Abstract
Background and rationale
Some studies of hospitalized patients suggested that the risk of death and/or severe illness due to COVID-19 is not associated with the use of angiotensin-converting enzyme inhibitors (ACEIs) and/or angiotensin II receptor type 1 blockers (ARBs). Nevertheless, some controversy still exists and there is limited information of the ACEIs/ARBs effect size on COVID-19 prognosis.
Aim and Methods
We aimed to measure the effect of ACEIs and/or ARBs on COVID-19 severe clinical illness by a meta-analysis. Literature search included all studies published since the COVID-19 outbreak began (December 2019) until May 9, 2020. We analyzed information from studies that included tested COVID-19 patients with arterial hypertension as comorbidity prior to hospital admission and history of taking ACEIs, ARBs, or ACEIs/ARBs.
Results
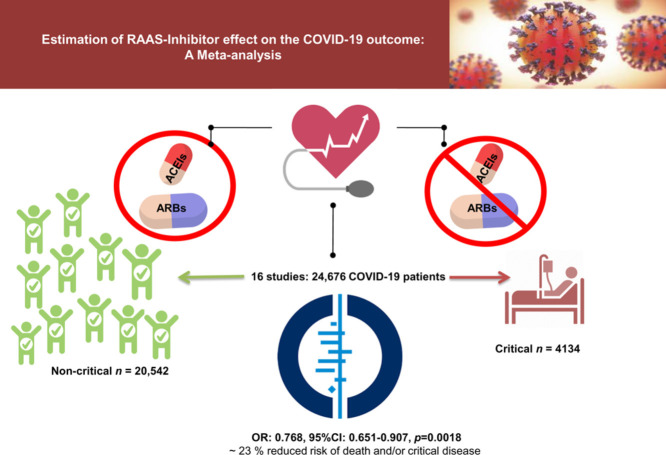
We included 16 studies that involved 24,676 COVID-19 patients, and we compared patients with critical (n = 4134) vs. non-critical (n = 20,542) outcomes. The overall assessment by estimating random effects shows that the use of ACEIs/ARBs is not associated with higher risk of in-hospital-death and/or severe illness among hypertensive patients with COVID-19 infection. On the contrary, effect estimate shows an overall protective effect of RAAS inhibitors/blockers (ACEIs, ARBs, and/or ACEIs/ARBs) with ∼ 23 % reduced risk of death and/or critical disease (OR: 0.768, 95%CI: 0.651-0.907, p=0.0018). The use of ACEIs (OR:0.652, 95%CI:0.478-0.891, p=0.0072) but not ACEIs/ARBs (OR:0.867, 95%CI:0.638-1.179, p =NS) or ARBs alone (OR:0.810, 95%CI:0.629-1.044, p=NS) may explain the overall protection displayed by RAAS intervention combined.
Conclusion
RAAS inhibitors might be associated with better COVID-19 prognosis.
Keywords: COVID-19, hypertension, diabetes, cardiovascular disease, prognosis, RAAS inhibitors, Angiotensin II-converting enzyme inhibitors, Angiotensin II receptor type 1 blockers
Graphical abstract
Introduction
Although COVID-19 pandemic is only a few months old, the magnitude of clinical information regarding the disease spectrum is overwhelming. ACE2 (angiotensin-converting enzyme 2) is presumably the host receptor of the novel SARS-CoV-2 coronavirus.1 Although the effect is significantly reduced by adjusting by age,2 arterial hypertension seems to be one of the most common risk factor associated with COVID-19 mortality,3 , 4. In fact, 56.6% of a case series of 5700 patients with COVID-19 admitted to 12 hospitals in New York City3 and 30% of patients with COVID-19 in Wuhan, China4 presented arterial hypertension as comorbidity.
