Summary
Background
The use of aspirin in the primary prevention of cardiovascular events remains controversial. We aimed to assess the efficacy and safety of aspirin versus placebo in patients with a moderate estimated risk of a first cardiovascular event.
Methods
ARRIVE is a randomised, double-blind, placebo-controlled, multicentre study done in seven countries. Eligible patients were aged 55 years (men) or 60 years (women) and older and had an average cardiovascular risk, deemed to be moderate on the basis of the number of specific risk factors. We excluded patients at high risk of gastrointestinal bleeding or other bleeding, or diabetes. Patients were randomly assigned (1:1) with a computer-generated randomisation code to receive enteric-coated aspirin tablets (100 mg) or placebo tablets, once daily. Patients, investigators, and others involved in treatment or data analysis were masked to treatment allocation. The primary efficacy endpoint was a composite outcome of time to first occurrence of cardiovascular death, myocardial infarction, unstable angina, stroke, or transient ischaemic attack. Safety endpoints were haemorrhagic events and incidence of other adverse events, and were analysed in the intention-to-treat population. This study is registered with ClinicalTrials. gov, number NCT00501059.
Findings
Between July 5, 2007, and Nov 15, 2016, 12 546 patients were enrolled and randomly assigned to receive aspirin (n=6270) or placebo (n=6276) at 501 study sites. Median follow-up was 60 months. In the intention-to-treat analysis, the primary endpoint occurred in 269 (4·29%) patients in the aspirin group versus 281 (4·48%) patients in the placebo group (hazard ratio [HR] 0·96; 95% CI 0·81–1·13; p=0·6038). Gastrointestinal bleeding events (mostly mild) occurred in 61 (0·97%) patients in the aspirin group versus 29 (0·46%) in the placebo group (HR 2·11; 95% CI 1·36–3·28; p=0·0007). The overall incidence rate of serious adverse events was similar in both treatment groups (n=1266 [20·19%] in the aspirin group vs n=1311 [20·89%] in the placebo group. The overall incidence of adverse events was similar in both treatment groups (n=5142 [82·01%] vs n=5129 [81·72%] in the placebo group). The overall incidence of treatment-related adverse events was low (n=1050 [16·75%] vs n=850 [13·54%] in the placebo group; p<0·0001). There were 321 documented deaths in the intention-to-treat population (n=160 [2·55%] vs n=161 [2·57%] of 6276 patients in the placebo group).
Interpretation
The event rate was much lower than expected, which is probably reflective of contemporary risk management strategies, making the study more representative of a low-risk population. The role of aspirin in primary prevention among patients at moderate risk could therefore not be addressed. Nonetheless, the findings with respect to aspirin’s effects are consistent with those observed in the previously published low-risk primary prevention studies.
Funding
Bayer.
Introduction
The role of aspirin (acetylsalicylic acid) in inhibiting platelet aggregation has been well established in the secondary prevention of coronary and cerebrovascular diseases.1 The benefit of low-dose aspirin in patients with acute coronary syndromes or previous myocardial infarction, stroke, or transient ischaemic attacks is supported by more than 200 studies involving more than 200 000 patients.2 Numerous professional societies and governmental agencies recommend the use of 75–100 mg of aspirin in patient subgroups with overt cardiovascular disease with a 10-year risk of myocardial infarction or stroke that exceeds 20%.3–5
The role of aspirin in the primary prevention of myocardial infarction and stroke in groups with a moderate estimated risk of a first cardiovascular event has been controversial, despite 30 years of randomised trials. A major issue complicating the interpretation of these studies is a low but well described risk of bleeding, ranging from more common episodes of easy bruising and epistaxis, to less frequent but life-endangering gastrointestinal haemorrhage and haemorrhagic stroke. These deleterious effects of aspirin limit its use in people with low 10-year risk of myocardial infarction or stroke, or intolerance to aspirin. Six large randomised studies published from 1988 to 2005, included 100 000 participants who contributed 700 000 person-years of follow-up.6–11 Most of these patients had a 10-year risk of less than 10%. The results of these studies were generally supportive of the use of 75–150 mg aspirin per day to prevent incident myocardial infarction and stroke. Since 2005, there have been six additional studies of aspirin (81–100 mg daily) in the primary prevention setting; four have been pub-lished.12–15 These studies had less consistent results. This outcome has led to inconsistent guidelines, with recommendations both for and against aspirin use in primary prevention.4,5,16 Subsequently, a trend of decreasing aspirin use for primary prevention has been observed in the USA, including 10-year risk groups of both 10–20% and greater than 20%, presumably because of uncertainty of the risks and benefits of low-dose aspirin.17 Recent evidence about the potential benefits of aspirin in colon cancer prophylaxis also need to be considered in the overall benefit-risk assessment of low-dose aspirin use in the primary prevention setting.
We designed the Aspirin to Reduce Risk of Initial Vascular Events (ARRIVE) study to investigate the efficacy of 100 mg enteric-coated aspirin daily versus placebo in the reduction of incident myocardial infarction, stroke, and related cardiovascular conditions in people at moderate risk (defined as 10–20% 10-year coronary heart disease, with the exclusion of patients with diabetes). An additional objective of ARRIVE was to assess the safety and tolerability of aspirin in these patients in the setting of decreasing population-wide cardiovascular risk.
Research in context
Evidence before this study
Numerous studies have investigated the benefits and risks of aspirin in the acute management of cardiovascular events and in longer-term secondary prevention among people with cardiovascular disease. Fewer large-scale trials were done in the primary prevention of cardiovascular disease before the design of the ARRIVE trial. There remains a gap in the understanding of benefits and risks of aspirin use in patients at moderate risk of cardiovascular disease. We considered six key aspirin primary prevention studies before doing this study (the only published large-scale trials), which showed a reduction in risk of cardiovascular disease, largely due to a reduction in the risk of myocardial infarction among people treated with aspirin. Four additional studies of aspirin (81–100 mg daily) in the primary prevention setting have been published since the initial design of ARRIVE. These studies, and the meta-analyses that followed, were also considered in the implementation of ARRIVE and for the interpretation of the ARRIVE findings.
