To the Editor
Since January 2020 when it was first isolated in China, coronavirus disease 2019 (COVID-19) has spread throughout the world and caused substantial morbidity and mortality.[1] Despite the rapidly growing knowledge base on the clinical course of the disease, no therapeutic agents have been proven to be effective for COVID-19. Further clarification of the clinical course of the disease could help in the development of effective treatment strategies. Wang and colleagues in their recent elegant study to investigate characteristics and prognostic factors in 339 elderly patients with COVID-19, observed a high proportion of severe and critical cases as well as high fatality rates.[2] Common complications included bacterial infection, acute respiratory distress syndrome as well as liver enzyme abnormalities. In their analyses to explore prognostic factors for fatal outcomes, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (known markers of liver injury) were not found to be independently associated with the risk of mortality. Though it has been reported that liver injury is more prevalent in severe cases of COVID-19,[3, 4] whether circulating levels of markers of liver injury at admission could predict clinical outcomes in COVID-19 patients is uncertain. In this context, we aimed to determine the nature of the relationships of admission levels of five main markers of liver injury (ALT, AST, gamma-glutamyltransferase (GGT), alkaline phosphatase (ALP) and total bilirubin) with the risk of clinical outcomes in patients with COVID-19 using a systematic meta-analysis.
We conducted this review using PRISMA and MOOSE guidelines (Supplementary Materials 1-2) and in accordance with a registered protocol in the PROSPERO International prospective register of systematic reviews (CRD42020183672). MEDLINE, Embase, and The Cochrane library were searched from 2019 to 17 May 2020 for published studies reporting on relationships between admission levels of markers of liver injury (GGT, ALT, AST, ALP and total bilirubin) and clinical outcomes in patients with COVID-19. The detailed search strategy has been reported in Supplementary Material 3. Outcomes were categorised into severe illness and mortality. Mean differences (95% CIs) for comparing mean levels of circulating markers across outcomes and relative risks (RRs) (95% confidence intervals, CIs) for associations between markers and outcomes were used as summary measures across studies.[5] The inverse variance-weighted method was used to effect estimates using random-effects models to minimize the effect of heterogeneity. STATA release MP 16 (StataCorp LP, College Station, TX, USA) was used for all statistical analyses.
Sixteen retrospective cohort studies comprising 10,540 COVID-19 patients were eligible (Table 1 ; Supplementary Materials 4-5). All studies were based in China. The average age at baseline ranged from approximately 38 to 71 years. Comparing elevated vs low levels of ALT and AST respectively, the RRs (95% CIs) of severe illness were 1.03 (0.23-2.15) and 2.09 (0.44-9.9) respectively. Pooled analyses of 9 studies each showed significantly higher levels of ALT and AST in COVID-19 patients who developed severe illness compared to patients who did not deveop severe illness: mean differences (95% CIs) of 9.15 U/L (1.47, 16.82; p=0.02) and 12.60 U/L (8.43, 16.77; p<0.001) respectively (Fig. 1 A)
Fig. 1.
(Continued)
Table 1.
