantages of microwave pyrolysis/gasification over conventional gasification include uniform temperature profile, ability to handle large biomass particles, cleaner product output with high heating value and cost effectiveness (Chen et al., 2015). However, the role of microwave radiation on chemical reactions, particularly the non-thermal effects are not well understood (Chen et al., 2015, Kuhnert, 2002). Xie et al. studied the microwave gasification of biomass in the presence of Fe, Co and Ni-based catalysts at laboratory scale (Xie et al., 2014). The results revealed that Ni is the most effective catalyst in terms of syngas yield and tar reduction. The optimum catalyst to biomass ratio was determined to be 1:5–1:3 with a syngas yield over 80%. The addition of steam in the reaction was found to be imperative. The concept of a microwave assisted dual fluidized bed gasifier was proposed by Xie et al. (2014).
While the application of microwaves may hold promise for biomass and waste gasification in the long term, to date, the technology has only been implemented at laboratory scale.
3. Syngas conditioning and cleaning
The main contaminants found in syngas are: particulate matter, tars, sulphur compounds, nitrogen compounds, alkali metals, chlorine and carbon dioxide. However, the level of contaminants largely depends on the feedstock and gasification process. Table 2 shows the general syngas applications and related syngas cleaning requirements (Ephraim et al., 2020, Prabhansu et al., 2015, Richardson et al., 2015, Woolcock and Brown, 2013). For aviation fuel production from syngas, the syngas quality must meet the strict requirements of the FT synthesis process. Recent work on biomass derived aviation fuel production has been conducted by Larson et al. (2020), who evaluated co-gasification of pine wood log and lignite to produce low carbon jet fuel. The proposed design, which is typical of those applied with the FT process, was composed of the following steps: syngas cooling, filtering and scrubbing prior to a partial sour water gas shift adjustment to alter the H2/CO ratio in the syngas. Acid gases, CO2, H2S, and trace impurities were removed using a chilled methanol solvent (Rectisol®), with the captured H2S being converted to wet sulfuric acid and sold. CO2 would also be captured and sold for use in enhanced oil recovery. The syngas cleaning section in this project was designed to reduce technological risk by using proven technologies and reduce the cost of CO2 captured, while making value added products from the contaminants.
Table 2.
General syngas application and related cleaning requirements (Dayton et al., 2019, Ephraim et al., 2020, Prabhansu et al., 2015, Richardson et al., 2015, Woolcock and Brown, 2013).
| Contaminant | Syngas application |
|||||||
|---|---|---|---|---|---|---|---|---|
| Steam cycle power station | Gas engine | Gas turbine | Solid oxide fuel cell | Molten carbonate fuel cell | Proton exchange membrane fuel cell | Methanol synthesis | Fischer-Tropsch synthesis | |
| Particles | Minimal requirements | <50 mg/Nm3 | <30 mg/Nm3 | <1 ppmw | <0.01 nm | n.d. | <0.02 mg/Nm3 | <0.5 mg/Nm3 |
| Tar | Of no importance, but condensation must be avoided | <100 mg/Nm3 | <50 mg/Nm3 | Several tens to few hundred ppmv | <2000 ppmw | <100 ppmv | <0.1 mg/Nm3 | <1 ppmv |
| Sulfur (H2S, COS) | Final SOx emissions limited by regulation | Final SOx emissions limited by regulation | <20 ppmv | Few ppmv | <0.1 ppmv (H2S) | <1 ppm | <1 mg/Nm3 | <0.01 ppmv |
| Nitrogen (HCN, NH3) | Final NOx emissions limited by regulation | Final NOx emissions limited by regulation | <50 ppmv | n.d. | <0.1 ppmw (HCN) <1%vol (NH3) |
n.d. | <0.1 mg | <0.02 ppmv |
| Alkali (primary K and Na) | n.d. | n.d. | <0.02 ppmv | 1 ppmv | n.d. | n.d. | n.d. | <0.01 ppmv |
| Halides (primary HCl) | n.d. | n.d. | <1 ppmv | Few ppmv | <0.1 ppmw | n.d. | <0.1 mg/Nm3 | <0.01 ppmv |
| Heavy metals | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | <0.001 ppmv |
n.d. = not detected
The following sections provides a brief description of currently available syngas cleaning technologies, their performance, advantages and drawbacks within the context of producing aviation fuels from the FT process.
3.1. Particulate matter
Based on the feedstock characteristics and gasification process, the size and the composition of particulate matter can vary broadly. The major constituents of particulate matters are residual solid carbon and inorganic compounds such as alkali and alkaline earth metals, silica, and iron, whereas, the minor constituents are arsenic, selenium, antimony, zinc and lead (Courson and Gallucci, 2019). Particulate matter can cause severe corrosion, erosion and fouling if not removed properly. Particulate matter can be removed using a variety of methods, including warm and hot gas removal, inertial separation, filters, scrubbing with a liquid and electrostatic precipitator. Table 3 summarises the hot gas particle matter removal technologies, their efficiency and operating conditions (Courson and Gallucci, 2019, Dayton et al., 2019, Prabhansu et al., 2015, Woolcock and Brown, 2013).
Table 3.
Hot gas particle matter removal technologies, their efficiency and operating conditions (Courson and Gallucci, 2019, Dayton et al., 2019, Prabhansu et al., 2015, Woolcock and Brown, 2013).
| Dust separator | Temperature range (℃) | Removal efficiency | Pressure drop (kPa) |
|---|---|---|---|
| Cyclone | 100–900 | Dust > 5 μm, 80% | <10 |
| Fabric bag filters | 60–250 | Dust > 0.3 μm, 99–99.8% | 1–2.5 |
| Wet scrubbers (venturi) | 20–100 | Dust > 0.1–1 μm, 85–95, otherwise 90–99% | 5–20 |
| Fibrous ceramic filters | 200–800 | Dust > 0.1 μm, 99.5–99.99% | 1–5 |
| Metallic foam filters | 200–800 | Dust > 1 μm, 99–99.5% | <1 |
| Granular bed filters | 200–800 | Highly depends on regime and surface cake filtration | <10 |
Inertial separation technology is based on the mass and acceleration principle of separation. The cyclone is the most common device in this category. However, other options like dust agglomerates and impact separator are also available. One of the most advanced inertial separation technologies is a reverse flow gas cyclone, which operates using partial recirculation and has a removal efficiency of 99.6%, which has even higher efficiency than the long-established Stairmand high-efficiency designs (Sakin et al., 2019).
A filter is known as barrier filter when a gas stream flows through granules or through porous monolithic solids. Particulate matter can be extracted in four stages during filtration, which are diffusion, inertial impaction, gravitational settling and aggregation of particles. Ceramic or metallic materials are the most popular ingredients for the construction of rigid filters. These are capable of removing 99.99% of particulate matter (<100 mm) at operating temperatures above 400 °C (Prabhansu et al., 2015). However, ceramic filters are fragile in nature, which leads to the production of sintered metal barrier filter in which operating temperatures can be increased to 1000 °C and the removal efficiency can be nearly 100%. Another viable barrier filtration option is moving or fixed bed granular filters, which achieve more than 99.9% efficiency even at high temperatures (Prabhansu et al., 2015).
In electrostatic separations particles are charged due to strong electric field and are extracted according to the difference in dielectric properties. The electrostatic forces acting on particles (<30 μm) are 100 times more powerful than the gravitational force. Consequently, electrostatic precipitators are very effective in the removal of particulates and have traditionally been used in coal/biomass fired power plants for the removal of fly ash up to 200℃ temperature (Prabhansu et al., 2015). They are also popular for removing particulates and tars from syngas produced in biomass gasification.
Wet scrubbing is used to clean the particulate matter from cold gas. Based on its operating principle and removal efficiency cold gas wet scrubbing can be ranked in the following order: spray scrubber > wet dynamic scrubber > cyclonic spray scrubber > impactor scrubber > venturi scrubber > electrostatic scrubber. Table 4 summarises the cold gas particulate matter removal technologies and their removal efficiency and working principle (Courson and Gallucci, 2019, Dayton et al., 2019, Prabhansu et al., 2015, Woolcock and Brown, 2013).
Table 4.
