Abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a highly contagious life-threatening condition with unprecedented impacts for worldwide societies and health care systems. Since the first detection in China, it has spread rapidly worldwide. The increased burden has substantially affected neurosurgical practice and intensive modifications have been required in surgical scheduling, inpatient and outpatient clinics, management of emergency cases, and even in academic activities. In some systems, nonoverlapping teams have been created to minimize transmission among health care workers. In cases of a massive burden, neurosurgeons may need to be reassigned to COVID-19 wards, or teams from other regions may need to be sent to severely affected areas. Recommendations are as following. In outpatient practice, if possible, appointments should be undertaken via telemedicine. All staff assigned to the non-COVID treatment unit should be clothed in level 1 personal protective equipment. If possible, postponement is recommended for operations that do not require urgent or emergent intervention. All patients indicated for surgery must receive COVID-19 screening, including a nasopharyngeal swab and thorax computed tomography. Level 2 protection measures are appropriate during COVID-19–negative patients' operations. Operations of COVID-19–positive patients and emergency operations, in which screening cannot be obtained, should be performed after level 3 protective measures. During surgery, the use of high-speed drills and electrocautery should be reduced to minimize aerosol production. Screening is crucial in all patients because the surgical outcome is highly mortal in patients with COVID-19. All educational and academic conferences can be undertaken as virtual webinars.
Key words: Central nervous system, Coronavirus disease 2019, Operation room, Scheduling, Severe acute respiratory syndrome coronavirus 2, Telemedicine, Viral exposure
Abbreviations and Acronyms: ASIA, American Spinal Injury Association; CNS, Central nervous system; COVID-19, Coronavirus disease 2019; CSF, Cerebrospinal fluid; FFP, Filtering face piece; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
Introduction
Coronavirus disease 2019 (COVID-19) is an exceedingly infectious, life-threatening condition. The outbreak has created unprecedented extraordinary threats and difficulties for societies and health care systems worldwide.1, 2, 3 Since the first detection in China in late December 2019, it has spread rapidly to 213 countries around the globe and had reached approximately 3,000,000 confirmed cases with >200,000 deaths on April 28, 2020.4 The increased burden of this pandemic disease has substantially affected the entire health system, including neurosurgical practice in most countries.5, 6, 7
In neurosurgical practice, intensive modifications have been required in surgical scheduling, administration of inpatient and outpatient clinics, management of emergency cases, and even in academic and educational activities. The major goal of this review is to compose a comprehensive guide using existing guides and recommendations for reorganizing daily practice and the academic routine of neurosurgery during the COVID-19 pandemic. This study also aims to refine the substantial information for neurosurgical practice about this pandemic disease.
Terminology
An outbreak of pneumonia of unknown origin showed up in Wuhan, the capital of Hubei province in the People's Republic of China, in late December 2019.8 , 9 On 7 January 2020, China isolated a new coronavirus called 2019 novel coronavirus (2019-nCoV) and presented virus genome data to the international community.10 Later, coincidentally, on 11 February 2020, the Coronavirus Study Group of the International Committee on Taxonomy of Viruses gave a new name to the virus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2])11 and the World Health Organization named the epidemic disease coronavirus disease 2019 (COVID-19).12
Virology
SARS-CoV-2, a positive-sense single-stranded RNA virus, a member of the subgenus betacoronaviruses, is the seventh determined coronavirus that infects humans.8 , 13 , 14 The genetic sequence of the SARS-CoV-2 presents approximately 80% analogy to SARS-CoV.13 SARS-CoV-2 comprises 4 structural proteins: N (nucleocapsid), E (envelope), M (membrane), and S (spike) (Figure 1 ).15 The N protein supports the RNA genome, and E, M, and S proteins comprise the viral envelope. The S protein is also responsible for binding to the angiotensin-converting enzyme 2 receptor on the human cell membrane.15
Figure 1.
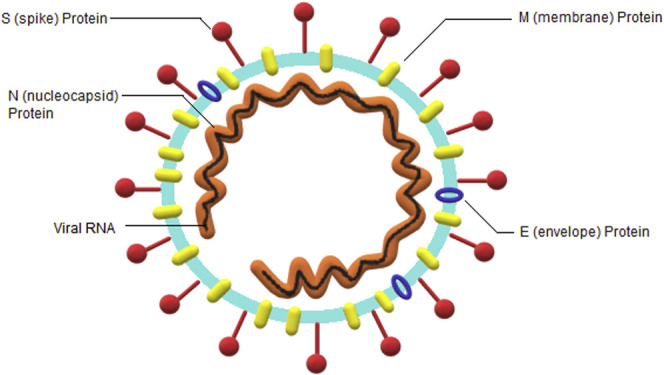
Severe acute respiratory syndrome coronavirus 2 virion.