Therefore, the effect/s of angiotensin-converting enzyme inhibitors (ACEIs) and/or angiotensin II receptor type 1 blockers (ARBs) on the clinical course of the disease have been on the top of clinical debates owing to the putative regulation of ACE2 exerted by these drugs.5
Four large studies, including hospitalized patients from Europe and USA6, 7, 8, 9 convincingly demonstrated that the risk of severe COVID-19 and/or in-hospital death among those infected is not associated with the use of ACEIs and/or ARBs. Likewise, results from large studies from Asia suggested that it is unlikely that in-hospital use of ACEIs/ARBs is associated with increased COVID-19 mortality risk.10 , 11
While the evidence shows consistent results, there is limited information on the ACEIs/ARBs effect size on the COVID-19 prognosis. Hence, the primary objective of the current study is to provide a quantitative estimation of the effect of ACEIs and/or ARBs, alone or ACEIs/ARBs (undistinct drug) on COVID-19 severe clinical illness in patients with arterial hypertension by a meta-analysis.
Methods
We followed the appropriate method for conducting a meta-analysis of observational studies (MOOSE) (Supplementary Table S1).
Search strategy
The literature search included all studies published since the COVID-19 outbreak began (December 2019) until May 9, 2020, with no country restrictions imposed. To identify studies for inclusion in the meta-analysis, we searched for published studies on PubMed, Ovid-Medline and Google Scholar using the following query: (RAAS OR ACE OR angiotensin-converting enzyme OR ACE OR ATR1 OR angiotensin II receptor type 1 OR ATR OR AGTR1 OR AGT1R) AND (inhibitor* OR blocker*) OR (ACEI* OR ARBs OR lisinopril OR fosinopril OR losartan OR irbesartan OR ramipril OR olmesartan OR perindopril OR captopril OR telmisartan) OR hypertension AND (coronavirus OR SARS OR COVID-19 OR SARS-CoV*) AND (clinical OR outcomes OR death* OR hospitalization*) AND (2019 OR 2020). Besides, we performed a search in online repositories under the following terms: “COVID-19 AND hypertension AND RAAS”.
Details of the search strategy and included studies are shown in Supplementary Figure S1. The authors (CJP and SS) reviewed all abstracts independently to determine the alignment with the eligibility criteria, or to establish the appropriateness of the research topic. If these criteria were met, the article was retrieved and reviewed in its entirety. There were no discrepancies in this process.
Inclusion and Exclusion Criteria and Data Collection
The following meta-analysis inclusion criteria were considered when assessing the eligibility of the identified studies:
Observational studies of hospitalized patients with confirmed COVID-19 infection that: 1) included COVID-19 patients with arterial hypertension as comorbidity prior to hospital admission and history of taking ACEIs, ARBs or ACEIs/ARBs (the Authors did not disclose individual drug information) at the time of COVID-19 testing, and 2) disclosed information on clinical outcomes defined as critical or fatal versus non-critical disease.
Statistical Analysis
A random effect model was adopted when summarising statistical synthesis; this model assumes that the treatment effect is not the same across all studies included in the analysis.
For each analysis, a forest plot was generated to display results.
Heterogeneity was evaluated via the Q statistic and I 2 statistic, which is a transformation of Q that estimates the percentage of the variation in effect sizes that is due to heterogeneity. As an I 2 value of 0% indicated no observed heterogeneity, greater values denoted increasing heterogeneity. Subgroup analyses were performed to determine the presence of potential heterogeneity sources. We identified characteristics that allowed the studies to be stratified into subsets with homogeneous effects. As we hypothesized that the RAAS inhibitor class, ethnicity, and peer-reviewed process may provide an important source of variability, the estimate of the average effect of the studies was additionally stratified by these moderator variables.
To identify studies yielding findings that had a disproportionately significant influence on the effect estimate, we repeated the analysis after removing one study at a time.
We performed a visual inspection of funnel plots, but publication bias was formally tested by using the Begg and Mazumdar's rank correlation test and Egger's method.
Statistical significance was assumed for p ≤ 0.05.
All calculations were performed using the Comprehensive Meta-Analysis computer program (Biostat, Englewood, NJ, USA).