Added value of this study
Findings of the ARRIVE trial add to our understanding of the use of aspirin in primary prevention in several ways. First, the study was designed to assess the benefit of 100 mg per day of enteric-coated aspirin versus placebo in the reduction of incident myocardial infarction, stroke, and related cardiovascular conditions in people considered to be at moderate risk, with the exclusion of patients with diabetes. An additional objective of ARRIVE was to assess the safety and tolerability of aspirin in these patients in the setting of decreasing population-wide cardiovascular risk. Finally, ARRIVE assessed the role of aspirin with a background of modern preventive and therapeutic strategies. The findings of ARRIVE are generally consistent with the collective results of the previous primary prevention studies.
Implications of all the available evidence
While ARRIVE sought to add relevant information about the cardiovascular benefits and bleeding risks of aspirin among people at moderate cardiovascular risk, it shows some of the challenges of doing long-term prevention studies in the current era. Findings from ARRIVE are generally consistent with many other studies that tended to show aspirin’s ability to lower the risk of first non-fatal myocardial infarction without affecting the risk of total stroke. With respect to safety, as expected, rates of gastrointestinal bleeding events and some other minor bleeding events were higher in the aspirin treatment group, but there was no difference in the incidence of fatal events. The use of aspirin remains a decision that should involve a thoughtful discussion between a clinician and a patient, given the need to weigh the cardiovascular as well as possible cancer prevention benefits against the bleeding risks, patient preferences, cost, and other factors. ARRIVE contributes useful information in relation to the efficacy and safety of aspirin in the intermediate term. The ARRIVE study adds additional relevant data to the body of evidence that can help the clinician with the decision as to when to use aspirin. The ARRIVE data must be interpreted in the context of other studies, which have tended to demonstrate a reduction primarily in myocardial infarction, but less of an effect on total stroke (including both ischaemic and haemorrhagic stroke). The overall decision to use aspirin should be based on individual patient–physician discussion.
Methods
Study design and participants
The ARRIVE study is a randomised, double-blind, placebo-controlled, multicentre primary prevention study done in seven countries (Germany, Italy, Ireland, Poland, Spain, the UK, and the USA). The study setting was largely primary care offices.
Eligible male patients were aged 55 years and older and had between two and four risk factors; eligible female patients were aged 60 years or older and had three or more risk factors. Risk factors were high cholesterol (total cholesterol >200 mg/dL [5·180 mmol/L] or LDL >130 mg/dL [3·367 mmol/L] for men; total cholesterol >240 mg/dL [6·126 mmol/L] or LDL >160 mg/dL [4·144 mmol/L] for women) irrespective of current treatment, current smoking (any cigarette smoking in the past 12 months), low HDL cholesterol (<40 mg/dL), high blood pressure (systolic blood pressure >140 mm Hg), receiving medication to treat high blood pressure, and a positive family history of cardiovascular heart disease.
Participants had an average cardiovascular risk (10-year risk of coronary heart disease of 10–20%), deemed to be moderate on the basis of these risk factors. This proportion corresponds to a patient population mean 10-year cardiovascular disease risk of approximately 20–30%. The eligibility criteria were based on various European anr US risk calculators. To estimate overall cardiovascular disease risk for the study population, we assessed each risk component of the composite. Each of these assessments were combined to provide a composite estimate of study population risk. Additional details regarding estimation of risk are provided in the appendix (p 5).18–20
Patients were excluded if they had a history of a vascular event, such as stroke, myocardial infarction, coronary artery angioplasty or stenting, coronary artery bypass graft, relevant arrhythmias, congestive heart failure, or vascular intervention. Patients were also ineligible if they required antiplatelet therapy. Similar to previous studies of aspirin in primary prevention, we excluded participants at high risk of gastrointestinal and other bleeding, including those with a history of gastric or duodenal ulcers or gastrointestinal bleeding, and those requiring concomitant use of anticoagulants or frequent use of non-steroidal anti-inflammatory drugs. In light of the complexity of the various considerations relating to the effectiveness of aspirin in patients with diabetes, we decided not to include patients with diabetes in the study; people with diabetes are often considered to be at higher risk of cardiovascular disease. Additionally, a large trial of aspirin among people with diabetes was underway (NCT00110448).
The study was done according to Good Clinical Practice guidelines and under the principles detailed in the Declaration of Helsinki. The study protocol was approved by the relevant ethics committees and appropriate competent authorities in accordance with applicable laws and regulations. Before inclusion, participants provided written informed consent. The study was monitored by an independent data safety monitoring board that reviewed all data to identify any undue risk to the safety of the patients and to do planned interim efficacy analyses.
Randomisation and masking
Eligible patients were randomly assigned (1:1) to receive aspirin or placebo, according to a computer-generated randomisation code using balanced permuted blocks of treatment group allocations. Randomisation was stratified by sex and study centre. Eligible patients were selected to receive either aspirin or placebo starting at visit 2 (baseline) and the study investigator was informed of treatment assignment by an automated telephone system. Patients, investigators and their staff, the sponsor, and others involved in treating the patients or data collection and analysis were masked to the identity of the treatment. A patient for whom a primary endpoint occurred was considered to have reached the end of study.
Procedures
Patients were assigned to receive enteric-coated aspirin tablets (100 mg) or placebo tablets once daily. Follow-up was done by primary care physicians at face-to-face visits, through phone calls, and by obtaining medical records which were submitted for adjudication. Key variables were collected every 6 months during yearly visits and during yearly phone contact. Participants were followed up until their last contact; outcome ascertainment was attempted for 30 days after discontinuation.
Outcomes
The primary efficacy endpoint was a composite outcome consisting of time to first occurrence of confirmed myocardial infarction, stroke, cardiovascular death, unstable angina, or transient ischaemic attack. Myocardial infarction was confirmed if two of the three following factors were present: a consistent clinical history, electrocardiogram, or cardiac biomarkers. Time to event was defined as the number of days from the date of randomisation to the confirmed date of the event. Secondary endpoints were a composite of the time to first occurrence of cardiovascular death, myocardial infarction, or stroke; time to individual components of this composite secondary outcome; time to first occurrence of unstable angina; time to first occurrence of transient ischaemic attack; and time to and incidence of all-cause mortality. Additionally, the study provided an opportunity to examine the effects of aspirin on the incidence of all cancers in this patient population, excluding non-melanoma skin cancer. The results about effects of aspirin on cancer incidence will be reported elsewhere.
To monitor safety, adverse events were recorded throughout the study treatment period; haemorrhagic events were graded (severe, moderate, mild) according to GUSTO criteria.21 Relatedness of treatment-related adverse events was determined by site investigators.