Clinical Characteristics
The median incubation period is approximately 5 days and practically all patients experience symptoms in 14 days after exposure to SARS-CoV-2.16 Transmission occurs mainly through direct contact with the infected material or via droplets spread by sneezing or coughing.17 SARS-CoV-2 primarily targets the respiratory system.18 The main clinical symptoms of COVID-19 are fever, cough, myalgia or fatigue, expectoration, and dyspnea.18 , 19 Minor symptoms include headache or dizziness, diarrhea, and nausea and vomiting.18 , 19 Dyspnea may be observed in critical patients and may proceed to severe acute respiratory syndrome, sepsis, and multiple organ dysfunction syndrome.18 Reduced total leukocyte and lymphocyte counts and increased levels of C-reactive protein and lactate dehydrogenase are common results in laboratory tests.18 , 19 The typical appearance on thorax computed tomography is bilateral, subpleural, ground-glass opacities with air bronchograms.20 The viral load is increased throughout the upper respiratory tract mucosa, including the nasal cavity and naso-oropharynx.9 Viral RNA can be identified in the sputum and saliva, as well as in the serum.9
COVID-19 and Central Nervous System
The blood-brain barrier works as a natural barrier against pathogenic microorganisms and reduces the risk of intracranial infection.21 Some human coronaviruses can invade the central nervous system (CNS) through hematogenous or neuronal retrograde dissemination, leading to encephalitis and exacerbation of existing neurologic diseases.22 The brainstem involvement of SARS-CoV has been described in both clinical and experimental studies.23, 24, 25 Given the high analogy between SARS-CoV and SARS-CoV-2, the CNS spread of SARS-CoV-2 may be partly responsible for acute respiratory failure in COVID-19 disease.26 A recent study from Wuhan, China27 reported that some patients with severe COVID-19 developed neurologic manifestations, such as acute cerebrovascular diseases (5.7%) and impaired consciousness (14.8%). Cerebrovascular accidents may occur because of systemic highly prothrombotic state of COVID-19.28 Furthermore, SARS-CoV-2 was isolated in cerebrospinal fluid (CSF) by gene sequencing from a patient with COVID-19 in Beijing Ditan Hospital, China on March 4, 2020.29
Because results of encephalitis are highly mortal, early diagnosis is essential.26 Severely affected and comatose patients with neurologic symptoms should undergo brain imaging and CSF examination. Magnetic resonance imaging shows the definitive signs of the presence of infectious intracranial processes. The regional hyperintense abnormalities on T1-weighted, T2-weighted, fluid-attenuated inversion recovery, and diffusion-weighted images are considered suspicious for viral encephalitis.30 In cases of suspected CNS infection, lumbar puncture is indicated.31 The CSF obtained can be investigated to detect viral genetic material through a polymerase chain reaction examination or using antibody testing.32
Viral encephalitis may also present with neurologic deterioration related to massive cerebral edema.33 , 34 If conservative management (corticosteroids, hyperventilation, hypertonics, hypothermia, and barbiturate coma) fails, decompressive craniectomy can be considered as an option for last-chance therapy in selected cases.35 , 36
Faculty Planning
The regional disease burden has surged during the pandemic and the disease has also shown significant transmission to health care professionals. In the algorithm proposed by the University of California at San Francisco, surgical scheduling is organized according to the ''surge level'' which correlates with the increasing viral transmission among the local community.37 Using this system, green, yellow, red, and black levels represent the lowest, moderate, high, and highest levels of the surge, respectively. At the green level (<6 COVID-19–positive inpatients and no staffing shortages), all elective operations proceed as scheduled. At the yellow level (7–16 COVID-19–positive inpatients or <20% staffing shortages), the schedule is rearranged to yield a 25% reduction in the capacity of all elective procedures and all outpatient procedures are designated to an off-site (COVID-19–free) hospital. At the red level (>17 COVID-19–positive inpatients or >21% staffing shortages), a 50% reduction is introduced in elective scheduling. At the black level, in which significant assistance is required from outside institutions to resist the outbreak, only emergent surgical cases are performed.
The University of California at San Francisco recommends a system based on the “paired coverage model” designed to minimize patient and provider viral exposure and to provide continuous inpatient coverage for neurosurgical emergencies.37 In this model, each department is covered by 2 nonoverlapping teams (rotating in 3-day cycles: 3 days on and 3 days off), in which members have contact only within the same team. This model is activated by a red level of surge and includes an assigned alternative pool of providers to replace those who show COVID-19 prodrome.
If there is a massive increase in COVID-19 cases, nonspecialized physicians in respiratory or infectious diseases, including neurosurgeons, may need to be reassigned to the COVID-19 wards to initiate supplementary emergency responses. Remodeling the hospital system by identifying concentration centers for neurosurgical activities is necessary to manage emergent and urgent cases. This circumstance is exemplified in Lombardy, northern Italy. The Lombardy health system was rearranged as a “spoke-and-hub system.”38, 39, 40, 41 The local neurosurgical network was assembled in 4 hub hospitals (3 for cranial or spinal emergencies and 1 for oncologic emergencies41). All other neurosurgery departments served as spokes. In this way, hub hospitals are available to manage neurosurgical emergencies, whereas spoke hospitals concentrate on patients with COVID-19.38, 39, 40, 41 In this system, a huge increase may be expected in the number of patients treated in hub hospitals. According to an early report from the University of Insubria, Italy, there was an increase of 265% and 144% of hospitalized and surgically treated neurosurgical emergencies, respectively.42
Health care professionals from other areas may need to be sent to regions that are heavily affected by pandemics. During the outbreak, >30,000 medical staff including 74 teams, of which 9 were neurosurgical teams, from other regions of China have been dispatched to Hubei Province.43 Thanks to the newly appointed teams, emergency operations could be performed even during the worst times of the epidemic peak.43
Robertson et al.44 suggested a ''task shifting and task sharing'' method that involves training, practice, and maintenance phases for increasing workforce capacity during the pandemic. According to this method, the most experienced neurosurgeons, who are also from the most vulnerable age-groups, may practice on telemedicine encounters, guiding ethical decisions on appropriate neurosurgical interventions, or neurosurgery-specific cases.44 Residents skilled in neurocritical care may receive intensive skills training in endotracheal intubation and mechanical ventilator management.44 , 45 Residents may serve remotely when possible to perform virtual visits, record notes, give orders, and call consults.44
Outpatient Departments
According to the physicians' preference, patient follow-up and appointments should be performed by telemedicine, if possible.46 , 47 In particular, remote examinations are often suited to this strategy. According to the Harvard Medical School experience, >80% of outpatient visits have been switched to telemedicine.47 Also, in a validation study by Neumarkt Clinic, Germany, remote neurologic examination consisting of 22 items performed via audiovisual telemedicine presented comparable results to bedside examination.48 Sometimes, the assistance of a person may be required for the patient to perform some parts of the examination, such as the Lasègue test for spinal examination.49 Transmission of the patient's radiologic images to the outpatient team via a data transfer method before a telemedicine appointment is beneficial.50 Actual visits should be reserved for selected patients, such as patients requiring wound control and stitch removal.47 Also, the use of absorbable sutures in neurosurgical surgery could be considered to decrease contacts among clinicians and patients after discharge.51 In addition, patients aged >65 years should be encouraged to avoid visiting the outpatient clinic.
Outpatient facilities and personnel should be separated in non-COVID and COVID treatment units during the pandemic.46 The work schedule should be organized with as minimal staff as possible using the appropriate personal protective equipment (PPE).46 Physicians and staff assigned in outpatient facilities should be clothed in level 1 PPE during their practice. Details of PPE according to the Handbook of COVID-19 Prevention and Treatment 52 are presented in Table 1 . Companions for pediatric or nonambulatory patients should be limited to 1 person.46 Ambulatory individuals should visit outpatient clinics alone.