Assessment of Study Quality
The quality of the studies included in the meta-analysis was assessed using The Newcastle-Ottawa Scale (NOS) (Supplementary Table S2).
Results
Study selection
Following the previously described search strategy, 29 articles were initially identified as potentially relevant for the present investigation, based on the assessment of the titles and abstracts. We excluded thirteen studies because they did not meet all the inclusion criteria (Supplementary Figure S1). Thus, the remaining 16 studies were included in the meta-analysis,3 , 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 which scored well in terms of the selection criteria, comparability of critical and non-critical COVID-19 on the basis of the design or analysis, and ascertainment of exposure (Supplementary Table S2).
Study Characteristics
We included 16 studies that involved 24,676 COVID-19 patients, and we compared patients with critical (n = 4134) vs. non-critical (n = 20,542) outcomes. The study characteristics, including the clinical criterion used for the differentiation between critical and non-critical patients, are shown in Table 1 . All 16 studies included adult patients of both sexes, mean/median estimated age ranging from 50 to >70 years. Eight studies included patients from China,10 , 11 , 15, 16, 17, 18 , 20 , 21 four studies included patients from North America,3 , 8 , 9 , 12 three studies included patients from Europe,6 , 13 , 14 and one study included data extracted from an international registry.7 Complete details of the study design and sample sizes are fully disclosed in Table 1.
Table 1.
Characteristics of the studies included in the meta-analysis
| Author, Country. Journal | Diagnosis of COVID-19 (details as specified by the authors) | Setting and study design. | Total sample size (n) | Inclusion criterion for critical or fatal infection | Non-severe/ Death or critical illness (n) |
|---|---|---|---|---|---|
| Mancia G, Italy. NEJM |
Positive nasopharyngeal swab specimens tested with real-time reverse-transcriptase–polymerase-chain-reaction assays | Population-based case–control study of patients older than 40 years; Lombardy region. | 6272 | Received assisted ventilation or died | 5655/617 |
| Mehra MR Asia, Europe, and North America. NEJM |
Positive result on high throughput sequencing or real-time reverse-transcriptase–PCR assay of nasal or pharyngeal swab specimens. | Data extracted from an international registry involving 169 hospitals in 11 countries. | 8910 | Recorded in the registry as having died in the hospital | 8395/515 |
| Reynolds HR North America. NEJM |
Positive for SARS-CoV-2 RNA | Observational study; inpatients in the NYU Langone Health system. | 2408 | Admission to the intensive care unit; use of invasive or noninvasive mechanical ventilation, or death. | 1195/1213 |
| Li J China. JAMA Cardiol |
Real-time reverse transcription polymerase chain reaction |
Retrospective, single-center case series Central Hospital of Wuhan (Hubei Province, China) | 362 | One of the following: blood oxygen saturation levels of 93% or less, respiratory frequency of 30/min or greater, a partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, lung infiltrates more than 50% within 24 to 48 hours, septic shock, respiratory failure, and/or multiple organ dysfunction or failure. | 247/115 |
| Yang G China. Hypertension |
Confirmed COVID-19 according to the guideline of SARS-CoV-2 (The Fifth Trial Version of the Chinese National Health Commission) | Retrospective, single-center study. | 126 | One of the following: Respiratory failure and mechanical ventilation; shock; other organ failure that requires intensive care unit care | 83/43 |
