Abstract
Oesophageal adenocarcinoma (OAC) has increased dramatically in Western countries, including the UK, over the past 30 years. It usually presents de novo, but is often preceded by Barrett’s oesophagus (BO), a premalignant condition whereby the normal squamous epithelium is replaced by columnar lined epithelium with intestinal metaplasia. The main risk factors for BO include male sex, obesity and chronic gastro-oesophageal reflux of acid and bile. The estimated annual risk of BO progression is 0.3%, increasing substantially, up to 30%, when dysplasia is present. Endoscopic surveillance is recommended to detect neoplastic changes at an early stage and considerable evidence supports endoscopic treatment for confirmed low- and high-grade dysplasia, and intramucosal adenocarcinoma. Most OACs are diagnosed at a more advanced stage requiring CT-PET assessment and multi-modal treatment. Surgical treatment is performed in specialist centres, increasingly combined with cytotoxic chemotherapy and radiotherapy, involving close liaison between members of the multidisciplinary team. Molecular targeted therapies, such as HER2 and VEGFR-inhibitors, are beginning to penetrate clinical practice, but high molecular heterogeneity has impeded progress. In view of the overall dismal survival (<20%) for advanced OAC, there is renewed interest in screening techniques for early detection and intervention of dysplastic BO.
Keywords: Ablation, adenocarcinoma, Barrett’s oesophagus, biomarkers, dysphagia, endoscopic mucosal resection, endoscopy, MRCP, oesophagectomy, radiofrequency, screening, staging, surveillance
Barrett’s oesophagus
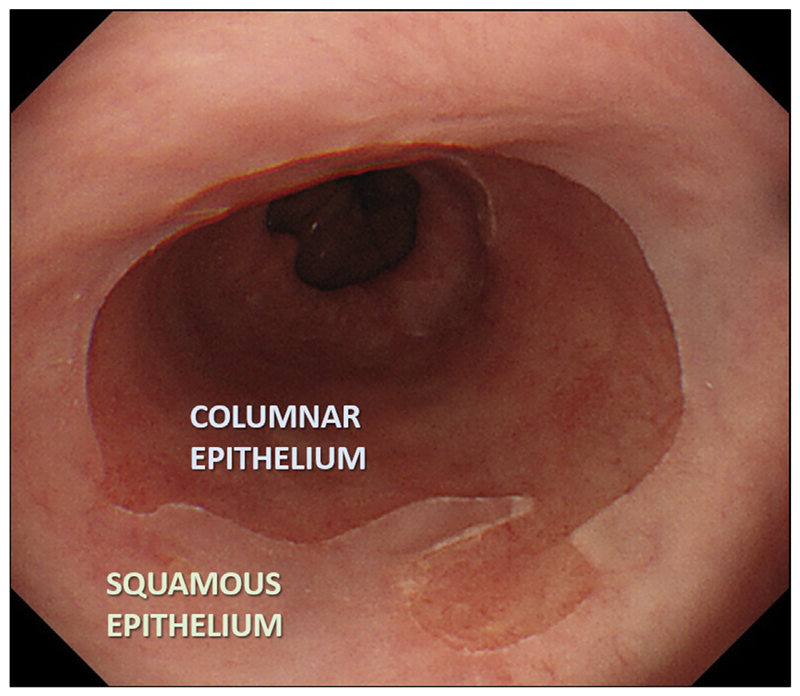
Barrett’s oesophagus (BO) is a well-established precursor of oesophageal adenocarcinoma (OAC). It is defined as an endoscopically visible segment (≥1 cm) of metaplastic columnar epithelium that replaces the normal stratified squamous lining of the distal oesophagus (Figure 1). BO segments are measured from columnar mucosa extending proximally from the upper limit of the gastric folds, which is an anatomical landmark of the gastro-oesophageal junction (GOJ). Long-standing bile and acid reflux from the stomach promotes the development of BO, which in some individuals progresses to adenocarcinoma, via low- and high-grade dysplasia (LGD and HGD).
Figure 1.
Endoscopic appearance of BO. The transition between the pale pink squamous oesophagus and the darker columnar metaplasia that has replaced it in the lower oesophagus can easily be detected endoscopically.
Epidemiology
The prevalence of BO is difficult to establish, as most patients are asymptomatic and therefore undiagnosed. Nevertheless, the estimated prevalence of BO is 1–5% in developed countries. Gastro-oesophageal reflux disease (GORD), particularly of prolonged duration, is the main risk factor. It is more common in white patients than Hispanic and black individuals. Its prevalence increases with age, particularly after 50 years old, until the 70s when it reaches a plateau. The incidence of BO differs significantly between the sexes, being 2–4 times higher in men across all age groups.
Obesity is another well-established risk factor, although the causal relationship between obesity, GORD and BO is not clear. As well as having mechanical effects on reflux, obesity may contribute to metaplastic oesophageal conversion by the release of adipokines. In relation to obesity, some studies indicate that waist-to-hip ratio is a better predictor of BO than increased body mass index. There is no evidence that alcohol consumption increases risk of BO, and smoking is thought to be only a moderate risk factor. The risk of progression to HGD and cancer is, however, significantly increased in smokers with BO.
There appears to be familial clustering in approximately 7% of BO cases, suggesting a genetic predisposition. Although monogenic mutations responsible for familial BO and adenocarcinoma have not been identified, recent genome-wide association studies identified 16 independent risk loci for development of BO (and/or OAC). The risk locus with the strongest association was found within the CFTR gene.2 Interestingly, there is an inverse relationship between Helicobacter pylori infection and risk of BO. Helicobacter pylori may lower acid production in the stomach by causing gastric inflammation and atrophy of the acid-producing parietal cells, hence diminishing the frequency of GORD and consequently BO. Table 1 summarizes the risk factors for BO.
Table 1. Risk factors for BO and their association.
| Factor | Relative strength of association |
|---|---|
| History of reflux symptoms | +++ |
| Male sex | ++ |
| Obesity | ++ |
| White race | ++ |
| Age | ++ |
| Family history | + |
| Smoking | + |
| Alcohol | − |
| Helicobacter pylori infection | Inverse |
Diagnosis
Endoscopy, typically via the trans-oral route, is the preferred method for diagnosing BO. Accurate assessment can be hampered by excessive motility of the gastrointestinal tract during endoscopy and the common coexistence of a hiatus hernia, which can confound precise localization of the GOJ.