All cases of myocardial infarction, stroke, cardiovascular death, unstable angina, transient ischaemic attack, and haemorrhagic events were adjudicated by an endpoint adjudication committee. During the study, the Executive Committee reviewed the cases while masked to treatment allocation, and determined whether the predefined criteria for the endpoint were met.
Statistical analysis
We did intention-to-treat and per-protocol analyses of the composite endpoint using a two-sided log-rank test stratified for treatment, country, and sex. We analysed the per-protocol population to assess the robustness of the data. The per-protocol population included all eligible patients who were at least 60% compliant with the study drug during their time in the study. Compliance was self-reported by patients and was assessed by investigators at each visit. We considered results to be significant and the primary objective of the study to be met if the two-sided p value was 0·05 or less. We used a Cox proportional hazards model, adjusted for country and sex, to estimate the hazard ratio (HR) and the corresponding two-sided 95% CIs for the intention-to-treat and perprotocol analyses. Because the primary efficacy variable is a composite endpoint, no adjustment for multiplicity was required. The analysis of secondary efficacy variables followed a similar survival analysis approach to that used for the primary efficacy variable. We did safety analyses using the intention-to-treat population. Safety was assessed by the incidence of observed and reported adverse events and by changes in the physical examination findings and vital signs. We compared treatment groups using the χ2 test where appropriate. We planned to study the effects of treat ment, sex, country, and hypertension on adjudicated gastrointestinal bleeding.
In a subgroup analysis, we used a Cox proportional hazards model to estimate unstratified HRs by sex, age (<65 years vs ≥65 years), smoking in past 12 months (yes vs no), body-mass index (≤25 vs >25), cardiovascular disease risk score quantile (≤10·5 vs 10·5 to ≤15·1, 15·1 to ≤21·6, >21·6), treatment compliance (yes vs no), hypertension at screening (yes vs no), hyperlipidaemia at baseline (yes vs no), use of anti-hypertensives (yes vs no), use of statins (yes vs no), and country.
Based on a log-rank test with two-sided α of 0·05 and the assumption that all patients would be followed up for a duration of 5 years, we estimated that 1488 events would provide 91% power to detect a relative risk re duction of 14·9%, assuming a placebo event rate of 13·4% and an aspirin event rate of 11·4%. With an event-driven design, a sample size of 12 000 patients (6000 per group) was expected to yield a total of 1488 events. However, because of the lower than expected event rate observed, there were several protocol amendments to expand study endpoints (we included unstable angina and transient ischaemic attack in the primary com posite endpoint), to add person-years of observation (we extended the study follow-up from 60 months to approximately 72 months, to result in a planned total exposure time of 60 000 patient-years). The trial became time-driven rather than event-driven. On the basis of these protocol amendments, the estimated event rate was changed from 2·48% to 1·5% per year, and the expected relative risk reduction was revised to 17·5%. This would provide approximately 80% power with the amount of follow-up time actually observed. This study is registered with ClinicalTrials.gov, number NCT00501059.
Role of the funding source
The funder of the study had a role in study design, data collection, data analysis, and data interpretation. The Executive Committee had access to an independent statistician, who had full access to all the data in the study, and the Executive Committee had final responsibility for writing the report and for the decision to submit for publication.
Results
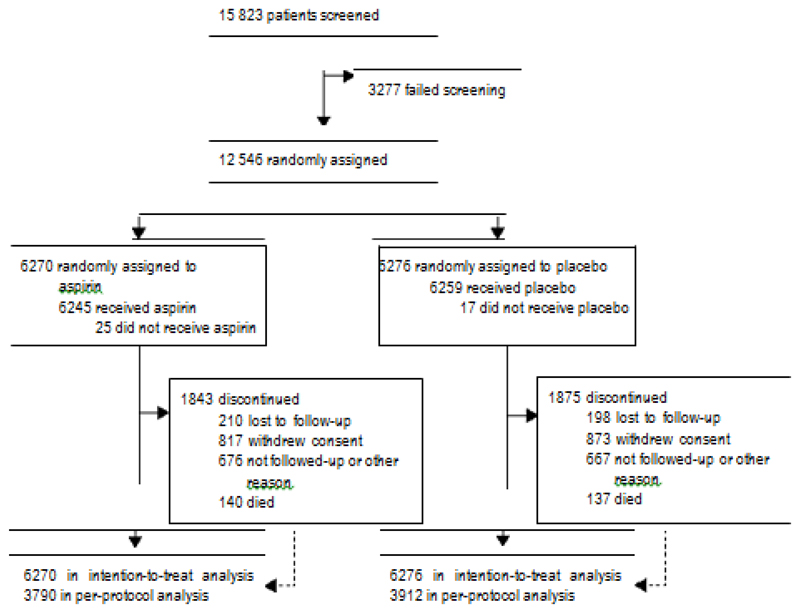
Between July 5, 2007, and Nov 15, 2016, 12 546 participants were enrolled and assigned to receive aspirin (n=6270) or placebo (n=6276) at 501 study sites (figure 1). Each participant completed up to a total of nine visits over an approximate 6-year period. Over the course of the study, which lasted 60 months on average (median follow-up 1858 days; IQR 1475–2209), 29·6% of patients terminated the study prematurely (1843 [29·4%] in the aspirin group and 1875 [29·9%] in the placebo group). The five most frequently documented reasons for pre mature termination were withdrawal (1690 [13·5%]), other reasons (1343 [10·7%]), lost to follow-up (408 [3·3%]), death (277 [2·2%]) without relevant differences between treatment groups. Additional details are provided in the appendix.
Figure 1.
Trial profile
Table 1 provides an overview of the relevant baseline characteristics of the enrolled participants in the intention-to-treat population. The baseline characteristics of the per-protocol population are shown in the appendix. The mean age of participants in the intention-to-treat popula tion (n=12 546) was 63·9 years (SD 7·1), 3708 (29·7%) were female, 3594 (28·7%) were current smokers, 7304 (58·2%) had high total cholesterol, and 5644 (45·0%) had high LDL cholesterol; more than 60% of participants had high systolic blood pressure or were treated for hypertension (table 1). Mean body-mass index was 28·37 (SD 4·34). About 90% of study participants were recruited in Germany (n=3050, 24·3%), Poland (n=3095, 24·7%), and the UK (n=5028, 40·1%).