| Zhang P China Cir Res |
Reverse transcription polymerase chain reaction according to the guideline of SARS-CoV-2 (The Fifth Trial Version of the Chinese National Health Commission) | Retrospective, multi-center study; patients aged from 18 to 74 years. | 1650 | All-cause death. ARDS and septic shock | 1288/362 |
| Andrew Ip North America. medRxiv |
Confirmed SARS-CoV-2 (methods not specified). | Retrospective, multicenter study; Hackensack Meridian Health network New Jersey. | 1129 | Death | 669/460 |
| Feng Y China. Am J Respir Crit Care Med |
Throat-swab specimens from the upper respiratory tract; real-time reverse transcription polymerase chain reaction assay. | Multi-center retrospective study involving three hospitals in Wuhan, Shanghai and Anhui. | 97 | One of the following conditions: (1) Respiratory failure and mechanical ventilation is required;(2) Shock;(3) Patients with other organ dysfunction needing intensive care unit | 62/35 |
| Guo T China. JAMA Cardiol |
Interim guidance of the World Health Organization. | Retrospective single-center case series; electronic medical records | 187 | Death | 168/19 |
| Liu Y China. medRxiv |
Guidelines of 2019-CoV infection from the National Health Commission of the People's Republic of China. | Multicentre retrospective study; medical records of three cohorts (adult patients ≥18 years old). | 46 | The guidelines of 2019-nCoV infection from the National Health Commission of the People's Republic of China. | 18/28 |
| Mehta N North America. JAMA Cardiol |
Nasopharyngeal and oropharyngeal swab specimens with SARS-CoV-2 confirmed by laboratory testing using the Centers for Disease Control and reverse transcription–polymerase chain reactionSARS-CoV-2 assay. | Retrospective cohort analysis of a prospective, observational study; Cleveland Clinic Health System in Ohio and Florida | 1705 | Patients admitted to an ICU; patients who required mechanical ventilation/ death | 1494/211 |
| Meng J China. Emerg Microbes Infect |
A commercial real-time PCR kit (GeneoDX Co., Ltd., Shanghai, China) | Retrospective analysis of medical records; Shenzhen Third People's Hospital |
42 | Guidelines established by the National Health Commission of the People's Republic of China. | 25/17 |
| Richardson S North America. JAMA |
Positive result on polymerase chain reaction testing of a nasopharyngeal sample. | Case series COVID-19 hospitalized patients; Northwell Health academic health system in New York. | 1366 | Death | 982/384 |
| Zeng Z China. medRxiv |
Clinically confirmed COVID-19 and RT-PCR assay | Single-center, retrospective, observational study (Hankou Hospital, Wuhan) | 75 | Death | 47/28 |
| Conversano A Italy. Hypertension |
Confirmed diagnosis of SARS-CoV-2 pneumonia by chest x-ray-or CT-scan and real-time PCR | Retrospective, observational study from a single tertiary center (Milan); data obtained from electronic medical records. | 96 | Non-survivors | 62/34 |
| Bean D London. medRxiv |
Inpatients testing positive for SARS-Cov2 by RT-PCR | Study cohort | 205 | Death or admission to a critical care unit for organ-support within 21 days of symptoms onset. | 152/53 |
Estimation of effect sizes of RAAS inhibitors/blockers
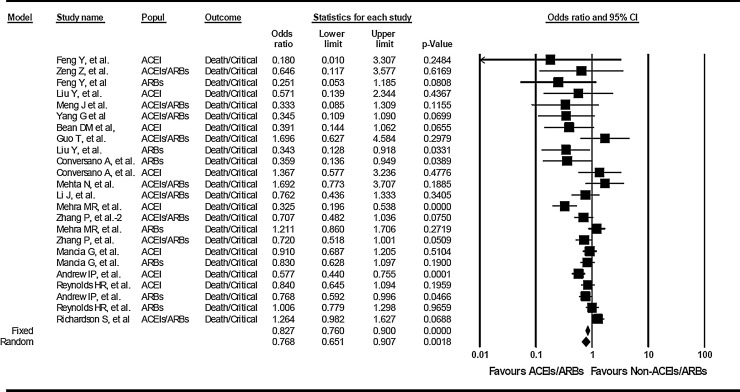
The overall assessment by estimating random effects shows that the use of ACEIs/ARBs is not associated with a higher risk of in-hospital death and/or severe illness among hypertensive patients with COVID-19 infection. On the contrary, the effect estimates show an overall protective effect by RAAS inhibition (ACEIs, ARBs, or ACEIs/ARBs) of ∼ 23 % reduced risk of death and/or critical disease (OR: 0.768, 95%CI: 0.651-0.907, p=0.0018) (Figure 1 ).