Table 1.
Personal Protective Equipment According to Handbook of COVID-19 Prevention and Treatment52
| Level 1 |
| Disposable surgical cap |
| Disposable surgical mask |
| Work uniform |
| Disposable latex gloves |
| Disposable isolation clothing |
| Level 2 |
| Disposable surgical cap |
| Medical protective mask (N95/FFP3) |
| Work uniform |
| Disposable latex gloves |
| Disposable medical protective uniform |
| Goggles |
| Level 3 |
| Disposable surgical cap |
| Medical protective mask (N95/FFP3) |
| Work uniform |
| Disposable medical protective uniform |
| Disposable latex gloves |
| Full-face respiratory protective devices or powered air-purifying respirator |
FFP, Filtering face piece.
Surgical Volume and Scheduling
The lockdown and the stay-at-home strategies during the pandemic have dramatically reduced spinal and cranial trauma, allowing medical professionals to focus on patients with COVID-19.53 , 54 Also, there has been a reduction in surgical treatments for degenerative diseases. The decrease in traumatic events can be explained by reduced traffic and work activities.53 Two potential reasons have been suggested by Dobran et al. for the decrease in demand for surgical treatment for spinal degenerative diseases: 1) the fear prevalent in the community that hospitals are a risky place for a possible infection and 2) patients overrating their impairments and pains, which results in surgical overtreatment.53
A global study on the impact of COVID-19 on neurosurgeons that generated an acuity index for the triage strategy for nonemergent operations surveyed 494 respondents from 60 countries.55 Of respondents, 53% reported that all elective cases had been cancelled and clinics were closed down. Of respondents, 46% reported that their operative density had decreased by >50%, and this rate was 55% in the most affected countries.55 A strong agreement was found that postponement of fast-evolving neuro-oncologic cases and unstable vascular cases has a higher risk according to the acuity index.55
Several clinical guides and recommendations for the scheduling of neurosurgery patients during the pandemic have been announced. In this study, a comprehensive proposal chart for scheduling has been formed using recommendations from the European Association of Neurosurgical Societies, American Association of Neurological Surgeons, British Neurosurgical Society, and Turkish Neurosurgical Society (Table 2 ). All plans for scheduling of neurosurgical interventions should rely on an expert opinion form a board-certified neurosurgeon based on the patient's condition. Case-specific decision should be made in each patient. For example, elective surgery can be postponed for patients with asymptomatic benign tumors, low-grade gliomas, degenerative spinal disease without neurologic deficits, and peripheral nerve entrapments. For patients with benign tumors with severe symptoms, higher-grade gliomas, or spinal degenerative diseases with acute neurologic deficits, an urgent surgical intervention should be scheduled. Emergency surgery must be arranged for patients with cerebral herniation or acute hydrocephalus.
Table 2.
Proposal Chart for Scheduling Formed Using Recommendations Announced by the European Association of Neurosurgical Societies, American Association of Neurological Surgeons, British Neurosurgical Society, and Turkish Neurosurgical Society
| Neuro-oncology | Neurovascular | Spine | Pediatric | Functional | Hydrocephalus | Trauma | Peripheral Nerves | ||
|---|---|---|---|---|---|---|---|---|---|
| Low acuity surgery | Asymptomatic benign intracranial tumors (e.g., meningioma, schwannoma, pituitary adenoma) | Microvascular decompression of cranial nerves | Degenerative spinal disease (lumbar stenosis, spinal deformity) without neurologic deficits | Deep brain stimulation | Carpal tunnel release Ulnar nerve decompression |
Postpone surgery | |||
| Intermediate acuity surgery | Symptomatic benign intracranial tumors Low-grade glioma |
Unruptured aneurysm Arteriovenous malformation | Craniosynostosis Tethered cord Spina bifida occulta Chiari decompression Baclofen pump placement |
Deep brain stimulation for progressive parkinsonism Refractory epilepsy |
Normal-pressure hydrocephalus | Postpone surgery if possible | |||
| High acuity surgery | Malignant primary tumors Metastases Benign or low-grade tumors with progressive neurologic deficits Pituitary tumors with cranial nerve deficits, visual impairment or endocrine deficiency Posterior fossa tumors |
Subarachnoid hemorrhage Malignant cerebral artery infarction Space-occupying intracerebral hematoma Arteriovenous malformation hemorrhage Higher-grade dural arteriovenous fistulas Procedures including revascularization in patients with evidence of relevant vascular occlusive disease Unstable aneurysms |
Progressive cervical and thoracic myelopathy Infectious conditions with abscess formation, instability, and compression Unstable or compressive spinal metastases Degenerative spine conditions with acute onset of motor deficits Unstable spinal fractures |
Myelomeningocele | Battery depletion in deep brain stimulation patients Infection of implanted devices |
Progressive increase of intracranial pressure with hydrocephalus Shunt material Infection Shunt dysfunction |
Acute subdural hematoma Acute epidural hematoma Uncontrolled intracranial pressure increase during traumatic brain injury Chronic subdural hematoma with neurologic symptoms |
Malignant peripheral nerve tumors Benign nerve tumors with motor deficits Acute injuries Brachial plexus injury |
Urgency Emergency Do not postpone surgery |
Although only emergency and urgency operations are performed during the pandemic, it is necessary to shorten the length of hospital stay of these operated patients to minimize the risk of exposure. The spinal team of the Great Metropolitan Hospital Niguarda, Milan, Italy reported an organization protocol for emergency spinal surgery that indicates timing of surgery according to neurologic status.56 Based on this protocol, in which neurologic status is assessed using the American Spinal Injury Association (ASIA) impairment scale, 1) early urgent (<12 hours) surgery is performed for patients with cervical displacement or progressive neurologic worsening in ASIA grading; 2) urgent (<24–36 hours) surgery is performed for patients with ASIA grade B–D; 3) middle urgent (<36–48 hours) surgery is performed for patients with spinal cord injury with previous cervical spondylosis; and 4) planned (<72–96 hours) surgery is performed for patients with ASIA grade A and E.56
If possible, medical care methods requiring less invasive interventions (e.g., endovascular treatment in neurovascular conditions and radiosurgery in certain neuro-oncologic diseases) may be considered.57 Endotracheal intubation or high-speed drill use is not required during stereotactic radiosurgery, which reduces the risk of exposure to aerosols compared with open surgery.58
Protection of Operating Room Staff
A summary of measures during the COVID-19 pandemic is presented in Table 3 . Health care personnel, including operating room staff, are at high-level risk of exposure to Sars-Cov-2. Up to 29% of the confirmed cases were in health care staff in the initial cohort reports.59 Later, according to the report of the Chinese Center for Disease Control and Prevention, which included >72,000 cases, 3.9% of the confirmed cases were medical staff, and 14.8% of them were in a critical or severe condition.60 Minimal or no symptoms are observed during the incubation period (first 3–6 days) in most cases.61 However, these asymptomatic patients are able to spread the virus.62 All patients indicated for surgery must receive COVID-19 screening, including measuring body temperature, symptom investigation, SARS-CoV-2 polymerase chain reaction and antibody test, nasopharyngeal swab, and thorax computed tomography scan.63
Table 3.