Summary of cold gas particulate matter removal technologies (Courson and Gallucci, 2019, Dayton et al., 2019, Prabhansu et al., 2015, Woolcock and Brown, 2013).
| Device | Removal efficiency | Working principle |
|---|---|---|
| Spray scrubber | Particle > 5 mm; 90% Submicron particle; 40% |
Spray nozzles or atomisers disperse liquid into a moving gas stream simultaneously or counterfactually |
| Dynamic wet scrubber | Particle > 5 mm; up to 95% | Use the mechanical motion of fan blades to turbulently mix the water droplets with the gas stream and increase the chances of inertial impaction of particles with water |
| Cyclonic scrubber | Submicron particle; 60–75% | |
| Impactor scrubber | Large particles; >98% | Dirty gas moves through perforated plates or trays on a smaller plate that is regularly washed with water for impaction |
| Venturi scrubber | Submicron particle; >50% | Scrubbers work by reducing the flow area based on the principle of increasing the gas flow, as a results water splits into fine drops |
| Electrostatic scrubber | Submicron particle; around 99% | Water is sprayed into the stream before or after applying an electric charge |
For warm gas particulate matter removal, the most commonly used technologies are cyclones, electrostatic precipitator and fabric filters (Jaworek et al., 2019). Fabric filters use fabrics that are made from temperature-resistant fibres. Fabric filter gathers particles into the filter media by inertial impaction, capture and diffusion. The most common type of fabrics that are used in fabric filter are polyester, wool, polypropylene.
3.2. Tars
Tars are organic compounds composed of hydrocarbons and free carbon (Park et al., 2018). Depending on the process parameters and feedstock characteristics, various types of tar are formed during thermochemical conversion processes, and can be classified as: (i) primary tar, (ii) secondary tar, (iii) tertiary tar (Liu, 2019). Organic compounds (such as furfural and levoglucosan) released during devolatilising stages are known as primary tars; phenolics and olefins are examples of secondary tar and polycyclic aromatic hydrocarbon (PAHs) are examples of tertiary tars (Benedikt et al., 2019).
There are four principal methodologies for removing tar from hot gas namely thermal cracking, catalytic cracking, non-thermal plasma, and physical separation (Saleem et al., 2020). Based on the prospective application of syngas and gasifier types, these methodologies are applicable for both primary (in-situ) and secondary tar removal (post-gasifier). Table 5 tabulates the working principal, advantages and disadvantages of these processes (Chen et al., 2019, Courson and Gallucci, 2019, Islam, 2020, Prabhansu et al., 2015, Saleem et al., 2020).
Table 5.
Summary of hot gas tar removal technologies (Chen et al., 2019, Courson and Gallucci, 2019, Islam, 2020, Prabhansu et al., 2015, Saleem et al., 2020).
| Method | Working principle | Remarks |
|---|---|---|
| Thermal cracking |
|
|
| Catalytic cracking |
|
|
| Non-thermal plasma |
|
|
| Physical separation |
|
|
Wet scrubber cleans both particulate matter and tars in the cold gas cleaning process. By dropping the gas temperature adequately, wet scrubbers condense tarry vapours, which is easily absorbed by the water. Water leaving the wet scrubber, which is heavily contaminated with tar compounds, reaches a settling tank where water-insoluble tar compounds are isolated from water so that the water can be recirculated to the scrubber (Brown, 2019).
A process named OLGA (an acronym for the oil-based gas washer in Dutch) has been recently developed by the Energy Research Centre of the Netherlands for warm gas tar cleaning (Rueda and Helsen, 2019). This process aims to combine the advantages of hot gas clean-up (HGC) and cold gas clean-up (CGC) system without taking their disadvantages. Compared to typical HGC and CGC process OLGA technique offers several added benefits such as low operating cost, catalysis cost and low-temperature requirement. OLGA process has been successfully used in several gasification facilities and is currently on the verge of commercialization (Rueda and Helsen, 2019).
3.3. Sulphur compounds
Sulphur contaminants are mostly found in the form of hydrogen sulphide (H2S) or carbonyl sulphide (COS) in the syngas and may be removed separately or together with other acid gases such as CO2. Several processes which use physical or chemical adsorption or a combination are available for acid gas removal. For the production of aviation fuels, physical adsorption (e.g. methanol) or chemical adsorption (e.g. amines) can be used for bulk sulphur removal, with solid adsorption used for ensuring no sulphur slip into the Fischer-Tropsch catalyst unit.
Solvents like methanol and dimethyl ether are generally used in physical absorption processes, because of their ability to consume hydrocarbons (Korens et al., 2002). Liquid redox is a promising method for direct H2S removal and the recovery of sulphur from syngas streams. In this method a dissolved vanadium catalyst is passed through the wet scrubbing phase to the gas stream. Biological and chemo-biological techniques can also be used for the removal of sulphur. Various types of microorganisms such as Chlorobiaciae and Thiobacillus have been examined in this regard (Jensen and Webb, 1995). Commercially available processes like Thiopaq and Biopuric, use standard chemical or physical techniques in order to extract H2S from a gas stream, however to date, none have been used together with the FT process in a large scale facility (Fortuny et al., 2008).
Hot gas sulphur removal mainly concentrates on hydrogen sulphide and/or sulphur dioxide removal with the majority of hot gas disposal techniques using adsorption. Metal oxides show the best chemical properties for adsorption of sulphur at elevated temperatures, and the most propitious metal oxides for desulphurisation are Fe, Cu, Zn, Co, Mo, V and Mn (Vamvuka et al., 2004). Another commonly used adsorption material is a mixed metal oxide(such as CuO and ZnO) and it can achieve >99% sulphur removal efficiency (Vamvuka et al., 2004). Conoco-Phillips has produced a commercial sorbent blends with ZnO, which can successfully remove >99% of sulphur compounds (Sánchez-Hervás et al., 2005).
3.4. Carbon dioxide
The removal of CO2 from syngas is necessary before it can be used in the Fischer-Tropsch process. In commercial projects acid gas removal with physical and chemical adsorption will be the most likely choice for bulk removal of the CO2 from syngas. The major technologies which are used for CO2 capture and separation are solvent, sorbent and membrane. Different types of material such as activated carbon, zeolites, lime, alkali oxides, silver oxides, silica gel, alumina, and metal-organic framework have been typically used as CO2 sorbents (Dayton et al., 2019). Some pros of this process are that no heat is required to reverse a chemical reaction and sorbents can simultaneously recover H2S and CO2. Some of the cons of this process are: some H2 may be lost with CO2 and CO2 may lose some pressure during flash recovery. Another promising method in this regard is ion pump technology, which is neither temperature-dependent nor pressure (Taheri et al., 2019). Moreover, they drastically increase the carbonate ion concentration by dissolving CO2 in the solution. CO2 removal using membrane is energy efficient process; however, there are challenges in the selection of membrane materials and in the design of membranes for effective CO2 removal (Hatab et al., 2019). Hollow fiber membranes (HFM) are one of the best performing in this regard. Hatab et al. (2019) reported that the CO2 removal efficiency can be further enhanced by 21%, when the shell compartment of HFM is packed with the glass beads.
3.5. Nitrogen compounds
Nitrogen compounds in the syngas are mostly found as ammonia (NH3) or hydrogen cyanide (HCN). Generally, NH3 is the governing form of nitrogen contaminants. For hot gas nitrogen clean-up process, selective catalytic oxidation or thermal catalytic decomposition is necessary (Nelson et al., 2018). An oxidiser such as NO is a promising option for selective catalytic oxidation. Thermal catalytic degradation of NH3 occurs primarily through the opposite NH3 formation mechanism. Dolomite and iron-based low-cost catalysts can successfully remove up to 70–80% of NH3 from syngas (Palma et al., 2017). Ni-based low-cost catalyst has also shown promising results, and capable of removing up to 75% of NH3 (Palma et al., 2017). However, deactivation of sulphur is a big concern associated with these catalysts. Tungsten based catalyst such as tungstate zirconia and tungsten carbide could be a possible option to avoid this situation (Palma et al., 2017).
During cold gas clean up, nitrogen pollutants are mainly removed from the syngas using water absorption. Even the condensation of water vapour found in the syngas can remove nitrogen compounds considerably. More than 90% of ammonia removal was achieved by using a chilled condenser for sewage sludge derived syngas (Pinto et al., 2007). The removal rate can further be enhanced by using additional water in the wet scrubber.
3.6. Alkali metals
Alkali compounds causes severe fouling and corrosion in the downstream process. Thus, removing alkali compounds from the syngas is very crucial for combustion/gasification process. There are two ways to remove alkali metals from syngas at high temperatures. The first option is condensation, and the second option is hot adsorption onto a solid sorbent. As the temperatures of the gas stream falls below alkali condensation points, alkali vapours will nucleate and agglomerate in the gas stream to form or add to particulate matter. Sorbents like kaolinite and bauxite are useful for the high-temperature alkali removal process (Adhikari et al., 2017). For low-temperature alkali removal process emathlite are useful (Punjak et al., 1989). Other sorbents which can remove alkali from syngas at high temperatures are alumina and silica (Adhikari et al., 2017).
