Abstract
The interaction between tetranectin and high mobility group box-1 may be manipulated via monoclonal antibodies to improve survival in sepsis.
Sepsis is life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. Recent data indicate that the morbidity and mortality from sepsis are much greater than previously understood, as sepsis is responsible for approximately 20% of deaths worldwide [2]. In addition, forty percent of Medicare patients in the United States with septic shock die while in the hospital or within a week of discharge, and 75% die within three years [3]. The cost estimate for inpatient hospital and skilled nursing care for sepsis in U.S. Medicare beneficiaries may exceed $62 billion, more than double previous estimates [3]. Despite a robust increase in the understanding of the pathophysiology of sepsis, advances in sepsis treatment remain elusive. Current therapy for sepsis consists of antibiotics, fluid resuscitation, and supportive care [4]. Although early aggressive therapy of sepsis has been associated with decreased mortality [3], no adjunctive pharmacologic treatment has been demonstrated to improve survival. A recent meta-analysis of over 200 randomized clinical trials in critical care showed that only a tiny fraction reported a reduction in mortality [5]. Even more sobering, the very few positive trials in sepsis have generally failed to be replicated in subsequent studies. The reasons for this depressing lack of progress in translating new sepsis therapies to the bedside are assuredly multifactorial. In part, this likely arises from treating patients with a heterogeneous syndrome as having a single homogeneous disease. For instance, treating septic patients with a single agent designed to manipulate the inflammatory response presumes that all patients have either increased or decreased inflammation throughout their ICU stay– a supposition that is almost certainly incorrect. Additionally, there are both strengths and limitations to animal models of sepsis [6], and attempting to take findings from murine studies without carefully considering when and how (or if) the findings can be translated to the bedside has resulted in multiple false starts. Rather than despair, however, the sepsis field is ripe for mechanistic breakthroughs with the potential to be manipulated for ultimate patient benefit.
Tetranectin (TN) and high mobility group box-1 (HMGB1) in sepsis
A study by Chen et al. demonstrates how a bedside to bench to in vitro approach can identify unexpected biological targets that are altered in septic patients and hold notable translational promise [7]. This study reports a previously unrecognized interaction between the proteins TN and HMGB1, which is implicated in the septic response, as well as a potential means to manipulate this interaction with a monoclonal antibody.
The authors began their study in the intensive care unit with a search for differences in the blood of septic patients and healthy controls. The authors identified a 20-kDa protein – ultimately determined to be TN -- that was different between the groups. Further analysis of a modest-sized patient cohort demonstrated that TN concentrations were lower in septic adults, regardless of their age or gender. This was an unexpected finding because the link between TN and sepsis was not previously apparent. TN is a plasma protein secreted by myeloid cells. Its expression is highest in the lungs, although it is also present in the bloodstream of humans. The biologic functions of TN are largely unknown, although it has been implicated in multiple bone disorders and impaired wound healing.
Identifying a difference in a protein’s concentrations between septic patients and healthy controls cannot determine the importance of this association. Blood concentrations cannot distinguish a marker of disease from a mediator and further cannot distinguish a protein that plays a critical role from one which is redundant and can be deleted with no obvious phenotype. With this in mind, Chen et al. reverse translated their finding from the bedside to the bench and examined the role of TN in mice. Using a well-accepted preclinical model of sepsis (cecal ligation and puncture) and a model of sterile inflammation (endotoxemia), they found a similar decrease in TN concentrations to that seen in patients. To determine the functional relevance of this decrease, they performed survival experiments demonstrating that TN augmentation improved survival whereas TN knockout had the opposite effect. Further, genetic deletion of TN in septic animals exacerbated lung injury, enhanced expression of proinflammatory genes, and increased serum markers of liver injury, whereas low-dose exogenous TN conferred a survival benefit and attenuated lung and liver injury.
The authors then generated a number of polyclonal antibodies against human TN, and administration of subset of these antibodies also improved survival in septic mice. To characterize the beneficial component of the polyclonal antibodies, the authors screened a library of peptides spanning the entire sequence of human TN. This strategy led to identification of peptide P5, which forms stable α-helical epitopes either alone in synthetic peptides or being carried by TN proteins. Further work in generating monoclonal antibodies against P5 identified a smaller epitope sequence (P5-5) that is 100% identical between TN proteins in humans and numerous other species ranging from mice to monkeys. Notably, survival was improved when this monoclonal antibody was given to mice, even if it was initiated 24 hours after sepsis. The improved survival was associated with attenuation of TN depletion as well as a decrease in sepsis-induced lung and liver injury. Antibody treatment also decreased the severity of bacteremia in septic animals.
This has marked translational potential and overcomes a limitation in many murine sepsis studies. In most murine studies of sepsis, the therapeutic agent is started very shortly after sepsis is induced. This strategy has direct clinical relevance for acute diseases when the exact time of injury is known (such as trauma). However, in sepsis, patients are often sick for a number of hours or even days before presenting for care. As such, it is nearly impossible to treat patients in the earliest stages of disease, and initiating therapy in a septic patient in the first hour after recognition is likely to be similar to treating a mouse 12-24 hours after a septic insult.