Summary of COVID-19 Pandemic Measures
| Academic activities |
| All in-person conferences should be cancelled |
| All conferences can occur via video teleconferences |
| Outpatient department |
| Appointments switched to telemedicine |
| Actual visits reserved for selected patients, such as wound control |
| Use absorbable sutures |
| Use level 1 PPE in non-COVID-19 facilities |
| Single companion for pediatric or nonambulatory patients |
| Lone visits for ambulatory individuals |
| Social distancing measures during appointments |
| Operation theater staff prevention |
| COVID-19 screening for all patients (nasopharyngeal swab, and thorax computed tomography scan) |
| COVID-19–negative operations: level 2 PPE |
| COVID-19–positive or emergency operations: level 3 PPE |
| Routine training about wearing and removing PPE |
| General considerations for COVID-19–positive operation |
| Clear the route during transfers |
| Separate negative pressure operating room with independent access |
| Separate mechanical ventilator |
| Endotracheal intubation with video-laryngoscope |
| Level 3 PPE is obligatory for all operating room staff |
| Powered air-purifying respirators for the surgical team |
| Minimum operating room staff number |
| Procedures performed by experienced neurosurgeons |
| Surgical considerations for COVID-19–positive operation |
| Reduced use of high-speed drills |
| More meticulous irrigation and reduction of drill speed |
| Increased use of traditional hand drills and rongeurs |
| Avoid breaching frontal or ethmoidal sinuses |
| Reduced use of electrocautery with reduced power setting |
PPE, Personal protective equipment.
Different recommendations are present for COVID-19–negative patients for the protection of medical staff in the operating theater.63 According to a surgical neuro-oncology team perspective, patients from low-risk areas who are verified COVID-19 negative can be operated on following level 1 precautions.63 Other perspectives from Tongji Medical College, Wuhan, China64 and Heinrich-Heine University, Düsseldorf, Germany57 recommend that medical staff should take level 2 protection measures because of the long incubation period.57 , 64 For patients who are suspected or confirmed COVID-19 positive, or patients from a high-risk area, operations should be performed under level 3 precautions.63 , 64 In emergency cases, the results of SARS-CoV-2 tests may not be obtained before surgery, and therefore, the surgery should be performed following strict measures (level 3 protective measures) to reduce potential exposure.65
General Precautions During Operation of COVID-19–Positive Patients
The route from the ward to the operating room, including the elevators, should be cleared during the transfer of a COVID-19–positive patient. The transfer should be performed by COVID-19 ward nurses in full level 3 PPE.66 The operating room of COVID-19–positive patients should be separate.67 An operating room with a negative atmospheric pressure setting and with independent access should be designated for all confirmed or suspected COVID-19–positive patients.66 , 67 During the pandemic, the same operating room, and the same continuous flow anesthetic machine, should be used only for COVID-19–positive patients.66 Because endotracheal intubation can generate aerosols,68 intubation should be performed via the method with the maximum possibility of first-time success, using a video-laryngoscope to avoid multiple attempts.69 During the operation of patients with confirmed or suspected COVID-19, all operating room staff must wear level 3 PPE52 under a surgical gown to prevent contamination.70 PPE is obligatory for all interventions involving close contacts, such as surgery, endotracheal intubation, intravenous cannulation, cardiac catheterization, and regional anesthesia. Use of powered air-purifying respirators by the surgical team is recommended.57 To prevent contamination, all personnel should be trained in wearing and removing PPE.67 After extubation, it is recommended that a patient wears a surgical mask as soon as possible.67
The viral exposure load of operating room staff can be considered to be proportional to the duration of the surgery. During the pandemic, the staff number in the operating theater must be reduced to the minimum.67 Also, all neurosurgical procedures ought to be designed to reduce operating theater time.57 If possible, only a single experienced neurosurgeon beyond their learning curve ought to carry out the procedure to reduce operation time and to prevent exposure of other physicians.57
Surgical Considerations During Operation of COVID-19–Positive Patients
Powered instruments such as high-speed drills, which are commonly used tools for cranial and spinal procedures, produce blood-containing aerosols and hemoglobin has been identified in the ambient air.71 Viruses such as human immunodeficiency virus 1 survive in aerosol produced by surgical power instruments.72 Because coronaviral RNA can be determined in plasma or lymphocytes of confirmed or asymptomatic patients,73 the aerosols produced during neurosurgical operations can be contagious. Also, a recent study used the Bayesian regression model (a statistical model that uses probability to represent all uncertainty within the model) indicated that aerosol transmission of SARS-CoV-2 is plausible.74 Attention should be paid to minimizing aerosol generation in operations performed during the pandemic. Upholding the increase in using traditional hand drills and rongeurs is beneficial.75 More meticulous irrigation and reduction of drill speed are some precautions that may be taken if cranial or spinal drilling is necessary.76 Special caution should be taken during anterior skull base surgery to avoid breach frontal or ethmoidal sinuses.76
The use of electrocautery creates a gaseous by-product containing aerosol commonly referred to as surgical smoke.77 Viral transmission of human papillomavirus from patients to treating physicians through surgical smoke has been shown.78 , 79 Because of potential transmission risk, the duration of use of monopolar and bipolar electrocautery should be reduced and their power settings minimized to decrease aerosol dispersal during the pandemic.57