In cold gas clean-up systems most of the alkali compounds are removed with tar using a wet scrubber due to the low condensation temperature (<300℃) of the alkali compounds. Another option for alkali removal is pre-treatment of biomass (Cummer and Brown, 2002). Biomass washing using water is a viable option for alkali removal as most of the alkali compounds are either water-soluble or ion exchangeable. Washing with acid instead of water could be another logical option as it could remove 70% alkali compounds from biomass (Cummer and Brown, 2002).
3.7. Chlorine
Chlorine is generally found as hydrochloric acid (HCl) in the syngas. In the gas phase, HCl reacts to form other contaminants such as ammonium chloride (NH4Cl) and sodium chloride (NaCl). These contaminants cause heavy deposition and fouling on the downstream processes (Li et al., 2020). Furthermore, chlorides deactivate the catalysts used for chemical synthesis.
Sorbents like alumina and activated carbon are most commonly used for HCl removal in a hot gas environment. Due to the chemical balance between the gases and solids involved, high-temperature removal of HCl is most active between 500 °C − 550 °C (Dou et al., 2001). Other efficient methods include alkali-based multi-oxides; however, these could be expensive compared to typical sorbents. Direct injection of sorbent in the hot gas stream at elevated temperature (600℃ – 1000℃) could be another promising option for HCl clean up as the experimental results show 80% removal efficiency for calcium-based sorbents (Shemwell et al., 2001).
Wet scrubbing is generally used to clean chlorine compounds in the cold gas cleaning process. It is done either by adsorption of HCl vapour or by the deposition of ammonium chloride salts (Woolcock and Brown, 2013). Gasification produces HCl and NH3, which by reacting with each other form ammonium chloride, and deposit on the downstream process and causes fouling. Thus, it is recommended to maintain a syngas temperature over 300 ℃ until the cleaning process is done (Chan et al., 2019). In a wet scrubber, lower amounts of ammonium chloride are usually formed due to fast cooling process. However, the wet scrubber can successfully absorb all form of chlorides.
For removing HCl from warm gas stream, semi-wet removal process is applied aloft the water condensation temperature. Lime slurry is used in this process, which by reacting with HCl forms CaCl2 and H2O. Using this method, more than 99.5% HCl can be removed from the process. Another option could be a Mg-Al oxide based scrubbing process as it is regenerable and could remove up to 97% of the chlorine compounds (Kameda et al., 2008).
3.8. Other contaminants
A number of other contaminants such as mineral and metallic trace elements can be found in the syngas in addition to the above-mentioned contaminants. However, the concentration of these trace contaminants is low. For the Fischer-Tropsch process trace contaminants such as Hg, As, Se and Zn should be reduced to very low levels, preferably in the ppb range. Lime, activated carbon, zeolite, silica, bauxite and kaolinite are currently used as a solid sorbent for trace metal removal. Earlier, activated carbon has been used for Hg removal, which has an efficiency between 90 and 95%. At present, some projects are using a new variety of activated carbon developed by Calgon Carbon Corporation, which has 99.99% Hg removal efficiency (Mimna and Tramposch, 2016). Another typical adsorbent used for mercury removal is zeolite. Due to their high mercury removal rate and regeneration capabilities they are a preferred option in commercial gasification processes. TDA Research Inc. has developed a state-of-the-art regenerable sorbent which can work even at high temperature and pressure (Alptekin et al., 2016). The sorbent not only showed higher mercury removal (95%) from syngas but also removed other trace metals from syngas. Furthermore, the sorbent successfully removed the residual sulphur as well, by at least three times by working as a guard bed.
4. Product synthesis and purification
4.1. Production pathways for renewable aviation fuel
As mentioned before, the FAA has approved five different routes for the production of aviation fuels from renewable carbon resources i.e. biomass (Morgan et al., 2019, Pearlson et al., 2013) and two of these methods are of relevance to this review namely, the pathways FT-SPK (Synthetic paraffin kerosene) and FT-SKA (Synthetic kerosene with aromatics). FT-SPK is produced by gasification of biomass followed by FT synthesis. On the other hand, in FT-SKA some alkylated benzenes of non-petroleum origin are added to the FT-SPK (European Technology and Innovation Platform, 2017). SPK can be blended in variable amounts of up to 50%, depending on the fuel type with conventional commercial and military jet (or aviation turbine) fuel. Blending is required with SPK fuels because they lack sufficient aromatic hydrocarbons, which are present in conventional jet fuel. Aromatic hydrocarbons are limited in jet fuel to prevent smoke formation during combustion, yet a minimum aromatic content is needed to cause elastomer swell in aircraft fuel systems and increase fuel density. On the other hand, synthetic kerosene with aromatics (SKA) fuels can be used interchangeably with fossil fuels (US Department of Energy, 2020).
The companies developing projects using the FT-SPK route include Fulcrum Bioenergy and Red Rock Biofuels, whereas Sasol and Rentech are focused on the FT-SKA route.
4.2. Fischer-Tropsch synthesis for the production of jet fuel
Long chain paraffinic hydrocarbons can be produced in a Fischer-Tropsch (FT) unit using syngas with a H2/CO ratio of ~ 2, according to:
| (1) |
Generally, the synthesis occurs at a pressure of 40 – 80 bar, with a cobalt or iron based catalyst. The products obtained by the Fischer-Tropsch synthesis process are governed by the Anderson‐Schulz‐Flory (ASF) distribution. It is highly essential to have a suitable catalyst in the process which has high selectivity towards jet range hydrocarbons. Cobalt has proved to be a suitable candidate when used with certain promoters, as has iron, albeit with generally lower selectivity.
Low temperature FT synthesis is carried out in the temperature range of 200 to 240 °C using Fe or Co based catalysts. Alkenes are favoured when Fe based catalysts are used at higher temperatures than Co based catalysts. On the other hand, high temperature FT synthesis is carried out in the range of 300 to 350 °C using Fe based catalysts. Aromatics are formed in significant quantities only in high temperature FT synthesis (de Klerk, 2016).
Cobalt based zeolite catalysts have shown better performance than others, with Co/ZSM-34 showing up to 30% selectivity towards jet range hydrocarbons. The accessibility to active sites and porous structure plays a greater role than the acidity of the catalyst (Bessell, 1995). USY zeolite with 10% cobalt metallic phase was shown to favour production of jet fuel range hydrocarbons due to its three‐dimensional system of channels and large micropores favouring the accessibility to catalytic sites (Zola, 2007). The zeolitic structure along with the material acidity is seen to narrow the carbon number distribution. Co on USY and Co on ZSM-5 was found to be favourable by another group of researchers as well (Ngamcharussrivichai et al., 2007). Several such studies have emphasized the importance of pore structure and acidity of the catalyst to produce narrow range of jet fuel hydrocarbons. In addition, promoters also have positive effects on the product yields by increasing the reaction rate, improved stability, and higher C5+ selectivity. Mn, K, Mb etc. are most commonly used as promoters. Hybrid catalysts have also shown significant improvements in the product quality and yields (da Silva et al., 2016). Researchers at NASA have obtained around 28 wt% of C5 - C11 hydrocarbons using Co on Alumina catalyst (De La Ree, 2011). In another study by Li et al. (2016), Co/ZrO2–SiO2 catalyst with specific bimodal structure and varied 1-olefins as additives was used during FT synthesis. They found that 1-decene and 1-tetradecene mixed in the volume ratio of 1:1 showed highest selectivity towards jet-fuel-like hydrocarbons (Li et al., 2016).
FT reactions are highly exothermic in nature and hence, heat removal is the one of the most important factors to be considered when designing a catalyst/reactor system for the process. Initially, Arge reactors (multi-tubular fixed bed reactors) jointly developed by Lurgi and Ruhrchemie were used for the low temperature FT process. Shell uses multi-tubular reactors in their commercial GTL plants in Malaysia and Qatar.
BP and Johnson Matthey (JM) have co-operated to develop a new technology which they call the “cans tech” where the reactors resemble the baked beans cans and they are filled with a new recipe catalyst. This new technology claims to treble the productivity at half the cost of traditional FT reactors (BP, 2018). This has been licensed to Fulcrum Bioenergy for their Sierra Biofuels Plant in Nevada, USA.
Emerging Fuels Technology has developed its own gas-to-liquids process using multi-tubular reactor technology followed by hydrotreating to produce HEFA fuels (hydro processed esters and fatty acids). They have worked with Red Rock Biofuels and sold the first license of their second generation TL8a catalyst/reactor system which requires half as much catalyst volume for the same output capacity as their first generation TL8 catalyst (EFT, 2018, Lane, 2015).
Fluidized bed slurry reactors offer better temperature control and higher conversion than fixed bed multi-tubular reactors. They also enable the catalyst inventory to be continuously replaced and regenerated offline in a separate system. Fluidized bed reactors have been used by SASOL for their high temperature FT synthesis process. Initially they were operated in circulating mode known as Synthol reactors and have now been converted to a fixed fluidized bed type of design called Advanced Synthol reactors which can handle high throughputs (NETL, 2020). However, fluidized bed slurry reactors are designed for very large production capacities which are probably only feasible when using natural gas or coal as feedstocks. The economic viability of fluidized bed slurry reactors for smaller projects typical of biomass and waste (<500 ktpa) is questionable.