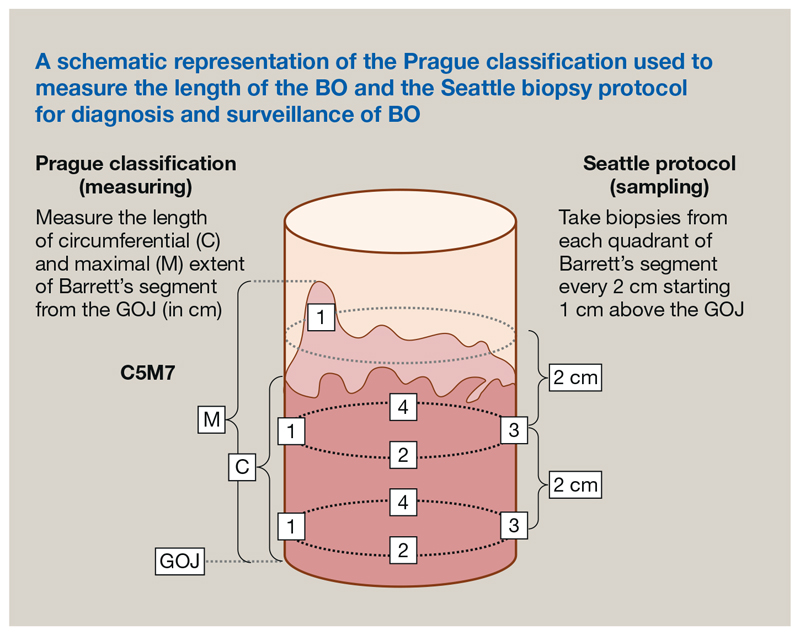
Endoscopic measurement of the BO segment has been standardized using the Prague classification, which involves documenting the maximum circumferential extension of the columnar epithelium from the GOJ in centimetres (C score) and the maximal total extension of the segment from the GOJ (M score) (Figure 2). A major benefit of this standardized nomenclature lies in communicating the endoscopic findings between clinicians, particularly when treatment is envisaged. The area of metaplasia should be systematically biopsied using the Seattle protocol. This involves quadrantic biopsies taken every 2 cm along the length of the metaplastic epithelium (Figure 2), starting distally to prevent bleeding obscuring the endoscopist’s view. This technique is used for baseline diagnosis and subsequent surveillance.
Figure 2.
A schematic representation of Prague classification used to measure the length of the Barrett's oesophagus and the Seattle biopsy protocol for diagnosis and surveillance of Barrett's oesophagus
Histologically, there are three types of columnar metaplasia: gastric, which contains mucus-secreting parietal and chief cells; cardiac (transitional), composed almost entirely of mucus-secreting cells; and intestinal, defined by the presence of goblet cells. The latter, also termed intestinal metaplasia (IM), is more likely to progress to OAC. American guidelines require the presence of goblet cells for a diagnosis of BO, but this differs in the UK because of concern that random sampling might miss the presence of the goblet cells. However, in order to distinguish between low and high risk of progression the UK surveillance protocol varies according to the length of the segment and whether or not IM is present.
Screening and diagnostic referral pathways for Barrett’s oesophagus
Screening interventions for BO seem appealing because >80% patients with this condition are not diagnosed. However, the overall low prevalence of OAC does not justify a population-based endoscopic screening programme. Currently the British Society of Gastroenterology guidelines suggest that screening can be considered in patients with chronic GORD symptoms and multiple risk factors (at least three of age 50 years or older, white race, male sex and obesity). This threshold should be lowered for patients who have a first-degree relative with BO or OAC. Although high-definition white light endoscopy remains the gold standard in diagnosing BO, new technologies to improve the accuracy, feasibility and cost-effectiveness of screening are being widely investigated.
Ultrathin transnasal endoscopy is more cost-effective than traditional endoscopy as it does not require sedation. The scope has a diameter of <6 mm and has been shown to have a similar technical success rate to standard endoscopy, with higher acceptability and preference for patients. It could be performed in primary care, although the equipment is expensive and a skilled operator is still required. In addition, the biopsies are very small compared with standard endoscopy, which could compromise the histopathological review. While suitable for diagnosis, transnasal endoscopy is therefore not recommended for surveillance.
Non-endoscopic tools which could be readily performed in primary care are ideal for screening and there are promising data on the Cytosponge®-TFF3 test. The Cytosponge® comprises a small capsule tethered to a string. The capsule is swallowed in a sitting position, its coating disintegrating on reaching the stomach. This reveals a 3 cm diameter spherical sponge, which is then withdrawn by pulling the string. On withdrawal, the device samples the entire oesophageal lining. The collected cells can be analysed for BO using the immunohistochemical biomarker trefoil factor 3 (TFF3, a marker of columnar epithelium), and for features suggestive of dysplasia (using other biomarker assays, including TP53). Cytosponge® sampling has been proven to be a safe procedure with high acceptability ratings in several trials and a large-scale randomized trial is underway. Similar non-endoscopic approaches coupled with methylation markers are also being investigated.
Surveillance and dysplasia diagnosis
Routine surveillance for patients with BO remains controversial due to the high cost and low cancer conversion rate. The rationale for repeated endoscopic monitoring is to allow for early, pre-symptomatic detection of dysplasia or cancer at a point when treatment can be preventive or curative. Several population-based studies suggest that it results in earlier detection of cancer and may provide a survival benefit although lead time bias may confound the results.3,4 Randomized controlled trials (RCTs) analysing the effect of surveillance compared with symptomatic referral in BO patients are underway (e.g. the BOSS trial).