Endonasal procedures, using debriders and drills inside the nasal cavity, generate highly hazardous aerosols.76 Otolaryngologists are among the worst affected medical professionals in Wuhan, China, and even N95/level 3 filtering face piece (FFP3) masks do not prevent transmission.76 , 80 Also, a patient with a mass lesion in the sellar region who underwent endonasal endoscopic surgery in the Department of Neurosurgery, Tongji Medical College, Wuhan, China was diagnosed with COVID-19 after surgery, and disease was confirmed in 14 health care professionals in the same clinic afterward.81 According to an initial perspective from the Society of British Neurological Surgeons, endonasal transsphenoidal endoscopic surgical approaches should be avoided during the pandemic. Alternatives routes to endoscopic surgery should be considered for patients whose surgery cannot be postponed: 1) craniotomy and 2) microscopic endonasal transsphenoidal surgery, with the submucosal approach and nondrill techniques used during the endonasal and sellar phase.76 Another recent perspective from Singapore suggests that endonasal procedures should be managed according to the COVID-19 test results.82 These investigators suggested wearing N95/FFP3 mask, eye protection (goggles and full-face shield), and standard level 2 PPE (gown and gloves) in treatment of patients whose test results are negative.82 In patients with positive test results, the entire surgical and anesthesia team, including the circulating nurse and operating room attendant, are recommended to don additional powered air-purifying respirators.82 Using rongeurs and chisels instead of power instruments is recommended during surgical exposure.82 And avoiding the use of nasal pledgets, the removal of which may stimulate gagging or coughing in the postoperative phase is recommended.82 Also, use of gowns, N95/FFP3 masks, and face shelters is recommended during all outpatient nasal endoscopies.83
Effect of COVID-19 on Neurosurgical Outcome
Because the disease is asymptomatic in some patients, COVID-19 screening is crucial in all patients before operation. In addition to protecting health care professionals, high mortality risk is present in patients with COVID-19 who have undergone surgical intervention. The University of Brescia, Italy reported that the mortality of COVID-19–positive patients with chronic subdural hematoma was 80%.84 This rate was reported as 3.7% in a control group treated before the pandemic.84 A meta-analysis including nearly 1800 patients with COVID-1985 showed that lower platelet count was associated with severe COVID-19. Thrombocytopenia can lead to rebleeding, resulting in a poor outcome. Also, in subclinical patients with COVID-19, surgical intervention could impair the immune system, leading to the emergence of COVID-19 disease.86 , 87 Interstitial pneumonia progression after surgical intervention may worsen the outcome. A conservative strategy should be preferred whenever surgery can be postponed.84
This situation may be different for babies and children. The general observation is that newborns, infants, and children are relatively resistant to COVID-19.88 A case report from Milan, Italy showed that an 8-month-old infant with complex hydrocephalus underwent 2 consequent shunt revision interventions while his nasopharyngeal swab was positive for SARS-CoV-2.89 The baby, who underwent 2 operations under general anesthesia without respiratory complications, showed a favorable neurologic course.89
Academic and Educational Activities
Web-based conferencing systems have emerged and reached primacy.5 , 90 All in-person conferences such as resident education lectures, multidisciplinary board meetings, and weekly morbidity and mortality conferences should be converted to video teleconferences, with an individual person participating in the conference from one site.47 , 91 , 92 Many elements of medical students' lectures may be converted into virtual webinars.93 Involving students interested in neurosurgery in departmental educational video teleconferences would intensify student learning and provide accessibility of the department to students.93
Experiences from Different Countries During COVID-19 Pandemic
Our country, Turkey, is among the 10 countries most affected by the pandemic. In Turkey, the pandemic burden is being managed by collaboration of state and private health institutions. A substantial or all part of the many hospitals were modified to COVID-19 wards. When necessary, some operating rooms were used as intensive care units. During the pandemic, many neurosurgeons have attended in the front lines. Urgent and emergent surgery is performed and the schedule for elective procedures has been postponed. In India, one of the most affected countries in Asia, a consensus was suggested for neurointerventional teams to switch their coverage model, including cycles of 14 days of work and 14 days of self-isolation.94 In this consensus statement, the categorization of the patients based on priority and postponing nonessential elective surgeries and outpatient visits are advocated.94 In a report of the experience from Iran, one of the 10 most affected countries, it was reported that outpatient clinics had been shut down, elective surgeries were cancelled or postponed, and neurosurgery residents were reassigned to COVID-19 wards.95 According to the Iran University of Medical Sciences and Health Services experience, a significant decrease (56%) was noticed in elective and emergency neurologic surgery.95 A report of the experience from Germany declared that 64.4% decrease was observed in spine cases according to baseline (2019) levels.96
Conclusions
With the increasing burden of the COVID-19 pandemic worldwide, the need for various modifications to neurosurgical practice will continue. During the pandemic, strict measures are essential for both medical staff safety and patient care. In this study, we outline substantial information and recommendations for daily outpatient and inpatient practice, management of surgical cases, and additional strategies in the circumstances of the COVID-19 pandemic. The data summarized are beneficial for documentation of measures in terms of both the ongoing pandemic and future outbreaks. The common principles defined in this review should be considered in the light of the available resources and the local burden surge of the COVID-19 pandemic.