TN/HMGB1-induced pyroptosis in sepsis
Mechanistically, Chen et al. then further reverse translated their findings by studying TN in vitro. Through an elegant series of experiments, the investigators determined that TN binds HMGB1 and facilitates its cellular uptake by endocytosis of TN/HMGB1 complexes. This process, in turn, induces macrophage pyroptosis .Importantly, TN appears to selectively bind to HMGB1 and does not interfere with the release of other cytokines. A P5-5-reacting monoclonal antibody prevents this TN/HMGB1 interaction and cellular uptake. Although there are multiple different ways in which inhibition of TN/HMGB1 binding and preservation of TN could potentially improve mortality, prevention of pyroptosis is a plausible mechanism. Cell death (apoptosis, necroptosis, pyroptosis) is common after sepsis and is associated with worsened prognosis, whereas preventing cell death is associated with improved outcomes in pre-clinical trials. When properly regulated, pyroptosis is an effective host defense mechanism that can minimize tissue damage. However, when pyroptosis is dysregulated, it can become a driver of the life-threatening hyperinflammatory phase of sepsis and can also promote immunosuppression by depletion of immune cells necessary for pathogen clearance. Pyroptosis occurs in response to the inflammasome -- cytosolic protein complexes that form in response to both host-derived and pathogen-derived mediators -- to release proinflammatory cytokines [8]. Through a series of complex steps, this ultimately causes activation of the gasdermin D protein, which forms plasma membrane pores, resulting in cell rupture and the release of additional inflammatory mediators. Macrophage pyroptosis in sepsis can thus result in both propagation of inflammation and ineffective pathogen elimination, in a feed-forward mechanism ultimately resulting in death of the host. An antibody that interferes with the interaction between TN and HMGB1 thus represents a rational approach to sepsis treatment by preventing downstream cell death and its resultant sequelae.
Unlike TN, HMGB1 has been studied extensively in sepsis for the past 20 years [9]. HMGB1 is an evolutionarily conserved intranuclear protein with important homeostatic roles that can also initiate and perpetuate the inflammatory response when released in response to cellular stressors. Extracellularly, HGMB1 can act as a cytokine/chemokine, which is released primarily from hepatocytes during the acute septic response. It can remain increased for weeks after the acute event and is associated with late mortality. HMGB1 release can be protective or pathologic depending on the cell it acts upon, where on the cell it acts, and any posttranslational modifications to the protein itself. For example, the function of HMGB1 in macrophages is multifaceted: extracellular HGMB1 induces release of inflammatory cytokines, whereas intracellular signaling can induce protective autophagy and promote cell survival. However, extracellular HMGB1 also binds lipopolysaccharide, a key component of the outer membrane of gram negative bacteria, and promotes its endocytosis, ultimately activating the noncanonical inflammasome pathway that culminates in pyroptosis [10].
A potential schematic for the complex interplay between TN and HMGB1 in sepsis is presented in Figure 1. Having used a bedside to bench to in vitro paradigm to arrive at this point, numerous complementary directions can be taken to translate this back to the bedside. P5-5-reacting monoclonal antibodies represent an approach that can potentially proceed to Phase I testing. However, efficacy should first be demonstrated in complementary animal models (and perhaps in the setting of co-morbidities) because no matter how strikingly positive a single murine study is, it is insufficient to proceed to human testing. Further, although human TN concentrations were similar regardless of severity of illness, age, or gender, this result was obtained in a single healthcare system in a relatively small patient population. Given the inherent heterogeneity of septic patients, repeating this analysis in a larger patient population with different infection types at different time points and at multiple sites should give additional insights into specific endotypes where TN might be a therapeutic target in sepsis. Additionally, understanding additional roles of TN (both related to and independent of HMGB1) would be important. Finally, given the discovery of the relationship between TN and HMGB1, the role of TN in other inflammatory diseases should be explored.
Fig 1. Potential consequences of TN-HMGB1 interaction in sepsis.