Table 1. Baseline characteristics of the intention-to-treat population.
| Aspirin (n=6270) | Placebo (n=6276) | |
|---|---|---|
| Mean age, years | 63·9 (7·1) | 63·9 (7·1) |
| Sex | ||
| Female | 1851 (29·5%) | 1857 (29·6%) |
| Male | 4419 (70·5%) | 4419 (70·4%) |
| Race | ||
| White | 6133 (97·8%) | 6146 (97·9%) |
| Other | 137 (2·2%) | 130 (2·1%) |
| Current cigarette smoker* | 1808 (28·8%) | 1786 (28·5%) |
| Median weight, kg | 82·0 (35–163) | 82·0 (43–177) |
| Mean body-mass index | 28·3 (4·3) | 28·5 (4·3) |
| High total cholesterol† | 3647 (58·2%) | 3657 (58·3%) |
| High LDL‡ | 2775 (44·3%) | 2869 (45·7%) |
| Low HDL§ | 857 (13·7%) | 875 (13·9%) |
| High systolic blood pressure¶ | 3916 (62·5%) | 3950 (62·9%) |
| Median systolic blood pressure | 145·0 (80–199) | 145·0 (95–215) |
| Taking anti-hypertensive medications | 4038 (64·4%) | 4097 (65·3%) |
| Country | ||
| Germany | 1525 (24·3%) | 1525 (24·3%) |
| Italy | 164 (2·6%) | 171 (2·7%) |
| Ireland | 54 (0·9%) | 58 (0·9%) |
| Poland | 1550 (24·7%) | 1545 (24·6%) |
| Spain | 212 (3·4%) | 200 (3·2%) |
| UK | 2518 (40·2%) | 2510 (40·0%) |
| USA | 247 (3·9%) | 267 (4·3%) |
| Mean Framingham 10-year coronary heart disease risk score | 13·9% (6·4) | 14·1% (6·4) |
| Mean estimate ACC/AHA 10-year ASCVD risk score at baseline | 17·3% (9·8) | 17·4% (9·7) |
Data are mean (SD), n (%), or median (range). ACC=American College of Cardiology. AHA=American Heart Association. ACSVD=atherosclerotic cardiovascular disease.
Any cigarette smoking in the past 12 months or continuing smoker at randomisation.
Defined as concentrations greater than 200 mg/dL (5·180 mmol/L) in men, and greater than 240 mg/dL (6·216 mmol/L) in women.
Defined as concentrations greater than 130 mg/dL (3·367 mmol/L) in men, and greater than 160 mg/dL (4·144 mmol/L) in women.
HDL concentrations less than 40 mg/dL (1·036 mmol/L) at screening for both sexes.
Systolic blood pressure greater than 140 mm Hg at screening.
There was no significant difference in estimated baseline 10-year risk of cardiovascular disease by study group, as shown by the American College of Cardiology/ American Heart Association cardiovascular disease risk score (table 1). Although this estimated risk was somewhat lower than the goal, the actual event rate in each group, transformed to a 10-year rate, was con siderably lower: 8·43% in the aspirin group and 8·80% in the placebo group.
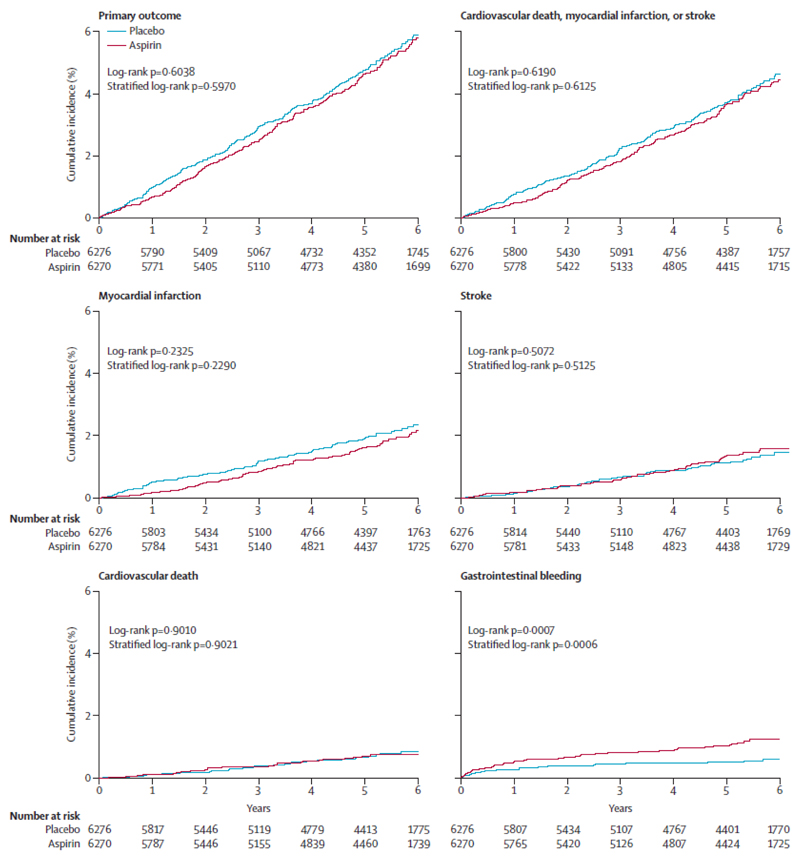
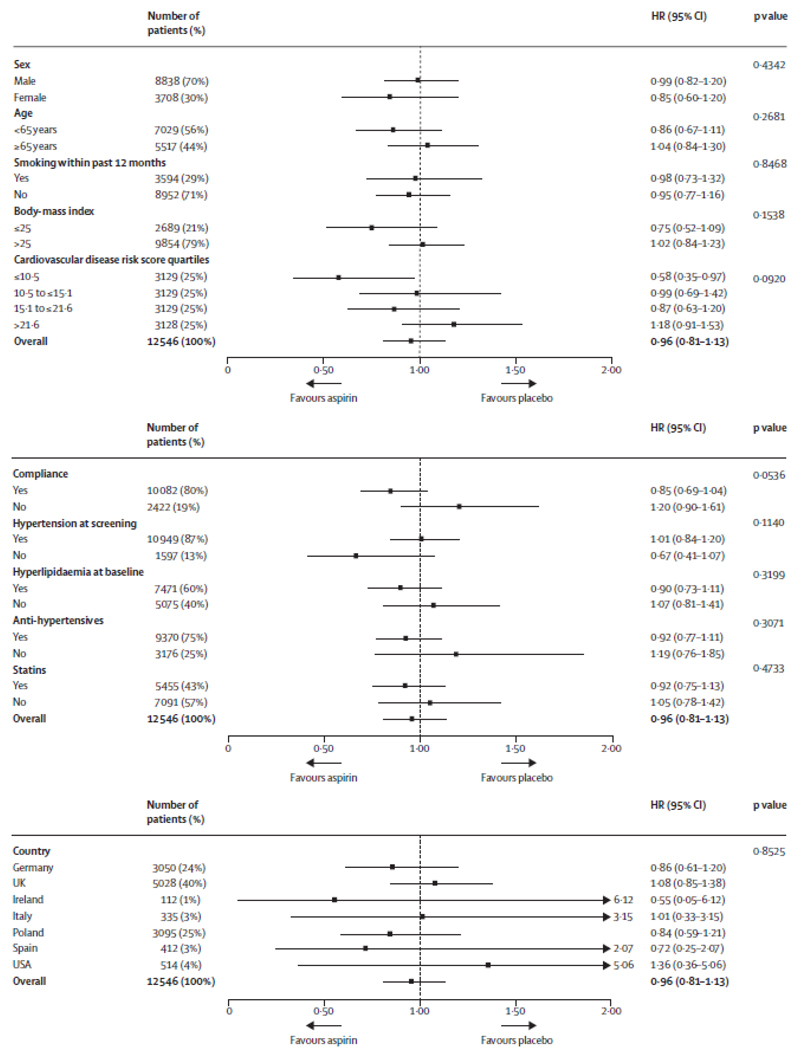
An overview of the efficacy endpoints in the intention-to-treat population is shown in table 2 and figure 2. In the intention-to-treat population, the primary endpoint occurred in 269 (4·29%) of 6270 patients in the aspirin group and 281 (4·48%) of 6276 patients in the placebo group (HR 0·96, 95% CI 0·81–1·13; p=0·6038; table 2; figure 2). The time to the first event of the primary efficacy endpoint in 1%, 2%, 3%, and 4% of the patients is