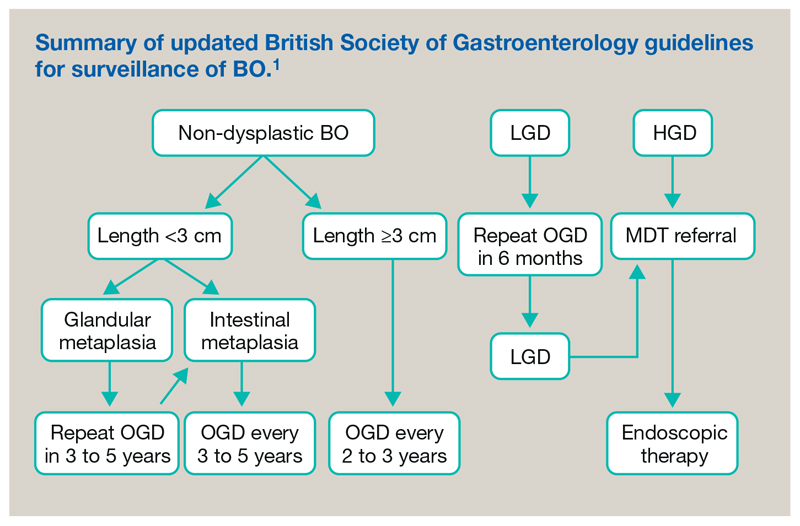
The British guidelines currently recommend surveillance with regular endoscopic monitoring and histopathological assessment with a tailored approach based on clinical and pathological features.1 Short lengths (<3 cm) of BO that do not contain IM have a particularly low risk of progression to dysplasia or cancer (0.05%/year); hence the algorithm is adjusted according to the presence of IM or dysplasia and the segment length (Figure 3).
Figure 3.
Summary of updated British Society of Gastroenterology guidelines for surveillance of Barrett's oesophagus 2
Dysplasia is a histopathological diagnosis in which the epithelial cells lose their polarity and develop nuclear crowding, with increasingly elongated and hyperchromatic nuclei. Endoscopic detection of dysplasia, particularly LGD, can be challenging because the affected epithelium is often flat and indistinct. The histological differentiation between degrees of dysplasia (LGD versus HGD) can also be difficult, with significant inter-observer variability. Therefore these samples should be reviewed by two specialist pathologists, discussed at a specialist multidisciplinary team (SMDT) meeting and reported using the Revised Vienna Classification (2000) (Table 2). It is hoped that molecular features can help to provide a more objective measure of the propensity for malignancy, and p53 immunostaining is currently recommended as an adjunct to histopathological diagnosis of dysplasia.
Table 2. Vienna histological classification.
| Category | Classification |
|---|---|
| 1 | Negative for dysplasia |
| 2 | Indefinite for dysplasia |
| 3 | Low-grade dysplasia |
| 4a | High-grade dysplasia |
| 4b | Intramucosal carcinoma |
| 5 | Submucosal invasion by adenocarcinoma |
BO with changes classified as indefinite for dysplasia constitutes a separate histological category, relating to atypical cytological changes considered too severe to be termed negative for dysplasia but falling short of the criteria for true dysplasia. Care should be used when interpreting this finding because underlying LGD or HGD could be obscured by inflammation. These patients should be treated with optimal anti-reflux therapy and rescoped after 6 months and managed accordingly.
The development of advanced endoscopic techniques has raised questions over which method is best for identifying dysplasia. Regular surveillance with high-resolution endoscopy and careful examination of the mucosal surface is the current standard of care. Data demonstrate that there is a direct correlation between the time spent on inspecting the BO segment and detection of HGD/OAC. Other methods have been advocated to help localize areas of dysplasia that are not easily visible. Standard chromoendoscopy agents, such as methylene blue, acetic acid and indigo carmine, have previously been used, with inconsistent results, and imaging technology is advancing which negates the need for topical dyes.
Narrow-band imaging (NBI) is an example of virtual chromoendoscopy, one of the most widely studied tools for detecting and characterizing early neoplasia in BO. NBI enhances light of a short wavelength (blue and green in the visible spectrum) that penetrates only superficially into the mucosa. This improves imaging of the mucosal surface and related vascular pattern. A simple validated system to identify dysplasia and OAC within BO segments using NBI has recently been developed (the BING criteria). This is based on a simple evaluation of the BO surface and vascular patterns (regular versus irregular). In a recent study, the BING criteria had an overall accuracy of 85% in identifying patients with dysplasia. Moreover, NBI can be combined with magnifying systems to improve the detailed characterization of the vascular microarchitecture and glandular pit pattern.
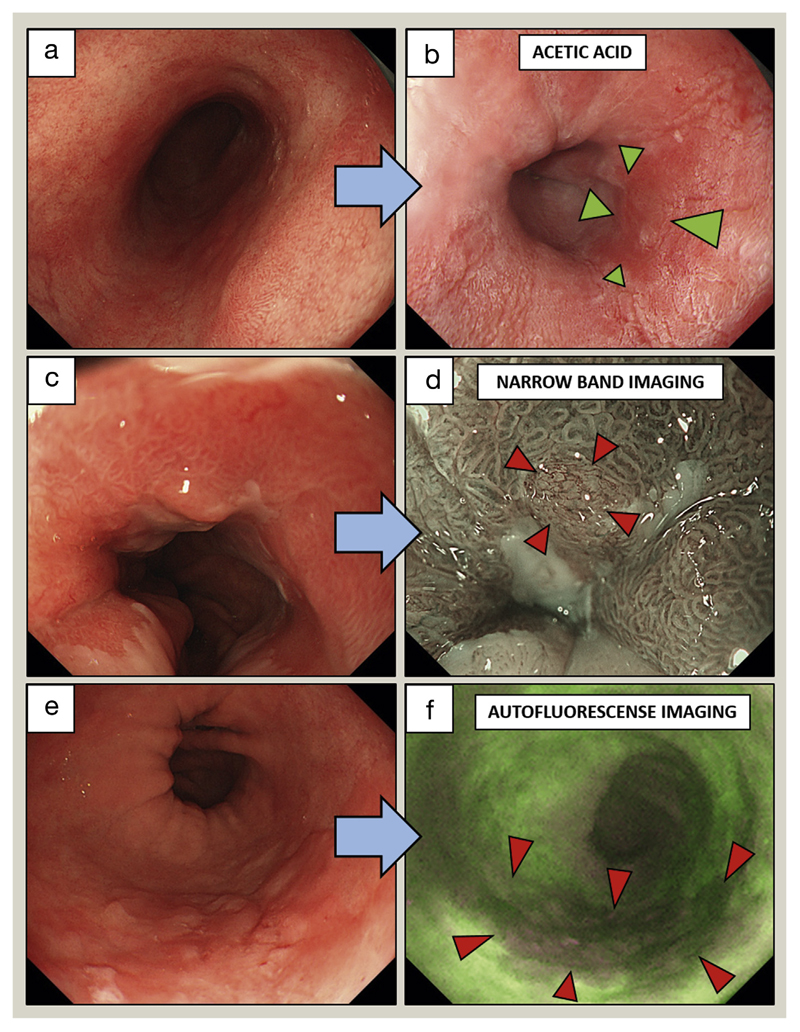
Autofluorescence imaging, which relies on endogenous fluorophores being excited by short-wavelength light has a high rate of false-positive diagnosis of dysplasia. Confocal endomicroscopy provides cellular resolution but is limited by a narrow field of view and difficulties with image interpretation. An ideal endoscopic tool should be able to rapidly examine the entire BO segment and provide an accurate, automated analysis to localize areas of concern for biopsy and treatment. This remains an area of active investigation. Examples of advanced imaging techniques are presented in Figure 4.