Baseline characteristics of included studies of COVID-19 patients
| Author, year ofpublication | Source of participants | Country | Date of datacollection | Mean/medianAge (yrs) | Male % | Total participants | No. of outcomes | Outcomes | NOSscore |
|---|---|---|---|---|---|---|---|---|---|
| Zhou, 2020 | Jinyintan Hospital and Wuhan Pulmonary Hospital | China | Dec 2019 - Jan 2020 | 56.0 | 62.0 | 191 | 54 | In-hospital mortality | 5 |
| Huang, 2020 | Jin Yintan Hospital | China | Dec 2019 - Jan 2020 | 49.0 | 73.0 | 41 | 13 | ICU care | 4 |
| Ruan, 2020 | Jin Yin-tan Hospital and Tongji Hospital | China | NR | 57.7 | 68.0 | 150 | 68 | Mortality | 4 |
| Guan, 2020 | National Health Commission | China | Dec 2019 - Jan 2020 | 47.0 | 58.1 | 1099 | 173 (67) | Severe disease (Composite outcome of ICU admission, the use of mechanical ventilation, or death) | 4 |
| Liu, 2020 | 3 tertiary hospitals in Wuhan | China | Dec 2019 - Jan 2020 | 38.0 | 50.0 | 78 | 11 | Severe disease | 5 |
| Qian, 2020 | 5 hospitals in Zhejiang province | China | Jan - Feb 2020 | 50.0 | 40.7 | 91 | 9 | Severe disease | 4 |
| Zheng, 2020 | North Hospital of Changsha first Hospital | China | Jan - Feb 2020 | 45.0 | 49.7 | 161 | 30 | Severe disease | 4 |
| Wang, 2020 | Zhongnan Hospital of Wuhan University | China | Jan, 2020 | 56.0 | 54.3 | 138 | 36 | ICU care | 4 |
| Wang, 2020b | Union Hospital in Wuhan | China | Jan - Feb 2020 | 42.0 | 46.0 | 69 | 14 | SpO2<90% | 4 |
| Wang, 2020c | Renmin Hospital of Wuhan University | China | Jan – Feb 2020 | 71.0 | 49.0 | 339 | 65 | Mortality | 4 |
| Chen, 2020 | Tongji Hospital in Wuhan | China | Jan - Feb 2020 | 62.0 | 62.0 | 274 | 113 | Mortality | 4 |
| Chen, 2020b | National Health Commission | China | Dec 2019 - Jan 2020 | NR | NR | 1,590 | 50 | Mortality | 6 |
| Cai, 2020 | Third People's Hospital of Shenzhen | China | Jan - Feb 2020 | 47.0 | 47.5 | 417 | 91 | Severe disease | 6 |
| Yang, 2020 | Wuhan Jin Yin-tan hospital | China | Dec 2019 – Jan 2020 | 59.7 | 67.0 | 52 | 32 | Mortality | 4 |
| Lei, 2020 | 10 hospitals in Hubei Province | China | Dec 2019 – Mar 2020 | 56.0 | 47.2 | 5,771 | 1,186 | Severe disease | 5 |
| Xie, 2020 | Jinyintan Hospital | China | Feb 2020 | 60.0 | 55.7 | 79 | 28 | Severe disease | 4 |
ICU, intensive care unit; NOS, Newcastle-Ottawa Scale; NR, not reported
Fig. 1.
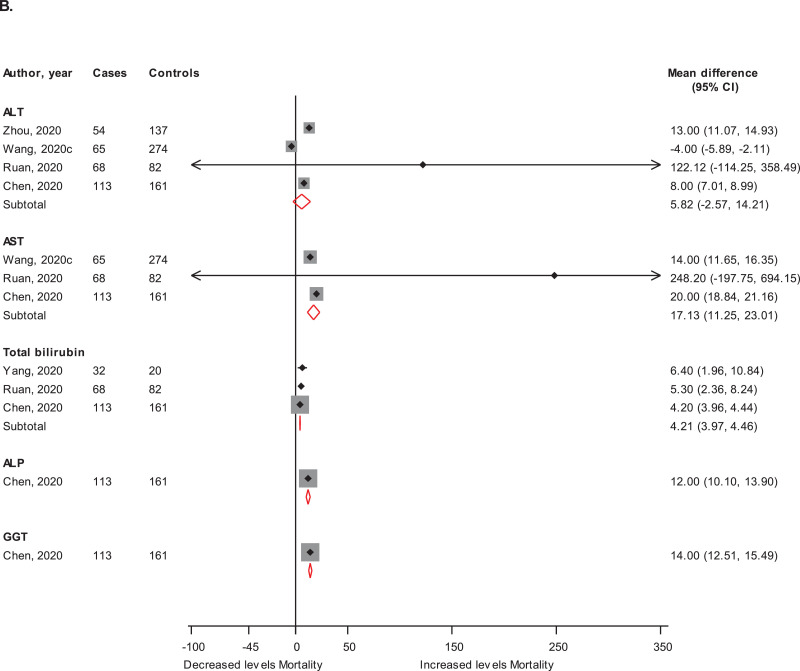
Admission levels of markers of liver injury in (A) patients with or without severe COVID-19 illness and in (B) patients who died or survived ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; CI, confidence interval (bars); GGT, gamma-glutamyltransferase
In pooled results of two studies each, the RRs (95% CIs) of mortality associated with elevated ALT and AST were 3.35 (2.37-4.75) and 10.42 (7.05-15.40) respectively. In results from single studies, increased levels of ALP and total bilirubin were each associated with an increased risk of mortality (Supplementary Material 6). Admission levels of AST and total bilirubin were higher in those who died; whereas ALT levels were not significantly different in both groups: mean differences (95% CIs) of 17.13 U/L (11.25, 23.01; p<0.001); 4.21 µmol/l (3.97, 4.46; p<0.001) and 5.82 U/L (-2.57, 14.21; p=0.17) respectively. In single reports, levels of ALP and GGT were higher in those who died compared with survivors (Fig. 1 B).