Figure 1.
Quantitative estimation of the effect of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor type 1 blockers (ARBs), alone or undistinct drug (ACEIs/ARBs) on COVID-19 severe clinical illness. Association analysis of death/critical illness vs. non-critical illness in COVID-19 patients receiving ACEIs, ARBs, or ACEIs/ARBs without discrimination. For the dichotomous variable (critical / non‐critical), the effect denotes odds ratio (OR) and corresponding 95% confidence interval (CI). Because of the presence of heterogeneity, a random effect model was adopted to estimate the pooled ORs. This model assumes that the treatment effect is not the same across all studies included in the analysis. The first author of the study is shown under the sub‐heading “study name.” Popul: indicates the use of ACEIs, ACEIs/ARBs, or ARBs. In the graph, the filled squares denote the effect of individual studies, and filled diamonds express combined fixed and random effects.
Of note, the use of ACEIs (OR:0.652, 95%CI:0.478-0.891, p=0.0072) but not ACEIs/ARBs (OR:0.867, 95%CI:0.638-1.179, p =NS) or ARBs alone (OR:0.810, 95%CI:0.629-1.044, p=NS) may confer a significant ∼ 35% reduction in the risk of death/critical disease (Supplementary Figure S2) and explain the overall protection displayed by RAAS inhibition combined.
Overall heterogeneity as assessed by the Q statistics (p = 0.0001, I2=63.1) was not mitigated by grouping studies by ACEI, ARBs, or the indistinct drug (ACEIs/ARBs) (p =0.004, I2=66.1; p =0.021, I2=59.8; p = 0.013, I2=58.6, respectively). However, stratification of studies by country of origin showed a significant heterogeneity in the results pertaining to studies involving patients from North America or Europe (p = 0.001, I2=66.8) but not from studies involving patients from China (p = 0.42, I2=2.3). Surprisingly, no significant heterogeneity was found in studies retrieved from online repositories (p = 0.42, I2=0). Conversely, the heterogeneity remained significant among all studies representing peer-reviewed contributions (p = 0.0001, I2=64.1).
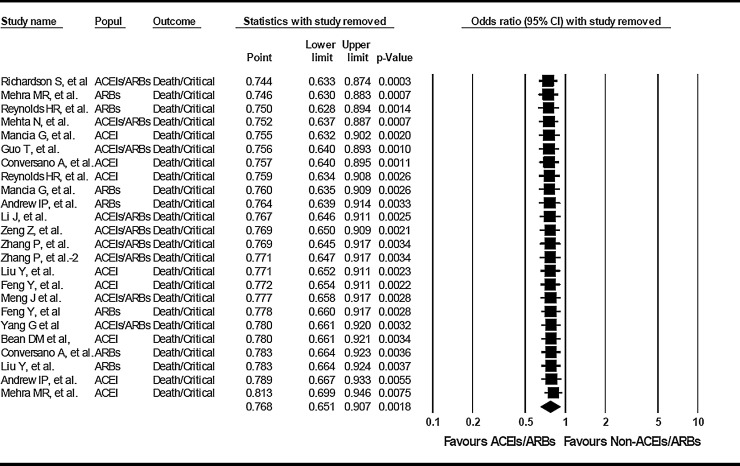
The removal of one study at a time shows robust estimation of the pooled effect (estimated ORs from 0.744 (95% CI: 0.633-0.874) to 0.813 (95% CI: 0.699-0.946), p=0.0003 to 0.0075) (Figure 2 ).
Figure 2.
Quantitative estimation of the effect of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor type 1 blockers (ARBs), alone or undistinct drug (ACEIs/ARBs) on COVID-19 severe clinical illness after removing the indicated study at a time. The first author of the removed study is shown under the sub‐heading “study name.” Popul: indicates the use of ACEIs, ACEIs/ARBs, or ARBs.