Figure 4.
Advanced endoscopic imaging techniques to identify dysplasia in BO. (a, b) 2.5% Acetic acid staining in a long segment of BO. Arrows indicate an area of HGD, characterized by earlier loss of acetowhitening compared with the surrounding Barrett’s mucosa. (c, d) NBI. Arrows indicate a slightly raised area of early adenocarcinoma with irregular vessels. (e, f) Autofluorescense imaging. Arrows indicate an area of early adenocarcinoma characterized by abnormal autofluorescense signal (dark red compared with bright green).
Treatment of Barrett’s oesophagus and management of dysplasia
Most patients with BO have reflux symptoms and should be given long-term acid suppression treatment. Proton pump inhibitors (PPIs) have the best clinical profile for symptomatic management. PPIs irreversibly block the H+/K+-ATPase pump in acid-secreting gastric parietal cells. The addition of an H2-receptor antagonist to a PPI can help with nocturnal symptoms. If inflammation is present during the endoscopic assessment of BO, medication should be introduced or the dose of existing medicine increased. This approach allows accurate histopathological assessment of the degree of dysplasia in repeated endoscopy. Anti-reflux surgery, usually laparoscopic Nissen fundoplication, should be considered for patients who have persistent reflux symptoms (confirmed by pH-monitoring) or are unable to take PPIs without significant adverse effects.
As with the regular use of aspirin and non-steroidal anti-inflammatory drugs, it is currently debated whether PPIs should be offered to all BO patients as a chemopreventive agent, irrespective of the presence of symptoms. PPIs effectively reduce acid reflux, which is the main driver of BO, and downregulate cyclooxygenase-2 expression, which might protect against neoplastic progression. Most recently, the AspECT RCT compared outcomes in BO patients treated with low- or high-dose PPI (esomeprazole 20 mg or 40 mg twice daily), with or without aspirin (300 mg/day). The study showed that high-dose PPI slightly reduced all-cause mortality and incidence for HGD/OAC compared with low-dose PPI after a follow-up time of nearly 9 years (p = 0.038). Interestingly, aspirin alone did not provide significant benefit, but a combination of high-dose PPI and aspirin had a trend towards a stronger effect.
All patients with confirmed LGD (in two subsequent examinations confirmed by pathologists) and HGD should be discussed at an SMDT meeting and undergo high-resolution endoscopy at a tertiary centre. Endoscopic abnormalities should be documented using the Paris classification, which assesses the likelihood of a lesion harbouring invasive disease. Endoscopic treatment is the current gold standard in management of dysplasia. With LGD or HGD without visible abnormality, endoscopic ablation is recommended, using argon plasma coagulation (APC) and, more recently, radiofrequency ablation (RFA). The aim of such treatment is to ablate (burn) the metaplastic mucosa and allow reepithelialization with squamous epithelium and usually involves several sessions.
The efficacy of APC in BO treatment has been demonstrated in numerous case-series reports; most recently, in a pilot RCT, it was found to be similarly effective to RFA in terms of BO eradication after endoscopic mucosal resection (EMR). Overall, APC is cheaper and more widely available than RFA; however, it is less standardized and more often associated with the presence of post-treatment ‘buried glands’, defined as the presence of residual Barrett’s epithelium under the neo-squamous epithelium. APC has recently been combined with submucosal sodium chloride injection (hybrid-APC) to improve its safety compared with conventional APC.
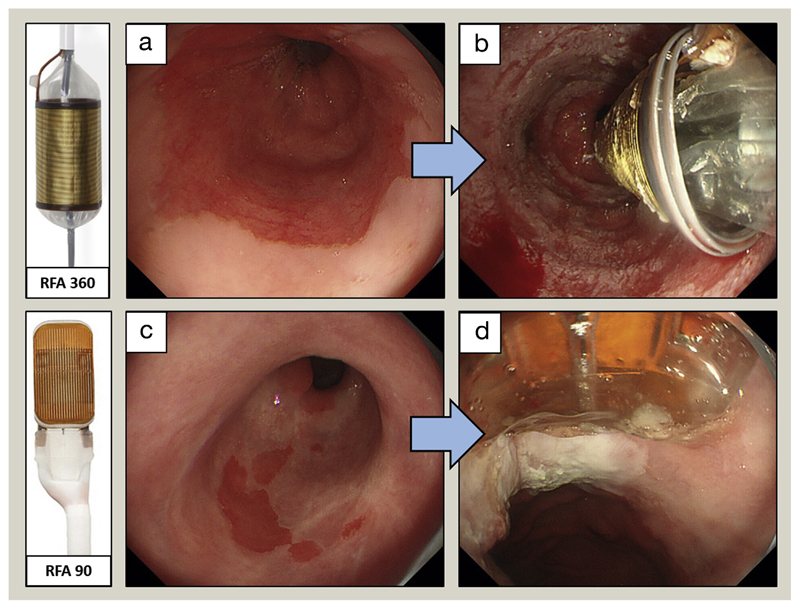
In many countries, RFA has become the method of choice to treat dysplastic BO. Two basic types of RFA are available – RFA 360 and RFA 90. In RFA 360, a balloon with electrodes is insufflated within the oesophageal lumen to deliver a shallow circumferential burn to the luminal surface. RFA 90 is used to focally burn small areas of Barrett’s epithelium (Figure 5). The initial multicentre, sham-controlled trial showed complete eradication of IM in 77.4% in patients with dysplastic BO treated with RFA; this resulted in lower disease progression rates (3.6% versus 16.3%, p = 0.03) and fewer cancers (1.2% versus. 9.3%, p = 0.045) compared with the control group (sham procedure). A more recent RCT of 68 patients with LGD treated with RFA and an equal number of patients undergoing annual endoscopic surveillance showed that, over 3 years’ follow-up, only 1% of patients in the treatment arm progressed to HGD or OAC, compared with 26.5% in the control arm (p < 0.001). Complete eradication of dysplasia and IM by RFA was achieved in 98% and 90% of cases, respectively. The most common complication was a stricture, which occurred in 12% of patients, but this was successfully resolved by endoscopic dilation in all cases. Of note, endoscopic monitoring is still recommended after ablative therapy because there is a significant risk of recurrence in these patients.