Microchannel reactors are compact reactors with many small channels on the scale of millimetres and several vendors have developed designs for the FT process (Konarova et al., 2020). The advantage of microchannel reactors is that they can be used to intensify chemical reactions and improve heat and mass transfer performance. The presence of water coolant channels makes it very effective for usage in FT reactions which are highly exothermic in nature. They also ensure better flow in the channels leading to reduced formation of side product and increased selectivity towards required products, better heat transfer increasing the efficiency of the process and reducing the utility requirement. In case of catalytic reactions, better heat transfer reduces the chance of hot spots thereby reducing the incidences of catalyst deactivation (Todić et al., 2015). The increased ease in heat dissipation helps in increased life of catalysts since the active sites are retained for a longer time. Though the microchannel technology has several advantages, it has faced obstacles in the area of commercialisation, as not many technologies are available using this type of reactor. A simulation study by Guettel and Turek showed that the microchannel reactor had the highest productivity per unit of catalyst volume. Yet, the productivity per unit of reactor volume was the same as a fixed bed reactor due to the low ratio of catalyst to reactor volume (Guettel and Turek, 2009). Konarova et al. have recently reported on using 3D printing to manufacture catalysts for the FT process (Konarova et al., 2020). Despite some of the potential technical advantages of microchannel reactors, it is not yet clear whether they are significantly cheaper than multi-tubular fixed bed reactors in commercial projects, due to the relatively large amount of infrastructure associated with each reactor, such as piping and control systems, steam delivery and quality requirements etc.
Velocys’ microchannel FT technology is the most advanced and has been implemented in relatively small project capacities of around 1,400 barrels per day (bpd) (around 19 million gallons per year). In these reactors, thousands of process channels with dimensions in the millimetre range and filled with catalyst are constructed immediately adjacent to water-filled coolant channels. The small-size channels dissipate heat more quickly than in conventional FT reactors making it easier to use more active catalysts (Green Car Congress, 2018). The main challenges with microchannel reactors are the need for many tens of them in a typical plant, and associated utility and catalyst handling facilities, and the requirement for very, very clean boiler feed water. Microchannel vendors argue that their FT process is much cheaper and more productive than conventional designs, however the actual savings are generally overstated. In the context of the overall plant, the FT unit typically represents only 10 – 20% of the total plant cost. More important is the unit performance in terms of syngas conversion and selectivity to the desired end products.
A new trend in FT plants is to use renewable energy sources for utilities. This would mean that the FT tail gas, which is often used for power generation, could be recycled to make more product. Life cycle analysis of the process for producing jet fuel from biomass though gasification followed by FT synthesis has been carried out by Li et al. (2019). They observed that reduction in electricity consumption and production of required electricity from renewable resources significantly reduces the GHG emissions of the process.
4.3. Product purification
Once the hydrocarbons are produced after FT synthesis, it is necessary to purify the product stream to test its suitability as aviation turbine fuel. Some of the reactions that are carried out are hydrocracking and isomerisation. The jet range hydrocarbons are obtained by distilling the liquid between boiling‐point range of 110 and 310 °C (da Silva et al., 2016). In a study carried out by Hanaoka et al., 0.1 wt% of Pt loaded on β-type zeolite gave a jet fuel yield of 21.5% at 250 °C and 1.5 MPa during hydrocracking of the FT product (Hanaoka et al., 2015).
Hybrid catalysts are gaining attention in the recent times for FT synthesis where zeolites are used in combination with conventional Co based catalysts. The zeolite catalyst helps in aiding the oligomerisation, hydrocracking, isomerisation, aromatisation and hydrogenation reactions which directly improves the end product quality thereby eliminating/reducing the severity of further purification steps. In some cases, the use of hybrid catalysts (Ru and Co, as active metals on ZSM‐12 and ZSM‐5 zeolites) during the FT synthesis stage has eliminated the requirement of further hydrocracking step (Adeleke et al., 2018, Kibby et al., 2013). Li et al. in their recent study, have developed catalysts that eliminate the hydrorefining step of FT products. They report 72% selectivity towards jet fuel only by using mesoporous Y-type zeolites in combination with cobalt nanoparticles (Li et al., 2018).
4.4. Waste to fuel projects
Waste to fuel projects have been gaining momentum in the past few years and several reports of different companies trying to commercialise their processes are emerging. These processes are in various levels of deployment such as pilot scale, demo scale or commercial scale in various parts of the world. Frontline Bioenergy LLC is developing a process along with SGC Energia that will use wood and other waste feedstocks to produce military fuels F-76 diesel and JP-5 and JP-8 jet (Smeenk, 2015). Southern Research is developing process intensification approaches to reduce the cost of CTL/CBTL for production of JP-8 jet fuel (Lucero, 2017). It involves autothermal reforming (ATR) of raw syngas from gasification followed by an advanced hybrid Fischer-Tropsch synthesis that does not produce waxes. The project team comprises of Southern Research (lead, ATR catalyst development), Chevron (Co-zeolite hybrid FT catalyst supplier), IntraMicron (FT heat exchange reactor technology), National Carbon Capture Center (testing host site) and Southwest Research Institute (product qualification support) (Lucero, 2017).
The economical viability of commercial projects requires the gate fees provided by using waste feedstocks and various subsidies or incentives for producing renewable fuels. Some of the commercial waste to aviation fuel projects that have been announced are discussed below.
4.4.1. Fulcrum bioenergy
Fulcrum Bioenergy is developing projects which apply MSW gasification followed by FT synthesis using a proprietary catalyst to produce aviation fuel and diesel (Fulcrum Bioenergy, 2019). The process heat is used to generate electricity for the plant and the process is scalable and flexible. The process has been reviewed by several third parties such as BP, United Airlines, the U.S. Department of Defense and independent engineers Leidos and Black & Veatch. The process is expected to reduce greenhouse gas emissions by >80% compared to conventional crude oil production (Fulcrum Bioenergy, 2019). The fuel has low nitrogen, no sulphur and has been tested, certified and approved for commercial and military aviation worldwide as it qualifies for the US Renewable Fuel Standard, California Low-Carbon Fuel Standard and the Roundtable for Sustainable Biomaterials. The first plant of Fulcrum Bioenergy is the Sierra BioFuels Plant located near Reno, Nevada, which may become operational in 2020. The location for its second plant, Centerpoint BioFuels Plant, has been chosen as Gary, Indiana. It is designed to handle 700,000 tons of waste from the Greater Chicago area producing approximately 33 million gallons of fuel annually (Fulcrum Bioenergy, 2018, Fulcrum Bioenergy [WWW Document], 2019).
4.4.2. Velocys
Altalto Immingham Limited is a collaborative venture between Velocys, British Airways and Shell which plans to build a plant for the conversion of commercial waste to fuel. Once the planning approvals are received, they plan to initiate the construction in 2021 and produce commercial volumes from 2024. Once produced, British Airways would purchase the jet fuel for use in its aircraft. This would reduce the airline’s carbon emissions towards the industry targets of carbon neutral growth from 2020 and a 50% reduction by 2050 from 2005 levels. Velocys is the supplier of the microchannel Fischer-Tropsch reactor with its proprietary Velocys Actocat catalyst. The plant is designed to process over half a million tonnes each year of household and commercial solid waste and convert it into sustainable aviation fuel and road transport fuels. These municipal solid wastes are now being incinerated or landfilled causing several environmental issues. The process claims to reduce the net greenhouse gases by 70% compared to its fossil fuel equivalent. This is equivalent to taking up to 40,000 cars per year off the road. It also claims to improve air quality by reducing up to 90% particulate matter (soot) from aircraft engine exhausts and almost 100% reduction in sulphur oxides. With millions of pounds of investment for the project the local employment in Immingham, North East Lincolnshire, close to the Humber Estuary is set to boom both during the construction phase and the plant operations phase (Business Business Traveller, 2019, Green Car Congress, 2019, Velocys, 2019).
Though there are several companies which are coming forward to commercialize the production of aviation fuels through the gasification followed by FT route, there still exists several challenges that need to be addressed for better economic returns and increased energy efficiencies. As mentioned earlier, the design of reactors is of utmost importance in these processes as heat dissipation is necessary considering the exothermicity of the reactions taking place. The development of catalysts with greater hydrothermal stability and lower deactivation rates are desired. Studies still need to be carried out to identify the most suitable promoters or additives to be used along with the catalysts that can eliminate the product purification step and produce jet fuel with considerably good selectivity towards jet fuel range hydrocarbons in the FT synthesis step itself.