shown in the appendix. Figure 3 shows the results of the subgroup analysis, which were consistent with the overall findings for the primary endpoint, except for the lowest cardiovascular risk quartile (0·58, 0·35–0·97; p=0·015). In the per-protocol analysis, the primary endpoint occurred in 129 (3·40%) of 3790 patients in the aspirin group and 164 (4·19%) of 3912 patients in the placebo group (HR 0·81, 95% CI 0·64–1·02; p=0·0756; table 2).
Table 2. Efficacy endpoints in the intention-to-treat and per-protocol populations.
| Number of events in the intention-to-treat population | Number of events in the per-protocol population | |||||
|---|---|---|---|---|---|---|
| Aspirin (n=6270) | Placebo (n=6276) | Hazard ratio (95% CI); p value | Aspirin (n=3790) | Placebo (n=3912) | Hazard ratio (95% CI); p value | |
| Myocardial infarction, stroke, cardiovascular death, unstable angina, or transient ischaemic attack | 269 (4·29%) | 281 (4·48%) | 0·96 (0·81–1·13); p=0·6038 | 129 (3·40%) | 164 (4·19%) | 0·81 (0·64–1·02); p=0·0756 |
| Myocardial infarction, stroke, or cardiovascular death | 208 (3·32%) | 218 (3·47%) | 0·95 (0·79–1·15); p=0·6190 | 103 (2·72%) | 135 (3·45%) | 0·79 (0·61–1·02); p=0·0661 |
| Myocardial infarction* | 95 (1·52%) | 112 (1·78%) | 0·85 (0·64–1·11); p=0·2325 | 37(0·98%) | 72 (1·84%) | 0·53 (0·36–0·79); p=0·0014 |
| Non-fatal myocardial infarction | 88 (1·40%) | 98 (1·56%) | 0·90 (0·67–1·20); p=0·4562 | 32 (0·84%) | 60 (1·53%) | 0·55 (0·36–0·84); p=0·0056 |
| Stroke* | 75 (1·20%) | 67 (1·07%) | 1·12 (0·80–1·55); p=0·5072 | 40 (1·06%) | 37 (0·95%) | 1·12 (0·71–1·75); p=0·6291 |
| Cardiovascular death | 38 (0·61%) | 39 (0·62%) | 0·97 (0·62–1·52); p=0·9010 | 26 (0·69%) | 26 (0·66%) | 1·03 (0·60–1·77); p=0·9161 |
| Unstable angina | 20 (0·32%) | 20 (0·32%) | 1·00 (0·54–1·86); p=0·9979 | 8 (0·21%) | 11 (0·28%) | 0·75 (0·30–1·87); p=0·5380 |
| Transient ischaemic attack | 42 (0·67%) | 45 (0·72%) | 0·93 (0·61–1·42); p=0·7455 | 19 (0·50%) | 19 (0·49%) | 1·03 (0·55–1·95); p=0·9181 |
| Any death | 160 (2·55%) | 161 (2·57%) | 0·99 (0·80–1·24); p=0·9459 | 108 (2·85%) | 101 (2·58%) | 1·10 (0·84–1·45); p=0·4796 |
Fatal or non-fatal.
Figure 2.
Kaplan-Meier cumulative incidence of primary outcome, original primary outcome, and components of the primary outcome (intention-to-treat population)
Figure 3.
Primary outcome by prespecified subgroups (intention-to-treat population) Hazard ratios are unstratified.
Similar to the primary endpoint, there were no significant differences between the two treatment groups in the secondary efficacy endpoints. In the intention-to-treat population, fatal or non-fatal myocardial infarction occurred in 95 (1·52%) patients in the aspirin group and 112 (1·78%) patients in the placebo group (HR 0·85, 95% CI 0·64–1·11; p=0·2325). The time to the first event (fatal or non-fatal myocardial infarction) in 1% and 2% of the patients is provided in the appendix.