Figure 5.
RFA treatment for BO with dysplasia. (a, b) RFA 360 balloon to treat a circumferential segment of BO. (c, d) RFA 90 to treat small areas of residual BO.
For dysplasia or early cancer within a visible lesion, endoscopic resection should be performed, with either EMR or endoscopic submucosal dissection (ESD). Band-ligation EMR is the most common technique used in the setting of BO. In this, a transparent suction cap with rubber bands is fitted to the tip of the endoscope so that the targeted tissue can be suctioned inside the cap, and a ligation band is then deployed. This creates a pseudopolyp that is then resected beneath the band by a metal polypectomy snare (Figure 6). EMR has a favourable safety profile. In large database studies, the rate of acute complications, such as bleeding and perforations, were 1.2–1.8% and 0–2.4%, respectively. More common were the late complications such as strictures, in 1.0% up to 49.7% of EMRs involving a large, circumferential resection. All strictures were, however, successfully treated with endoscopic dilation.
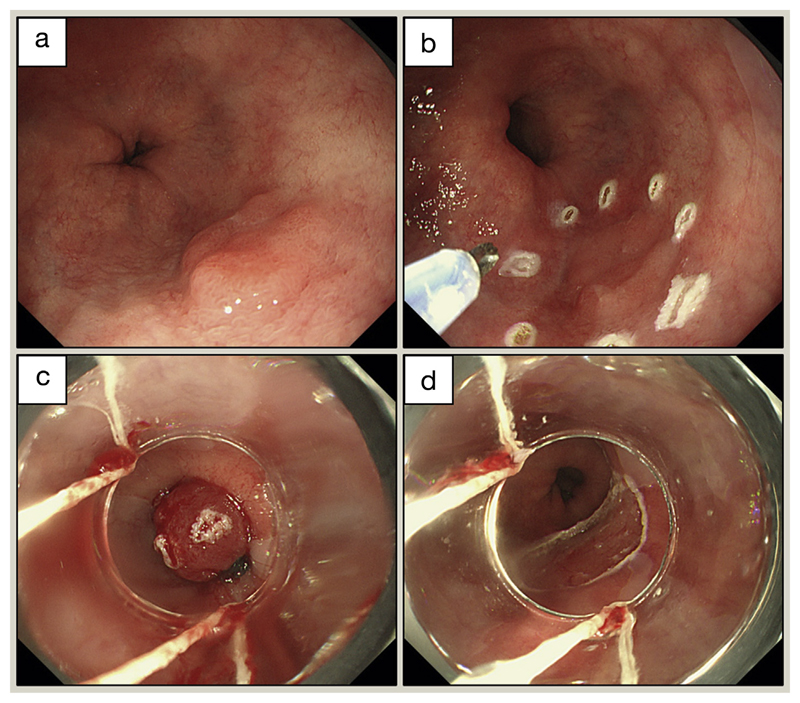
Figure 6.
EMR for early adenocarcinoma in BO. (a) Nodular lesion corresponding to early adenocarcinoma. (b) Marking the lesion before resection. (c) A transparent suction cap is fitted to the tip of the endoscope, the targeted tissue is sucked inside, and a ligation band is then deployed, creating a pseudopolyp. (d) Resection with a metal polypectomy snare.
EMR provides histological information including prognostic features such as degree of cancer differentiation, presence or absence of lymphovascular invasion, depth of cancer invasion and distance of cancer from the deep and lateral resection margins. These criteria aid in determining the suitability of curative endoscopic therapy. Lesions confined to the mucosa have a very low rate of lymphatic involvement, so EMR is considered curative for adenocarcinoma limited to the mucosal layer (T1a) in conjunction with low or moderate differentiation of cancer, no lymphovascular invasion and clear resection margins. EMR is frequently performed piecemeal, and histological complete resection (R0) is difficult to assess in such cases as this requires a tumour to be included in only one specimen.
ESD is a demanding procedure which has the advantage over EMR of allowing en bloc resection of larger neoplastic lesions. The procedure is initiated by injection of fluid (typically sodium chloride with epinephrine, and indigo carmine dye) underneath the target lesion; this is followed by circumferential cutting, dissection of the submucosal layer, coagulation of visible vessels and removal of the whole specimen. A recent RCT comparing EMR with ESD in the treatment of BO-related neoplasia showed that both methods were comparably effective in terms of the need for surgery, neoplasia remission and recurrence. ESD was, however, more time-consuming and led to more severe adverse events. Overall, ESD has not been shown to be superior to EMR, although it can be considered in selected cases, such as larger lesions (>15 mm in size), poorly lifting lesions and lesions at increased risk of submucosal invasion.
For adenocarcinoma with superficial submucosal invasion (T1b sm1), data are conflicting regarding the risk of lymph node involvement. A growing body of evidence shows that this risk is lower than previously reported, and some guidelines consider endoscopic therapy as an alternative to oesophagectomy in cases of low-risk T1b adenocarcinoma; this is defined as cancer limited to the superficial layer (500 micrometres) of the submucosa (Sm1) that is well differentiated, has clear resection margins and shows no lymphovascular invasion. This approach is especially recommended for poor surgical candidates. Such therapeutic decisions should be always made by a multidisciplinary team. After endoscopic resection for focal dysplasia, patients should be offered subsequent ablative therapy for the residual BO segment.