Some of the advantages of using such biomass derived FT jet fuel are that they are devoid of sulphur, have very low amounts of nitrogen and emit lower particulate matter during combustion. As of now, 50% blending of FT-SPK with petroleum jet fuels is allowed to meet the ASTM specifications of jet fuel (Bwapwa et al., 2019, ElGalad et al., 2018). On the flip side, the quantity of aromatic compounds is low leading to problems in aircraft fuel system seals (Ebbinghaus and Wiesen, 2001). It also leads to higher freezing points and lower densities, which are points of concern for aviation fuel that is used in high altitude low temperature zones.
5. Conclusions
This review has found that the preferred technology for gasification in recent aviation fuel projects has been the fluidized bed due to its flexibility and large processing capacity Multi-tubular and microchannel reactors using cobalt catalysts have been the preferred technology for Fischer-Tropsch synthesis. Further development of gasification technologies for converting untreated residual wastes and new catalysts to improve selectivity to jet fuel hydrocarbons are needed. The production of sustainable aviation fuels from biomass and residuals wastes is a new endeavour and pioneer plants can expect to face integration challenges and take time to achieve their design capacities.
CRediT authorship contribution statement
M. Shahabuddin: Writing - original draft. Md Tanvir Alam: Writing - original draft. Bhavya B. Krishna: Writing - original draft. Thallada Bhaskar: Writing - review & editing. Greg Perkins: Conceptualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adeleke A.A., Liu X., Lu X., Moyo M., Hildebrandt D. Cobalt hybrid catalysts in Fischer-Tropsch synthesis. Rev. Chem. Eng. 2018 doi: 10.1515/revce-2018-0012. [DOI] [Google Scholar]
- Adhikari, S., Abdoulmoumine, N., Nam, H., Oyedeji, O., 2017. Biomass gasification producer gas cleanup. In: Bioenergy Systems for the Future. Elsevier, pp. 541–557. https://doi.org/10.1016/B978-0-08-101031-0.00016-8.
- Ahmad A.A., Zawawi N.A., Kasim F.H., Inayat A., Khasri A. Assessing the gasification performance of biomass: a review on biomass gasification process conditions, optimization and economic evaluation. Renew. Sustain. Energy Rev. 2016;53:1333–1347. doi: 10.1016/j.rser.2015.09.030. [DOI] [Google Scholar]
- Alptekin, G., Jayaraman, A., Dietz, S., 2016. Low Cost, High Capacity Regenerable Sorbent for Carbon Dioxide Capture from Existing Coal-fired Power Plants (No. 1253138). doi: 10.2172/1253138.
- Alter NRG, 2018. Summary of Qualifications. AlterNRG.
- Azadi P., Farnood R. Review of heterogeneous catalysts for sub- and supercritical water gasification of biomass and wastes. Int. J. Hydrog. Energy. 2011;36:9529–9541. doi: 10.1016/j.ijhydene.2011.05.081. [DOI] [Google Scholar]
- Basu P. second. ed. Academic Press; London, United Kingdom: 2013. Biomass Gasification, Pyrolysis and Torrefaction. [Google Scholar]
- Bell D.A., Towler B.F., Fan M. Elservier; Oxford, UK: 2011. Coal Gasification and its Applications. [Google Scholar]
- Benedikt F., Kuba M., Schmid J.C., Müller S., Hofbauer H. Assessment of correlations between tar and product gas composition in dual fluidized bed steam gasification for online tar prediction. Appl. Energy. 2019;238:1138–1149. doi: 10.1016/j.apenergy.2019.01.181. [DOI] [Google Scholar]
- Bessell S. Investigation of bifunctional zeolite supported cobalt Fischer-Tropsch catalysts. Appl. Catal. Gen. 1995;126:235–244. doi: 10.1016/0926-860X(95)00040-2. [DOI] [Google Scholar]
- BP, 2018. First licence for new waste-to-fuel technology [WWW Document]. First Licence New Waste--Fuel Technol. URL https://www.bp.com/en/global/corporate/news-and-insights/bp-magazine/new-waste-to-fuel-technology.html (accessed 2.9.20).
- Brown R.C., editor. Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power. 2nd ed. Wiley; New York, NY, USA: 2019. [Google Scholar]
- Business Traveller, 2019. British Airways and Shell plan Europe’s first commercial waste to jet fuel plant [WWW Document]. Br. Airw. Shell Plan Eur. First Commer. Waste Jet Fuel Plant. URL https://www.businesstraveller.com/business-travel/2019/08/20/british-airways-and-shell-plan-europes-first-commercial-waste-to-jet-fuel-plant/ (accessed 2.9.20).
- Bwapwa J.K., Akash A., Trois C. Jet fuel blend from Algal Jet Fuel and Jet al in 50/50 volume ratio. Int. J. Low-Carbon Technol. 2019;14:234–240. doi: 10.1093/ijlct/ctz014. [DOI] [Google Scholar]
- Chan W.P., Veksha A., Lei J., Oh W.-D., Dou X., Giannis A., Lisak G., Lim T.-T. A hot syngas purification system integrated with downdraft gasification of municipal solid waste. Appl. Energy. 2019;237:227–240. doi: 10.1016/j.apenergy.2019.01.031. [DOI] [Google Scholar]
- Chen G., Li J., Liu C., Yan B., Cheng Z., Ma W., Yao J., Zhang H. Low-temperature catalytic cracking of biomass gasification tar over Ni/HZSM-5. Waste Biomass Valorization. 2019;10:1013–1020. doi: 10.1007/s12649-017-0107-7. [DOI] [Google Scholar]
- Chen P., Xie Q., Du Z., Borges F.C., Peng P., Cheng Y., Wan Y., Lin X., Liu Y., Ruan R. Microwave-assisted thermochemical conversion of biomass for biofuel production. In: Fang Z., Smith R.L., Qi X., editors. Production of Biofuels and Chemicals with Microwave, Biofuels and Biorefineries. Springer; Netherlands, Dordrecht: 2015. pp. 83–98. [DOI] [Google Scholar]
- Corella J., Toledo J.M., Molina G. A review on dual fluidized-bed biomass gasifiers. Ind. Eng. Chem. Res. 2007;46:6831–6839. doi: 10.1021/ie0705507. [DOI] [Google Scholar]
- Courson, C., Gallucci, K., 2019. Gas cleaning for waste applications (syngas cleaning for catalytic synthetic natural gas synthesis), in: Substitute Natural Gas from Waste. Elsevier, pp. 161–220. https://doi.org/10.1016/B978-0-12-815554-7.00008-8.
- Cummer K.R., Brown R.C. Ancillary equipment for biomass gasification. Biomass Bioenergy. 2002;23:113–128. doi: 10.1016/S0961-9534(02)00038-7. [DOI] [Google Scholar]
- da Silva, L.S., da Silva, V.L. dos S.T., de Barros, M.A.S.D., Arroyo, P.A., 2016. Zeolites as Potential Structures in Obtaining Jet Fuel Through the Fischer‐Tropsch Synthesis, in: Belviso, C. (Ed.), Zeolites - Useful Minerals. InTech. doi: 10.5772/63662.
- Dahmen, N., Henrich, E., Kruse, A., Raffelt, K., 2010. Biomass Liquefaction and Gasification, in: Verts, A.A., Qureshi, N., Blaschek, H.P., Yukawa, H. (Eds.), Biomass to Biofuels. Blackwell Publishing Ltd., Oxford, UK, pp. 89–122. doi: 10.1002/9780470750025.ch5.
- Dayton, D.C., Turk, B., Gupta, R., 2019. Syngas Cleanup, Conditioning, and Utilization, in: C Brown, R. (Ed.), Thermochemical Processing of Biomass. John Wiley & Sons, Ltd, Chichester, UK, pp. 125–174. doi: 10.1002/9781119417637.ch5.
- de Klerk, A., 2016. Aviation Turbine Fuels Through the Fischer–Tropsch Process, in: Biofuels for Aviation. Elsevier, pp. 241–259. https://doi.org/10.1016/B978-0-12-804568-8.00010-X.
- De La Ree, A., 2011. Fischer-Tropsch Catalyst for Aviation Fuel Production.
- Dou B., Gao J., Sha X. A study on the reaction kinetics of HCl removal from high-temperature coal gas. Fuel Process. Technol. 2001;72:23–33. doi: 10.1016/S0378-3820(01)00176-X. [DOI] [Google Scholar]
- Ebbinghaus A., Wiesen P. Aircraft fuels and their effect upon engine emissions. Air Space Eur. 2001;3:101–103. doi: 10.1016/S1290-0958(01)90026-7. [DOI] [Google Scholar]
- EFT, 2018. Emerging Fuels Technology Licenses its Fischer-Tropsch Technology to Red Rock Biofuels [WWW Document]. Emerg. Fuels Technol. Licens. Its Fisch.-Tropsch Technol. Red Rock Biofuels. URL https://www.prnewswire.com/news-releases/emerging-fuels-technology-licenses-its-fischer-tropsch-technology-to-red-rock-biofuels-300735494.html (accessed 2.9.20).