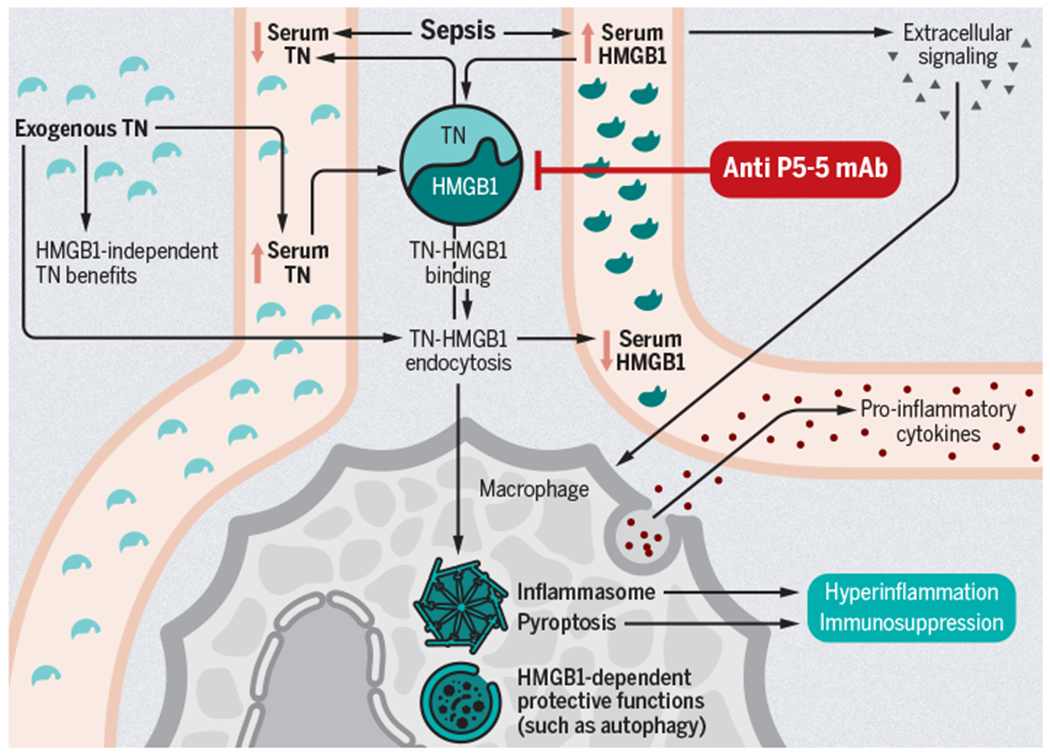
Sepsis promotes the release of HGMB1 into the serum, where it can bind TN and contribute to the decreased serum TN also seen in sepsis. Independent from TN, HGMB1 extracellular signaling can promote proinflammatory cytokine release, whereas its intracellular presence can facilitate protective autophagy or instead promote pyroptosis and further serum release of HGMB1. The HMGB1-independent functions of TN in sepsis are poorly understood but it can bind HGMB1 and promote endocytosis as a complex by macrophages, decreasing circulating availability of both proteins while also augmenting downstream consequences of intracellular HGMB1 presence, including pyroptosis. Pyroptotic macrophage death may exacerbate maladaptive hyperinflammation and may also cause immunosuppression as a result of cell loss. Anti-P5-5 TN mAb may prevent these downstream effects.
Acknowledgments
Funding: The authors’ work is supported by funding from the National Institutes of Health (GM072808, GM095442, GM104323, AA027396, AI149724).
Footnotes
Competing interests:
The authors declare that they have no competing interests
REFERENCES AND NOTES
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 2020, 395(10219):200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchman TG, Simpson SQ, Sciarretta KL, Finne KP, Sowers N, Collier M, Chavan S, Oke I, Pennini ME, Santhosh A et al. Sepsis Among Medicare Beneficiaries: 1. The Burdens of Sepsis, 2012-2018. Crit Care Med 2020, 48(3):276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 2017. [DOI] [PubMed] [Google Scholar]
- 5.Santacruz CA, Pereira AJ, Celis E, Vincent JL: Which Multicenter Randomized Controlled Trials in Critical Care Medicine Have Shown Reduced Mortality? A Systematic Review. Crit Care Med 2019, 47(12):1680–1691. [DOI] [PubMed] [Google Scholar]
- 6.Osuchowski MF, Ayala A, Bahrami S, Bauer M, Boros M, Cavaillon JM, Chaudry IH, Coopersmith CM, Deutschman CS, Drechsler S et al. Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS): An International Expert Consensus Initiative for Improvement of Animal Modeling in Sepsis. Shock 2018, 50(4):377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W; Qiang XWY, Zhu S, Li J, Babaev A, Yang H, Gong J. Becker L, Wang P, Tracey KJ, Wang H: Identification of tetranectin-targeting monoclonal antibodies to fight against lethal sepsis. Science translational medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hachim MY, Khalil BA, Elemam NM, Maghazachi AA: Pyroptosis: The missing puzzle among innate and adaptive immunity crosstalk. Journal of leukocyte biology 2020. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Liu H, Zeng Q, Imperato GH, Addorisio ME, Li J, He M, Cheng KF, Al-Abed Y, Harris HE et al. Inhibition of HMGB1/RAGE-mediated endocytosis by HMGB1 antagonist box A, anti-HMGB1 antibodies, and cholinergic agonists suppresses inflammation. Molecular medicine (Cambridge, Mass) 2019, 25(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng M, Tang Y, Li W, Wang X, Zhang R, Zhang X, Zhao X, Liu J, Tang C, Liu Z et al. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity 2018, 49(4):740–753.e747. [DOI] [PMC free article] [PubMed] [Google Scholar]