Oesophageal adenocarcinoma
Epidemiology
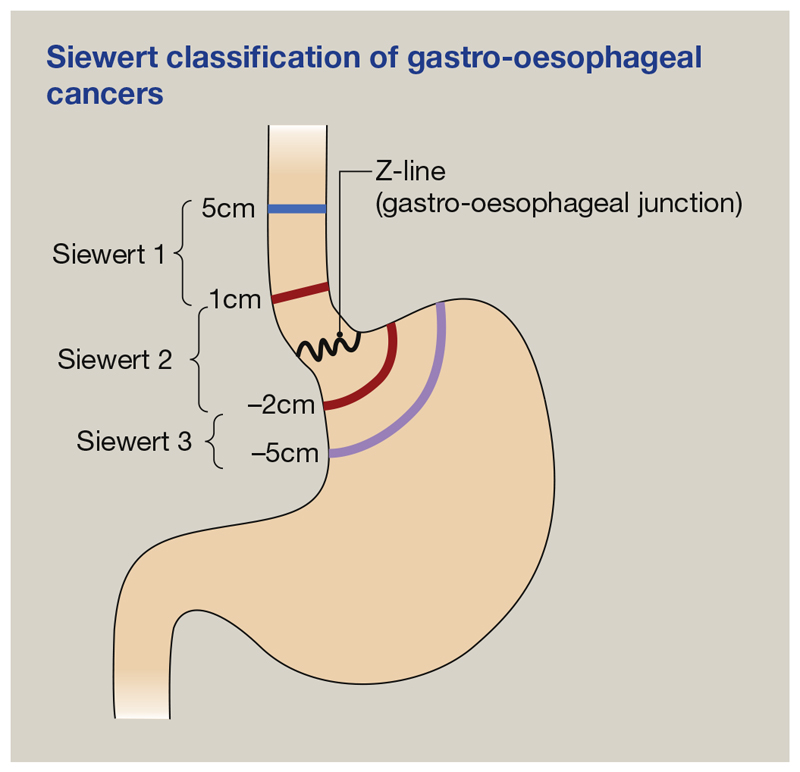
In the UK, the overall annual incidence of oesophageal cancer (14.1 per 100,000 population in 2015) is similar to the annual mortality because of the associated poor 5-year survival of <13%.5 It is the UK’s seventh most common cause of cancer death, accounting for 5% of all cancer deaths in 2016. Although squamous cell carcinoma of the oesophagus remains the dominant subtype of oesophageal cancer worldwide, the incidence of OAC, particularly in men, has been rapidly increasing in the Western world. Adenocarcinoma currently accounts for more than half of oesophageal cancers in England. The high mortality stems from the appearance of symptoms late in the disease when spread has already occurred beyond the primary site. Adenocarcinomas occurring within 5 cm of the junction are termed GOJ cancers. These are currently subdivided using the Siewert classification (Figure 7), based on whether the bulk of cancer lies in the stomach or the oesophagus. The precision and usefulness of this classification are, however, under debate.
Figure 7. Siewert classification of gastro-oesophageal cancers.
Siewert 1, cancer of the distal oesophagus; Siewert 2, gastric cardia cancer; Siewert 3, subcardial gastric cancer.
Diagnosis
Most patients with oesophageal cancer present with difficulty swallowing (dysphagia) or pain when swallowing (odynophagia). This starts with solid foods (e.g. bread, meat) and progresses until even liquids are difficult to swallow. In 2005, the National Institute for Health and Care Excellence issued guidelines indicating that patients of any age with dyspepsia and progressive dysphagia should have an urgent referral for endoscopy to exclude malignancy.
Dysphagia is associated with weight loss, anaemia and/or symptoms from metastatic disease. The oesophagus can distend to a remarkable degree before swallowing difficulties are noticed, and this contributes to the late diagnosis. Some patients present with haematemesis (vomiting blood) and melaena (dark appearance of stools from the presence of digested blood). Fatigue can occur as a consequence of anaemia related to chronic occult bleeding from a tumour. Endoscopy with biopsies remains the gold standard in the diagnosis of OAC. Typically, a set of at least six biopsies should be taken from suspicious lesions to provide adequate tissue material for histopathological assessment. Histology should be reported in accordance with the World Health Organization classification and include information on subtype of cancer and grade.
Staging of oesophageal cancer
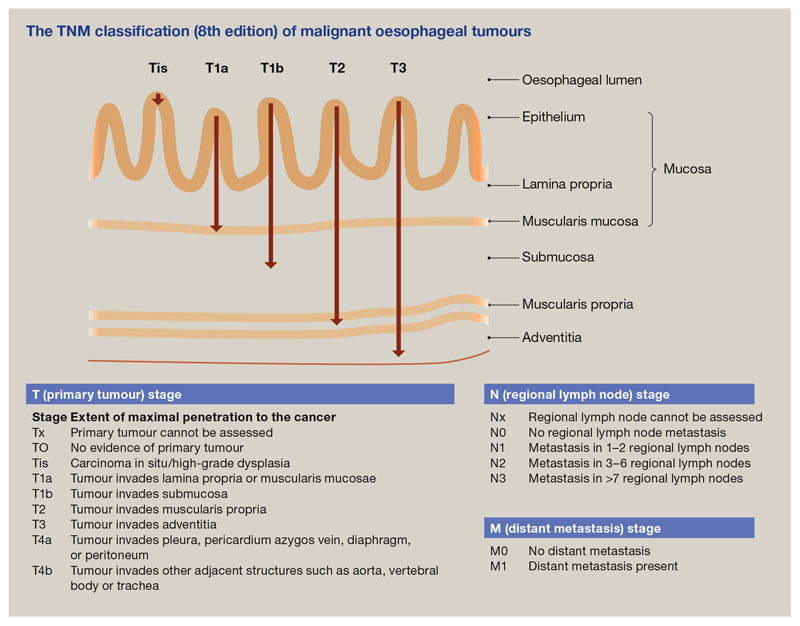
Once oesophageal cancer has been diagnosed, it is staged according to the tumour, node, metastasis (TNM) classification (8th edition; Figure 8). Most people who present with ‘alarm’ symptoms, such as dysphagia, already have locally advanced disease (T3) and lymph node involvement (N1). T1 lesions are determined most accurately by histological examination, obtained by EMR or ESD. The lesion should ideally be removed en bloc so the pathologist can accurately determine the depth of cancer penetration. Radiological investigations are useful for staging more advanced cancers to determine lymph node involvement and metastases. Recent attempts have been made to identify prognostic molecular biomarkers, but further work is required before this can be incorporated into routine practice in the way it is for breast cancer.
Figure 8.
The TNM classification (8th edition) of malignant oesophageal tumours
Computed tomography (CT) is very useful for staging nodes (N) and metastases (M) but has poor sensitivity in differentiating between the T stages (often understaging the tumour), particularly when there is no mediastinal invasion. To obtain the best views, CT should be performed after the stomach has been distended with water. The most common sites for metastases are the liver, lungs, adrenal glands and peritoneum. Magnetic resonance scanning has not been shown to have any additional benefit.