Acknowledgments
The authors would like to thank to Mrs. Senay Guner Ozoner for her illustrative figure work.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai C.C., Wang C.Y., Wang Y.H., Hsueh S.C., Ko W.C., Hsueh P.R. Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int J Antimicrob Agents. 2020;55:105946. doi: 10.1016/j.ijantimicag.2020.105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandel N., Chungong S., Omaar A., Xing J. Health security capacities in the context of COVID-19 outbreak: an analysis of International Health Regulations annual report data from 182 countries. Lancet. 2020;395:1047–1053. doi: 10.1016/S0140-6736(20)30553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(WHO) WHO Coronavirus disease 2019 (COVID-19) Situation Report–99. WHO Bull. 2020;14:e01218. [Google Scholar]
- 5.Kondziolka D., Couldwell W.T., Rutka J.T. Introduction. On pandemics: the impact of COVID-19 on the practice of neurosurgery [e-pub ahead of print] J Neurosurg. 2020:1–2. doi: 10.3171/2020.3.JNS201007. accessed May 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin-hanjani S., Bambakidis N.C., Ii F.G.B. COVID-19 and neurosurgical practice: an interim report [e-pub ahead of print] https://doi.org/10.3171/2020.4.JNS201099 J Neurosurg. accessed May 3, 2020. [DOI] [PMC free article] [PubMed]
- 7.Fontanella M., Saraceno G., Lei T. Neurosurgical activity during COVID-19 pandemic: an expert opinion from China , South Korea , Italy , United Stated of America , Colombia and United Kingdom [e-pub ahead of print] https://doi.org/10.23736/S0390-5616.20.04994-2 J Neurosurg Sci. accessed May 2, 2020. [DOI] [PubMed]
- 8.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J.F.W., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization Novel Coronavirus (2019-nCoV ) Situation Report–1 21 January 2020. WHO Bull. 2020;1:1–5. [Google Scholar]
- 11.Gorbalenya A.E., Baker S.C., Baric R.S. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization Coronavirus disease 2019 (COVID-19) Situation Report–23. WHO Bull. 2020;14:e01218. [Google Scholar]
- 13.Zhou P., Lou Yang X., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu F., Zhao S., Yu B. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C., Liu Y., Yang Y. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauer S.A., Grantz K.H., Bi Q. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L., Huang T., Wang Y. Novel coronavirus patients' clinical characteristics, discharge rate and fatality rate of meta-nalysis. J Med Virol. 2020;92:577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nassif X., Bourdoulous S., Eug E., Couraud P., Nassif X. How do extracellular pathogens cross the blood–brain barrier? Trends Microbiol. 2002;10:227–232. doi: 10.1016/s0966-842x(02)02349-1. [DOI] [PubMed] [Google Scholar]
- 22.Desforges M., Le Coupanec A., Stodola J.K., Meessen-Pinard M., Talbot P.J. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;194:145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCray P.B., Pewe L., Wohlford-Lenane C. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81:813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J., Zhong S., Liu J. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine Mig in pathogenesis. Clin Infect Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beyrouti R., Adams M.E., Benjamin L. Characteristics of ischaemic stroke associated with COVID-19 [e-pub ahead of print] https://doi.org/10.1136/jnnp-2020-323586 J Neurol Neurosurg Psychiatry. accessed May 2, 2020. [DOI] [PMC free article] [PubMed]
- 29.Sun T., Guan J. Novel coronavirus and central nervous system [e-pub ahead of print] https://doi.org/10.1111/ene.14227 Eur J Neurol. accessed April 30, 2020. [DOI] [PubMed]
- 30.Kiroğlu Y., Calli C., Yunten N. Diffusion-weighted MR imaging of viral encephalitis. Neuroradiology. 2006;48:875–880. doi: 10.1007/s00234-006-0143-7. [DOI] [PubMed] [Google Scholar]
- 31.Costerus J.M., Lemmens C.M.C., van de Beek D., Brouwer M.C. Cranial imaging and lumbar puncture in patients with suspected central nervous system infection. Clin Infect Dis. 2020;70:2469–2475. doi: 10.1093/cid/ciz694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costerus J.M., Brouwer M.C., van de Beek D. Technological advances and changing indications for lumbar puncture in neurological disorders. Lancet Neurol. 2018;17:268–278. doi: 10.1016/S1474-4422(18)30033-4. [DOI] [PubMed] [Google Scholar]
- 33.Solomon T., Hart I.J., Beeching N.J. Viral encephalitis: a clinician’s guide. Pract Neurol. 2007;7:288–305. doi: 10.1136/jnnp.2007.129098. [DOI] [PubMed] [Google Scholar]
- 34.Kumar G., Kalita J., Misra U.K. Raised intracranial pressure in acute viral encephalitis. Clin Neurol Neurosurg. 2009;111:399–406. doi: 10.1016/j.clineuro.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Pérez-Bovet J., Garcia-Armengol R., Buxó-Pujolràs M. Decompressive craniectomy for encephalitis with brain herniation: case report and review of the literature. Acta Neurochir (Wien) 2012;154:1717–1724. doi: 10.1007/s00701-012-1323-3. [DOI] [PubMed] [Google Scholar]
- 36.Pesce A., Palmieri M., Armocida D., Frati A., Santoro A. Neurosurgery and coronavirus (COVID-19) epidemic: doing our part. Neurosurgery. 2020;87:E48–E49. doi: 10.1093/neuros/nyaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke J.F., Chan A.K., Mummaneni V. Letter: The coronavirus disease 2019 global pandemic: a neurosurgical treatment algorithm. Neurosurgery. 2020;87:E50–E56. doi: 10.1093/neuros/nyaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zoia C., Bongetta D., Veiceschi P. Neurosurgery during the COVID-19 pandemic: update from Lombardy, northern Italy. Acta Neurochir (Wien) 2020;162:1221–1222. doi: 10.1007/s00701-020-04305-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veiceschi P., Brembilla C., Bernucci C. Effects of COVID-19 outbreak in Northern Italy. Perspectives from Bergamo Neurosurgery Department. World Neurosurg. 2020;137:465–468. doi: 10.1016/j.wneu.2020.03.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cenzato M., DiMeco F., Fontanella M., Locatelli D., Servadei F. Neurosurgery in the storm of COVID-19: suggestions from the Lombardy region, Italy (ex malo bonum) [e-pub ahead of print] https://doi.org/10.1001/jama.2020.4031 J Neurosurg. accessed May 1, 2020. [DOI] [PMC free article] [PubMed]