The Begg and Mazumdar's rank correlation test (Kendall`s tau -0.23, p = 0.11) shows no publication bias.
Discussion
Summary of main findings
Based upon the results yielded by a comprehensive analysis of the results reported by 16 studies, we presented robust evidence on the lack of association between the use of RAAS inhibitors/blockers and COVID-19 severe clinical illness. In addition, our findings demonstrated that the use of ACEIs/ARBs is associated with potential protective effects on the COVID-19 prognosis. The analysis focused on the estimation of the individual effect size of each group of drugs, including ACEIs, ARBs, or indistinct drug (ACEIs/ARBs) suggested that the protective effect of RAAS inhibitors against severe COVID-19 illness may be explained by the use of ACEIs. It is worth noting, however, that none of the studies included in this meta-analysis were randomized trials. Thus, many unmeasured confounding factors could not be assessed.
Furthermore, retrospective analysis and data extraction from electronic heath records might have introduced selection bias and /or treatment misclassification, which might have artificially favored the protective effects of ACEIs for over ARBs or indistinct drug (ACEIs/ARBs) against the development of severe COVID-19. Another unmeasured confounding factors, for example, obesity and severity of type 2 diabetes, are likely to influence the outcomes.
Finally, one could speculate on any key differential effect of ACEIs on the pathophysiology of severe COVID-19. Nevertheless, the lack of complete knowledge on the mechanism/s behind critical COVID-19 illness jeopardizes the plausibility of any biological hypothesis, including the question of whether the ACEIs or ARBs-mediated reduction of the angiotensin II production or the AT1R activation might explain the clinical observations. Both drug classes seem to up-regulate ACE2 expression in relevant organs,22 and its implications in COVID-19 outcomes have been largely discussed.5 , 23
There is one remarkable aspect that could not be specifically weighted in our meta-analysis, which is the analysis of comorbidities and effect sizes for the individual treatment or the co-administration of ACEIs and ARBs in elderly patients. Patients with suboptimal control of blood pressure with any of the drug classes, included those in the reference groups (non-RAAS inhibitors), might also influence the explored outcomes.
Limitations and strengths at study, outcome, and review level
Some limitations of our study, which are implicit in the studies included, have been mentioned but should be emphasized. Indeed, there are limitations and potential sources of heterogeneity imposed by the quality of the observational data. For instance, although many reports used age and sex-matched patients, potential confounders and selection bias, not only regarding the patients but also treatment comparisons could not be assessed because of insufficient information. By meta-regression, the average age of the studied populations did not explain the results, but a nondisclosure difference between the age of treated and untreated with RAAS inhibitor groups cannot be ruled out.
Notably, substantial heterogeneity was present within most studies from North America and Europe but not among studies from China. We could not identify the sources of heterogeneity among studies involving non-Asian COVID-19 patients. However, there are many potential explanations, from differences in doses of antiviral drugs and/or interventions for the treatment of severe COVID-19 to differences in recruitment and timing of outcomes measurements.
Furthermore, characteristics of the studies (for example, methodological differences in the study design), or even differences at the population level (such as unknown environmental factors and/or underlying disease comorbidities), are certainly highly important variables that may explain the heterogeneity of the dataset as a whole.
Unfortunately, as the authors of a large majority of studies included in the meta-analysis did not report the findings for male and female patients separately, we were unable to perform stratification of the results by sex. Consequently, the potential presence of sexual dimorphism could not be explored. Likewise, the effect of potential confounder risk factors, such as obesity and/or type 2 diabetes, which might probably co-exist with arterial hypertension, could not be assessed as potential source of heterogeneity because of lack of information in the original studies. Finally, the quality of the studies retrieved from online repositories might be compromised because preprints are preliminary reports of work that have not been certified by peer review. Surprisingly, sensitivity analysis of studies published in peer-reviewed journals vs. preprint reports showed no heterogeneity between the latter.