Positron emission tomography (PET) is currently used to detect the synthetic glucose analogue 2-fluoro-2-deoxyglucose, which contains the radioisotope 18F and is incorporated into tissue with a high rate of glycolysis. PET is useful for detecting distant lymph node involvement and metastases in patients being considered for surgery and is particularly useful in clarifying the involvement of nodes that were classified as indeterminate on CT by size. This is now most commonly performed in combination with a co-registered CT (CT-PET) to enable accurate localization of areas of high signal. It can also be used as a tool for monitoring the response to chemotherapy.
Endoscopic ultrasound (EUS) is more accurate than CT in discerning the degree of invasion through the layers of the oesophagus, as well as determining the size and malignant characteristics of local lymph nodes. However, it is currently poor for distinguishing between the T1 stages (T1a versus T1b). It also allows for EUS-guided fine needle aspiration of suspicious lymph nodes, but this may not be possible when the cancer has caused stricturing of the oesophagus.
Laparoscopy allows the detection of low-volume hepatic or peritoneal metastases or seeding that would not normally be detected on CT and is a useful aid in assessing tumour operability. This is particularly helpful for tumours of the GOJ or with gastric involvement.
Other ‘staging adjuncts’ should be used to address specific clinical queries. These include endobronchial ultrasound, which can detect airway infiltration in tumours of the upper oesophagus. It may also be useful for sampling mediastinal lymph nodes that are not accessible by EUS, especially in the paratracheal space. Thoracoscopy can also be required in specific circumstances.
Management of oesophageal cancer
Oesophageal cancer in the UK is now managed in regional specialist centres by an SMDT. This team should include surgeons, radiation and medical oncologists, endoscopists, nutritionists, radiologists, pathologists, palliative care physicians, specialist nurses and individuals with knowledge of clinical trials.
Endoscopic therapy: as mentioned earlier, endoscopic resection should be used to resect focal lesions, usually within an area of BO; this can be sufficient if the cancer is intramucosal or, in some circumstances, is penetrating only the superficial layers of the submucosa (T1b). The data show that patients with early oesophageal cancer managed with endoscopic therapy have comparable long-term survival to those treated with surgical resection.
Endoscopic therapy also has a role in palliative treatment and can be used to manage dysphagia, bleeding or fistula formation in more advanced cancers. Stents provide good palliation for patients with malignant tracheo-oesophageal fistulae. However, they cannot be used if a tumour extends to within 2 cm of cricopharyngeus, and tend not to function as well at the GOJ. Currently, self-expanding covered stents are being more commonly used, reducing the need for oesophageal dilatation before their placement and decreasing tumour overgrowth through the stent. Tumours can be downsized with techniques such as laser photocoagulation, local diathermy and sclerotherapy (with alcohol), or with brachytherapy (internally delivered radiotherapy); however, the requirement for repeat procedures and the lack of effect on overall survival make these techniques of limited usefulness. The patient’s overall fitness and risk of aspiration pneumonia, oesophageal perforation, bleeding or formation of tracheo-oesophageal fistulae must be considered when undertaking any of these procedures.
Surgery: this is generally undertaken only with the intent to cure, but even then median survival is only up to 46 months in tertiary care centres. For early oesophageal cancer (T1) after EMR or ESD staging, surgery should be reserved for cases with high risk of lymph node metastasis, as previously discussed. Surgery is generally combined with neoadjuvant oncological therapy, but for selected patients with T1 and T2 disease with low-risk features (size <2 cm and well-differentiation), surgery alone can be sufficient. The morbidity and mortality associated with oesophagectomy are improving, especially for early cancers, for which there are now minimally invasive laparoscopic and/or thoracoscopically assisted approaches.
The operative approach depends on the precise site of the lesion and the need for lymph node resection. Options include the following:
Two-phase Ivor Lewis oesophagectomy – the most widely practised operation, this entails a laparotomy followed by a right thoracic approach with an anastomosis high in the chest, and allows a thorough lymphadenectomy.
Laparoscopically assisted oesophagectomy or minimally invasive oesophagectomy – this permits lymph node resection and has the advantage of reduced recovery times. However, the technique is used primarily for early disease because of concerns about achieving adequate lymph node clearance.
Trans-hiatal oesophagectomy – this entails the formation of a high anastomosis in the neck without thoracotomy. A less radical lymph node dissection restricts this approach to early disease.
Merendino procedure – a more limited resection with a jejunal interposition is used for early disease because it has lower morbidity and mortality rates, and gives a good functional result.
Whichever approach is used, the most important objective is to achieve a clear resection margin. A recent meta-analysis has shown that patients with involved margins have a significantly poorer prognosis. Various neoadjuvant treatments are available to help downsize these tumours and aid complete resection.
Chemotherapy: this is used in different contexts in the management of OAC. For patients with operable disease, neoadjuvant or perioperative chemotherapy is used to improve overall survival. The Medical Research Council (MRC) OEO2 trial was the first study to demonstrate that chemotherapy in the form of cisplatin and 5-fluorouracil (5-FU) before oesophagectomy improved 5-year overall survival from 17.1% to 23.0%. The survival benefit was similar for oesophageal squamous cancer and adenocarcinoma.
The subsequent MRC ST02 (MAGIC) trial examined the use of the three-drug combination epirubicin, cisplatin and 5-FU (ECF) given before and after surgery. The trial studied gastric cancers, but the cohort contained many patients with lower oesophagus and GOJ tumours. This study demonstrated an increased 5-year overall survival of 36% for the ECF group, compared with the surgery group (23%; p = 0.009). 5-FU can be replaced by capecitabine – an oral form of the drug that has been shown to have equivalent efficacy and is often used as an alternative to infusional 5-FU for patient convenience (giving the chemotherapy regimen ECX). An improved pathological response, compared with ECF, was recently shown in patients treated with a docetaxel-based triplet chemotherapy (FLOT regimen – docetaxel, oxaliplatin, 5-FU) before surgical resection.
Chemotherapy is also used in patients with metastatic inoperable disease to improve quality of life and prolong survival. Similar drugs are used in the perioperative setting. The REAL 2 study suggests there might be a small advantage of replacing cisplatin with another member of the platinum family, oxaliplatin. EOX (epirubicin, oxaliplatin, capecitabine) is a common regimen for patients with metastatic disease. There is evidence for the use of other drugs (e.g. docetaxel) in the second-line setting, but patients should be carefully assessed and involved in discussions about treatment selection as the goal of therapy in this context is to maximize quality of life.