- 41.Perin A., Servadei F., DiMeco F., "Hub and Spoke" Lombardy Neurosurgery Group May we deliver neuro-oncology in difficult times (e.g. COVID-19)? J Neurooncol. 2020;148:203–205. doi: 10.1007/s11060-020-03496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agosti E., Giorgianni A., Pradella R., Locatelli D. Coronavirus disease 2019 (COVID-19) outbreak: single-center experience in neurosurgical and neuroradiologic emergency network tailoring. World Neurosurg. 2020;138:548–550. doi: 10.1016/j.wneu.2020.04.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Y., Mao Y. Response to COVID-19 in Chinese neurosurgery and beyond [e-pub ahead of print] https://doi.org/10.3171/2020.3.JNS20929.2 accessed April 30, 2020. [DOI] [PMC free article] [PubMed]
- 44.Robertson F.C., Lippa L., Broekman M.L.D. Editorial: Task shifting and task sharing for neurosurgeons amidst the COVID-19 pandemic [e-pub ahead of print] https://doi.org/10.3171/2020.4.JNS201056 J Neurosurg. accessed April 30, 2020. [DOI] [PMC free article] [PubMed]
- 45.Choi B.D. A neurosurgery resident’s response to COVID-19: anything but routine [e-pub ahead of print] https://doi.org/10.3171/2020.4.JNS201028 J Neurosurg. accessed April 29, 2020. [DOI] [PMC free article] [PubMed]
- 46.Iii J.C.W., Grant G., Krieger M.D. Early lessons in the management of COVID-19 for the pediatric neurosurgical community from the leadership of the American Society of Pediatric Neurosurgeons [e-pub ahead of print] https://doi.org/10.3171/2020.3.PEDS20215.2 J Neurosurg Pediatr. accessed April 28, 2020. [DOI] [PMC free article] [PubMed]
- 47.Arnaout O., Patel A., Carter B., Chiocca E.A. Letter: Adaptation under fire: two Harvard neurosurgical services during the COVID-19 pandemic [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa146 Neurosurgery. accessed April 28, 2020. [DOI] [PMC free article] [PubMed]
- 48.Awadallah M., Janssen F., Körber B., Breuer L., Scibor M., Handschu R. Telemedicine in general neurology: interrater reliability of clinical neurological examination via audio-visual telemedicine. Eur Neurol. 2019;80:289–294. doi: 10.1159/000497157. [DOI] [PubMed] [Google Scholar]
- 49.Szmuda T., Ali S., Słoniewski P. Telemedicine in neurosurgery during the novel coronavirus (COVID-19) pandemic. Polish J Neurol Neurosurg. 2020;54:1–2. doi: 10.5603/PJNNS.a2020.0038. [DOI] [PubMed] [Google Scholar]
- 50.Greven A.C.M., Rich C.W., Malcolm J.G. Letter: Neurosurgical management of spinal pathology via telemedicine during the COVID-19 pandemic: early experience and unique challenges [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa165 Neurosurgery. accessed April 28, 2020. [DOI] [PMC free article] [PubMed]
- 51.Santos E., Pailler J.I., Beynon C., Damaty A El. Letter: The use of absorbable sutures in neurosurgical procedures in the time of COVID-19 [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa181 Neurosurgery. accessed May 3, 2020. [DOI] [PMC free article] [PubMed]
- 52.Tingbo L., editor. Handbook of COVID-19 Prevention and Treatment. The First Affiliated Hospital, Zhejiang University School of Medicine; Zhejiang, China: 2020. [Google Scholar]
- 53.Dobran M., Paracino R., Iacoangel M. Letter to the editor by Dobran Mauro, Paracino Riccardo, and Iacoangeli Maurizio regarding "Neurosurgery during the COVID-19 pandemic: update from Lombardy, northern Italy". Acta Neurochir (Wien) 2020;162:1221–1222. [Google Scholar]
- 54.Borsa S., Bertani G., Pluderi M., Locatelli M. Our darkest hours (being neurosurgeons during the COVID-19 war) Acta Neurochir (Wien) 2020;162:1227–1228. doi: 10.1007/s00701-020-04333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jean W.C., Ironside N.T., Sack K.D., Felbaum D.R., Syed H.R. The impact of COVID-19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien) 2020;162:1229–1240. doi: 10.1007/s00701-020-04342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giorgi P.D., Villa F., Gallazzi E. The management of emergency spinal surgery during the COVID-19 pandemic in Italy. Bone Joint J. 2020;102:1–6. doi: 10.1302/0301-620X.102B6.BJJ-2020-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muhammad S., Tanikawa R., Lawton M.T., Niemelä M., Hänggi D. Safety instructions for neurosurgeons during COVID-19 pandemic based on recent knowledge and experience [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa184 Neurosurgery. accessed May 3, 2020. [DOI] [PMC free article] [PubMed]
- 58.Brettler S., Tuanquin L., Zacharia B.E., Letter Isildak H. COVID-19 pandemic: safety precautions for stereotactic radiosurgery [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa163 Neurosurgery. accessed April 30, 2020. [DOI] [PMC free article] [PubMed]
- 59.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 61.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rothe C., Schunk M., Sothmann P. Transmission of 2019-NCOV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu Y.J., Zhang J., Chen Z. Experiences of practicing surgical neuro-oncology during the COVID-19 pandemic. J Neurooncol. 2020;148:199–200. doi: 10.1007/s11060-020-03489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X., Wang M.-J., Jiang X.-B., Wang H.-J., Zhao H.-Y. Letter: Strategies for prevention and control of 2019 novel coronavirus infection among medical staff. Neurosurgery. 2020;87:E57–E62. doi: 10.1093/neuros/nyaa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tong X., Yang Y. Lessons learned: special precautions for performing emergency cerebrovascular procedures amid the COVID-19 pandemic [e-pub ahead of print] https://doi.org/10.3171/2020.4.JNS201018.2 J Neurosurg. accessed April 30, 2020. [DOI] [PMC free article] [PubMed]
- 66.Ti L.K., Ang L.S., Foong T.W., Ng B.S.W. What we do when a COVID-19 patient needs an operation: operating room preparation and guidance. Can J Anesth. 2020;67:756–758. doi: 10.1007/s12630-020-01617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grelat M., Pommier B., Portet S. Patients with coronavirus 2019 (COVID-19) and surgery: guidelines and checklist proposal [e-pub ahead of print] https://doi.org/10.1016/j.wneu.2020.04.155 World Neurosurg. accessed April 30, 2020. [DOI] [PMC free article] [PubMed]