- El Takriti, S., Pavlenko, N., Searle, S., 2017. Mitigating international aviation emissions risks and opportunities for alternative jet fuels. International Council of Clean Transportation.
- ElGalad M.I., El- Khatib K.M., Abdelkader E., El-Araby R., ElDiwani G., Hawash S.I. Empirical equations and economical study for blending biofuel with petroleum jet fuel. J. Adv. Res. 2018;9:43–50. doi: 10.1016/j.jare.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D.C., Hart T.R., Neuenschwander G.G. Chemical Processing in High-Pressure Aqueous Environments. 8. Improved Catalysts for Hydrothermal Gasification. Ind. Eng. Chem. Res. 2006;45:3776–3781. doi: 10.1021/ie060031o. [DOI] [Google Scholar]
- Enerkem [WWW Document], 2019. . Enerkem. URL https://enerkem.com/ (accessed 3.31.19).
- Ephraim A., Munirathinam R., Nzihou A., Pham Minh D., Richardson Y. Handbook on Characterization of Biomass, Biowaste and Related By-Products. Springer International Publishing; Cham: 2020. pp. 1113–1171. [DOI] [Google Scholar]
- European Technology and Innovation Platform, 2017. Aviation Biofuels [WWW Document]. Aviat. Biofuels. URL http://www.etipbioenergy.eu/images/ETIP_Bioenergy_Factsheet_Aviation_Biofuels.pdf (accessed 2.9.20).
- Fang Z. Catalytic hydrothermal gasification of cellulose and glucose. Int. J. Hydrog. Energy. 2008;33:981–990. doi: 10.1016/j.ijhydene.2007.11.023. [DOI] [Google Scholar]
- Favas J., Monteiro E., Rouboa A. Hydrogen production using plasma gasification with steam injection. Int. J. Hydrog. Energy. 2017;42:10997–11005. doi: 10.1016/j.ijhydene.2017.03.109. [DOI] [Google Scholar]
- Fortuny M., Baeza J.A., Gamisans X., Casas C., Lafuente J., Deshusses M.A., Gabriel D. Biological sweetening of energy gases mimics in biotrickling filters. Chemosphere. 2008;71:10–17. doi: 10.1016/j.chemosphere.2007.10.072. [DOI] [PubMed] [Google Scholar]
- Frank Campbell, 2008. Overview of the History and Capabilities of the Thermoselect Technology, in: Interstate Waste Technologies.
- Fulcrum Bioenergy, 2018. FULCRUM TARGETS NORTHWEST INDIANA FOR THE LOCATION OF ITS NEXT WASTE-TO-FUEL PLANT [WWW Document]. FULCRUM TARGETS NORTHWEST INDIANA Locat. ITS WASTE--FUEL PLANT. URL http://fulcrum-bioenergy.com/wp-content/uploads/2018/12/2018-12-13-Fulcrum-Centerpoint-Announcement-FINAL.pdf (accessed 2.9.20).
- Fulcrum Bioenergy [WWW Document], 2019. Fulcrum Bioenergy. URL http://fulcrum-bioenergy.com/ (accessed 3.31.19).
- Gersham, Brickner & Bratton, Inc., 2013. Gasification of Non-Recycled Plastics From Municipal Solid Waste In the United States. The American Chemistry Council, Fairfax, VA, USA.
- Green Car Congress, 2019. Altalto waste-to-jet fuel plant advances in UK; BA, Shell, Velocys [WWW Document]. Altalto Waste--Jet Fuel Plant Adv. UK BA Shell Velocys. URL https://www.greencarcongress.com/2019/08/20190822-altalto.html (accessed 2.9.20).
- Green Car Congress, 2018. Velocys sells its second commercial license for FT renewable diesel and jet technology to Red Rock Biofuels [WWW Document]. Velocys Sells Its Second Commer. License FT Renew. Diesel Jet Technol. Red Rock Biofuels. URL https://www.greencarcongress.com/2018/05/20180504-velocys.html (accessed 2.9.20).
- Guettel R., Turek T. Comparison of different reactor types for low temperature Fischer-Tropsch synthesis: a simulation study. Chem. Eng. Sci. 2009;64:955–964. doi: 10.1016/j.ces.2008.10.059. [DOI] [Google Scholar]
- Hanaoka T., Miyazawa T., Shimura K., Hirata S. Jet fuel synthesis from Fischer-Tropsch product under mild hydrocracking conditions using Pt-loaded catalysts. Chem. Eng. J. 2015;263:178–185. doi: 10.1016/j.cej.2014.11.042. [DOI] [Google Scholar]
- Hatab F.A., Abdullatif N., Marzouk S.A.M., Al-Marzouqi M.H. Experimental and modeling of CO2 removal from gas mixtures using membrane contactors packed with glass beads. Sep. Purif. Technol. 2019;217:240–246. doi: 10.1016/j.seppur.2019.01.081. [DOI] [Google Scholar]
- Higman, C., 2017. GSTC Syngas Database: 2017 Update.
- Higman C., van der Burgt M. second. ed. Gulf Professional Publishing; Burlington, MA, USA: 2008. Gasification. [Google Scholar]
- Hirschfelder, H., Olschar, M., 2010. Further Developments and Commercial Progress of the BGL Gasification Technology. Pap. Present. Gasif. Technol. Conf. Wash. DC.
- IATA, 2015. IATA Sustainable Aviation Fuel Roadmap. International Air Transport Association, Geneva, Switzerland.
- IEA, 2020. Task IEA Bioenergy [WWW Document]. Task Gasif. Biomass Waste. URL http://task33.ieabioenergy.com/ (accessed 3.14.20).
- Islam M.W. A review of dolomite catalyst for biomass gasification tar removal. Fuel. 2020;267 doi: 10.1016/j.fuel.2020.117095. [DOI] [Google Scholar]
- J. Schilli, 2004. The Fourth Dimension for Waste Management in the United States: Thermoselect gasification technology and the hydrogen energy economy, in: Proceedings of NAWTEC12. Presented at the 12th North American Waste to Energy Conference, ASME, Savannah, Georgia, USA.
- Jaworek, A., Sobczyk, A.T., Krupa, A., Marchewicz, A., Czech, T., Śliwiński, L., 2019. Hybrid electrostatic filtration systems for fly ash particles emission control. A review. Sep. Purif. Technol. 213, 283–302. doi: 10.1016/j.seppur.2018.12.011.
- Jensen A.B., Webb C. Treatment of H2S-containing gases: a review of microbiological alternatives. Enzyme Microb. Technol. 1995;17:2–10. doi: 10.1016/0141-0229(94)00080-B. [DOI] [Google Scholar]
- Kameda T., Uchiyama N., Park K.-S., Grause G., Yoshioka T. Removal of hydrogen chloride from gaseous streams using magnesium–aluminum oxide. Chemosphere. 2008;73:844–847. doi: 10.1016/j.chemosphere.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Kibby C., Jothimurugesan K., Das T., Lacheen H.S., Rea T., Saxton R.J. Chevron’s gas conversion catalysis-hybrid catalysts for wax-free Fischer-Tropsch synthesis. Catal. Today. 2013;215:131–141. doi: 10.1016/j.cattod.2013.03.009. [DOI] [Google Scholar]
- Konarova M., Jones G., Rudolph V. Enabling compact GTL by 3D-printing of structured catalysts. Results Eng. 2020;6 doi: 10.1016/j.rineng.2020.100127. [DOI] [Google Scholar]
- Korens, N., Simbeck, D., Wilhelm, D., 2002. PROCESS SCREENING ANALYSIS OF ALTERNATIVE GAS TREATING AND SULFUR REMOVAL FOR GASIFICATION. U.S. Department of Energy National Energy Technology Laboratory, Pittsburgh, PA, USA.
- Kruse A., Dahmen N. Water – A magic solvent for biomass conversion. J. Supercrit. Fluids. 2015;96:36–45. doi: 10.1016/j.supflu.2014.09.038. [DOI] [Google Scholar]
- Kruse A., Funke A., Titirici M.-M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013;17:515–521. doi: 10.1016/j.cbpa.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Kuhnert N. Microwave-assisted reactions in organic synthesis—are there any nonthermal microwave effects? Angew Chem Int Ed. 2002;41:1863–1866. doi: 10.1002/1521-3773(20020603)41:11<1863::aid-anie1863>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Lane, J., 2015. A new technology debuts for renewable jet fuel [WWW Document]. New Technol. Debuts Renew. Jet Fuel. URL http://www.biofuelsdigest.com/bdigest/2015/03/11/a-new-technology-debuts-for-renewable-jet-fuel/ (accessed 2.9.20).