As the molecular profile of OAC is being characterized through genome-wide sequencing efforts and systematic assessment of receptor tyrosine kinase expression, it is becoming more feasible to introduce molecular targeted therapy in curative as well as advanced disease settings. However, a personalized approach seems necessary because of heterogeneity of the somatic alterations. Overexpression of human epidermal growth factor receptor 2 (HER2) is common in junctional and oesophageal adenocarcinomas (32.2% in the ToGA study cohort and 30% in the TCGA cohort). This overexpression was first noted in breast cancer, and the targeted monoclonal antibody trastuzumab has been used to improve survival dramatically in these patients. The ToGA trial found that trastuzumab prolonged survival in HER2-overexpressing inoperable gastric and GOJ tumours. New agents including lapatinib, a dual epidermal growth factor receptor and HER2 tyrosine kinase inhibitor, and pertuzumab, another anti-HER2 antibody, which has been shown to improve response rates in other cancers (e.g. HER2-expressing breast cancer), are being examined in this patient cohort.
Tumours can only grow through recruitment of a blood supply and angiogenesis; hence the tumour vasculature, which is thought to have greater genomic stability and lead to a more predictable response, is also a molecular target. Ramucirumab, a monoclonal antibody against the vascular endothelial growth factor receptor (VEGFR) 2, given both as a single agent and in conjunction with paclitaxel, was shown to increase overall survival in patients with gastric and GOJ adenocarcinomas. Because recent molecular studies suggest that OACs resemble chromosomally unstable gastric cancers, these therapies also seem justified for OACs.
Patients with advanced metastatic disease need good palliative care to manage symptoms such as pain and address any psychological concerns. Evidence from studies in patients with tumours at other sites suggests that early palliative care can be as effective as chemotherapy in improving quality of life.
Radiotherapy: definitive treatment of oesophageal tumours with chemo-radiotherapy alone is limited to more radio-sensitive squamous cell cancers. However, it can be an option for patients with OAC who are unsuitable for surgical treatment or decline such treatment. Chemo-radiation has been used in the neoadjuvant context for the management of oesophageal cancer, and the CROSS trial has demonstrated this to be superior to surgery alone. These results, however, were predominantly driven by squamous cell carcinoma and were only marginally significant for adenocarcinomas. An ongoing trial comparing neoadjuvant chemotherapy and neoadjuvant chemo-radiotherapy in oesophageal and GOJ adenocarcinomas (Neo-AEGIS trial) should provide more evidence on the role of neoadjuvant chemo-radiation in OAC treatment.
Chemo-radiotherapy entails the use of cisplatin and 5-FU, alongside external-beam radiotherapy to a dose of 45 Gy in 25 fractions over a period of 5 weeks. In the UK, this intensive treatment is currently reserved for relatively fit patients with a good performance status. The optimal concurrent chemotherapy is being examined in the current NeoSCOPE trial of neoadjuvant chemo-radiotherapy in the UK. External-beam radiotherapy can also be used in the palliation of symptoms including dysphagia, bleeding and pain from bone metastases, and in the treatment of brain metastases.
Key points.
Chronic gastro-oesophageal acid and bile reflux can lead to Barrett’s oesophagus, a well-described precursor of oesophageal adenocarcinoma
The incidence of oesophageal adenocarcinoma has been dramatically increasing in developed countries
Regular endoscopic monitoring of Barrett’s oesophagus is recommended to detect neoplastic changes at an early curative stage
Endoscopic diagnostic and therapeutic technologies to detect and treat early lesions are rapidly advancing
Surgery, increasingly combined with chemotherapy and radiotherapy, remains the main treatment modality for locally advanced oesophageal adenocarcinoma
Emerging molecular targeted therapies, such as HER2 and VEGR inhibitors, can provide additional treatment avenues for oesophageal adenocarcinoma, and a number of combination small molecules and immunotherapy treatments are under evaluation
Footnotes
Competing interests: none declared.
Competing interests: Rebecca Fitzgerald developed the Cytosponge-TFF3 test, which has been licensed by the Medical Research Council to Covidien (now Medtronic).
Contributor Information
Wladyslaw Januszewicz, MRC Cancer Unit Hutchinson/MRC Research Centre, University of Cambridge and an Endoscopy Honorary Clinical Fellow in the Gastroenterology Department, Cambridge University Hospitals NHSFT, UK.
Rebecca C Fitzgerald, MRC Cancer Unit, Hutchinson/MRC Research Centre, University of Cambridge, and Honorary Consultant in Gastroenterology, Cambridge University Hospitals NHSFT, UK.
Key References
- 1.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 2.Gharahkhani P, Fitzgerald RC, Vaughan TL, et al. Genome-wide association studies in oesophageal adenocarcinoma and Barrett’s oesophagus: a large-scale meta-analysis. Lancet Oncol. 2016;17:1363–73. doi: 10.1016/S1470-2045(16)30240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterol. 2018;154:2068–86. doi: 10.1053/j.gastro.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population-based cohort study. Am J Gastroenterol. 2014;109:1215–22. doi: 10.1038/ajg.2014.156. [DOI] [PubMed] [Google Scholar]
- 5.Coleman HG, Xie SH, Lagergren J. The epidemiology of esophageal adenocarcinoma. Gastroenterol. 2018;154:390–405. doi: 10.1053/j.gastro.2017.07.046. [DOI] [PubMed] [Google Scholar]
Further Reading
- Poa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. J Am Med Assoc. 2014;311:1209–17. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- Quante M, Graham TA, Jansen M. Insights into the pathophysiology of esophageal adenocarcinoma. Gastroenterol. 2018;154:406–20. doi: 10.1053/j.gastro.2017.09.046. [DOI] [PubMed] [Google Scholar]
- Ross-Innes CS, Debiram-Beecham I, O’Donovan M, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multi-center case-control study. PLoS Med. 2015;12:e1001780. doi: 10.1371/journal.pmed.1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secrier M, Li X, de Silva N, et al. Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS) Consortium Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat Genet. 2016;48:1131–41. doi: 10.1038/ng.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Prim. 2017;3 doi: 10.1038/nrdp.2017.48. 17048. [DOI] [PMC free article] [PubMed] [Google Scholar]