In the intention-to-treat population, non-fatal myocardial infarction occurred in 88 (1·40%) patients in the aspirin group and 98 (1·56%) patients in the placebo group (HR 0·90, 95% CI 0·67–1·20; p=0·4562). The time to the first event (non-fatal myocardial infarction) in 1% of patients was 1309 days in the aspirin group and 1085 days in the placebo group (Kaplan-Meier estimates). In the per-protocol analysis, the HRs for total myocardial infarction (0·53, 0·36–0·79; p=0·0014) and non-fatal myocardial infarction (0·55, 0·36–0·84; p=0·0056) were lower for aspirin (table 2; appendix).
There were 321 documented deaths (n=160 [2·55%] of 6270 patients in the aspirin group and n=161 [2·57%] of 6276 patients in the placebo group; HR 0·99, 95% CI 0·80–1·24; p=0·9459). Of these deaths, 108 patients had fatal myocardial infarction, fatal stroke, or other vascular death (n=49 [0·78%] in the aspirin group and n=59 [0·94%] in the placebo group).
In the safety analysis, gastrointestinal bleeding events occurred in 61 (0·97%) of 6270 patients in the aspirin group and 29 (0·46%) of 6276 patients in the placebo group (HR 2·11, 95% CI 1·36–3·28; p=0·0007; table 3; figure 2). We tested the effects of treatment, sex, coun try, and hypertension on adjudicated gastrointestinal bleeding. The only significant effect was detected for treatment (data for sex, country, and hypertension not shown). Gastrointestinal bleeding events were predominantly mild (table 3).
Table 3. Serious adverse events in the intention-to-treat population.
| Aspirin (n=6270) | Placebo (n=6276) | |||
|---|---|---|---|---|
| Total number of serious adverse events | 1266 | (20·19%) | 1311 | (20·89%) |
| Bleeding serious adverse events by severity | ||||
| Any gastrointestinal bleed | 61 | (0·97%) | 29 | (0·46%) |
| Severe gastrointestinal bleed | 4 | (0·06%) | 2 | (0·03%) |
| Moderate gastrointestinal bleed | 15 | (0·24%) | 5 | (0·08%) |
| Mild gastrointestinal bleed | 42 | (0·67%) | 22 | (0·35%) |
| Haemorrhagic stroke | 8 | (0·13%) | 11 | |
| Most common non-bleeding serious adverse events* | ||||
| Osteoarthritis | 104 | (1·66%) | 103 | (1·64%) |
| Coronary artery disease | 46 | (0·73%) | 61 | (0·97%) |
| Prostate cancer† | 59 | (0·94%) | 44 | (0·70%) |
| Acute myocardial infarction | 43 | (0·69%) | 58 | (0·92%) |
| Atrial fibrillation | 37 | (0·59%) | 40 | (0·64%) |
| Myocardial infarction | 29 | (0·46%) | 38 | (0·61%) |
| Inguinal hernia | 35 | (0·56%) | 31 | (0·49%) |
| Transient ischaemic attack | 26 | (0·41%) | 30 | (0·48%) |
| Pneumonia | 26 | (0·41%) | 19 | (0·30%) |
| Cholelithiasis | 24 | (0·38%) | 17 | (0·27%) |
| Chest pain | 23 | (0·37%) | 19 | (0·30%) |
| Angina pectoris | 20 | (0·32%) | 14 | (0·22%) |
| Benign prostatic hyperplasia† | 10 | (0·16%) | 20 | (0·32%) |
| Unstable angina | 15 | (0·24%) | 19 | (0·30%) |
| Pulmonary embolism | 12 | (0·19%) | 16 | (0·25%) |
| Colon cancer | 14 | (0·22%) | 6 | (0·10%) |
| Ankle fracture | 13 | (0·21%) | 9 | (0·14%) |
| Cholecystitis | 13 | (0·21%) | 8 | (0·13%) |
| Rotator cuff syndrome | 6 | (0·10%) | 13 | (0·21%) |
| Number of serious adverse events per participant | ||||
| One | 873 | (13·92%) | 879 | (14·01%) |
| Two | 256 | (4·08%) | 281 | (4·48%) |
| Three or more | 137 | (2·18%) | 151 | (2·41%) |
Data are number of participants with the serious adverse event.
At least 0·2% in any treatment group.
Male patients only.
The overall incidence rate of serious adverse events was similar in both treatment groups (n=1266 [20·19%] in the aspirin group and n=1311 [20·89%] in the placebo group; table 3). The most frequently reported non-bleeding serious adverse events (≥0·50% in any treat ment group) were osteoarthritis (n=104 [1·66%] in the aspirin group vs n=103 [1·64%] in the placebo group), prostate cancer (59 [0·94%] of 4419 men vs 44 [0·70%] of 4419 men), coronary artery disease (46 [0·73%] vs 61 [0·97%]), acute myocardial infarction (43 [0·69%] vs 58 [0·92%]), atrial fibrillation (37 [0·59%] vs 40 [0·64%]), myocardial infarction (29 [0·46%] vs 38 [0·61%]), and inguinal hernia (35 [0·56%] vs 31 [0·49%]). The incidence of serious adverse events showing a substantial difference between groups by a factor of 2 or more (ie, occurring two times or more in one treatment group versus the other treatment group) was very low. Compared with the placebo group, fewer patients in the aspirin group had at least one cardiac disorder (n=216 [3·44%] in the aspirin group vs n=266 [4·24%] in the placebo group) or vascular disorder (59 [0·94%] vs 87 [1·39%]).
The overall incidence of adverse events in the intention-to-treat population was similar in both treatment groups (n=5142 [82·01%] in the aspirin group and n=5129 [81·72%] in the placebo group); there were no differences of 1% or more between the treatment groups for any of the adverse events. Overall, 4514 (71·99%) of 6270 patients in the aspirin group and 4514 (71·92%) of 6276 patients in the placebo group had at least one mild adverse event, 3098 (49·41%) and 3145 (50·11%) had at least one moderate adverse event, and 724 (11·55%) and 759 (12·09%) had at least one severe adverse event. The incidence of individual severe adverse events was usually 0·10% or less, apart from osteoarthritis (aspirin 0·75% vs placebo 0·65%), coronary artery disease (0·53% vs 0·62%), atrial fibrillation (0·26% vs 0·22%), back pain (0·24% vs 0·14%), fall (0·14% vs 0·22%), chest pain (0·16% vs 0·10%), sciatica (0·14% vs 0·10%), and hyper cholesterol-aemia (0·11% vs 0·08%). There were clinically relevant increased incidences of adverse events in the aspirin group for predominantly mild gastrointestinal bleeding events and some other minor bleeding events, such as epistaxis.