- Larson E.D., Kreutz T.G., Greig C., Williams R.H., Rooney T., Gray E., Elsido C., Martelli E., Meerman J.C. Design and analysis of a low-carbon lignite/biomass-to-jet fuel demonstration project. Appl. Energy. 2020;260 doi: 10.1016/j.apenergy.2019.114209. [DOI] [Google Scholar]
- Lemmens B., Elslander H., Vanderreydt I., Peys K., Diels L., Oosterlinck M., Joos M. Assessment of plasma gasification of high caloric waste streams. Waste Manag. 2007;27:1562–1569. doi: 10.1016/j.wasman.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Li G., Xu S., Zhao X., Sun R., Wang C., Liu K., Mao Q., Che D. Investigation of chemical composition and morphology of ash deposition in syngas cooler of an industrialized two-stage entrained-flow coal gasifier. Energy. 2020;194 doi: 10.1016/j.energy.2020.116901. [DOI] [Google Scholar]
- Li J., He Y., Tan L., Zhang P., Peng X., Oruganti A., Yang G., Abe H., Wang Y., Tsubaki N. Integrated tuneable synthesis of liquid fuels via Fischer-Tropsch technology. Nat. Catal. 2018;1:787–793. doi: 10.1038/s41929-018-0144-z. [DOI] [Google Scholar]
- Li J., Yang G., Yoneyama Y., Vitidsant T., Tsubaki N. Jet fuel synthesis via Fischer-Tropsch synthesis with varied 1-olefins as additives using Co/ZrO2–SiO2 bimodal catalyst. Fuel. 2016;171:159–166. doi: 10.1016/j.fuel.2015.12.062. [DOI] [Google Scholar]
- Li M., Zhao W., Xu Y., Zhao Y., Yang K., Tao W., Xiao J. Comprehensive life cycle evaluation of jet fuel from biomass gasification and Fischer-Tropsch synthesis based on environmental and economic performances. Ind. Eng. Chem. Res. 2019;58:19179–19188. doi: 10.1021/acs.iecr.9b03468. [DOI] [Google Scholar]
- Liu, Z., 2019. Gasification of municipal solid wastes: a review on the tar yields. Energy Sources Part Recovery Util. Environ. Eff. 41, 1296–1304. doi: 10.1080/15567036.2018.1548508.
- Lucero, A., 2017. Selective Conversion of Syngas to JP-8 Jet Fuel.
- Matveev I.B., Washcilenko N.V., Serbin S.I., Goncharova N.A. Integrated plasma coal gasification power plant. IEEE Trans. Plasma Sci. 2013;41:3195–3200. doi: 10.1109/TPS.2013.2289908. [DOI] [Google Scholar]
- Mazzoni L., Almazrouei M., Ghenai C., Janajreh I. A comparison of energy recovery from MSW through plasma gasification and entrained flow gasification. Energy Procedia. 2017;142:3480–3485. doi: 10.1016/j.egypro.2017.12.233. [DOI] [Google Scholar]
- Mimna R.A., Tramposch W.G. Methods and systems for reducing mercury emissions from fluid streams are provided herein, as are adsorbent materials having high volumetric iodine numbers. US. 2016;2016(0339385):A1. [Google Scholar]
- Minutillo M., Perna A., Di Bona D. Modelling and performance analysis of an integrated plasma gasification combined cycle (IPGCC) power plant. Energy Convers. Manag. 2009;50:2837–2842. doi: 10.1016/j.enconman.2009.07.002. [DOI] [Google Scholar]
- Molino A., Chianese S., Musmarra D. Biomass gasification technology: the state of the art overview. J. Energy Chem. 2016;25:10–25. doi: 10.1016/j.jechem.2015.11.005. [DOI] [Google Scholar]
- Molino A., Larocca V., Chianese S., Musmarra D. Biofuels production by biomass gasification: a review. Energies. 2018;11:811. doi: 10.3390/en11040811. [DOI] [Google Scholar]
- Morgan T.J., Youkhana A., Turn S.Q., Ogoshi R., Garcia-Pérez M. Review of biomass resources and conversion technologies for alternative jet fuel production in Hawai’i and Tropical regions. Energy Fuels. 2019;33:2699–2762. doi: 10.1021/acs.energyfuels.8b03001. [DOI] [Google Scholar]
- Mountouris A., Voutsas E., Tassios D. Solid waste plasma gasification: equilibrium model development and exergy analysis. Energy Convers. Manag. 2006;47:1723–1737. doi: 10.1016/j.enconman.2005.10.015. [DOI] [Google Scholar]
- Moustakas K., Fatta D., Malamis S., Haralambous K., Loizidou M. Demonstration plasma gasification/vitrification system for effective hazardous waste treatment. J. Hazard. Mater. 2005;123:120–126. doi: 10.1016/j.jhazmat.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Nair S.A., Yan K., Safitri A., Pemen A.J.M., van Heesch E.J.M., Ptasinski K.J., Drinkenburg A.A.H. Streamer corona plasma for fuel gas cleaning: comparison of energization techniques. J. Electrost. 2005;63:1105–1114. doi: 10.1016/j.elstat.2005.02.004. [DOI] [Google Scholar]
- Nelson L., Park S., Hubbe M.A. Thermal depolymerization of biomass with emphasis on gasifier design and best method for catalytic hot gas conditioning. BioResources. 2018;13:4630–4727. [Google Scholar]
- NETL, 2020. Fischer-Tropsch Synthesis [WWW Document]. Fisch.-Tropsch Synth. URL https://www.netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/ftsynthesis (accessed 2.9.20).
- Ngamcharussrivichai C., Liu X., Li X., Vitidsant T., Fujimoto K. An active and selective production of gasoline-range hydrocarbons over bifunctional co-based catalysts. Fuel. 2007;86:50–59. doi: 10.1016/j.fuel.2006.06.021. [DOI] [Google Scholar]
- Osada M., Sato T., Watanabe M., Shirai M., Arai K. Catalytic gasification of wood biomass in subcritical and supercritical water. Combust. Sci. Technol. 2006;178:537–552. doi: 10.1080/00102200500290807. [DOI] [Google Scholar]
- Palma, V., Ruocco, C., Martino, M., Meloni, E., Ricca, A., 2017. Catalysts for conversion of synthesis gas, in: Bioenergy Systems for the Future. Elsevier, pp. 217–277. doi: 10.1016/B978-0-08-101031-0.00007-7.
- Park S.-W., Lee J.-S., Yang W.-S., Alam M.T., Seo Y.-C., Lee S.-Y. Gasification characteristics of biomass for tar removal by secondary oxidant injection. J. Mater. Cycles Waste Manag. 2018;20:823–831. doi: 10.1007/s10163-017-0642-0. [DOI] [Google Scholar]
- Pearlson M., Wollersheim C., Hileman J. A techno-economic review of hydroprocessed renewable esters and fatty acids for jet fuel production. Biofuels Bioprod. Biorefining. 2013;7:89–96. doi: 10.1002/bbb.1378. [DOI] [Google Scholar]
- Pemen A.J.M., Nair S.A., Yan K., van Heesch E.J.M., Ptasinski K.J., Drinkenburg A.A.H. Pulsed corona discharges for tar removal from biomass derived fuel gas. Plasmas Polym. 2003;8:209–224. doi: 10.1023/A:1024813306111. [DOI] [Google Scholar]
- Perkins, G., 2020. Production of electricity and chemicals using gasification of municipal solid wastes, in: Waste Biorefinery. Elsevier, pp. 3–39. doi: 10.1016/B978-0-12-818228-4.00001-0.
- Pinto, F., Lopes, H., André, R.N., Dias, M., Gulyurtlu, I., Cabrita, I., 2007. Effect of Experimental Conditions on Gas Quality and Solids Produced by Sewage Sludge Cogasification. 1. Sewage Sludge Mixed with Coal. Energy Fuels 21, 2737–2745. doi: 10.1021/ef0700836.
- Pourali M. Application of plasma gasification technology in waste to energy—challenges and opportunities. IEEE Trans. Sustain. Energy. 2010;1:125–130. doi: 10.1109/TSTE.2010.2061242. [DOI] [Google Scholar]
- Prabhansu, Karmakar, M.Kr., Chandra, P., Chatterjee, P.Kr., 2015. A review on the fuel gas cleaning technologies in gasification process. J. Environ. Chem. Eng. 3, 689–702. doi: 10.1016/j.jece.2015.02.011.