Perspectives
More studies are needed to ensure that our results can be generalizable to all populations. It seems relevant to replicate and confirm these findings in well-controlled studies with clear disclosure of co-variables to provide not only accurate clinical recommendations for patients with COVID-19 but also precise estimates of the treatment effects.
Source of funding: This study was supported by grant numbers PICT 2015-0551, and PICT 2016-0135 (Agencia Nacional de Promoción Científica y Tecnológica, FONCyT), CONICET Proyectos Unidades Ejecutoras 2017, grant number PUE 0055.
Declaration of Competing Interest
CJP: no conflict of interest to declare. SS: no conflict of interest to declare
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2020.05.052.
Appendix. Supplementary materials
References
- 1.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pirola CJ, Sookoian S. Age but not sex may explain the negative effect of arterial hyptertension and diabetes on COVID-19 prognosis. J Infect. 2020 May 11 doi: 10.1016/j.jinf.2020.05.010. pii: S0163-4453(20)30284-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.6775. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, Fan G, Liu G, Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danser AHJ, Epstein M, Batlle D. Renin-Angiotensin System Blockers and the COVID-19 Pandemic: At Present There Is No Evidence to Abandon Renin-Angiotensin System Blockers. Hypertension. 2020;75(6):1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-Angiotensin-Aldosterone System Blockers and the Risk of Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2006923. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007621. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P. Association of Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Testing Positive for Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1855. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2008975. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Wang X, Chen J, Zhang H, Deng A. Association of Renin-Angiotensin System Inhibitors With Severity or Risk of Death in Patients With Hypertension Hospitalized for Coronavirus Disease 2019 (COVID-19) Infection in Wuhan. China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1624. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J. Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ Res. 2020 doi: 10.1161/CIRCRESAHA.120.317134. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ip Andrew, K Parikh, Parrillo J, Mathura S, Hansen E, Sawczuk I, Goldberg S. Hypertension and Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. medRxiv preprint. 2020 doi: 10.1101/2020.04.24.20077388. [DOI] [Google Scholar]
- 13.Bean D, Kraljevic Z, Searle T, Bendayan R, Pickles A, Folarin A. ACE-inhibitors and Angiotensin-2 Receptor Blockers are not associated with severe SARSCOVID19 infection in a multi-site UK acute Hospital Trust. medRxiv. 2020 doi: 10.1002/ejhf.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conversano A, Melillo F, Napolano A, Fominskiy E, Spessot M, Ciceri F, Agricola E. RAAs inhibitors and outcome in patients with SARS-CoV-2 pneumonia. A case series study. Hypertension. 2020 doi: 10.1161/HYPERTENSIONAHA.120.15312. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J. COVID-19 with Different Severity: A Multi-center Study of Clinical Features. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202002-0445OC. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Huag F, Xu J, Yang P, Qin Y, Cao M. Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv preprint. 2020 doi: 10.1101/2020.03.20.20039586. [DOI] [Google Scholar]
- 18.Meng J, Xiao G, Zhang J, He X, Ou M, Bi J. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J. Effects Of ARBs And ACEIs On Virus Infection, Inflammatory Status And Clinical Outcomes In COVID-19 Patients With Hypertension: A Single Center Retrospective Study. YANG2020. Hypertension. 2020 doi: 10.1161/HYPERTENSIONAHA.120.15143. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Zeng Z, Sha T, Zhang Y, Wu F, Hu H, Li H. Hypertension in patients hospitalized with COVID-19 in Wuhan, China: A single-center retrospective observational study. medRxiv. 2020 doi: 10.1101/2020.04.06.20054825. [DOI] [Google Scholar]
- 21.Yang P, Gu H, Zhao Z, Wang W, Cao B, Lai C. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep. 2014;4:7027. doi: 10.1038/srep07027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 23.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 doi: 10.1002/ddr.21656. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.