The overall incidence of treatment-related adverse events was low, considering the duration of the study, and differed significantly between the two treatment groups (n=1050 [16·75%] in the aspirin group vs n=850 [13·54%] in the placebo group; p<0·0001). The most frequent ly reported treatment-related adverse events (≥1·0% in any treatment group) were dyspepsia (226 [3·60%] vs 197 [3·14%]), epistaxis (116 [1·85%] vs 56 [0·89%]), gastro -oesophageal reflux disease (70 [1·12%] vs 60 [0·96%]), and upper abdominal pain (68 [1·08%] vs 58 [0·92%]). Drug-related gastrointestinal bleeding was infrequently re ported and, as expected, was more frequent in the aspirin group (n=15 [0·24%] vs n=2 [0·03%]).
Discussion
In the ARRIVE study, we aimed to address the role of aspirin in primary prevention of cardiovascular disease in patients at moderate risk of a first cardiovascular event, in a pragmatic, primary care-based randomised trial. The findings add important information to the body of evidence and are generally consistent with previous primary prevention studies. This study also showed the challenges of doing long-term primary prevention studies in an era of aggressive management of risk factors among higher-risk individuals.
In ARRIVE, aspirin treatment did not lower risk of major cardiovascular events in the enrolled patients, despite the presence of multiple cardiovascular risk factors. Stroke incidence did not differ by treatment group. Similar to other published low-risk primary prevention studies, the risk of myocardial infarction was lower among patients taking aspirin than placebo, but this was not significant; however, this difference was significant in the per-protocol analysis. Of note, none of the p values or secondary endpoints were significant.
When determining whether low-dose aspirin is appropriate for an individual patient, the cardiovascular benefit must be weighed against the potential risk for clinical events such as gastrointestinal bleeding (or other bleeds) that can be associated with aspirin use. ARRIVE contributes meaningful clinical information about the risk of bleeding in middle-aged and older patients. The increased risk of gastrointestinal bleeding was in line with what would be expected, because the events were predominantly mild in severity and there was no difference in fatal bleeding rates, consistent with previous primary prevention studies.
ARRIVE provides valuable lessons about the challenges of carrying out large-scale primary prevention studies when there are multiple widely available preventive and therapeutic interventions, resulting in lower observed cardiovascular risk than expected. Although the targeted estimated risk for enrolment in ARRIVE was achieved, the observed event rate was considerably less than anticipated. The estimated baseline risk of cardiovascular disease over 10 years, calculated with the American College of Cardiology/American Heart Association risk calculator, was modestly lower than intended, at 17·3%. However, the actual rate of cardiovascular disease events, defined by the number of events confirmed in the study (550 vs 1488), was much lower than estimated, at less than 10% over 10 years.
There are several possible explanations for this lower actual risk in the ARRIVE population. First, risk calculators developed with older data might overestimate risk in current practice. Second, as a large pragmatic study based in the primary care setting, the ability to obtain records for vascular events was a challenge for some providers that were often remote from the acute care setting. Additionally, patients were only seen for study follow-up once a year after the first year, potentially giving rise to a failure to reliably report temporally distant events. Both of these factors could have led to an
undercounting of possible vascular events. Third, cardiovascular risk in a population is not a static feature, and this has been seen in other studies. Patients are being treated for their risk factors to lower the risk of the development of disease. Better management of blood pressure, dyslipidaemia, and other risk factors is likely to lower the risk of developing disease, and these interventions are most aggressively used in patients at higher risk. ARRIVE reflects a contemporary cardio vascular risk prevention (therapy) study in which widespread statin use is the norm. 43% of ARRIVE participants were taking statins. The exclusion of patients with diabetes could also contribute to lower overall risk.
Finally, better management of cardiovascular disease when it is manifested by non-acute symptoms can reduce the risk of major acute events. If a patient develops stable angina and receives more intense management including non-study aspirin and close follow-up, the chance of developing a myocardial infarction or other cardiovascular disease event is lessened. This finding is suggested by the difference between the intention-to-treat and per-protocol analyses, particularly for myocardial infarction. It is also supported by the suggestion of a bigger effect early in the study, compared with later in the study.
ARRIVE sought to further assess the effects of aspirin on cancer outcomes during the study. However, the ARRIVE study duration was probably not sufficient to investigate cancer outcomes and to rule out the possibility of benefits of aspirin on long-term cancer outcomes; hence the ARRIVE study could not add information in this regard. Results about incidence of all cancers will be reported elsewhere.
Although the absolute event rates in ARRIVE were lower than expected, the relative effects on specific outcomes were generally similar to those in previous studies in primary prevention of vascular events. Meta-analyses of previous studies showed that aspirin reduces risk of nonfatal myocardial infarction and all myocardial infarction, but does not reduce total stroke or all-cause vascular death.22 The results of ARRIVE, which suggested a stronger effect of aspirin early in the study, compared with later in the study, are also consistent with previous analyses of the time-course of effects of daily aspirin on risk of major vascular events in randomised studies. Whether this atrophy of benefit would be seen in routine practice, when patients know that they are taking active drug rather than placebo, is unknown but it could be argued that in studies with high rates of discon tinuation of study treatment, the effects of aspirin during the first 3 years of follow-up provide the best estimate of the probable effects in routine practice in patients who take the drug.
A strength of the ARRIVE study was the inclusion of a large number of older individuals and women with risk of cardiovascular disease. The study was a pragmatic study done in the primary care setting. As such, it was challenging to identify a population at true moderate risk throughout the long treatment period, given the preventive and therapeutic care participants were receiving, thereby reducing the overall power of the study to detect an effect on the primary outcome. Furthermore, it was challenging to capture all efficacy and safety events in this setting.
Compliance represents another major challenge in contemporaneous studies given the ongoing discussions about aspirin among providers, patients, and the public. By contrast with the null finding for the intention-to-treat analysis, the significant treatment differences observed in the per-protocol analysis (ie, approximately 60% of the study population; n=7702) suggests that compliance played a role. During the study, it appears that there was an impact of discussions about the use of aspirin in clinical practice in UK. In the UK subset, results seemed to be different from the rest of the study population, possibly reflecting coverage in the UK medical and general news media after 2009, regarding uncertainty about the effectiveness of aspirin in primary prevention.2 High rates of discontinuation of study treatment in the UK subset of the study are consistent with this possibility.