Taking the overall evidence together, the data supports a higher prevalence of elevated admission levels of markers of liver injury in severe or mortality due to COVID-19 disease, which suggests that patients with elevated levels of liver markers at baseline (during admission) had higher risks of developing worse outcomes in COVID-19. The likely explanation for the worse outcomes observed in patients with baseline elevated markers of liver injury (as seen in chronic liver disease) could be attributed to compromised immune status.[3, 4]
Irrespective of the fact that about 2-11% of patients with COVID-19 have liver comorbidities,[3] COVID-19 also causes liver injury. However, there is controversy regarding the causes of liver injury in COVID-19.[3, 4] Proposed explanations include (i) drug-induced liver injury; (ii) direct injury to the liver due to COVID-19 hepatitis [4]; (iii) COVID-19 induced myositis [4]; (iv) binding of SARS CoV-2 directly to angiotensin-converting enzyme 2 (ACE2) positive rich cholangiocytes and causing liver damage;[6] (v) hepatic congestion due to high levels of positive end expiratory pressure during mechanical ventilation; [4] and (vi) aggravation of liver injury by SARS CoV-2 in patients with pre-existing viral hepatitis.[7, 8] In the absence of robust association studies and formal risk prediction analyses, the overall evidence suggests that increased baseline levels of markers of liver injury could predict poor outcomes. The global prevalence of chronic liver disease remains high and continues to increase. Treatment options for COVID-19 are currently supportive; hence, there should be more intensive monitoring of levels of markers of liver injury during admission so that therapeutic approaches can be individually tailored.
There are several limitations which deserve mention. First, the heterogeneous reporting of severe illness outcomes prompted the use of composite measures. Second, the possibility of patient overlap as all 16 studies were reported from China; there have been concerns with duplicate reporting of study participants in articles.[9] Third, due to the limited sample sizes and low events, some studies were unable to assess risk ratios to quantify the relationships. Finally, though we extracted data on baseline (admission) levels of these markers, studies were not very specific regarding the exact time of blood sampling in relation to the disease status; hence, these results may have some biases.
In conclusion, elevated admission levels of markers of liver injury particularly the aminotransferases, may be associated with progression to severe disease or death in COVID-19. Monitoring levels of these markers could assist in the optimum management of patients.
Declaration of Competing Interest
None.
Acknowledgements
SKK acknowledges support from the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care. These sources had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2020.05.045.
Appendix. Supplementary materials
References
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Mar 28PubMed PMID: 32171076. Epub 2020/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020Mar 30. PubMed PMID: 32240670. Pubmed Central PMCID: PMC7118526. Epub 2020/04/03. [DOI] [PMC free article] [PubMed]
- 3.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. PubMed PMID: 32145190. Pubmed Central PMCID: PMC7129165. Epub 2020/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020Mar 20. PubMed PMID: 32203680. Epub 2020/03/24. [DOI] [PMC free article] [PubMed]
- 5.Kunutsor SK, Apekey TA, Laukkanen JA. Association of serum total osteocalcin with type 2 diabetes and intermediate metabolic phenotypes: systematic review and meta-analysis of observational evidence. Eur J Epidemiol. 2015;30(8):599–614. doi: 10.1007/s10654-015-0058-x. AugPubMed PMID: 26085114. Epub 2015/06/19. [DOI] [PubMed] [Google Scholar]
- 6.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv. 2020:2020.02.03.931766.
- 7.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. doi: 10.1111/liv.14435. MayPubMed PMID: 32170806. Epub 2020/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, et al. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020Apr 16. PubMed PMID: 32298473. Epub 2020/04/17. [DOI] [PMC free article] [PubMed]
- 9.Bauchner H, Golub RM, Zylke J. Editorial Concern-Possible Reporting of the Same Patients With COVID-19 in Different Reports. JAMA. 2020Mar 16. PubMed PMID: 32176775. Epub 2020/03/17. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.