- 68.Cheung J.C.H., Ho L.T., Cheng J.V., Cham E.Y.K., Lam K.N. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir Med. 2020;8:e19. doi: 10.1016/S2213-2600(20)30084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng P.W.H., Ho P.L., Hota S.S. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020;124:e497–e501. doi: 10.1016/j.bja.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wen J., Qi X., Lyon K.A. Lessons from China when performing neurosurgical procedures during the coronavirus disease 2019 (COVID-19) pandemic. World Neurosurg. 2020;138:e955–e960. doi: 10.1016/j.wneu.2020.04.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perdelli F., Spagnolo A.M., Cristina M.L. Evaluation of contamination by blood aerosols produced during various healthcare procedures. J Hosp Infect. 2008;70:174–179. doi: 10.1016/j.jhin.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 72.Johnson G.K., Robinson W.S. Human immunodeficiency virus-1 (HIV-1) in the vapors of surgical power instruments. J Med Virol. 1991;33:47–50. doi: 10.1002/jmv.1890330110. [DOI] [PubMed] [Google Scholar]
- 73.Chang L., Yan Y., Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev. 2020;34:75–80. doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chan D.Y.C., Chan D.T.M., Mak W.K., Wong G.K.C., Poon W.S. Letter: Rongeurs, neurosurgeons, and COVID-19: how do we protect health care personnel during neurosurgical operations in the midst of aerosol-generation from high-speed drills [e-pub ahead of print]? https://doi.org/10.1093/neuros/nyaa139 Neurosurgery. accessed April 30, 2020. [DOI] [PMC free article] [PubMed]
- 76.Jenkins A. Letter. Transmission of COVID-19 during neurosurgical procedures — some thoughts. Neurosurgery. 2020;87:E68. doi: 10.1093/neuros/nyaa126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barrett W.L., Garber S.M. Surgical smoke–a review of the literature. Is this just a lot of hot air? Surg Endosc Other Interv Tech. 2003;17:979–987. doi: 10.1007/s00464-002-8584-5. [DOI] [PubMed] [Google Scholar]
- 78.Hallmo P., Naess O. Laryngeal papillomatosis with human papillomavirus DNA contracted by a laser surgeon. Eur Arch Otorhinolaryngol. 1991;248:425–427. doi: 10.1007/BF01463570. [DOI] [PubMed] [Google Scholar]
- 79.Gloster H.M., Roenigk R.K. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436–441. doi: 10.1016/0190-9622(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 80.Vukkadala N., Qian Z.J., Holsinger F.C., Patel Z.M., Rosenthal E. COVID-19 and the otolaryngologist–preliminary evidence-based review [e-pub ahead of print] https://doi.org/10.1002/lary.28672 Laryngoscope. accessed April 27, 2020. [DOI] [PubMed]
- 81.Zhu W., Zhao H., Jiang X. A COVID-19 patient who underwent endonasal endoscopic pituitary adenoma resection: a case report [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa147 Neurosurgery. accessed May 3, 2020. [DOI] [PMC free article] [PubMed]
- 82.Lo Y.T., Wei N., Teo Y., Ang T. Endonasal neurosurgery during the COVID-19 pandemic: the Singapore perspective [e-pub ahead of print] https://doi.org/10.3171/2020.4.JNS201036 J Neurosurg. accessed May 2, 2020. [DOI] [PMC free article] [PubMed]
- 83.Patel Z.M., Fernandez-Miranda J., Hwang P.H. Letter: Precautions for endoscopic transnasal skull base surgery during the COVID-19 pandemic. Neurosurgery. 2020;87:E66–E67. doi: 10.1093/neuros/nyaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Panciani P.P., Saraceno G., Zanin L., Renisi G., Signorini L., Fontanella M.M. Letter: COVID-19 infection affects surgical outcome of chronic subdural hematoma [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa140 accessed May 3, 2020. [DOI] [PMC free article] [PubMed]
- 85.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oâ Dwyer M.J., Owen H.C., Torrance H.D.T. The perioperative immune response. Curr Opin Crit Care. 2015;21:336–342. doi: 10.1097/MCC.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 87.Torrance H.D.T., Pearse R.M., O’Dwyer M.J. Does major surgery induce immune suppression and increase the risk of postoperative infection? Curr Opin Anaesthesiol. 2016;29:376–383. doi: 10.1097/ACO.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 88.Hong H., Wang Y., Chung H.T., Chen C.J. Clinical characteristics of novel coronavirus disease 2019 (COVID-19) in newborns, infants and children. Pediatr Neonatol. 2020;61:131–132. doi: 10.1016/j.pedneo.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carrabba G., Tariciotti L., Guez S., Calderini E., Locatelli M. Correspondence: Neurosurgery in an infant with COVID-19. Lancet. 2020;6736:30927. doi: 10.1016/S0140-6736(20)30927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carter B.S., Chiocca E.A. Editorial. COVID-19 and academic neurosurgery [e-pub ahead of print] https://doi.org/10.3171/2020.4.JNS201013 J Neurosurg. accessed May 3, 2020. [DOI] [PMC free article] [PubMed]
- 91.Eichberg D.G., Shah A.H., Luther E.M. Letter: Academic Neurosurgery Department Response to COVID-19 Pandemic: The University of Miami/Jackson Memorial Hospital. Neurosurg. 2020;87:E63–E65. doi: 10.1093/neuros/nyaa118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bray D.P., Stricsek G.P., Malcolm J. Letter : Maintaining neurosurgical resident education and safety during the COVID-19 pandemic [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa164 Neurosurgery. accessed May 2, 2020. [DOI] [PMC free article] [PubMed]
- 93.Chae J.K., Haghdel A., Guadix S.W. Letter: COVID-19 impact on the medical student path to neurosurgery [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa187 Neurosurgery. accessed May 2, 2020. [DOI] [PMC free article] [PubMed]
- 94.Gupta P., Muthukumar N., Rajshekhar V. Neurosurgery and Neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68:246–254. doi: 10.4103/0028-3886.283130. [DOI] [PubMed] [Google Scholar]
- 95.Khosravi M.H., Sisakht A.M., Kiani D., Ahmadi S. Effects of COVID-19 pandemic on neurological surgery care and education; our experience from Iran. World Neurosurgery. 2020;139:376. doi: 10.1016/j.wneu.2020.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mehta A.I., Chiu R.G. COVID-19 Non-essential surgery restrictions and spine surgery : a German experience [e-pub ahead of print] https://doi.org/10.1097/BRS.0000000000003571 Spine (Phila Pa 1976) accessed May 19, 2020. [DOI] [PMC free article] [PubMed]