Another related compliance issue was the potential use of aspirin by patients who became higher risk during the study. For example, if a patient developed chest pain or had a transient ischaemic event, they might well have been withdrawn from the study and started taking aspirin. To address this issue, the Executive Committee amended the protocol to add transient ischaemic events and unstable angina to the primary endpoint, given that these are two conditions biologically related to the primary composite endpoint, and for which clinicians would probably have selectively withdrawn the affected patient from the study. Redefinition of the primary composite endpoint is a strategy that has been used in other contemporary studies to link biologically related primary endpoints, compensate for selective withdrawals, and increase the number of primary endpoints. However, the results of the redefined primary endpoint were not different from those of the original primary endpoint.
While ARRIVE attempted to add relevant information about the cardiovascular benefits and bleeding risks of aspirin in patients at moderate cardiovascular risk, it showed some of the challenges in doing long-term prevention studies in the current era. ARRIVE is generally consistent with many other studies that show aspirin’s ability to lower the risk of first non-fatal myocardial infarction without affecting risk of total stroke. With respect to safety, as expected, rates of gastrointestinal bleeding events and some other minor bleeding events were higher in the aspirin treatment group, but there was no significant difference in the incidence of fatal events.
The use of aspirin remains a decision that should involve a thoughtful discussion between a clinician and a patient, given the need to weigh cardiovascular and possible cancer prevention benefits against the bleeding risks, patient preferences, cost, and other factors. The ARRIVE data must be interpreted and used in the context of other studies, which have tended to show a reduction primarily in myocardial infarction, with less of an effect on total stroke (including both ischaemic and haemor-rhagic stroke). The overall decision to use aspirin for cardiovascular effects should be done with the help a clinician, given the complex calculus needed to balance all potential benefits and risks.
Supplementary Material
Acknowledgments
We wish to thank the study investigators, site staff, and the participants and their families who participated in this study. We would also like to acknowledge the contributions of the members of the data safety monitoring board and adjudication committee: Ralph D’Agostino PhD, Felicita Andreotti MD, Charles H Hennekens MD, Loren Laine MD, and Martin Brown MD (data safety monitoring board); Paul A Gurbel MD, Thomas F Luscher MD, Dara Jamieson MD, Hans Christian Diener MD, Angel Lanas MD, and Christopher Hawkey MD (adjudication committee).
Footnotes
Contributors
All members of the ARRIVE Executive Committee (JMG, CB, RC, CC, HD, PBG, GH, TAP, PMR, LMR, MT, and GT) contributed to the writing and editing of the manuscript. The Executive Committee was responsible for the development of the study protocol.
Declaration of interests
All voting members of the ARRIVE Executive Committee (JMG, CB, CC, HD, PBG, GH, TAP, PMR, LMR, MT, and GT) received personal fees from Bayer during the conduct of the study. RC is an employee of Bayer and was a non-voting member of the Executive Committee. PMR reports personal fees from Bristol-Myers Squibb. LMR reports personal fees from Novartis, Sanofi, Medtronic, Daiichi-Sankyo, and grant funding from AstraZeneca. MT reports personal fees from Celyad, Janssen Cilag, Kowa, Perfuse Group, and Servier, outside the submitted work.
References
- 1.Guirguis-Blake JM, Evans CV, Senger CA, O’Connor EA, Whitlock EP. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804–13. doi: 10.7326/M15-2113. [DOI] [PubMed] [Google Scholar]
- 2.Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–73. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 4.Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:836–45. doi: 10.7326/M16-0577. [DOI] [PubMed] [Google Scholar]
- 5.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–81. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988;296:313–16. doi: 10.1136/bmj.296.6618.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 8.The Medical Research Council’s General Practice Research Framework. Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet. 1998;351:233–41. [PubMed] [Google Scholar]
- 9.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 10.Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa H, Nakayama M, Morimoto T, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134–41. doi: 10.1001/jama.2008.623. [DOI] [PubMed] [Google Scholar]
- 13.Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowkes FG, Price JF, Stewart MC, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841–48. doi: 10.1001/jama.2010.221. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510–20. doi: 10.1001/jama.2014.15690. [DOI] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration. Citizen Petition Denial Response From FDA to Bayer Healthcare LLC. [accessed March 3, 2016];2014 Docket ID: FDA-1977-N0018. www.regulations.gov/#!documentDetail;D=FDA-1977-N-0018-0101.
- 17.Van’t Hof JR, Duval S, Walts A, Kopecky SL, Luepker RV, Hirsch AT. Contemporary primary prevention aspirin use by cardiovascular disease risk: impact of US Preventive Services Task Force recommendations, 2007-2015: a serial, cross-sectional study. J Am Heart Assoc. 20173;6:e006328. doi: 10.1161/JAHA.117.006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Task Force for Prevention of Coronary Heart Disease. Pocket guide to prevention of coronary heart disease. [accessed Aug 13, 2018];2003 http://www.sisalombardia.it/pdfs/pocket_guide_engl.pdf.
- 19.National Cholesterol Education Program. Detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). Executive summary. [accessed Aug 13, 2018];2001 doi: 10.1001/jama.285.19.2486. https://www.nhlbi.nih.gov/files/docs/guidelines/atp3xsum.pdf. [DOI] [PubMed]
- 20.European Association of Preventive Cardiology. HeartScore quick calculator. [accessed Aug 13, 2018]; https://heartscore.escardio.org/2012/calc.aspx? model=europelow.
- 21.Berkowitz SD, Granger CB, Pieper KS, et al. Incidence and predictors of bleeding after contemporary thrombolytic therapy for myocardial infarction. The Global Utilization of Streptokinase and Tissue Plasminogen activator for Occluded coronary arteries (GUSTO) I Investigators. Circulation. 1997;95:2508–16. doi: 10.1161/01.cir.95.11.2508. [DOI] [PubMed] [Google Scholar]
- 22.Raju N, Sobieraj-Teague M, Bosch J, Eikelboom JW. Updated meta-analysis of aspirin in primary prevention of cardiovascular disease. Am J Med. 2016;129:e35–36. doi: 10.1016/j.amjmed.2015.10.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.