- Punjak W.A., Uberoi M., Shadman F. High-temperature adsorption of alkali vapors on solid sorbents. AIChE J. 1989;35:1186–1194. doi: 10.1002/aic.690350714. [DOI] [Google Scholar]
- Richardson, Y., Drobek, M., Julbe, A., Blin, J., Pinta, F., 2015. Biomass Gasification to Produce Syngas, in: Recent Advances in Thermo-Chemical Conversion of Biomass. Elsevier, pp. 213–250. doi: 10.1016/B978-0-444-63289-0.00008-9.
- Rodriguez Correa C., Kruse A. Supercritical water gasification of biomass for hydrogen production – Review. J. Supercrit. Fluids. 2018;133:573–590. doi: 10.1016/j.supflu.2017.09.019. [DOI] [Google Scholar]
- Rueda, Y.G., Helsen, L., 2019. The role of plasma in syngas tar cracking. Biomass Convers. Biorefinery. https://doi.org/10.1007/s13399-019-00461-x.
- S. Zola, A., Bidart, A.M.F., do C. Fraga, A., E. Hori, C., F. Sousa-Aguiar, E., A. Arroyo, P., 2007. Cobalt supported on different zeolites for Fischer-Tropsch synthesis. In: Studies in Surface Science and Catalysis. Elsevier, pp. 129–134. doi: 10.1016/S0167-2991(07)80120-0.
- Sakin, A., Karagoz, I., Avci, A., 2019. Performance analysis of axial and reverse flow cyclone separators. Chem. Eng. Process. – Process Intensif. 144, 107630. doi: 10.1016/j.cep.2019.107630.
- Saleem F., Harris J., Zhang K., Harvey A. Non-thermal plasma as a promising route for the removal of tar from the product gas of biomass gasification – a critical review. Chem. Eng. J. 2020;382 doi: 10.1016/j.cej.2019.122761. [DOI] [Google Scholar]
- Sánchez-Hervás J.M., Otero J., Ruiz E. A study on sulphidation and regeneration of Z-Sorb III sorbent for H2S removal from simulated ELCOGAS IGCC syngas. Chem. Eng. Sci. 2005;60:2977–2989. doi: 10.1016/j.ces.2005.01.018. [DOI] [Google Scholar]
- Schmieder H., Abeln J., Boukis N., Dinjus E., Kruse A., Kluth M., Petrich G., Sadri E., Schacht M. Hydrothermal gasification of biomass and organic wastes. J. Supercrit. Fluids. 2000;17:145–153. doi: 10.1016/S0896-8446(99)00051-0. [DOI] [Google Scholar]
- Seo, Y.-C., Alam, M.T., Yang, W.-S., 2018. Gasification of municipal solid waste. In: Yun, Y. (Ed.), Gasification for Low-Grade Feedstock. InTech. doi: 10.5772/intechopen.73685.
- Shahabuddin M., Krishna B.B., Bhaskar T., Perkins G. Advances in the thermo-chemical production of hydrogen from biomass and residual wastes: Summary of recent techno-economic analyses. Bioresour. Technol. 2020;299 doi: 10.1016/j.biortech.2019.122557. [DOI] [PubMed] [Google Scholar]
- Shemwell B., Levendis Y.A., Simons G.A. Laboratory study on the high-temperature capture of HCl gas by dry-injection of calcium-based sorbents. Chemosphere. 2001;42:785–796. doi: 10.1016/S0045-6535(00)00252-6. [DOI] [PubMed] [Google Scholar]
- Sierra Energy, 2019. Types of Gasification [WWW Document]. Types Gasif. URL https://www.sierraenergy.com/technology/knowledge-base/gasification/types-of-gasification/ (accessed 2.10.20).
- Sikarwar V.S., Zhao M., Clough P., Yao J., Zhong X., Memon M.Z., Shah N., Anthony E.J., Fennell P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016;9:2939–2977. doi: 10.1039/C6EE00935B. [DOI] [Google Scholar]
- Sikarwar V.S., Zhao M., Fennell P.S., Shah N., Anthony E.J. Progress in biofuel production from gasification. Prog. Energy Combust. Sci. 2017;61:189–248. doi: 10.1016/j.pecs.2017.04.001. [DOI] [Google Scholar]
- Smeenk, J., 2015. Innovative Gasification to Produce Fischer-Tropsch Jet and Diesel Fuel.
- Stapf, D., Giovanni Ceceri, Inge Johansson, Kevin Whitty, 2019. Biomass pre-treatment for bioenergy - Case study 3: Pretreatment of municipal solid waste (MSW) for gasification. International Energy Agency.
- Taheri M., Huang S., Lei Z. IL-Emitsol: ionic liquid based [EMIM][Tf2N] solvent process for selective removal of CO2 and H2S from syngas. Ind. Eng. Chem. Res. 2019;58:10007–10017. doi: 10.1021/acs.iecr.9b01656. [DOI] [Google Scholar]
- Tanigaki N., Fujinaga Y., Kajiyama H., Ishida Y. Operating and environmental performances of commercial-scale waste gasification and melting technology. Waste Manag. Res. 2013;31:1118–1124. doi: 10.1177/0734242X13502386. [DOI] [PubMed] [Google Scholar]
- Tanigaki, N., Ishida, Y., 2014. Waste Gasification Technology with Direct Melting for Energy and Material Recovery. In: Waste Management. TK Verlag, pp. 365–378.
- Tanigaki, N., Kashiwabara, T., 2017. Operating Experience from Japanese Waste Gasification Plants with Direct Melting System, in: Waste Management. TK Verlag, pp. 379–388.
- Tanigaki N., Manako K., Osada M. Co-gasification of municipal solid waste and material recovery in a large-scale gasification and melting system. Waste Manag. 2012;32:667–675. doi: 10.1016/j.wasman.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Tanksale A., Wong Y., Beltramini J., Lu G. Hydrogen generation from liquid phase catalytic reforming of sugar solutions using metal-supported catalysts. Int. J. Hydrog. Energy. 2007;32:717–724. doi: 10.1016/j.ijhydene.2006.08.009. [DOI] [Google Scholar]
- ThermoChem Recovery International Inc., 2020. Gasification [WWW Document]. Gasification. URL https://tri-inc.net/steam-reforming-gasification/ (accessed 2.10.20).
- Todić B., Ordomsky V.V., Nikačević N.M., Khodakov A.Y., Bukur D.B. Opportunities for intensification of Fischer-Tropsch synthesis through reduced formation of methane over cobalt catalysts in microreactors. Catal. Sci. Technol. 2015;5:1400–1411. doi: 10.1039/C4CY01547A. [DOI] [Google Scholar]
- US Department of Energy, 2020. Renewable Hydrocarbon Biofuels [WWW Document]. Renew. Hydrocarb. Biofuels. URL https://afdc.energy.gov/fuels/emerging_hydrocarbon.html (accessed 2.9.20).
- Valmet . Valmet; Finland: 2017. CFB’s Evolution from Coal-only Boiler to Biomass – or Anything in Between. [Google Scholar]
- Vamvuka D., Arvanitidis C., Zachariadis D. Flue gas desulfurization at high temperatures: a review. Environ. Eng. Sci. 2004;21:525–548. doi: 10.1089/1092875041358557. [DOI] [Google Scholar]
- Velocys, 2019. Plans submitted for the first waste to jet fuel plant in the UK and Europe [WWW Document]. Plans Submitt. First Waste Jet Fuel Plant UK Eur. URL https://www.velocys.com/2019/08/20/plans-submitted-for-the-first-waste-to-jet-fuel-plant-in-the-uk-and-europe/ (accessed 2.9.20).
- Woolcock P.J., Brown R.C. A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy. 2013;52:54–84. doi: 10.1016/j.biombioe.2013.02.036. [DOI] [Google Scholar]
- Xie Q., Borges F.C., Cheng Y., Wan Y., Li Y., Lin X., Liu Y., Hussain F., Chen P., Ruan R. Fast microwave-assisted catalytic gasification of biomass for syngas production and tar removal. Bioresour. Technol. 2014;156:291–296. doi: 10.1016/j.biortech.2014.01.057. [DOI] [PubMed] [Google Scholar]
- Yoshikawa, K., 2013. Gasification Gasification and Liquefaction liquefaction Alternatives incineration alternatives to Incineration incineration in Japan, in: Kaltschmitt, M., Themelis, N.J., Bronicki, L.Y., Söder, L., Vega, L.A. (Eds.), Renewable Energy Systems. Springer New York, New York, NY, pp. 728–743. doi: 10.1007/978-1-4614-5820-3_419.
- Zhang Q., Dor L., Fenigshtein D., Yang W., Blasiak W. Gasification of municipal solid waste in the Plasma Gasification Melting process. Appl. Energy. 2012;90:106–112. doi: 10.1016/j.apenergy.2011.01.041. [DOI] [Google Scholar]